Abstract
Disease or malformation of heart valves is one of the leading causes of morbidity and mortality in both children and adults. These congenital anomalies can remain undetected until cardiac function is compromised, making it important to understand the underlying nature of these disorders. Here we show that ephrin-A1, a ligand for class A Eph receptor tyrosine kinases, regulates cardiac valve formation. Exogenous ephrin-A1-Fc or overexpression of ephrin-A1 in the heart inhibits epithelial-to-mesenchymal transformation (EMT) in chick atrioventricular cushion explants. In contrast, overexpression of wild-type EphA3 receptor promotes EMT via a kinase-dependent mechanism. To analyze ephrin-A1 in vivo, we generated an ephrin-A1 knockout mouse through gene targeting. Ephrin-A1 null animals are viable but exhibit impaired cardiac function. Loss of ephrin-A1 results in thickened aortic and mitral valves in newborn and adult ephrin-A1-deficient mice. Analysis of early embryonic hearts revealed increased cellularity in outflow tract endocardial cushions and elevated mesenchymal marker expression, suggesting that excessive numbers of cells undergo EMT. Taken together, these data indicate that ephrin-A1 regulates cardiac valve development, making ephrin-A1-deficient mice a novel model for congenital heart defects.
Keywords: ephrin, Eph receptor, heart valve development
Introduction
Congenital heart valve malformation is a leading cause of birth defects and childhood mortality (15, 20). Valve injury also occurs in adults due to infection, drug side effects, or tumorigenesis (8, 22, 24, 32). Dissection of molecular mechanisms regulating heart valve morphogenesis provides the basis for regenerating valves or repairing valvular injury from progenitor stem cells [reviewed in (35)].
The primordia of valvuloseptal tissue in the heart are the endocardial cushions. The cushions are localized swellings of the inner heart wall that arise due to production of extracellular matrix by myocardial cells. In response to signals from myocardium, a subpopulation of endocardial endothelial cells delaminates, transdifferentiates into mesenchymal cells, and invades the cardiac jelly. This critical step is termed epithelial-to-mesenchymal transformation (EMT). After completion of EMT, endocardial cushions undergo further remodeling to form thin elongated valve leaflets for maintaining unidirectional blood flow.
Multiple soluble growth factors have been identified that regulate endocardial cushion transformation, including TGF-β, EGF-related growth factors, and VEGF [reviewed in (1, 29)]. Studies using neutralizing antibodies and antisense RNA demonstrated that TGF-β signaling is required for EMT in chick (30, 31). Although EMT initiates in knockout models of TGF-β [reviewed in (1, 29)], many of these mice do not form normal endocardial cushions (2, 18). EGF-related growth factors and their receptors also regulate heart valve morphogenesis. Loss of endocardial HB-EGF or EGFR causes abnormal hyperplasia of cushion mesenchyme (7, 17). However, ablation of endocardial neuregulin-1 or its receptor within cushion mesenchyme, ErbB3, results in severe cushion hypoplasia (11, 21). VEGF plays a dual role in cushion transformation: low levels of VEGF are required for EMT initiation, whereas high levels of VEGF at later stages inhibit EMT.
More recently, membrane-bound molecules that mediate cell-cell communication, such as Notch and Eph molecules, have also been implicated in modulation of cardiac valve morphogenesis. Embryos that have impaired Notch signaling exhibit reduced endocardial EMT, whereas ectopic expression of activated Notch1 results in hypercellular cardiac valves (37). Lethal defects in heart valve formation are also observed in mice lacking ephrin-B2 ligand and EphA3 receptor. Cytoplasmic truncation of ephrin-B2 leads to thickened cardiac valves (9), but mechanisms by which ephrin-B2 regulates valve formation remain to be elucidated. Ablation of EphA3 receptor, however, results in hypoplastic valves due to reduced endocardial cushion transformation (36).
Ephrins are ligands for Eph receptor tyrosine kinases. Originally identified as axonal guidance cues, it was subsequently recognized that these molecules also regulate other biological processes, including cardiovascular development and angiogenesis (28). Although the Eph family contains multiple ligands and receptors, the only A class ligand and receptor pair expressed during endocardial cushion development is ephrin-A1 in the endocardium and the EphA3 receptor in the adjacent cushion mensenchyme (36). To understand how ephrin-A1 functions in cardiac valve development, we examined the role of ephrin-A1 in both chick explants and ephrin-A1-null mice. We found that ephrin-A1 inhibited EMT in the chick and loss of ephrin-A1 resulted in thickened aortic and mitral valves in mice. These results indicate a critical role of ephrin-A1 in regulation of cardiac valve morphogenesis.
Results
Ephrin-A1 inhibits EMT in chick AV cushion explants
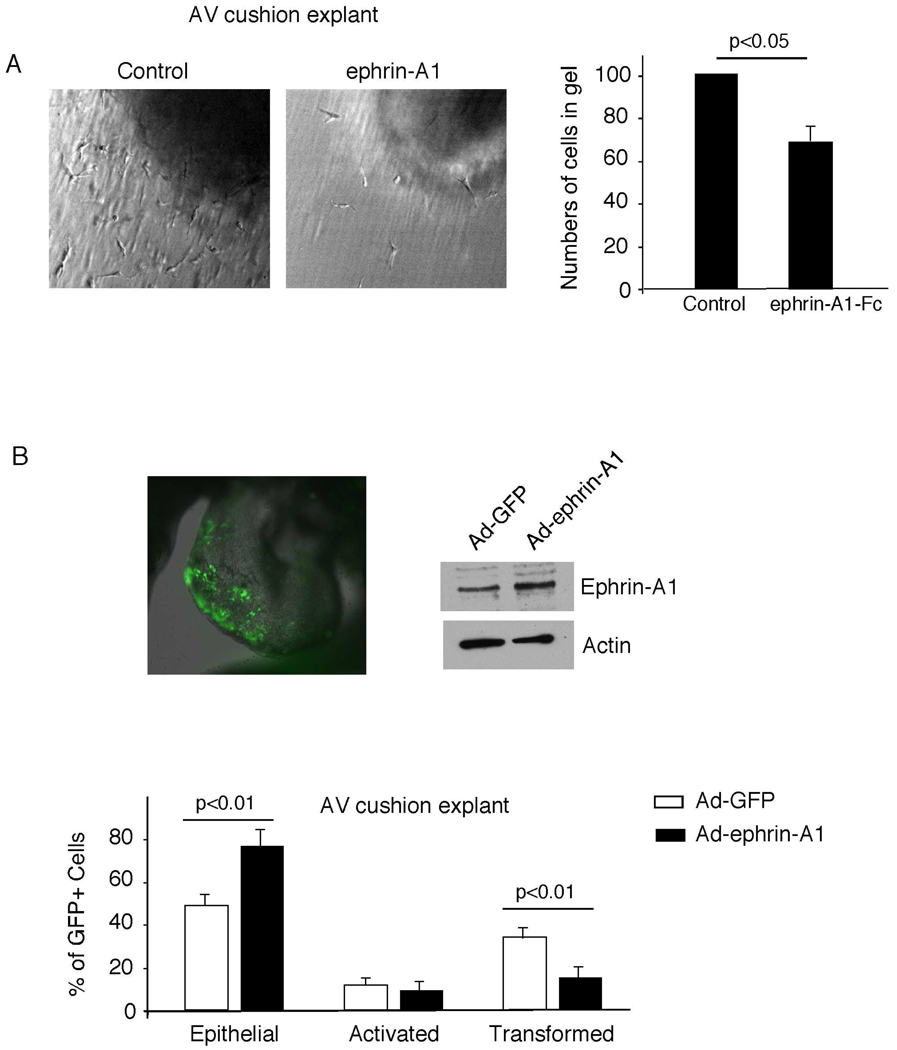
To determine the role of ephrin-A1 in endocardial cushion EMT, we first tested the effect of exogenous ephrin-A1 in an in vitro assay of early valve development. AV cushions from chick embryos were harvested and placed on a collagen gel containing either ephrin-A1-Fc or vehicle control. After 48 hours, in explants placed on top of control collagen gels, the endocardial cells formed a monolayer and, under the influence of the myocardium, underwent a specific activation, invasion of the matrix, and subsequent migration. In contrast, explants incubated on gels containing ephrin-A1-Fc demonstrated fewer migrating mesenchymal cells within the collagen matrix and an abundance of endocardial cells on the gel surface (Figure 1A). Quantification of this data revealed a 29% decrease in the number of transformed cells. These results suggest that exogenous ephrin-A1 reduces EMT in chick AV cushion explants.
Figure 1. Effects of Ephrin-A1 on EMT in chick cushion explants.
(A) Ephrin-A1-Fc inhibits EMT in chick cushion explants. AV cushion explants were cultured on collagen gel in the presence of 5 µg/ml ephrin-A1 or vehicle control. AV cushion explants were scored for the total number of transformed cells by optically sectioning the collagen gels using Hoffman Optics. The number of explants in each group, total numbers of transformed cells per condition, and the mean number of transformed cells ± SEM, expressed as a percentage of control, were as follows: ephrin-A1 (n=26; 1832; 71 ± 8%), control (n=24; 2370; 100 ± 5%). (B) Ephrin-A1 inhibits endocardial cushion transformation. Adenoviruses expressing ephrin-A1 and GFP, or control GFP only, were injected into the lumen of the chick heart tube at stage 10–12 (top left panel). Embryonic hearts were harvested 24 hours later and ephrin-A1 expression confirmed by western blot analysis (top right panel). AV cushion explants were cultured on collagen gels for 48 hours. GFP expressing cells were scored as epithelial, activated, and transformed. Results for each category, derived from 3 independent experiments, are presented as a percentage of total GFP positive cells ± SEM (bottom panel). Unpaired two-tailed student's t test was used to analyze data. For total explant numbers and total numbers of GFP cells per category, see Table S1.
As ephrin-A1 is expressed endogenously in the endothelial lining of the endocardium, but not cushion mesenchyme, we asked whether overexpression of ephrin-A1 in endocardial cells could inhibit EMT. Adenoviruses expressing both ephrin-A1 and a GFP marker controlled by separate promoters were injected into developing chick heart tubes and GFP positive cells were scored for EMT in a cell autonomous fashion in an AV cushion collagen gel assay. We injected viruses into the lumen of HH stage 10–12 chick hearts in order to infect endocardial cells prior to the initiation of EMT (10). Twenty-four hours after infection, AV cushions were harvested. AV cushion explants infected either with Ad-ephrin-A1 or control Ad-GFP viruses were incubated for 48 hours on top of collagen gel. Overexpression of ephrin-A1 resulted in fewer GFP positive mesenchymal cells within the collagen matrix. In order to quantify this phenotype, GFP-expressing cells were scored morphologically as epithelial (rounded cells that are tightly packed on the surface), activated (elongated cells with loss of cell-cell contact), or transformed (elongated cells that invade the matrix). By measuring the proportions of GFP expressing cells in each category as a percentage of total GFP expressing cells (Supplemental Table 1), we confirmed that ephrin-A1 overexpression led to a significant decrease in transformed cells with a concomitant increase in epithelial cells (Figure 1B). Taken together, these results suggest that ephrin-A1 inhibits the ability of endocardial cells to undergo EMT.
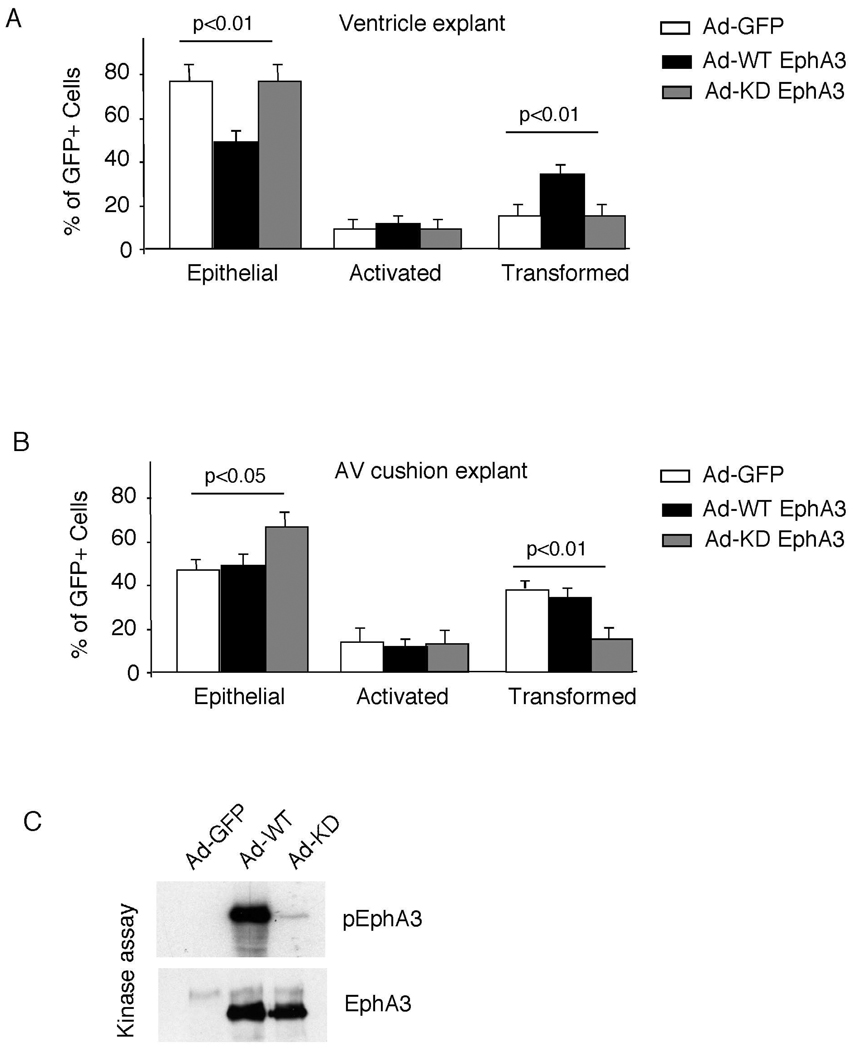
The ephrin-A1 receptor, EphA3, is expressed in endocardial cushion mesenchyme and mice lacking the EphA3 receptor have hypoplastic valves and reduced EMT (36). Accordingly, we asked whether, in contrast to ephrin-A1, EphA3 could promote a gain-of-function in the ability to undergo EMT in the endocardium. Adenoviruses overexpressing either wild-type EphA3 or control GFP were injected into the heart tube, and AV cushions and ventricular explants were harvested 24 hours later. Ventricular endothelial cells normally do not undergo EMT due to the absence of both inductive signals from myocardium and a lack of competence to respond (30). However, overexpression of EphA3 in the heart was sufficient to promote EMT in ventricular explants (Figure 2A)(Supplemental Table 1). EMT was unchanged by EphA3 overexpression in AV cushion explants.
Figure 2. Effects of wild-type and kinase dead EphA3 on EMT.
(A&B) Wild-type EphA3, kinase dead EphA3, or control GFP adenoviruses were injected into the lumen of the chick heart tube at stage 10–12. AV cushions and ventricles were harvested and subjected to collagen gel assay. Kinase dead EphA3 virus significantly inhibited the numbers of transformed cells. Three independent experiments were conducted and analyzed as described in Figure 1B. See Table S1 for complete results. (C) 293T cells were infected with adenovirus carrying either wild-type or kinase dead EphA3. Cell lysates were immunoprecipitated with anti-EphA3 and subjected to in vitro kinase assay.
Eph receptors are known to function via kinase dependent and independent mechanisms. To determine whether EphA3 regulated EMT via a kinase dependent mechanism, we performed parallel experiments in AV cushion and ventricular explants using an adenovirus expressing a kinase dead mutant of EphA3 (Figure 2C). Overexpression of the kinase dead EphA3 mutant not only failed to promote EMT within ventricular explants, but also inhibited EMT within AV cushion endocardial cells, suggesting that the EphA3 mutant inhibited EMT through a dominant negative mechanism. (Figure 2B)(Supplemental Table 1). Taken together, our results indicate that while ephrin-A1 inhibits endocardial cushion EMT, EphA3 promotes EMT via a kinase dependent mechanism.
Generation of an ephrin-A1 knockout mouse
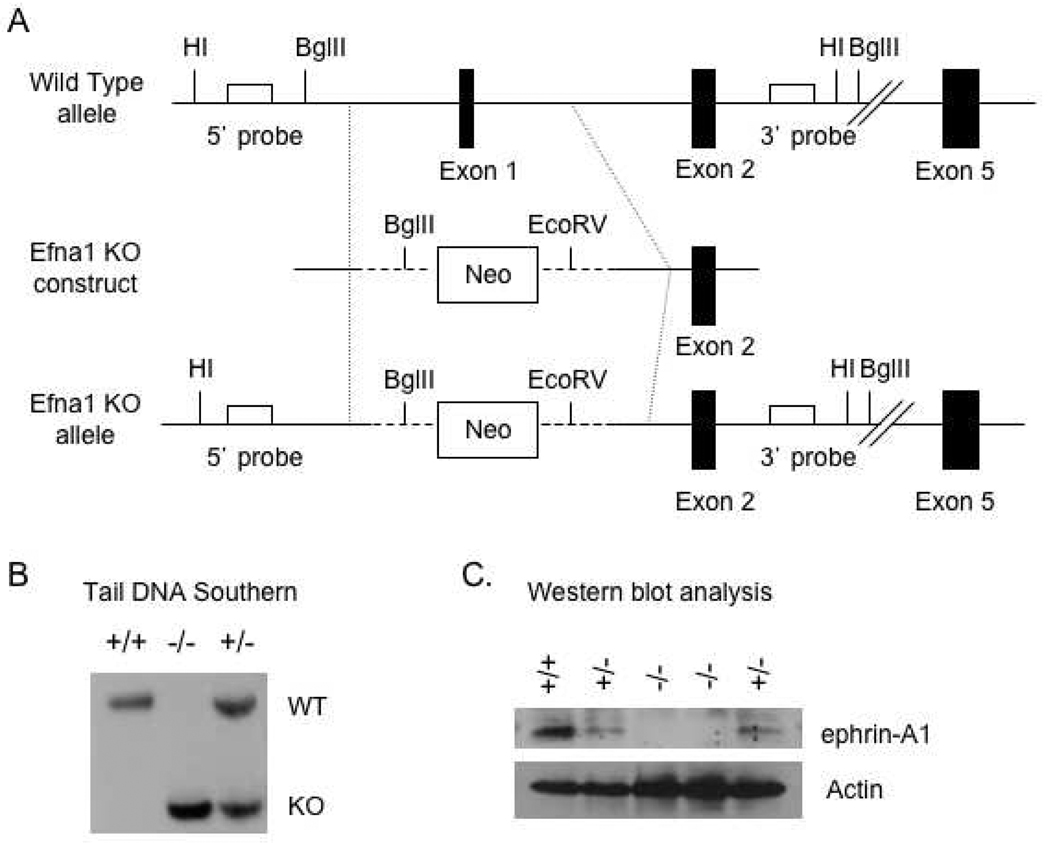
To study the role of ephrin-A1 in vivo, we generated an ephrin-A1 knock mouse by gene targeting through homologous recombination in embryonic stem (ES) cells. To create a null allele, exon 1 of the gene encoding ephrin-A1 (Efna1) was deleted in order to remove the initiating methionine codon and cause a frame-shift in translation (Figure 3A). Standard techniques were then used to produce germline homozygous mice (Figure 3B). As shown in Figure 3C, expression of ephrin-A1 protein is absent in homozygous mice, compared to that in wild-type or heterozygous control littermates, demonstrating that deletion of exon 1 results in a null allele. Efna1−/− mice are viable and fertile, displaying no overt phenotype in the pathogen-free animal facility.
Figure 3. Targeted disruption of the Efna-1 gene.
(A) Map of ephrin-A1 targeting vector, ephrin-A1 wild-type allele and ephrin-A1 knockout (KO) allele. Black boxes represent ephrin-A1 exon sequences. HI=BamHI. (B) Tail DNA Southern blot analysis confirmed the presence of the wild-type allele (WT) in wild-type (+/+) and heterozygous (+/−) mice and presence of the targeted allele (KO) in heterozygous (+/−) and null (−/−) mice. (C) Ephrin-A1 expression is abolished in ephrin-A1-null mice. Cell lysates prepared from P0 embryonic lungs were subjected to western blot analysis. The blot was stripped and re-probed with anti-actin for loading control.
Cardiac dysfunction in ephrin-A1-null mice
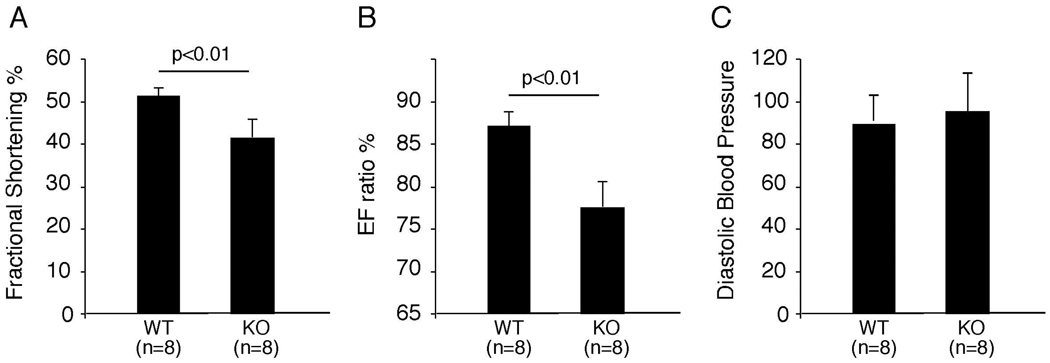
As a first step to identify the phenotype of ephrin-A1-deficient animals, we performed M mode echocardiography on 8-week-old wild-type and ephrin-A1 knockout mice. We found that fractional shortening and ejection fraction ratios were significantly decreased in ephrin-A1-deficient mice (Figure 4, A&B)(Supplemental Table 2). These results indicate that Efna1−/− hearts perform less efficiently than wild-type hearts. To investigate whether this phenotype is due to increased systemic resistance, we measured systolic and diastolic blood pressure from these mice. As shown in Figure 4C, there is no significant difference in blood pressure between ephrin-A1-deficient animals and their wild-type littermate controls, suggesting that the abnormal heart function in ephrin-A1-deficient animals is due to cardiac dysfunction rather than increased systemic blood pressure.
Figure 4. Echocardiogram and blood pressure in ephrin-A1-null mice.
8-week old ephrin-A1-deficient (KO) mice and their wild-type (WT) littermate controls were subjected to echocardiography and measurement of blood pressure. Fractional shortening and ejection fraction (EF) ratio were significantly decreased in KO mice, compared to WT controls (panel A&B), whereas there is no change in blood pressure between WT and KO animals (panel C). Data were pooled from three separate experiments and presented as mean ± SD.
Valve defects in ephrin-A1-deficient mice
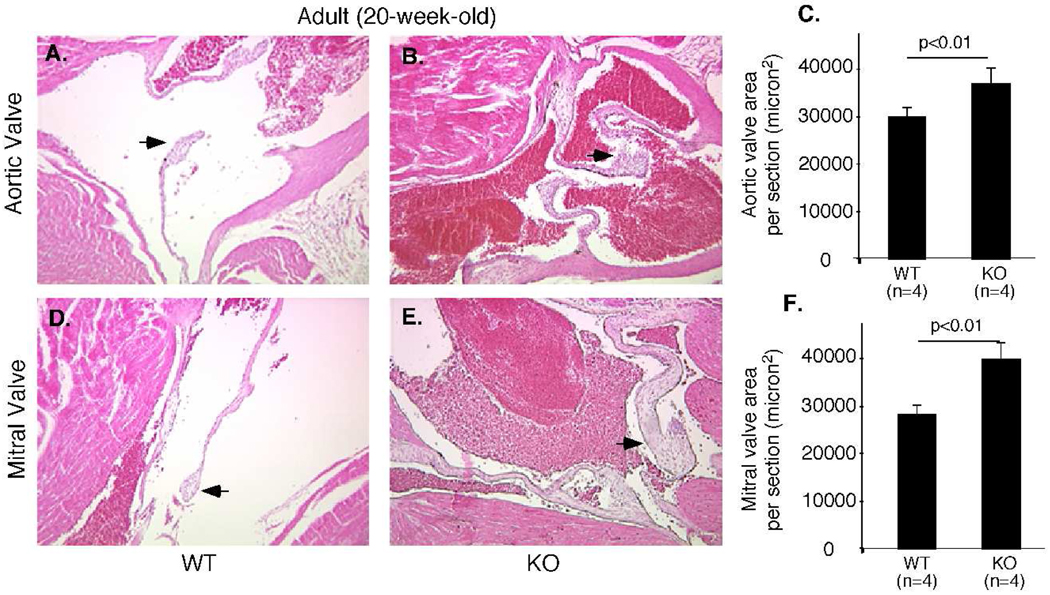
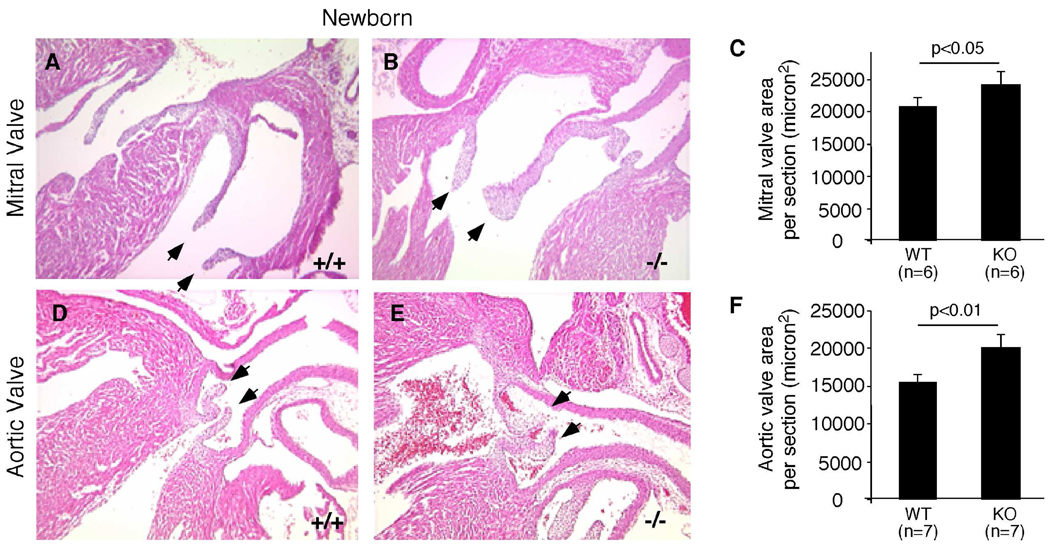
To determine the cause of heart dysfunction, we performed histological analysis on hearts from 20-week-old adult mice. Aortic and mitral valves of Efna1−/− mice are significantly thicker than those of wild-type controls (Figure 5). However, there was no evidence of increased heart weight versus body weight, indicating that cardiac defects at 20 weeks were not severe enough to cause hypertrophy (data not shown). In order to determine whether the increased valve thickness in Efna1−/− mice was a congenital defect, newborn mice were sacrificed and cardiac valve thickness measured (Figure 6). Histological examination revealed that aortic valves of ephrin-A1-null mice were significantly thicker than wild-type valves at birth. To ensure that the thickening aortic valve in ephrin-A1 knockout mice was not due to the plane of section, we examined serial sections from wild-type and ephrin-A1-null animals and quantified five representative sections using Image J. In addition to the aortic valve, mitral valve leaflet area was also increased, whereas pulmonary and tricuspid valve leaflet areas were not significantly affected (data not shown). These data suggest that ephrin-A1 is required in regulation of heart valve development.
Figure 5. Valve defects in Efna1−/− adult hearts.
Hearts dissected from 20-week-old wild-type (+/+) mice or knockout littermates (−/−) were processed for histological analyses. Increased thickness of aortic (B) and mitral (E) valves in (−/−) mice, compared to (+/+) controls (A, D). Arrows indicate mitral or aortic valve leaflets. (C,F) Valve areas were quantified using Image J software.
Figure 6. Valve abnormalities in ephrin-A1-deficient newborns.
Hearts from P0 wild-type (+/+) mice or knockout littermates (−/−) were processed for histological analyses. Increased thickness of mitral (B) and aortic (E) valves in (−/−) mice, compared to (+/+) controls (A,D). Arrows indicate mitral or aortic valve leaflets. (C,F) Valve areas were quantified using Image J software.
Increased cellularity and mesenchymal gene expression in ephrin-A1-deficient endocardial cushions
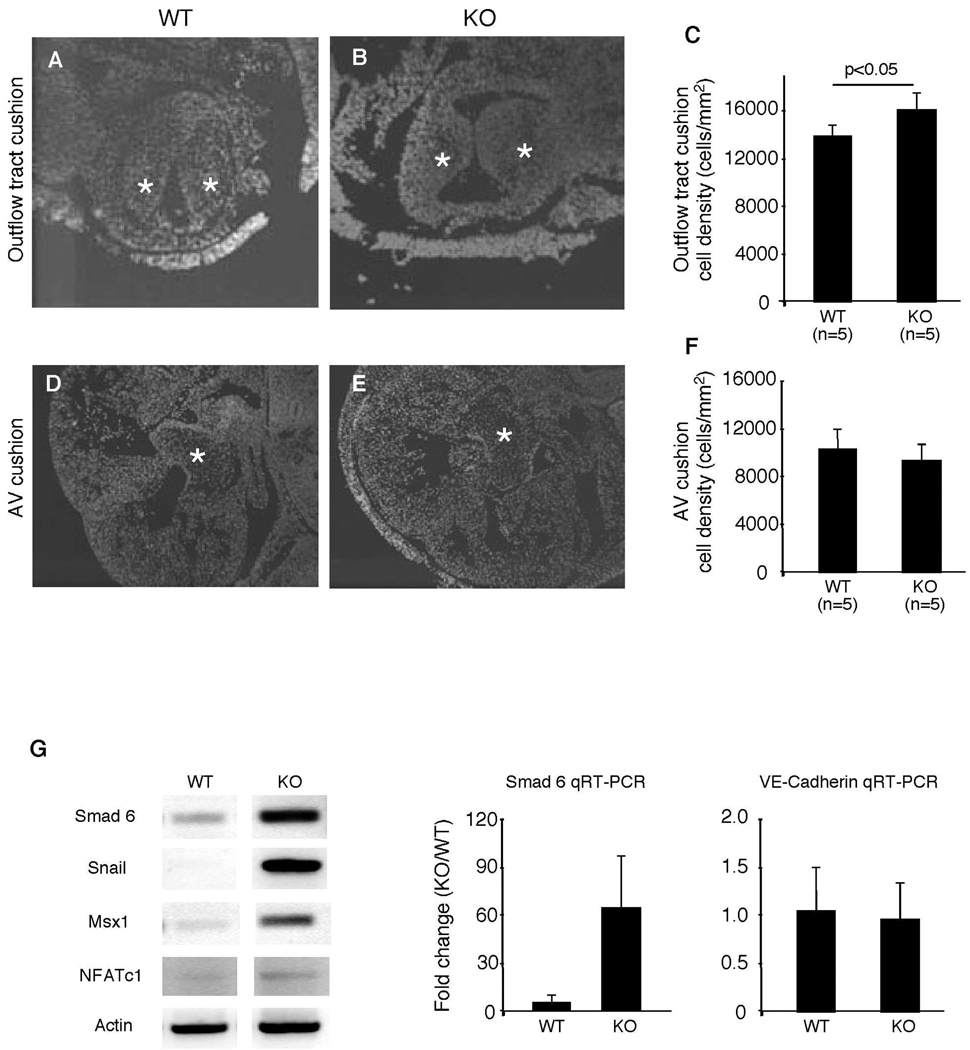
Heart valves and septum are derived from embryonic endocardial cushions. To determine whether thickening of heart valve in ephrin-A1-null animals was caused by increased EMT or by later stage valve remodeling, we chose to first examine the cellularity of ephrin-A1 knockout mouse endocardial cushions. Cushion sections from E12.5 mice were stained with DAPI and cell numbers in the endocardial cushion were quantified using Metamorph software. Cushion cell density was calculated by dividing the total nuclei per cushion by the area of the cushion. Outflow tract endocardial cushions of Efna1−/− mice exhibit significantly increased cell density in comparison to wild-type controls (Figure 7A–C). As outflow tract endocardial cushions are also populated by cells of cardiac neural crest origin, we performed in situ hybridization for plexinA2, a marker of cardiac neural crest (4). The expression pattern of plexinA2 in outflow tract is not changed between wild-type and Efna1−/− embryos (data not shown), suggesting no gross alteration in neural crest migration into the outflow tract.
Figure 7. Reduced cellularity in Efna1−/− outflow tract cushion.
Endocardial cushions from E12.5 embryos were stained with DAPI to visualize nuclei. Outflow tract (A–C) and AV (D–F) cushion cell numbers and areas were quantified using Image J software. Cell density was calculated by dividing the number of nuclei by cushion area. Stars indicate endocardial cushion. (G) RT-PCR of mesenchymal and endocardial marker expression on mRNA from hearts of E9.5 mouse embryos. Expression of mesenchymal markers is increased in the embryonic heart. Quantitative real-time PCR of a mesenchymal and a endocardial gene, Smad6 and VE cadherin, confirm these findings.
Next, we measured expression of cushion mesenchyme genes by RT- PCR at the initiation of EMT in E9.5 embryonic hearts. Expression levels of Smad6, Msx1, and Snail1, markers of cushion mesenchyme, are up-regulated in Efna1−/− hearts (Figure 7G). Expression of NFATc1, a gene localized to the endothelial lining of the endocardium appears unchanged Efna1−/− hearts. To confirm these findings, we performed real-time quantitative PCR using selected markers of mesenchymal and endothelial genes. Smad6, a marker of cushion mesenchyme is elevated in the hearts of E9.5 knockout mice, while VE-cadherin, a marker of endothelium, is unchanged between wild-type and ephrin-A1-null hearts. To determine whether changes in endocardial cushion cellularity were caused by factors other than EMT, we measured cellular proliferation and apoptosis in E12.5 endocardial cushions. We compared the percentage of BrdU- and TUNEL- positive cells between wild-type and ephrin-A1 knockout endocardial cushions at E12.5, respectively. There was no significant difference in cell proliferation and apoptosis between wild-type and Efna1−/− endocardial cushions (data not shown). These data, together with results in chick cushion explants, suggest that ephrin-A1 inhibits EMT in heart valve morphogenesis.
Discussion
Ephrin-A1 is a prototypic ligand for class A Eph receptor tyrosine kinases. Originally discovered as a TNF-α-inducible gene (16), it was subsequently found that ephrin-A1 induced corneal angiogenesis and tumor neovascularization (3, 26, 27). During embryonic development, ephrin-A1 is expressed in the endothelium of the developing blood vessels and in the endocardium as early as E8.5 (13, 33). At E12.5, ephrin-A1 expression is restricted in the endothelial lining of the endocardium, whereas EphA3 receptor is expressed in a complementary pattern within endocardial cushion mesenchyme (36). EphA3 knockout mice are perinatal lethal and exhibit abnormal AV valves and hypoplastic septum (36). If ephrin-A1 only functions as a ligand to activate EphA3, loss of ephrin-A1 would be expected to result in a phenotype similar to EphA3-null mice. However, ephrin-A1 knockout mice are viable and have thickened valves, raising the possibility that ephrin-A1 regulates heart valve morphogenesis through independent or additional mechanisms. Interestingly, mice homozygous for the cytoplasmic domain deletion mutant of ephrin-B2 are embryonic lethal with thickened cardiac valves, a phenotype reminiscent of the ephrin-A1 knockout mice (9). It remains to be determined whether the relatively mild phenotype of ephrin-A1-null mice is due to functional compensation by ephrin-B2.
Heart valve morphogenesis initiates with endocardial cushion transformation in which endocardial cells delaminate, transdifferentiate into mesenchymal cells, and invade into cardiac jelly. Transformed endocardial cushions then undergo subsequent remodeling through proliferation and apoptosis, giving rise to precisely formed cardiac valves that direct blood flow in heart. Three lines of evidence suggest that ephrin-A1 may regulate heart valve formation through, at least in part, inhibition of endocardial cushion transformation. First, outflow tract endocardial cushion cellularity is significantly increased in ephrin-A1 knockout embryos in the absence of changes in neural crest cell contribution. Second, increased cellularity in ephrin-A1-deficient endocardial cushions is accompanied by upregulation of the expression of mesenchymal markers in the embryonic heart. Third, exogenous ephrin-A1-Fc or overexpression of ephrin-A1 inhibited EMT in chick explant collagen gel assays, consistent with an inhibitory role of ephrin-A1 on EMT in vivo.
In addition to a role in inhibiting EMT, ephrin-A1 may also regulate valve morphogenesis through other mechanisms. After completion of EMT, endocardial cushions undergo remodeling, a process that is dependent upon proliferation and apoptosis, cellular migration and reorganization of the extracellular matrix. We observed a moderate reduction in apoptosis in AV cushion mesenchyme and an increase in cellularity in E14.5 Efna1−/− animals (data not shown), suggesting an additional role of ephrin-A1 in regulation of mesenchymal cell survival in the endocardial cushion. Furthermore, Movat's pentachrome staining failed to reveal any gross abnormalities in the distribution of collagen, elastin and glycoproteins within aortic and mitral valves (data not shown), indicating that valve thickness is not due to abnormal ECM deposition or abnormal development of valve layers.
What are the molecular mechanisms of ephrin-A1 action in heart valve development? As ephrin-A1 and EphA3 are expressed complementarily in juxtaposed tissue in endocardial cushion and EphA-ephrin-A interactions are known to mediate repulsive signal during neural development (39), one possibility is that the EphA3-expressing cells undergoing EMT may be repelled by the ephrin-A1-expressing endocardial cells. However, our data does not support this hypothesis as EphA3-null animals exhibited hypoplastic endocardial cushion (36) whereas ephrin-A1 knockout mice display hyperplastic valves. Alternatively, ephrin-A1 activation has been shown to increase cell adhesiveness (6). Ephrin-A1 could positively regulate cell-cell adhesion. Therefore, loss of ephrin-A1 would decrease cell adhesiveness and allow cell detachment from the endothelial layer. In addition, ephrin-A1 could also inhibit the expression of EphA3 receptor, as ephrin-A1 has been shown to negatively regulate the levels of EphA2 receptor in tumor cells (19). Ablation of ephrin-A1 would then upregulate expression of EphA3, resulting in increased EMT. Our data derived from overexpression of EphA3 in chick heart tube is consistent with this hypothesis.
In summary, we have shown that ephrin-A1 is critical in regulating proper formation of the mammalian heart valve. As ephrin-A1 is also expressed in blood vessels, the role of ephrin-A1 in vascular development and adult angiogenesis remains to be determined. Thus, the ephrin-A1 knockout mouse provides a valuable animal model for studying congenital heart defects as well as angiogenesis in adult.
Experimental Procedures
Cushion explant collagen gel assay
Atrioventricular (AV) cushions were dissected from Hamburger-Hamilton (HH) stage 14 chick embryos and cultured on collagen gels as described (31, 34). Adenoviral expression experiments were conducted by placing HH stage 10–12 embryos on Whatman paper rings. Adenoviruses expressing GFP only, ephrin-A1, EphA3, or kinase dead EphA3 (K657M) were injected into the lumen of the chick heart and explants were cultured for 48 hours as described (10). AV cushion and ventricular explants were cultured on collagen and GFP positive green cells were scored as epithelial, activated, or transformed 48 hours later. Seven to eleven explants were scored per condition for each experiment and experiments were repeated three times. Results from these three experiments were pooled, and data were expressed as mean±SEM. Group comparisons were performed using unpaired, two-tailed Student's t test. p <0.05 were considered statistically significant.
Generation of ephrin-A1 knockout mice
PCR was used to amplify genomic regions flanking exon 1 of the gene encoding ephrin-A1 (Efna1) (NM_010107). The targeting construct was electroporated into 129SV/J ES cells. ES cell clones were screened for homologous recombination using southern blotting and microinjected into C57/BL6 blastocysts. Chimeric founders were crossed to C57/BL6 females. Genotyping was performed by multiplex PCR using a common forward primer (5’CCCAACAAAAACAAACAGCCG3’) and two allele specific reverse primers, WT (5’GAGGTGGAGGAAGGGAAAAAGAC3’) and KO (5’TGGATGTGGAATGTGTGCGAGG3’). Mice were maintained on a mixed 129/SVJ/C57/BL6 background.
Echocardiography analysis
Mice were placed in a left lateral decubitus position and imaged at ambient temperature with a 15 MHz transducer. M mode imaging was used to measure left ventricular internal dimensions. Shortening fraction was calculated using the formula 100 X (LVIDD-LVIDS)/LVIDD. Ejection fraction was calculated as (LVIDD3-LVIDS3)/LVIDD3. The presence of arrhythmias was determined by detecting irregularities in the intervals between recorded heartbeats.
Histological analysis
Adult and embryonic hearts were fixed in formalin or 4% PFA overnight at 4°C, and 8–10 micron paraffin sections were stained in hematoxylin and eosin. Valve area was measured using Image J Software, version 1.38x. Five sections per animal/embryo were analyzed.
For analysis of endocardial cushion cell density, 8 micron sections of atrioventricular and outflow tract cushions from E12.5 embryos were stained with DAPI. Five sections per embryo were analyzed. Nuclei were counted using Metamorph software, 7.1 (Molecular Devices). Cushion area was measured using Image J. Cellular density was calculated by dividing nuclear number by cushion area.
Apoptosis was assessed by TUNEL assay using the Apo Tag in situ Apoptosis detection kit (Millipore), and counterstained with DAPI. Apoptotic indices were quantified as TUNEL positive nuclei/total nuclei (5 sections per embryo).
Analysis of gene expression in developing heart
RNAs was isolated from E9.5 embryonic hearts using Trizol reagents (Invitrogen). cDNA was prepared using the Superscript III 1st strand cDNA synthesis kit (Invitrogen). PCRs were performed using published primer sequences [Msx1 and Smad6, (12); β-actin, and Snail, (37); NFATc1, (38) and VE cadherin (25)]. Experiments were repeated three times. Real time PCR was performed on the Biorad icycler using iQ Sybr Green supermix (Biorad). Separate reactions were performed in duplicate for both experimental primers and GAPDH controls (5’CCTGCACCACCAACTGCTTA, 5’TCATGAGCCCTTCCACAA). Results were calculated using the ΔΔCT method.
Supplementary Material
Acknowledgements
This work was supported by Department of Veterans Affairs through a VA Merit Award (to J. Chen), NIH grants CA95004 and CA114301 (J. Chen), HL0952551(J. Barnett & D. DeLaughter), and T32 HD07390 (L. Frieden) and T32 GM007628 (T. Townsend). Special thanks to Drs. Chris Brown, Ron Emerson, Jeff Rottman, M. Jay Campbell, Scott Baldwin, Justin Cates and Jim Atkinson for invaluable advice on gene targeting technology, echocardiography, and patho-histological analysis. We acknowledge the Vanderbilt Transgenic and ES cell Core, Cardiovascular Physiology and Complications Core and Vanderbilt Small Animal Imaging Core for assistance in generation of ephrin-A1 deficient mice and echocardiography analysis. We also thank Tyson Foods, Inc. for chick eggs.
References
- 1.Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective. Birth Defects Res C Embryo Today. 2003;69:58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- 2.Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, Speer CP, Poelmann RE, Gittenberger-de Groot AC. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001;103:2745–2752. doi: 10.1161/01.cir.103.22.2745. [DOI] [PubMed] [Google Scholar]
- 3.Brantley DM, Cheng N, Thompson EJ, Lin Q, Brekken RA, Thorpe PE, Muraoka RS, Cerretti DP, Pozzi A, Jackson D, Lin C, Chen J. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–7026. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]
- 4.Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, Epstein JA. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development. 2001;128:3071–3080. doi: 10.1242/dev.128.16.3071. [DOI] [PubMed] [Google Scholar]
- 5.Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 6.Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas) Nat Cell Biol. 2002;4:565–573. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- 7.Chen B, Bronson RT, Klaman LD, Hampton TG, Wang JF, Green PJ, Magnuson T, Douglas PS, Morgan JP, Neel BG. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat Genet. 2000;24:296–299. doi: 10.1038/73528. [DOI] [PubMed] [Google Scholar]
- 8.Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 9.Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev Biol. 2004;271:263–271. doi: 10.1016/j.ydbio.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Desgrosellier JS, Mundell NA, McDonnell MA, Moses HL, Barnett JV. Activin receptor-like kinase 2 and Smad6 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2005;280:201–210. doi: 10.1016/j.ydbio.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Erickson SL, O'Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu LH, Moore MW. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 12.Flagg AE, Earley JU, Svensson EC. FOG-2 attenuates endothelial-to-mesenchymal transformation in the endocardial cushions of the developing heart. Dev Biol. 2007;304:308–316. doi: 10.1016/j.ydbio.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flenniken AM, Gale NW, Yancopoulos GD, Wilkinson DG. Distinct and overlapping expression patterns of ligands for Eph-related receptor tyrosine kinases during mouse embryogenesis. Dev Biol. 1996;179:382–401. doi: 10.1006/dbio.1996.0269. [DOI] [PubMed] [Google Scholar]
- 14.Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Holzman LB, Marks RM, Dixit VM. A novel immediate-early response gene of endothelium is induced by cytokines and encodes a secreted protein. Mol Cell Biol. 1990;10:5830–5838. doi: 10.1128/mcb.10.11.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. Embo J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, Gray JW, McCormick F. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Meberg A, Lindberg H, Thaulow E. Congenital heart defects: the patients who die. Acta Paediatr. 2005;94:1060–1065. doi: 10.1111/j.1651-2227.2005.tb02046.x. [DOI] [PubMed] [Google Scholar]
- 21.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 22.Misch KA. Development of heart valve lesions during methysergide therapy. Br Med J. 1974;2:365–366. doi: 10.1136/bmj.2.5915.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murai KK, Pasquale EB. 'Eph'ective signaling: forward, reverse and crosstalk. J Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- 24.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl Med. 2001;345:1318–1330. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson I, Rolny C, Wu Y, Pytowski B, Hicklin D, Alitalo K, Claesson-Welsh L, Wennstrom S. Vascular endothelial growth factor receptor-3 in hypoxia-induced vascular development. Faseb J. 2004;18:1507–1515. doi: 10.1096/fj.03-1276com. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–6052. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 27.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science. 1995;268:567–569. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- 28.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 29.Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- 30.Potts JD, Runyan RB. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol. 1989;134:392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- 31.Potts JD, Vincent EB, Runyan RB, Weeks DL. Sense and antisense TGF beta 3 mRNA levels correlate with cardiac valve induction. Dev Dyn. 1992;193:340–345. doi: 10.1002/aja.1001930407. [DOI] [PubMed] [Google Scholar]
- 32.Robiolio PA, Rigolin VH, Wilson JS, Harrison JK, Sanders LL, Bashore TM, Feldman JM. Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation. 1995;92:790–795. doi: 10.1161/01.cir.92.4.790. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz JC, Robertson EJ. The expression of the receptor-protein tyrosine kinase gene, eck, is highly restricted during early mouse development. Mech Dev. 1994;46:87–100. doi: 10.1016/0925-4773(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 34.Runyan RB, Markwald RR. Invasion of mesenchyme into three-dimensional collagen gels: a regional and temporal analysis of interaction in embryonic heart tissue. Dev Biol. 1983;95:108–114. doi: 10.1016/0012-1606(83)90010-6. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava D. Genetic regulation of cardiogenesis and congenital heart disease. Annu Rev Pathol. 2006;1:199–213. doi: 10.1146/annurev.pathol.1.110304.100039. [DOI] [PubMed] [Google Scholar]
- 36.Stephen LJ, Fawkes AL, Verhoeve A, Lemke G, Brown A. A critical role for the EphA3 receptor tyrosine kinase in heart development. Dev Biol. 2007;302:66–79. doi: 10.1016/j.ydbio.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 37.Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, Molkentin JD. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol. 2002;22:7603–7613. doi: 10.1128/MCB.22.21.7603-7613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.