Abstract
Release of marrow cells may be determined primarily by the restrictive barrier that separates marrow hematopoietic cords from sinusoids, and by the ability of the cell to negotiate the barrier pores which are of smaller diameter than the cell. This critical interrelationship may be further modulated by humoral agents (releasing factors).
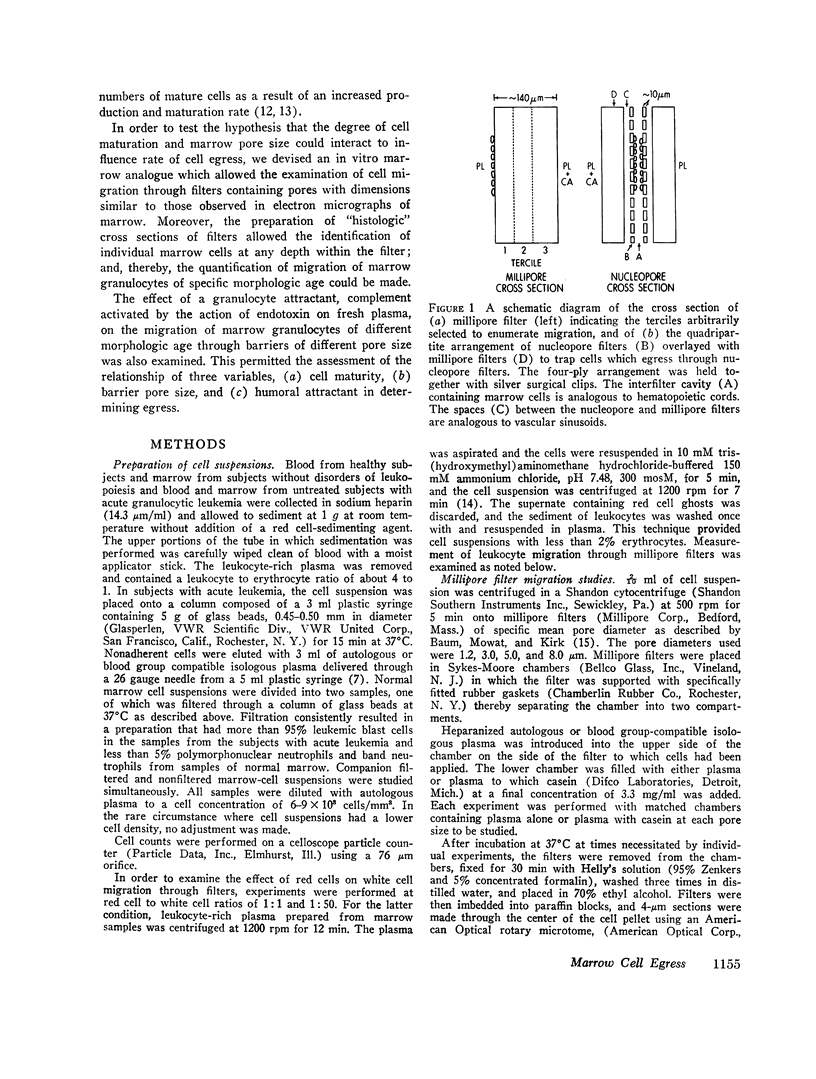
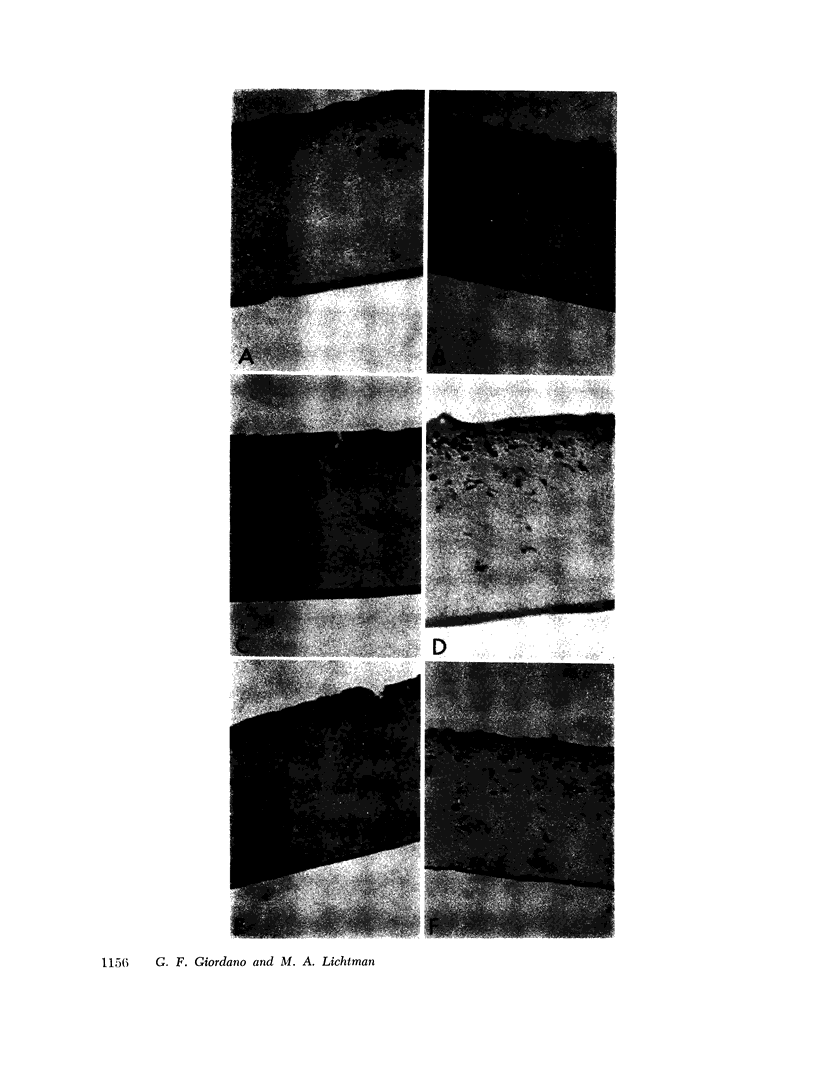
To test this hypothesis, we placed human marrow cells in a chamber between millipore or nucleopore filters with pore diameters of 1-8 μm. Fixed, stained, cross sections of the filters allowed histological examination of the penetration of cells and quantification of egress of age-specific cell types. The rate of marrow granulocyte egress was highly correlated with (a) barrier pore diameter. (b) morphological age of cells, and (c) the presence of chemical attractant. Immature granulocytes would not exit through a restrictive barrier even after protracted periods and were not responsive to chemoattractants. Intermediate-aged granulocytes showed a slight ability to respond to attractants and to exit if pore diameters were large. Mature granulocytes exited through the restrictive barrier at all pore diameters studied, however, this egress was accelerated by increasing pore diameter and by the presence of an attractant. Leukemia blast cells were incapable of traversing pore diameters of 1-8 μm.
These studies support the hypothesis that the development of deformability, motility, and surface receptors for chemoattractants at the later stages of granulocyte development allow the egress of cells through the marrow sinusoid wall which appears by electron microscopy to be a porous barrier with aperture diameters smaller than cell diameters; and that this process can be modulated by humoral agents which enhance directed movement of cells and may also increase pore size. Moreover, on the basis of our observations, the egress of leukemia cells is best explained by destruction of the normal sinusoid barrier of marrow indicating that manifestations of the disease are dependent on alteration in stromal as well as parenchymal marrow cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum J., Mowat A. G., Kirk J. A. A simplified method for the measurement of chemotaxis of polymorphonuclear leukocytes from human blood. J Lab Clin Med. 1971 Mar;77(3):501–509. [PubMed] [Google Scholar]
- Calvo W., Forteza-Vila J. Schwann cells of the bone marrow. Blood. 1970 Aug;36(2):180–188. [PubMed] [Google Scholar]
- De Bruyn P. P., Michelson S., Thomas T. B. The migration of blood cells of the bone marrow through the sinusoidal wall. J Morphol. 1971 Apr;133(4):417–437. doi: 10.1002/jmor.1051330406. [DOI] [PubMed] [Google Scholar]
- FISHER J. W., LAJTHA L. G., BUTTOO A. S., PORTEOUS D. D. DIRECT EFFECTS OF ERYTHROPOIETIN ON THE BONE MARROW OF THE ISOLATED PERFUSED HIND LIMBS OF RABBITS. Br J Haematol. 1965 May;11:342–349. doi: 10.1111/j.1365-2141.1965.tb06594.x. [DOI] [PubMed] [Google Scholar]
- Holland J. F., Senn H., Banerjee T. Quantitative studies of localized leukocyte mobilization in acute leukemia. Blood. 1971 May;37(5):499–511. [PubMed] [Google Scholar]
- Kass L., De Bruny P. P. Chemotaxis of mature and immature blood cells in tissue cultures. Anat Rec. 1967 Sep;159(1):115–125. doi: 10.1002/ar.1091590115. [DOI] [PubMed] [Google Scholar]
- Katz R., Gordon A. S., Lapin D. M. Mechanisms of leukocyte production and release. VI. Studies on the purification of the leukocytosis-inducing factor (LIF). J Reticuloendothel Soc. 1966 Jun;3(2):103–116. [PubMed] [Google Scholar]
- King-Smith E. A., Morley A. Computer simulation of granulopoiesis: normal and impaired granulopoiesis. Blood. 1970 Aug;36(2):254–262. [PubMed] [Google Scholar]
- Lapin D. M., LoBue J., Gordon A. S., Zanjani E. D., Schultz E. F. Mechanisms of leukocyte production and release. IX. Kinetics of leukocyte release in leukocytapheresed rats. Proc Soc Exp Biol Med. 1969 Jul;131(3):756–758. doi: 10.3181/00379727-131-33970. [DOI] [PubMed] [Google Scholar]
- Leblond P. F., LaCelle P. L., Weed R. I. Cellular deformability: a possible determinant of the normal release of maturing erythrocytes from the bone marrow. Blood. 1971 Jan;37(1):40–46. [PubMed] [Google Scholar]
- Lichtman M. A. Cellular deformability during maturation of the myeloblast. Possible role in marrow egress. N Engl J Med. 1970 Oct 29;283(18):943–948. doi: 10.1056/NEJM197010292831801. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A. Rheology of leukocytes, leukocyte suspensions, and blood in leukemia. Possible relationship to clinical manifestations. J Clin Invest. 1973 Feb;52(2):350–358. doi: 10.1172/JCI107191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Alteration of the cell periphery during granulocyte maturation: relationship to cell function. Blood. 1972 Mar;39(3):301–316. [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Peripheral cytoplasmic characteristics of leukocytes in monocytic leukemia: relationship to clinical manifestations. Blood. 1972 Jul;40(1):52–61. [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. The monovalent cation content and adenosine triphosphatase activity of human normal and leukemic granulocytes and lymphocytes: relationship to cell volume and morphologic age. Blood. 1969 Nov;34(5):645–660. [PubMed] [Google Scholar]
- Marsh J. C., Levitt M. Neutrophilia-inducing activity in plasma of neutropenic human beings. Blood. 1971 Jun;37(6):647–656. [PubMed] [Google Scholar]
- McCuskey R. S. Dynamic microscopic anatomy of the fetal liver. 3. Erythro poiesis. Anat Rec. 1968 Jul;161(3):267–279. doi: 10.1002/ar.1091610301. [DOI] [PubMed] [Google Scholar]
- McCuskey R. S. Sphincters in the microvascular system. Microvasc Res. 1971 Oct;3(4):428–433. doi: 10.1016/0026-2862(71)90045-8. [DOI] [PubMed] [Google Scholar]
- REIFF R. H., NUTTER J. Y., DONOHUE D. M., FINCH C. A. The relative number of marrow reticulocytes. Am J Clin Pathol. 1958 Sep;30(3):199–203. doi: 10.1093/ajcp/30.3.199. [DOI] [PubMed] [Google Scholar]
- Rytömaa T., Kiviniemi K. Regression of generalized leukaemia in rat induced by the granulocytic chalone. Eur J Cancer. 1970 Oct;6(5):401–410. doi: 10.1016/0014-2964(70)90038-1. [DOI] [PubMed] [Google Scholar]
- Stohlman F., Jr Some aspects of erythrokinetics. Semin Hematol. 1967 Oct;4(4):304–314. [PubMed] [Google Scholar]
- Trubowitz S., Masek B. The structural organization of the human marrow matrix in thin sections. Am J Clin Pathol. 1970 Jun;53(6):908–913. doi: 10.1093/ajcp/53.6.908. [DOI] [PubMed] [Google Scholar]
- WEISS L. An electron microscopic study of the vascular sinuses of the bone marrow of the rabbit. Bull Johns Hopkins Hosp. 1961 Mar;108:171–199. [PubMed] [Google Scholar]
- Weiss L. The structure of bone marrow. Functional interrelationships of vascular and hematopoietic compartments in experimental hemolytic anemia: an electron microscopic study. J Morphol. 1965 Nov;117(3):467–537. doi: 10.1002/jmor.1051170308. [DOI] [PubMed] [Google Scholar]
- Weiss L. Transmural cellular passage in vascular sinuses of rat bone marrow. Blood. 1970 Aug;36(2):189–208. [PubMed] [Google Scholar]