Abstract
Fetal cell microchimerism is defined as the persistence of pluripotent fetal cells in the maternal body long after delivery. The exact process by which fetal cells cross the placental barrier and enter maternal circulation is still being investigated. We reported that fetal cells persist only in the maternal bone marrow and may give rise to subpopulations with the ability to differentiate into the tissue-specific mature cells within injured maternal organs. Moreover, most of the fetal cells enter the maternal circulation during the early stages of pregnancy. These results indicate that the fetal cells with a multilineage potential, which were detected in a variety of maternal organs during pregnancy did not pass through the placental barrier; rather, they were derived from the fetal cells that entered maternal circulation early after implantation, and sustained their population long after delivery.
Key words: fetal cell microchimerism, stem cell, green fluorescent protein, implantation
Fetal cell microchimerism is defined as the persistence of fetal cells in the maternal blood and tissues long after delivery.1,2 Microchimeric fetal cells are thought to have the ability to colonize multiple tissues and organs.3–6 Little is known about the exact process by which fetal cells cross the placental barrier to enter maternal circulation. Previous studies have revealed that migration of fetal cells into maternal circulation starts during early gestation, and the frequency of fetal cell-associated DNA and cell free DNA in maternal blood increases with gestational age in humans.7 In murine pregnancy both cell-free DNA and fetal cells have similarly been identified.8,9
Fetal blood contains significant numbers of a variety of stem cell types during the first-trimester of pregnancy. However, the frequency of these fetal cells in fetal circulation declines with advancing gestational age.10 There is a possibility that most of the microchimeric fetal cells enter the maternal circulation before placenta formation. We tested this hypothesis by inducing injuries in specific maternal organs after performing hysterectomies in female mice at different times during pregnancy. Fetal cell residence was then estimated in various maternal organs. Wild female mice were crossbred with male transgenic mice, expressing enhanced green fluorescent protein (EGFP).11 Hysterectomies were performed on days 6, 13 and 19 of pregnancy. Fetal cells were then detected and quantified in a variety of maternal organs via fluorescent microscopy and quantitative polymerase chain reaction (PCR) amplification of the gfp transgene.
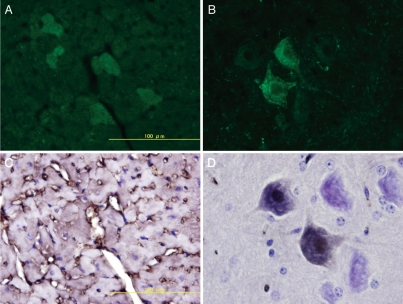
On post-hysterectomy day 60, fetal cells were detected only in the maternal bone marrow, regardless of when the hysterectomy was performed. Histological analysis revealed that most of the detected microchimeric fetal cells were morphologically mature hematopoietic cells of the neutrophil lineage, however, some immature hematopoietic cells (having a high nucleus-cytoplasm [N/C] ratio) also detected. We created models of injury by injecting streptozotocin and models of ischemic injury by ligating the left main coronary artery and the carotid artery to induce myocardial and cerebral infarction, respectively. Quantitative real-time PCR revealed that fetal DNA was present only in the injured organs and that their frequency was consistent in number, irrespective of the stage when hysterectomy was performed. In the models of ischemic injury, fetal DNA was detected specifically at ischemic sites. EGFP positive cells were detected in the injured organs by epifluorescence microscopy. Histological analysis with hematoxylin-eosin staining revealed that the morphological features of the fetal cells were similar to that of the following organ specific cells: pancreatic acinar cells, hepatocytes, tubular epithelial cells of the kidney, myocardial cells and neuronal cells. These fetal cells were mononuclear; cells with two or more separate nuclei were not detected. The myocardial and neuronal fetal cells expressed troponin T and neuronal nuclear antigen (NeuN) respectively (Fig. 1).
Figure 1.
Ischemic injury by ligating the left main coronary artery and the carotid artery to induce myocardial and cerebral infarction, respectively. Green fetal cells are observed only at the ischemic site of the maternal heart (A) and brain (B). Their morphological features were identical to that of the maternal myocardial cells [expressed troponin T, (C)] and neural cells [expressed neuronal nuclear antigen, (D)].
Our results are in partial agreement with those of previous studies that showed the presence of fetal cells in maternal tissue following injuries using rodent models.12–14 However, these studies did not examine the stage of pregnancy during which the fetal cells start migrating, because the injuries in maternal organs were induced only after term delivery. Our results show that fetal stem cells, enter maternal circulation during the early stages of pregnancy and the frequency of these cells was constant, regardless of the stage at which the hysterectomies were performed. Moreover, because the hysterectomies were performed with the fetus in situ, the origin of the microchimeric fetal cells is not feto-maternal hemorrhage following termination of pregnancy or delivery, but rather, migration of the fetal cells into maternal circulation before placenta formation. These results suggest that most of the fetal cells capable of multilineage differentiation may have entered the maternal circulation early after implantation.15
The biological significance of this physiological phenomenon is not yet known, however, many studies have demonstrated that fetal cells tend to concentrate in clinically affected tissues.16,17 Therefore, fetal cell microchimerism may mediate tissue repair, have a beneficial effect on postnatal lifespan, and protect against some diseases during pregnancy.
Footnotes
Previously published online: www.landesbioscience.com/journals/chimerism/article/14301
References
- 1.Liégeois A, Escourrou J, Ouvre E, Charreire J. Microchimerism: a stable state of low-ratio proliferation of allogeneic bone marrow. Transplant Proc. 1977;9:273–276. [PubMed] [Google Scholar]
- 2.Liégeois A, Gaillard MC, Ouvre E, Lewin D. Microchimerism in pregnant mice. Transplant Proc. 1981;13:1250–1252. [PubMed] [Google Scholar]
- 3.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- 5.O'Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnanacy. Lancet. 2004;364:179–182. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 6.Mikhail MA, M'Hamdi H, Welsh J, Levicar N, Marley SB, Nicholls JP, et al. High frequency of fetal cells within a primitive stem cell population in maternal blood. Hum Reporod. 2008;23:928–933. doi: 10.1093/humrep/dem417. [DOI] [PubMed] [Google Scholar]
- 7.Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, Lee TH. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal dignosis. Transfusion. 2001;41:1524–1530. doi: 10.1046/j.1537-2995.2001.41121524.x. [DOI] [PubMed] [Google Scholar]
- 8.Khosrotehrani K, Wataganara T, Bianchi DW, Johnson KL. Fetal cell-free DNA circulates in the plasma of pregnant mice: relevance for animal models of fetomaternal trafficking. Hum Reprod. 2004;19:2460–2464. doi: 10.1093/humrep/deh445. [DOI] [PubMed] [Google Scholar]
- 9.Bonney EA, Matzinger P. The maternal immune system's interaction with circulating fetal cells. J Immunol. 1997;158:40–47. [PubMed] [Google Scholar]
- 10.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 11.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Iwatani H, Ito T, Horimoto N, Yamato M, Matsui I, et al. Fetal cells in mother rats contribute to the remodeling of liver and kidney after injury. Biochem Biophys Res Commun. 2004;325:961–967. doi: 10.1016/j.bbrc.2004.10.105. [DOI] [PubMed] [Google Scholar]
- 13.Tan XY, Liao H, Sun L, Okabe M, Xiao ZC, Dawe GS. Fetal microchimerism in the maternal brain: A novel population of fetal progenitor or stem cells able to cross the blood-brain barrier? Stem Cells. 2005;23:1443–1452. doi: 10.1634/stemcells.2004-0169. [DOI] [PubMed] [Google Scholar]
- 14.Khosrotehrani K, Reyes RR, Johnson KL, Freeman RB, Salomon RN, Peter I, et al. Fetal cells participate over time in the response to specific types of murine maternal hepatic injury. Hum Reprod. 2007;22:654–661. doi: 10.1093/humrep/del426. [DOI] [PubMed] [Google Scholar]
- 15.Sunami R, Komuro M, Yuminamochi T, Hoshi K, Hirata S. Fetal cell microchimerism develops through the migration of fetus-derived cells to the maternal organs early after implantation. J Reprod Immunol. 2010;84:117–123. doi: 10.1016/j.jri.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi DW. Fetomaternal cell trafficking: a story that begins with prenatal diagnosis and may end with stem cell therapy. J Pediatr Surg. 2007;42:12–18. doi: 10.1016/j.jpedsurg.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 17.Lapaire O, Hosli I, Zanetti-Daellenbach R, Huang D, Jaggi C, Gatfield-Mergenthaler S, Hahn S, Holzgreve W. Impact of fetal-maternal chimerism on women's health-a review. J Matern Fetal Neonatal Med. 2007;20:1–5. doi: 10.1080/14767050601144834. [DOI] [PubMed] [Google Scholar]