Abstract
Non-coding regions of the human ngenome contain vast regulatory potential that contributes to the coordination of gene expression. Indeed, regulatory elements can reside large genomic distances from the promoters of genes they control. Here we describe approaches recently used to identify functional elements within the complex CFTR locus.
Key words: CFTR, DNase-chip, tissue-specific enhancers, chromosome conformation capture (3C), DNase I hypersensitivity
Transcriptional control of genes is mediated by the combined action of transcription factors and chromatin modifiers interacting with DNA and nucleosomes at discrete regions of the genome, often referred to as regulatory elements. Modern genomics tools have afforded molecular biologists the capability to discover putative regulatory elements on an unprecedented scale. These tools take advantage of complete genome sequences with technologies such as high-density tiled microarrays and next-generation high-throughput DNA sequencing. Chromatin immunoprecipitation (ChIP), in which specific antibodies recognizing transcription factors or histone modifications are used to determine enriched sites of the genome, is one such technique that has been scaled up with tiled arrays (ChIP-chip)1 and next-generation sequencing (ChIP-seq)2 in order to determine association of factors or histone characteristics genome-wide. Similar developments have been made with mapping DNase I hypersensitive sites (DHS), regions of the genome that are depleted of nucleosomes and thus amenable to factor binding. Techniques such as DNase-array,3 DNase-chip4 and DNase-seq5 have enabled the mapping of DHS across the human genome. Other technologies developed to identify functional units of the genome by formaldehyde-cross-linked chromatin analysis include FAIRE6 and Sono-Seq.7
While these technologies have allowed for significant annotation of the genome's functional elements, it is another challenge to determine the mechanism by which these sequences coordinate gene expression. Particularly, as the elements lie scattered throughout the non-coding regions of the genome, how can we determine which gene or genes a given sequence regulates? Given that genes can be organized in looped conformations to generate physical interactions between distal regulatory elements, methods have been developed that measure the in vivo conformations of the genome. Dekker and colleagues first described chromosome conformation capture (3C),8 which has proved a robust method for determining the interaction frequency between two distal genomic sites in a population of cells. Other approaches to capture de novo interacting regions with a selected element have also been developed.9
We and others have used several of the above techniques in order to define regulatory mechanisms of the cystic fibrosis transmembrane conductance regulator gene (CFTR). CFTR, which when mutated causes the common hereditary disease cystic fibrosis, is expressed in specialized epithelial cells of the airway, sweat glands, pancreas, intestine and male genital ducts among other sites.10 The CFTR locus occupies 189 kb of human chromosome 7 and is flanked roughly 50 kb upstream and downstream by genes (ASZ1, 5′; CTTNBP2, 3′) that display different spatial expression patterns than that of CFTR. While several critical elements activating CFTR are found within the promoter region,11–16 they do not confer the cell-type specific expression pattern of the gene. Thus, regulatory elements likely exist within the locus outside of the promoter, within intergenic and intronic regions.
Identification of Regulatory Elements of the CFTR Locus
Efforts to understand the transcriptional regulatory mechanisms of the CFTR locus have been ongoing since the discovery of the gene in 1989.17 Initial studies were appropriately concentrated on the promoter, which revealed several elements capable of binding factors such as C/EBP, Sp1, AP-1, NFκB, CREB, among others. However, the elements of the promoter failed to explain the restricted expression pattern of the gene and the promoter was classified as “housekeeping”-like.12 Spurred on by the success of discovering distal regulatory elements by DHS mapping in gene clusters such as the α and β-globin loci,18,19 our group set out to probe the CFTR locus for DHS outside of the promoter region, in the 5′ and 3′ flanking regions and within the introns of the gene, with the hypothesis that regulatory elements that conferred the gene's strict cell-type specific expression lie in one or more of these regions. These initial DHS mapping experiments relied on Southern blotting, in which specific restriction fragments are probed for DNase I hypersensitivity. This pursuit yielded numerous DHS that were deemed to contribute to the transcriptional regulation of the gene20–25 (Fig. 1). Three flanking DHS were shown to contain elements with insulator activity, two of which bound the insulator-binding protein CTCF.26,27 A DHS within the first intron of the gene detected in cells of colonic, pancreatic and genital duct origin was found to encompass an element that augmented CFTR promoter activity in a cell-type specific manner and bound the transcription factor HNF1α.21,28–30 Other DHS were also found to bind tissue-specific transcription factors in vitro or in vivo.31,32
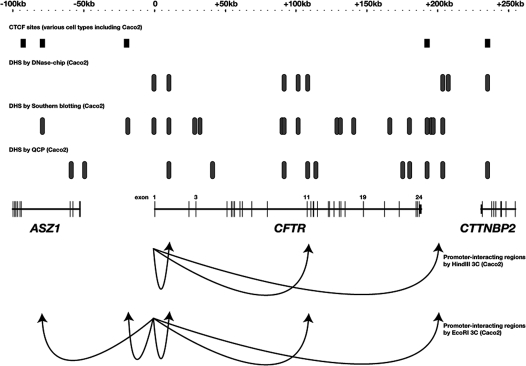
Figure 1.
A compiled map of regulatory elements of the CFTR locus. CTCF sites found in various cell types including the high-CFTR expressing colon carcinoma cell line Caco2 (reviewed in refs. 26, 27 and ENCODE Consortium data, http://genome.ucsc.edu/ENCODE); DHS measured by DNase-chip in Caco2 cells;33 DHS measured by Southern blotting in Caco2 cells20–25 and DHS measured by QCP in Caco2 cells (http://genome.ucsc.edu/ENCODE). CFTR promoter-interacting regions measured by 3C using promoter fragments as “bait” are indicated by arrows.33,35
While the traditional Southern blotting approach to mapping DHS of the CFTR locus yielded important insights into the organization of the transcriptionally active gene, the more recently developed genomics technologies allow for a more comprehensive analysis of its chromatin structure. Moreover, these approaches are likely to reveal important details of the transcription factors and associated proteins controlling the locus and the specific cell-type coordination of CFTR expression. We recently employed the DNase-chip technique to re-interrogate the CFTR locus. The purpose for this additional analysis was two-fold: first, we were curious to analyze the entire locus in a single experiment, which the tiled microarrays afforded us, so as to avoid any bias or oversights given by the restriction fragments analyzed previously; and second, DNase-chip allowed us to analyze chromatin from cells available in relatively limited supply, such as primary human airway epithelial cells. DNase-chip involves digesting chromatin with DNase I, followed by biotinylating the digested ends and capturing them with streptavidin beads.4 The captured material is amplified and hybridized to tiled microarrays. For our experiments, we utilized Nimblegen ENCODE arrays, which includes approximately 1% of the human genome with 50 bp tiles spaced by an average of 38 bp. We were able to detect many of the DHS that we had previously mapped in the CFTR locus, along with others that had not been previously identified33 (Fig. 1). The most significant of these, which was found in several CFTR-expressing cell types, lies within intron 11 of CFTR. Interestingly, a BamHI restriction site (the restriction enzyme used in the previous DHS mapping experiments of this region) lies within this newly mapped DHS, revealing an advantage of the DNase-chip technique. This intronic DHS contains a strong enhancer of CFTR promoter activity that associates with HNF1α and the histone acetyltransferase p300. The intron 11 DHS was also detected by others: by mapping DHS of the locus by quantitative chromatin profiling (QCP) with quantitative PCR,34 as part of the ENCODE Consortium, the Stamatoyannopoulos group measured DNase-hypersensitivity in the same location (http://genome.ucsc.edu/ENCODE, Fig. 1).
An important observation was that several DHS readily measured by the Southern blotting DHS mapping were not observed in our DNase-chip results, including those encompassing the insulators −20.9 kb upstream and +6.8 kb downstream of the CFTR coding region. This is most likely the result of masked-out repetitive regions on the arrays, two of which lie close to these insulator regions and may disrupt the array signal.
The discovery of a previously unknown transcriptional enhancer 100 kb downstream of the CFTR promoter prompted us to determine whether this element directly interacts with the CFTR promoter in vivo. Using 3C, we determined that several fragments of the locus loop towards the promoter, resulting in a complex three-dimensional configuration of the active locus.33 A similar study, performed by Dekker and colleagues, utilized several different restriction enzymes and a “scanning” approach that interrogated potential promoter interactions with the entire locus in an unbiased manner.35 These studies showed that the CFTR promoter directly interacts with elements located −80 kb, −20.9 kb, +10 kb, +109 kb and +203 kb from the promoter, specifically in cells in which CFTR is transcriptionally active (Fig. 2). Thus, independently, the DHS elements that had previously been identified by Southern blotting at −80 kb, −20.9 kb, +10 kb and +203 kb were given additional functional relevance.
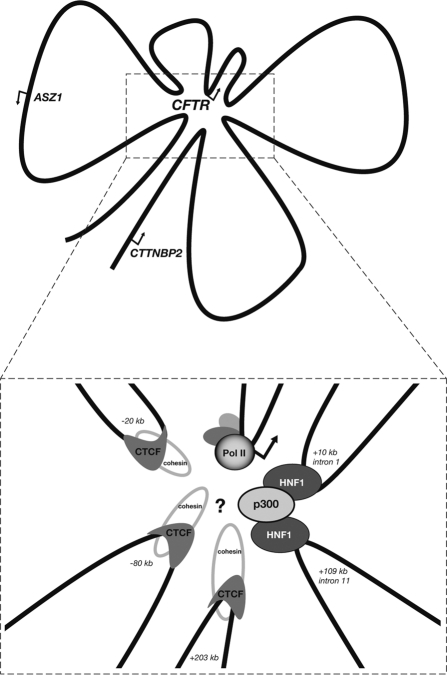
Figure 2.
The transcriptionally active chromatin hub of the CFTR locus. Included in the hub are each distal region shown to have relatively high interaction frequency with the promoter in 3C assays and known associated trans factors. The mechanism by which each of these elements and bound factors, including the insulator factors CTCF and cohesin, contribute to active gene transcription or maintain the hub conformation has yet to be determined.
It is important to note that not every 3C interaction was detected in every CFTR-expressing cell type or with every enzyme used. For example, Dekker and colleagues were able to readily detect an interaction between the promoter and the −20.9 kb insulator element with EcoRI digestion 3C, while we were unable to detect this interaction with HindIII digestion. Indeed, the EcoRI fragment around this region is larger than the HindIII fragment, which may explain this discrepancy. This illustrates the need for a comprehensive approach to 3C assays, utilizing several digestion enzymes. To reinforce their data, Dekker and colleagues performed complementary assays in which the interacting distal regions were used as “bait” (rather than the promoter region) in order to illustrate the direct interaction of all the elements in a single active chromatin hub. Recent similar experiments from our own group using primary male genital duct cells have shown high interaction frequencies between the −20.9 kb insulator and the +203 kb region (unpublished observations).
Future Analysis
These studies show how new genomics techniques can be used to comprehensively interrogate the chromatin structure and conformation of a large gene locus, with the purpose of inferring the transcriptional mechanisms that maintain expression of the gene. Other analyses, such as ChIP assays for specific enhancer-associated histone marks (H3K4me1)36 and the DHS-associated histone variants H3.3 and H2A.Z37 could also be used to identify novel regulatory elements. With respect to CFTR, additional questions remain: what are the chromatin modifiers and transcription factors that enable and maintain this three-dimensional “shape” of the active CFTR locus? The apparent interactions between the promoter and two known insulator regions are especially intriguing. These insulator regions are known to bind the classical insulator binding protein CTCF and the ring-like chromatin associated factor cohesin.33 May these factors somehow be playing a role in maintaining an active promoter? A recent study has shown that cohesin directly interacts with the Mediator complex at gene promoters in mouse embryonic stem cells, linking chromosome conformation and gene expression.38 It is intriguing to think that a similar mechanism may be working in human cells within the CFTR locus. Also, what are the factors at play in the newly characterized enhancer within intron 11? Newer techniques that can map nucleosome positions at a high-resolution may lead to more precise locations of important factor binding sites.39 Moreover, once these can be applied locus-wide or to the whole genome, they may reveal other regulatory elements within CFTR that are not associated with DHS but that play a critical role in the maintenance or silencing of gene expression. These might include specific DNA sequences that stabilize the interaction of the CFTR locus with structural networks of the nucleus. What about other elements that may act on the locus from an even farther distance, or in trans from another chromosome? Newly developed technologies that can interrogate the three-dimensional structure of a large genomic region in a high-throughput, unbiased manner (5C)40 or even the entire genome (Hi-C, ChIA-PET),41,42 may eventually be used to answer these questions.
By understanding the precise transcriptional mechanisms of important disease-causing genes such as CFTR, a reasonable hope is that these could represent avenues for gene-specific therapies. Small molecules and other potential pharmacological agents may be used to augment or repress transcriptional activity, by targeting chromatin modifiers or transcription factor complexes. Moreover, the cis elements responsible for endogenous control of a gene's expression could in theory be incorporated into novel vectors for use in gene therapy protocols.
Acknowledgements
We thank Drs. N.P. Blackledge and M.A. Lewandowska for their contributions. This work was funded in part by NIH R01 HL094585 and the Cystic Fibrosis Foundation (USA).
Abbreviations
- CFTR
- ChIP
- DHS
- FAIRE
- ASZ1
- CTTBBP2
- C/EBP
- Sp1
- AP-1
- NFκB
- CREB
- ENCODE
- QCP
- HNF1α
- Hi-C
- ChIA-PET
- CTCF
Footnotes
Previously published online: www.landesbioscience.com/journals/transcription/article/13693
References
- 1.Ren B, Robert F, Wyrick JJ, Aparicio O, Jennings EG, Simon I, et al. Genome-wide location and function of DNA binding proteins. Science. 2000;290:2306–2309. doi: 10.1126/science.290.5500.2306. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 3.Sabo PJ, Kuehn MS, Thurman R, Johnson BE, Johnson EM, Cao H, et al. Genome-scale mapping of DNase I sensitivity in vivo using tiling DNA microarrays. Nat Meth. 2006;3:511–518. doi: 10.1038/nmeth890. [DOI] [PubMed] [Google Scholar]
- 4.Crawford GE, Davis S, Scacheri PC, Renaud G, Halawi MJ, Erdos MR, et al. DNase-chip: a high-resolution method to identify DNase I hypersensitive sites using tiled microarrays. Nat Meth. 2006;3:503–509. doi: 10.1038/NMETH888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle AP, Davis S, Shulha HP, Meltzer P, Margulies EH, Weng Z, et al. High-resolution mapping and characterization of open chromatin across the genome. Cell. 2008;132:311–322. doi: 10.1016/j.cell.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giresi PG, Kim J, McDaniell RM, Iyer VR, Lieb JD. FAIRE (Formaldehyde-Assisted Isolation of Regulatory Elements) isolates active regulatory elements from human chromatin. Genome Res. 2007;17:877–885. doi: 10.1101/gr.5533506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auerbach RK, Euskirchen G, Rozowsky J, Lamarre-Vincent N, Moqtaderi Z, Lefrançois P, et al. Mapping accessible chromatin regions using Sono-Seq. Proc Nat Acad Sci USA. 2009;106:14926–14931. doi: 10.1073/pnas.0905443106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 9.Ohlsson R,, Göndör A. The 4C technique: the ‘Rosetta stone’ for genome biology in 3D? Curr Opin Cell Biol. 2007;19:321–325. doi: 10.1016/j.ceb.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy VA, Harris A. The CFTR gene and regulation of its expression. Pediatr Pulmonol. 2005;40:1–8. doi: 10.1002/ppul.20199. [DOI] [PubMed] [Google Scholar]
- 11.Chou JL, Rozmahel R, Tsui LC. Characterization of the promoter region of the cystic fibrosis conductance regulator gene. J Biol Chem. 1991;266:24471–24476. [PubMed] [Google Scholar]
- 12.Yoshimura K, Nakamura H, Trapnell BC, Chu CS, Dalemans W, Pavirani A, et al. The cystic fibrosis gene has a “housekeeping”-type promoter and is expressed at low levels in cells of epithelial origin. J Biol Chem. 1991;266:9140–9144. [PubMed] [Google Scholar]
- 13.Koh J, Sferra TJ, Collins FS. Characterization of the cystic fibrosis transmembrane conductance regulator promoter region; chromatin context and tissue-specificity. J Biol Chem. 1993;268:15912–15921. [PubMed] [Google Scholar]
- 14.McDonald RA, Matthews RP, Idzerda RL, McKnight GS. Basal expression of the cystic fibrosis transmembrane conductance regulator gene is dependent on protein kinase A activity. Proc Nat Acad Sci USA. 1995;92:7560–7564. doi: 10.1073/pnas.92.16.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittman N, Shue G, LeLeiko NS, Walsh MJ. Transcription of cystic fibrosis transmembrane conductance regulator requires CCAAT-like element for both basal adn cAMP-mediated regulation. J Biol Chem. 1995;270:28848–28857. doi: 10.1074/jbc.270.48.28848. [DOI] [PubMed] [Google Scholar]
- 16.Matthews RP, McKnight GS. Characterization of the cAMP response element of the cystic fibrosis transmembrane conductance regulator gene promoter. J Biol Chem. 1996;271:31869–31877. doi: 10.1074/jbc.271.50.31869. [DOI] [PubMed] [Google Scholar]
- 17.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 18.Higgs DR, Wood WG, Jarman AP, Sharpe J, Lida J, Pretorius IM, et al. A major positive regulatory region located far upstream of the human α-globin gene locus. Genes Dev. 1990;4:1588–1601. doi: 10.1101/gad.4.9.1588. [DOI] [PubMed] [Google Scholar]
- 19.Grosveld F, van Assendelft GB, Greaves DR, Kollias G. Position-independent, high-level expression of the human β-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 20.Smith AN, Wardle CJ, Harris A. Characterization of DNase I hypersensitive sites in the 120 kb 5′ to the CFTR gene. Biochem Biophys Res Commun. 1995;211:274–281. doi: 10.1006/bbrc.1995.1807. [DOI] [PubMed] [Google Scholar]
- 21.Smith AN, Barth ML, McDowell TL, Moulin DS, Nuthall HN, Hollingsworth MA, et al. A regulatory element in intron 1 of the cystic fibrosis transmembrane conductance regulator gene. J Biol Chem. 1996;271:9947–9954. doi: 10.1074/jbc.271.17.9947. [DOI] [PubMed] [Google Scholar]
- 22.Nuthall HN, Vassaux G, Huxley C, Harris A. Analysis of a DNase I hypersensitive site located −20.9 kb upstream of the CFTR gene. Eur J Biochem. 1999;266:431–443. doi: 10.1046/j.1432-1327.1999.00872.x. [DOI] [PubMed] [Google Scholar]
- 23.Nuthall HN, Moulin DS, Huxley C, Harris A. Analysis of DNase-I-hypersensitive sites at the 3′ end of the cystic fibrosis transmembrane conductance regulator gene (CFTR) Biochem J. 1999;341:601–611. [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DJ, Nuthall HN, Majetti ME, Harris A. Multiple potential intragenic regulatory elements in the CFTR gene. Genomics. 2000;64:90–96. doi: 10.1006/geno.1999.6086. [DOI] [PubMed] [Google Scholar]
- 25.Phylactides M, Rowntree R, Nuthall H, Ussery D, Wheeler A, Harris A. Evaluation of potential regulatory elements identified as DNase I hypersensitive sites in the CFTR gene. Eur J Biochem. 2002;269:553–559. doi: 10.1046/j.0014-2956.2001.02679.x. [DOI] [PubMed] [Google Scholar]
- 26.Blackledge NP, Carter EJ, Evans JR, Lawson V, Rowntree RK, Harris A. CTCF mediates insulator function at the CFTR locus. Biochem J. 2007;408:267–275. doi: 10.1042/BJ20070429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackledge NP, Ott CJ, Gillen AE, Harris A. An insulator element 3′ to the CFTR gene binds CTCF and reveals an active chromatin hub in primary cells. Nuc Acids Res. 2009;37:1086–1094. doi: 10.1093/nar/gkn1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mogayzel PJ, Ashlock MA. CFTR intron 1 increases luciferase expression driven by CFTR 5′-flanking DNA in a yeast artificial chromosome. Genomics. 2000;64:211–215. doi: 10.1006/geno.2000.6119. [DOI] [PubMed] [Google Scholar]
- 29.Rowntree RK, Vassaux G, McDowell TL, Howe S, McGuigan A, Phylactides M, et al. An element in intron 1 of the CFTR gene augments intestinal expression in vivo. Hum Mol Genet. 2001;10:1455–1464. doi: 10.1093/hmg/10.14.1455. [DOI] [PubMed] [Google Scholar]
- 30.Ott CJ, Suszko M, Blackledge NP, Wright JE, Crawford GE, Harris A. A complex intronic enhancer regulates expression of the CFTR gene by direct interaction with the promoter. J Cell Mol Med. 2009;13:680–692. doi: 10.1111/j.1582-4934.2008.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouchel N, Henstra SA, McCarthy VA, Williams SH, Phylactides M, Harris A. HNF1α is involved in tissue-specific regulation of CFTR gene expression. Biochem J. 2004;378:909–918. doi: 10.1042/BJ20031157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarthy VA, Ott CJ, Phylactides M, Harris A. Interaction of intestinal and pancreatic transcription factors in the regulation of CFTR gene expression. Biochim Biophys Acta. 2009;1789:709–718. doi: 10.1016/j.bbagrm.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ott CJ, Blackledge NP, Kerschner JL, Leir SH, Crawford GE, Cotton CU, et al. Intronic enhancers coordinate epithelial-specific looping of the active CFTR locus. Proc Nat Acad Sci USA. 2009;106:19934–19939. doi: 10.1073/pnas.0900946106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorschner MO, Hawrylycz M, Humbert R, Wallace JC, Shafer A, Kawamoto J, et al. High-throughput localization of functional elements by quantitative chromatin profiling. Nat Meth. 2005;1:219–225. doi: 10.1038/nmeth721. [DOI] [PubMed] [Google Scholar]
- 35.Gheldof N, Smith EM, Tabuchi TM, Koch CM, Dunham I, Stamatoyannopoulos JA, et al. Cell-type-specific long-range looping interactions identify distance regulatory elements of the CFTR gene. Nuc Acids Res. 2010;38:4325–4336. doi: 10.1093/nar/gkq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 37.Jin C, Zang C, Wei G, Cui K, Peng W, Zhao K, Felsenfeld G. H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat Genet. 2009;41:941–945. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schones DE, Cui K, Cuddapah S, Roh TY, Barski A, Wang Z, et al. Dynamic regulation of nucleosome positioning in the human genome. Cell. 2008;132:887–898. doi: 10.1016/j.cell.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dostie J, Richmond TA, Arnaout RA, Selzer RR, Lee WL, Honan TA, et al. Chromosome Conformation Capture Carbon Copy (5C): a massively parallel solution for mapping interactions between genomic elements. Genome Res. 2006;16:1299–1309. doi: 10.1101/gr.5571506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, et al. An oestrogen-receptor-α-bound human chromatin interactome. Nature. 2009;462:58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]