Abstract
It has been demonstrated that gastrointestinal extracts contain substances which react immunologically with antibodies prepared to pancreatic glucagon. These extracts have been termed intestinal GLI for glucagon-like immunoreactivity, or enteroglucagon. To determine whether GLI has specific biological effects, studies were designed using the criterion of effect with antiglucagon antibodies. These antibodies did not cross-react with either secretin or pancreozymin.
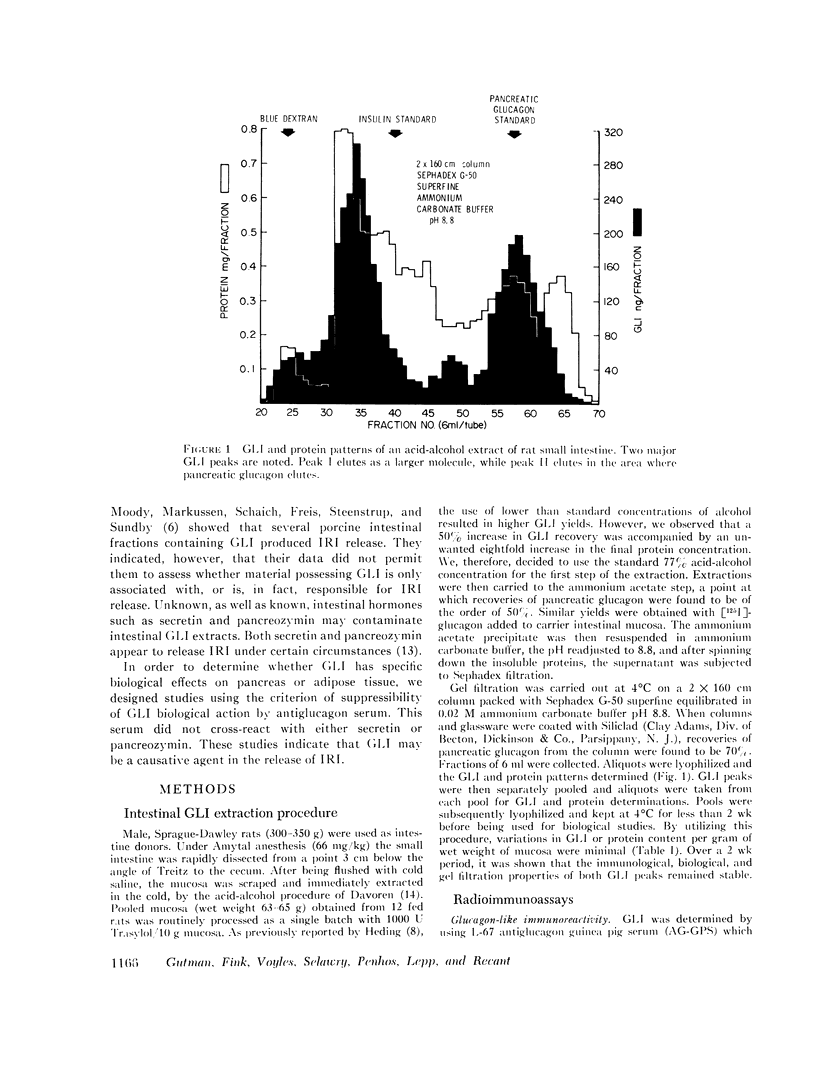
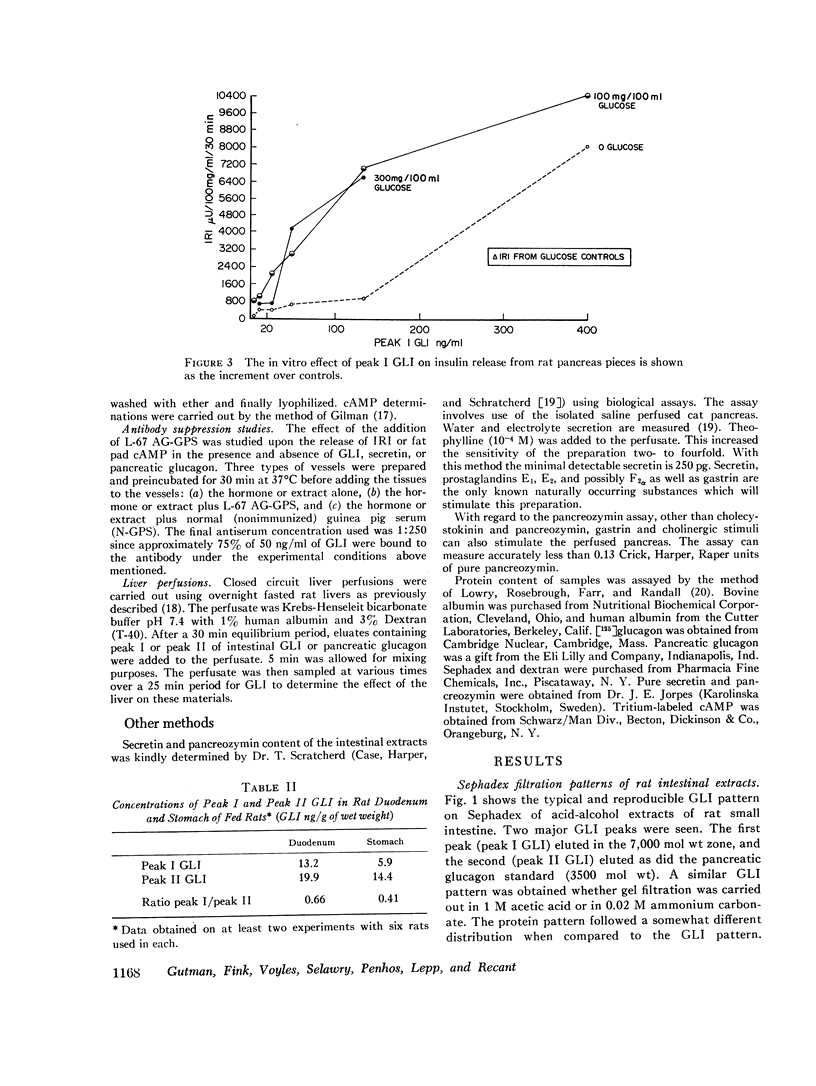
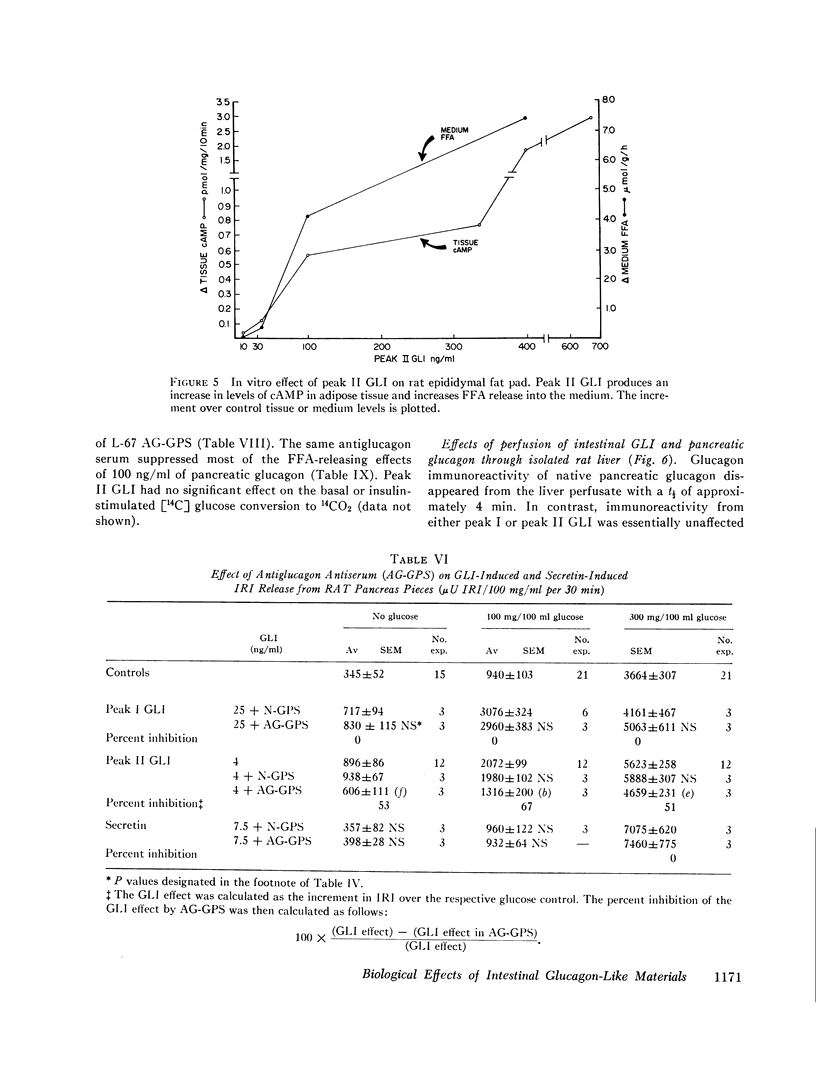
Rat intestinal extracts were prepared and filtered on Sephadex G-50 columns eluted in 0.02 M ammonium carbonate buffer pH 8.8. Two peaks of GLI (I, II) were consistently found, and the in vitro effects of these peaks on two biological systems were tested: (a) immunoreactive insulin (IRI) release by rat pancreas pieces, and (b) free fatty acid (FFA) release and 3′,5′-cyclic adenosine monophosphate (cAMP) levels in adipose tissue. Both GLI peaks increased IRI release in the absence of glucose and also enhanced the glucose effects. Antiglucagon antibody suppressed only peak II GLI activity. Both peaks increased FFA release and cAMP levels in adipose tissue. Only peak II GLI activity was suppressed by antibody.
These findings support a specific IRI-releasing and lipolytic action for Peak II GLI. Hypotheses are presented concerning the structure and possible physiologic role of peak II GLI.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assan R., Slusher N. Structure-function and structure-immunoreactivity relationships of the glucagon molecule and related synthetic peptides. Diabetes. 1972 Aug;21(8):843–855. doi: 10.2337/diab.21.8.843. [DOI] [PubMed] [Google Scholar]
- Buchanan K. D., Vance J. E., Williams R. H. Insulin and glucagon release from isolated islets of Langerhans. Effect of enteric factors. Diabetes. 1969 Jun;18(6):381–386. doi: 10.2337/diab.18.6.381. [DOI] [PubMed] [Google Scholar]
- Case R. M., Harper A. A., Scratcherd T. Water and electrolyte secretion by the perfused pancreas of the cat. J Physiol. 1968 May;196(1):133–149. doi: 10.1113/jphysiol.1968.sp008499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVOREN P. R. The isolation of insulin from a single cat pancreas. Biochim Biophys Acta. 1962 Sep 10;63:150–153. doi: 10.1016/0006-3002(62)90347-5. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of pancreatic and gut glucagon in plasma. Diabetologia. 1971 Feb;7(1):10–19. doi: 10.1007/BF02346248. [DOI] [PubMed] [Google Scholar]
- Imura H., Sparks L. L., Grodsky G. M., Forsham P. H. Immunologic studies of adrenocorticotropic hormone (ACTH): dissociation of biologic and immunologic activities. J Clin Endocrinol Metab. 1965 Oct;25(10):1361–1369. doi: 10.1210/jcem-25-10-1361. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarus N. R., Voyles N. R., Devrim S., Tanese T., Recant L. Extra-gastrointestinal effects of secretin, gastrin, and pancreozymin. Lancet. 1968 Aug 3;2(7562):248–250. doi: 10.1016/s0140-6736(68)92353-2. [DOI] [PubMed] [Google Scholar]
- Lefèbvre P. J., Unger R. H., Valverde I., Rigopoulou D., Luyckx A. S., Eisentraut A. Effect of dog jejunum "glucagon-like immunoreactive material" on adipose tissue metabolism. Horm Metab Res. 1969 May;1(3):143–144. doi: 10.1055/s-0028-1096834. [DOI] [PubMed] [Google Scholar]
- MAKMAN M. H., SUTHERLAND E. W., Jr USE OF LIVER ADENYL CYCLASE FOR ASSAY OF GLUCAGON IN HUMAN GASTRO-INTESTINAL TRACT AND PANCREAS. Endocrinology. 1964 Jul;75:127–134. doi: 10.1210/endo-75-1-127. [DOI] [PubMed] [Google Scholar]
- MCINTYRE N., HOLDSWORTH C. D., TURNER D. S. NEW INTERPRETATION OF ORAL GLUCOSE TOLERANCE. Lancet. 1964 Jul 4;2(7349):20–21. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- Marco J., Faloona G. R., Unger R. H. Effect of endogenous intestinal glucagon-like immunoreactivity (GLI) on insulin secretion and glucose concentration in dogs. J Clin Endocrinol Metab. 1971 Aug;33(2):318–325. doi: 10.1210/jcem-33-2-318. [DOI] [PubMed] [Google Scholar]
- Moody A. J., Markussen J., Fries A. S., Steenstrup C., Sundby F. The insulin releasing activites of extracts of pork intestine. Diabetologia. 1970 Apr;6(2):135–140. doi: 10.1007/BF00421441. [DOI] [PubMed] [Google Scholar]
- Penhos J. C., Basabe J. C., Lopez N., Levine R. Effect of diabetes or hyperglycemia on lipid metabolism and urea formation by the perfused rat liver. Horm Metab Res. 1971 Jan;3(1):10–20. doi: 10.1055/s-0028-1095040. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr, Graber A. L., Kuzuya T., Williams R. H. The effect of epinephrine on immunoreactive insulin levels in man. J Clin Invest. 1966 Feb;45(2):228–236. doi: 10.1172/JCI105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis S., Faulhaber J. D., Schröder K. E. The effect of intestinal hormones upon lipolysis of isolated human fat cells. Horm Metab Res. 1969 Jul;1(4):249–250. doi: 10.1055/s-0028-1096843. [DOI] [PubMed] [Google Scholar]
- Samols E., Tyler J., Megyesi C., Marks V. Immunochemical glucagon in human pancreas, gut, and plasma. Lancet. 1966 Oct 1;2(7466):727–729. doi: 10.1016/s0140-6736(66)92982-5. [DOI] [PubMed] [Google Scholar]
- Turner D. S. Intestinal hormones and insulin release: in vitro studies using rabbit pancreas. Horm Metab Res. 1969 Jul;1(4):168–174. doi: 10.1055/s-0028-1095149. [DOI] [PubMed] [Google Scholar]
- Turner D. S., Marks V. Enhancement of glucose-stimulated insulin release by an intestinal polypeptide in rats. Lancet. 1972 May 20;1(7760):1095–1097. doi: 10.1016/s0140-6736(72)91431-6. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Dupré J., Eisentraut A. M. The effects of secretin, pancreozymin, and gastrin on insulin and glucagon secretion in anesthetized dogs. J Clin Invest. 1967 Apr;46(4):630–645. doi: 10.1172/JCI105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ohneda A., Valverde I., Eisentraut A. M., Exton J. Characterization of the responses of circulating glucagon-like immunoreactivity to intraduodenal and intravenous administration of glucose. J Clin Invest. 1968 Jan;47(1):48–65. doi: 10.1172/JCI105714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde I., Rigopoulou D., Marco J., Faloona G. R., Unger R. H. Characterization of glucagon-like immunoreactivity (GLI). Diabetes. 1970 Sep;19(9):614–623. doi: 10.2337/diab.19.9.614. [DOI] [PubMed] [Google Scholar]
- Valverde I., Rigopoulou D., Marco J., Faloona G. R., Unger R. H. Molecular size of extractable glucagon and glucagon-like immunoreactivity (GLI) in plasma. Diabetes. 1970 Sep;19(9):624–629. doi: 10.2337/diab.19.9.624. [DOI] [PubMed] [Google Scholar]



