Abstract
This article examines the complex role of early stressful experiences in producing both vulnerability and resilience to later stress-related psychopathology in a variety of primate models of human development. Two types of models are reviewed: Parental Separation Models (e.g., isolate-rearing, peer-rearing, parental separations, and stress inoculation) and Maternal Behavior Models (e.g., foraging demands, variation in maternal style, and maternal abuse). Based on empirical evidence, it is argued that early life stress exposure does not increase adult vulnerability to stress-related psychopathology as a linear function, as is generally believed, but instead reflects a quadratic function. Features of early stress exposure including the type, duration, frequency, ecological validity, sensory modality, and developmental timing, within and between species, are identified to better understand how early stressful experiences alter neurobiological systems to produce such diverse developmental outcomes. This article concludes by identifying gaps in our current knowledge, providing directions for future research, and discussing the translational implications of these primate models for human development and psychopathology.
Keywords: development, early experiences, HPA axis, maternal care, primate models, psychopathology, resilience, risk, stress inoculation, stressor, vulnerability
1. Introduction
The long-term effects of early social experiences on developmental outcomes are of high significance. This is because early experiences occur during a time of extraordinary brain plasticity, when the brain is maximally capable of being programmed in an enduring way (Knudsen 2004). In an effort to understand the role of social relationships in fostering normative brain development, non-human primate (hereafter primate) models have historically focused on the developmental neuropathology of disrupted parent-offspring relationships. This research has shown that the stress of early parental loss, neglect, or abuse produces enhanced fear and anxiety, increased anhedonia, impaired cognition, abnormal brain neurochemistry and neurobiology, and alterations in baseline activity as well as stress reactivity of the hypothalamic-pituitary-adrenal (HPA) axis (Coplan et al 1998; Law et al 2009; Maestripieri et al 2005; Pryce et al 2005; Rosenblum et al 2001; Sanchez et al 1998; Stevens et al 2009; Suomi 1997). Parallel research in human populations likewise has established that traumatic experiences in childhood impair the acquisition of appropriate coping skills, impair corticolimbic brain systems that regulate stress and anxiety, produce abnormal baseline and stimulated HPA axis functioning, and increase the risk for the development of mood and anxiety disorders in the aftermath of subsequent stressors experienced in adulthood (Agid et al 1999; Heim et al 2004; Lupien et al 2009; McEwen 2007; Repetti et al 2002). These collective research efforts have produced profound scientific insights, but have also lead to the prevailing notion that the consequences of early life stress exposure are invariantly deleterious. This view of early life stress exposure is thus best conceptualized as a linear function, whereby each incremental “dose” of early life stress increases subsequent vulnerability to later psychopathology.
There is accumulating evidence, however, that early life stress exposure produces a diverse range of developmental outcomes, including resilience to subsequent stressors encountered in adulthood. For example, in humans, childhood stress has been linked to diminished increases in salivary cortisol responses to the Trier Social Stress Test (Gunnar et al 2009), lower cerebrospinal fluid (CSF) levels of corticotropin-releasing-factor (CRF) in healthy adults (Carpenter et al 2004), and diminished cardiovascular responses during stressful laboratory tests (e.g., mental arithmetic, videogame performance, hand submersion in ice water) (Boyce and Chesterman 1990). Prior stressful experiences also diminish emotional distress in day care settings and hospital admissions (Holmes 1935; Stacey et al 1970), and women and men are found to better cope with stressful events (e.g., spousal loss, major accident, illness, work stress) if they previously experienced and successfully coped with stressors in childhood (Forest 1991; Khoshaba and Maddi 1999). When early life stress exposure is examined across a continuum, adults exposed to moderate levels of early life stress exhibit lower levels of state anxiety (Edge et al 2009) and more resilient cardiovascular responses to a stressful motivated performance task (i.e., what subjects believed was an intelligence test) (Seery under review) compared to individuals exposed to either low or high levels of early life stress. These empirical studies suggest that early life stress exposure may be best conceptualized as a quadratic, rather than linear, function.
In the early 1980s, Garmezy and colleagues detailed a process-oriented “Challenge” framework by which to view the effects of early life stress exposure (Garmezy et al 1984). In this model, stress exposure is viewed as a potential enhancer of future competence, provided the degree of stress is not excessive. Whereas severe early life stress exposure generally undermines the development of resilience and leads to vulnerability (Bebbington et al 1993; Brown et al 1994; Frank et al 1994; Paykel 1978), mild or moderate early life stress exposure may protect against these deleterious effects. Specifically, milder forms of adversity may provide a challenge, that when overcome, produces competence in the management of, and enhanced resistance to, subsequent stressors (Boyce and Chesterman 1990; Fergus and Zimmerman 2005; Garmezy et al 1984; Haglund et al 2007; Huether et al 1999; O’Leary 1998; Rutter 1993). This phenomenon has been variously described in the literature as “inoculating” (Boyce and Chesterman 1990; Eysenck 1983; Parker et al 2004), “immunizing”(Levine et al 1989; Rutter 1987; Seligman et al 1975), “steeling” (Rutter 1985; Rutter 1993), “toughening” (Dienstbier 1989; Miller 1980), and “thriving” (O’Leary and Ickovics 1995).
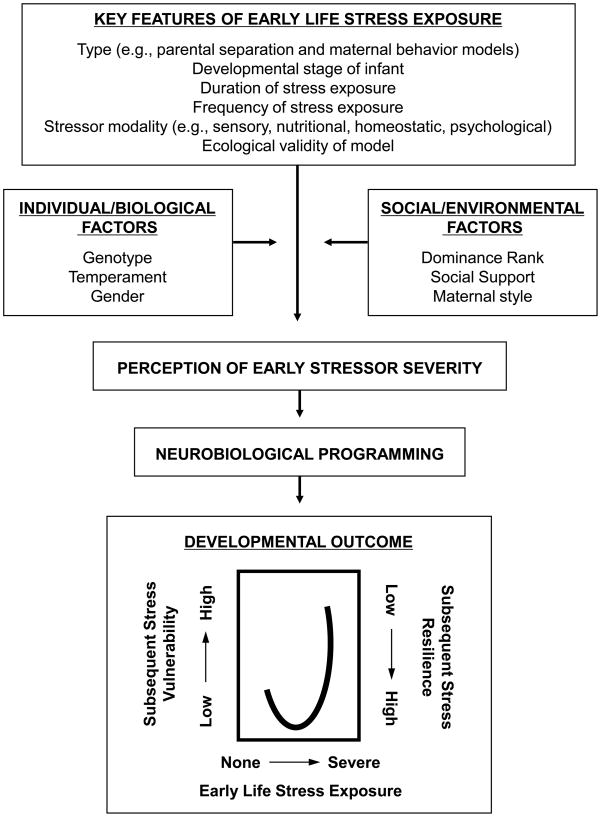
Despite a growing appreciation for the complexity of early life stress effects on developmental outcomes, progress in this research field has been hampered by a tendency to label early life stress exposure based on the outcomes it produces. Thus, early stressful experiences that produce vulnerability to stressors later in life are labeled “severe” or “adverse” and those that induce resilience to subsequent stressors are labeled “mild” or “moderate”. Not only are these definitions circular, they also preclude identification and detailed analyses of the key components of stressors, which contribute to whether stressful experiences produce deleterious or adaptive outcomes. Such detailed analyses may be difficult to do in human studies, in which early stress exposure is often documented retrospectively and through subjective rather than objective measures. Primate models of early stress and development therefore allow us to more clearly assess the relationships between the characteristics of early stressors (e.g., type, duration, frequency, ecological validity, sensory modality, developmental timing) and their developmental outcomes (i.e., stress vulnerability or resilience).
In this article we review various primate models of early stress and development within the broader context of animal model research. Examples from two types of animal models (maternal separation paradigms and maternal behavior paradigms) are reviewed. We discuss the benefits and limitations of each model in terms of its feasibility of use, its effectiveness in generating the expected developmental outcomes, its ecological validity, and its potential to enhance our understanding of how early stressful experiences produce stress vulnerability and resilience in human populations.
2. Necessity of animal models of early stress and development
Animal models of early stressful experiences are required because human studies have a variety of important limitations. Randomized longitudinal studies of stress exposure under otherwise identical conditions are rare in children. Because randomization to stress vs. no-stress control conditions is required for causal inference, and opportunities for randomization of children to stress exposure are limited due to ethical concerns, many studies of childhood stress exposure are thus necessarily correlational in nature. The collection of long-term follow-up data from longitudinal studies may take decades to complete, and cost considerations for such longitudinal studies are often prohibitive in humans. Given these constraints, animal research that examines how early experiences shape social, emotional, and cognitive capacities that have a lasting impact in adulthood is extremely valuable and provides a viable addition to human studies (Knudsen 2004).
The benefits of animal models of human development are numerous. Animal studies using randomization allow for strong causal inference. Early life environments can be rigorously controlled in animals, and this allows for the exclusion of extraneous sources of variation inherent in developmental or retrospective studies of humans (e.g., postnatal stress exposure in children is often confounded with adverse prenatal conditions such as maternal alcohol/drug abuse or poor maternal nutritional status). Animal studies are frequently more cost-effective, and allow for more mechanistic assessments and interventional approaches than those typically permitted in humans. Finally, the lifespan of most animals used in research is far shorter than that of humans, and animal studies therefore cover a fairly broad range of lifespan development within a short period of time (Kaplan et al 1991). This allows for comparatively rapid investigations of the long-term effects of early life experiences in the same subjects, and permits accelerated evaluation of interventions and treatments across the lifespan.
A variety of species are available to examine the long-term effects of early life stress effects, and all have advantages and disadvantages. Rodent models are limited because corticolimbic brain substrates involved in cognitive control of behavior and emotion regulation differ significantly in rats and mice compared to humans and other primates (Ongur and Price 2000; Preuss 1995). Moreover, HPA axis development is markedly different in rodents compared to primates and humans. Much of rodent development is characterized by the stress hyporesponsive period (SHRP) in which rodent pups do not respond to stress with HPA axis activation (Levine 2001; Schapiro et al 1962; Schmidt et al 2003), but this is not the case in primates (Bowman and Wolf 1965; Parker et al 2006; Pryce et al 2002; Pryce et al 2005), which exhibit robust HPA axis responses to stress early in development. Given that rodents exhibit an SHRP and primates do not, the mechanisms by which early life experiences affect the development of stress vulnerability and resilience are likely to differ in important ways between rodent and primate species.
3. Primate models
Primates are excellent animal models for investigating the effects of early experience on the development of brain and behavior (Lyons and Parker 2007; Maestripieri and Wallen 2003). Primates have an extended period of post-natal growth and maturation, in which developing neurobiological systems have ample opportunity to be influenced by experience (Nelson and Bloom 1997). Similar to humans, primate infants are immersed from birth in a rich social environment, which primarily consists of their mothers (infants are born in their mother’s arms, spend a considerable amount of their early postnatal life in direct ventral or dorsal contact, and thereafter in close proximity to her), and secondarily, the family or social group in which the mother-infant dyad is embedded. The infant’s early social environment thus has some basic species-specific features, which are shared by all infants and are presumably necessary for their “normal” development. There are also features of an infant’s early life which are unique to each individual (e.g., maternal availability, maternal style, dominance rank, effort required for mother to find adequate food). Clearly, variations in either the shared basic or unshared other features of the infant’s early social environment are potential sources of stress that may have long-lasting and significant consequences across lifespan development.
Two main types of primate models have been employed to examine these sources of variation in an infant’s early social environment. The first type of model (parental separation), involves complete or intermittent separation(s) of the infant from the parent(s). In the parental separation models, the effects of early stress on development are investigated by comparing developmental outcomes of infants that are exposed to some form of parental separation (either complete or intermittent) to infants that remain with their parent(s). The second type of model (maternal behavior) examines variation in the mother’s caregiving behavior. In one subtype of maternal behavior models, researchers can experimentally manipulate the quality of the mother’s behavior either directly (e.g., through pharmacological treatment; but this is rarely done in primate research) or indirectly (e.g., through alterations in the mother’s ecological or social environment) and compare these infants to infants of unmanipulated mothers. In another subtype of these models, researchers take advantage of naturally occurring variation in maternal behavior, which requires no experimental manipulation. In these models, groups of infants exposed to different kinds of maternal behavior are compared.
In the next sections, we first review various parental separation models. These include isolate-rearing, peer-rearing, parental separations, and stress inoculation paradigms. We then review various maternal behavior models, including subtypes in which early experience is altered through environmental manipulations (e.g., foraging demand paradigms), and those in which variations in early experience are produced through naturally occurring variability in maternal behavior (e.g., maternal style, maternal abuse). In all cases, we attempt to identify key features of early life stress exposure in each model - including the type, duration, frequency, ecological validity, sensory modality, and developmental timing of stress exposure – to understand how such diverse developmental outcomes occur, ranging from stress vulnerability to resilience.
3.1. Parental separation models
It has long been known that the responses of monkey infants to separation from their mothers share striking similarities with children’s responses to maternal separation (Bowlby 1969). Early mother-infant separation studies in monkeys were designed to evaluate the mechanisms underlying mother-infant attachment, such as the importance of the mother as a source of contact comfort versus nutrition (Harlow 1959). Interest in separation as a paradigm to investigate the nature of attachment has persisted over the years under the assumption that investigating the mechanisms underlying the disruption of the mother-infant bond could also provide useful information to understand the formation and maintenance of such a bond (Kraemer 1992; Reite 1987). Some researchers, however, have challenged these assumptions and argued that responses to maternal separation are better viewed within the framework of responsiveness to stress than within the framework of attachment (Insel 1992; Levine and Wiener 1988). Consequently, physiological responses to separation have attracted a great deal of interest as a prototype of the organism’s response to stress (e.g. Levine and Wiener 1988). Finally, investigation of responses to maternal separation has also been stimulated by the prospect that they could provide a primate model for human major depression (McKinney and Bunney 1969; Reite 1977). Whether the separation response was investigated from the perspective of attachment, stress, or as a model for major depression, reviewing the studies investigating the acute responses to maternal separation is beyond the scope of this article because such studies generally were not conducted within a clear developmental framework (see Mineka and Suomi, 1978, for a review) and have been reviewed elsewhere in detail (Levine and Wiener 1988). More relevant to the focus of this article, however, is the research that has investigated the long-term developmental effects of maternal separation. This research has developed along two independent lines: one investigating the long-term developmental effects of permanent maternal separation and social deprivation rearing, and the other investigating the long-term developmental effects of repeated, short parental separation-reunion experiences.
3.1.1. Long-term effects of early maternal and social deprivation
Many studies conducted in the 1960s and 1970s showed that separating primate infants permanently from their mothers and rearing them under conditions of total isolation can have devastating effects on subsequent development and behavior (see Suomi 1997 for review). These effects, usually subsumed under the label of “isolation syndrome”, or “isolate-rearing”, have been best studied in rhesus monkeys. Rhesus monkey infants typically spend the first few days of life in continuous physical contact with their mothers, and maintain close proximity to them for 1–2 years or longer. At the behavioral level, isolation-reared rhesus monkeys include displays of abnormal self-directed and stereotypic behavior and gross deficits in all aspects of social interactions, including affiliation, aggression, communication, mating, and parenting. Isolation effects are generally more pronounced when infants are isolated early in life and for at least 6 months, and when infants are denied access to any sensory stimuli from conspecifics. Denial of tactile contact appears to have more dramatic effects on infant development than denial of visual, auditory, and olfactory stimuli. Isolation-rearing, however, generally involves the simultaneous deprivation of social and nonsocial sensory stimuli as well as the deprivation of different types of social stimuli.
The behavioral effects of isolation-rearing generally persist into adulthood. Although some procedures, such as pairing a previously isolated infant with a non-isolated same-aged monkey “therapist” can reverse some of the social deficits displayed by isolates (Novak and Harlow 1975; Suomi and Harlow 1972), these individuals can rarely be successfully reintegrated into a complex social group with mother-reared individuals (Anderson and Mason 1974; 1978). Other environmental or pharmacological treatments may temporarily reduce abnormal behavior but the isolation syndrome usually returns if the treatment is withdrawn (Kraemer 1992). Recovery from isolation-rearing is considerably more effective in some species of monkeys and apes than in others (Dienske & de Jonge 1982). Although in some cases the differential effects of early isolation in different species can be accounted for by differences in life-history and social organization (e.g., solitary orangutans appear to be affected by maternal and social deprivation to a lesser extent than highly social chimpanzees), in other cases the causes of interspecific differences in vulnerability to early maternal and social deprivation are less clear (e.g., in the case of closely related macaque species; Sackett et al 1976).
Over time, research on the long-term effects of isolation-rearing has shifted its focus from behavior to physiology and neuroanatomy. Pioneering studies by Kraemer and colleagues reported that previously isolated rhesus monkeys showed considerable developmental variation in CSF concentrations of norepinephrine, dopamine, and serotonin metabolites relative to non-isolated control monkeys, as well as a lack of correlation between the different monoamine metabolites (Kraemer and McKinney 1979; Kraemer et al 1989). These effects persisted years after the isolation experience. Previously isolated and subsequently “rehabilitated” rhesus monkeys were also behaviorally and neurochemically hypersensitive to d-amphetamine. After the pharmacological treatment, they showed striking increases in aggressive/submissive behavior and in CSF concentrations of norepinephrine and the serotonin metabolite 5-hydroxy-indoleacetic acid (5-HIAA) (but not of the dopamine metabolite homovanilic acid, HVA; Kraemer et al 1983; 1984). On the basis of these findings, Kraemer (1992) hypothesized that isolation-rearing may produce cytoarchitectural changes in brain monoamine systems that result in a functional dysregulation of these systems.
Neuroanatomical alterations of isolation-reared monkeys have subsequently been reported in such brain areas as basal ganglia, hippocampus, cerebellum, corpus callosum, and neocortex. For example, isolates showed changes in dendritic branching in the neocortex (Struble and Riesen 1978), modifications in Purkinje cell structure in the cerebellum (Floeter and Greenough 1979), fewer tyrosine hydroxylase-immunoreactive fibers in the dopaminergic neurons of the neocortex (Morrison et al 1990), altered chemoarchitecture of some basal ganglia regions (but not of the amygdala and other basal forebrain regions; Martin et al 1991), cytoskeletal changes in the dentate gyrus cells of the hippocampus (Siegel et al 1993), and reduced size of the corpus callosum (Sanchez et al 1998). Several studies, however, reported lack of significant differences between isolates and controls in monoamine metabolite concentrations (Lewis et al 1990), tyrosine hydroxylase- and CRF- immunoreactive neurons in the paraventricular nucleus and arcuate nucleus of the hypothalamus (Ginsberg et al 1993a), noradrenergic innervation density of the paraventricular nucleus of the hypothalamus (Ginsberg et al 1993b), and overall brain volume (Sanchez et al 1998). In some cases, long-term developmental effects of isolation-rearing on brain structure or neurotransmitter systems were not directly observed but inferred from altered responses to pharmacological challenges. For example, Lewis et al. (1990) reported changes in dopamine receptor function following apomorphine challenge in previously isolated rhesus monkeys. Similarly, Coplan et al. (1992) reported differential effects of a low dose (but not of a high dose) of yohimbine in isolation-reared bonnet macaques and controls.
Further information on the long-term consequences of early maternal and social deprivation for behavioral, neuroendocrine, and neuroanatomical development has been provided by studies using the peer-rearing paradigm (Chamove et al 1973; Harlow 1969). In this paradigm, rhesus infants are permanently separated from their mothers within hours after birth, hand-raised in a nursery, and housed in small groups of same-aged infants, typically three, for the first six months of life. A longitudinal study of peer-reared versus mother-reared rhesus infants showed that, as neonates, the former were more awake, active, and irritable than the latter (Champoux et al 1991). From 1 to 5 months of age, the peer-reared infants exhibited a greater variety of behaviors relative to the mother-reared infants, which spent most of their time in contact with their mothers. As juveniles, the two groups of individuals were indistinguishable with the exception that the peer-reared individuals showed more self-directed behaviors. Other studies have found that peer-reared juveniles showed more distress in a novel environment than mother-reared juveniles (Chamove et al 1973; Higley et al 1992a; Kraemer and McKinney 1979; but see Shannon et al., 1998). Peer-reared monkeys also exhibit greater fear and anxiety as measured by acoustic startle response paradigms (Parr et al 2002).
Research investigating the long-term consequences of peer-rearing for physiological development, particularly the activity of the HPA axis, has produced some conflicting results. In various studies comparing cortisol levels, peer-reared individuals have been reported to have higher basal cortisol levels than mother-reared individuals (first month of life; Champoux et al 1989; first 2 years of life: Higley et al 1992b), lower basal cortisol levels (14–30 days of age: Shannon et al 1998), or lower basal ACTH but similar cortisol levels (1–6 months of age: Clarke 1993). Studies comparing peer-reared and isolation-reared animals have reported higher basal cortisol levels in peer-reared animals (19 months of age: Sackett et al 1973) or no differences in cortisol levels (4 years of age: Meyer and Bowman 1972). When physiological responses to stressful challenges were assessed, some studies found no differences in cortisol levels between peer-reared, mother-reared, and isolation-reared monkeys (Champoux et al 1989; Meyer and Bowman 1972; Sackett et al 1973), one study found that peer-reared monkeys had lower increases in both ACTH and cortisol than mother-reared monkeys (Clarke 1993), another study found that peer-reared infants had lower increases in cortisol but not in ACTH, when compared to mother-reared infants (Barr et al 2004b), and yet another study reported that the cortisol levels of peer-reared monkeys were higher than those of isolation-reared monkeys but similar to those of mother-reared monkeys (Shannon et al 1998). Shannon et al. (1998) suggested that these discrepancies in the activity of the HPA axis in relation to early rearing may have resulted from differences in the housing environment, type of stressful experiences, feeding regimen, or diurnal variation in HPA axis activity across studies.
More consistent findings have been obtained by studies investigating the consequences of peer-rearing on CSF concentrations of monoamine metabolites. In a study comparing rhesus monkeys that were peer-reared or mother-reared in the first 6 months of life, Higley et al (1991) reported that peer-reared males but not females had higher levels of 5-HIAA at 6 and 18 months than mother-reared males. Peer-reared subjects also had higher values of the norepinephrine metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG; see also Higley et al 1992b). No rearing effects on the dopamine metabolite HVA levels were found. In a related longitudinal study, no differences in CSF concentrations of 5-HIAA were found between peer-reared and mother-reared monkeys in the first 1.5 years of life (Higley et al 1992b). The higher levels of MHPG in peer-reared monkeys were consistent with previous findings by Kraemer et al. (1989) suggesting that rearing under conditions of maternal and social deprivation results in enhanced noradrenergic activity and responsivity to stress. In follow-up studies, peer-reared subjects exhibited lower CSF 5-HIAA concentrations at 50 months of age than mother-reared individuals (Higley et al 1996a). When peer-reared and mother-reared juveniles were observed in social groups, individuals with low CSF 5-HIAA and MHPG concentrations from both rearing groups exhibited reduced rates of social interaction and low dominance rank (Higley et al 1996b). In addition, peer-reared subjects with low CSF 5-HIAA concentrations exhibited inept social behaviors and were frequently removed from their social groups for excessive aggression and deviant social behaviors. Based on these findings, Higley (2003) has suggested that the peer-rearing paradigm exacerbates the negative social consequences associated with low CSF 5-HIAA such as high behavioral reactivity to the environment, high impulsivity, and high aggressiveness (see also Shannon et al 2005).
Consistent with this hypothesis, there is some evidence that lower serotonergic function in rhesus monkeys carrying the short allele of the serotonin transporter (SERT) gene is associated with differential responsiveness to stress, higher impulsivity, and higher aggressiveness in peer-reared than in mother-reared individuals. Specifically, Barr et al (2004a; 2004b) reported that in males, ACTH responses to social separation were higher in individuals carrying the short (s) SERT allele (l/s) than in individuals carrying two copies of the long (l) SERT allele (l/l) – both in mother-reared and in peer-reared infants. In females, however, higher ACTH responses to separation in l/s relative to l/l individuals were only observed in peer-reared and not in mother-reared individuals. Furthermore, peer-reared rhesus monkeys, in particular females, carrying the s allele showed lower cortisol responses to stress than mother-reared infants. Finally, the aggressiveness toward an unfamiliar conspecific of rhesus males carrying the s allele was greater in peer-reared than in mother-reared individuals (Schwandt et al 2010).
Behavioral and neurobiological differences between mother-reared and peer-reared infants have also been documented for other genotypes. For example, mother-reared male infants with the low-activity-associated allele of the MAO-A gene promoter were more aggressive than peer-reared infants (Newman et al 2005). Furthermore, monkey infants carrying the −248 T allele of the CRF gene showed reduced exploration and greater ACTH and cortisol responses to social separation than carriers of the −248 C allele, and more so in peer-reared than in mother-reared monkeys (Barr et al 2009). Another single nucleotide CRF gene polymorphism (−2232 C, −2232 G) was also associated with behavioral and physiological differences in response to a social challenge presented by an unfamiliar male: carriers of the G allele had lower CSF levels of CRF, higher levels of ACTH, and showed bolder and more exploratory behavior than carriers of the C allele, independent of rearing condition (Barr et al 2008). Differences in stress reactivity, and also in alcohol consumption, between peer-reared and mother-reared monkeys have been reported in relation to polymorphisms in the DRD1 receptor gene (Newman et al 2009) and in the Neuropeptide Y (NPY) gene (Lindell et al 2010).
Similar to isolate-rearing, research investigating the biology of peer-rearing has also been extended to examine structural brain differences. One recent study has reported neuroanatomical differences between peer-reared and mother-reared monkeys. Specifically, peer-reared monkeys showed reduced vermis, dorsomedial prefrontal cortex, and dorsal anterior cingulated cortex, but no differences in corpus callosum and hippocampus, when compared to mother-reared individuals (Spinelli et al 2009).
It is clear from the studies reviewed above that maternal and social deprivation in early life can result in abnormalities and deficits in behavioral development accompanied by alterations in neural structures and neurochemical/neuroendocrine function. These alterations may reflect a combination of the effects of the early maternal separation experience with those of the subsequent rearing environment. The neurobiological and endocrine effects of isolation-rearing and peer-rearing, however, have been variable among different studies. Variability in developmental outcomes probably reflects variation in the degree of sensory, social, and motor deprivation experienced by socially deprived infants (part of which is intentionally manipulated by researchers and part of which is unintentionally caused by them though variation in animal housing conditions and laboratory protocols) and also variation in the type and magnitude of stimulation provided by the human caretakers with whom the socially deprived monkeys necessarily interact. The alterations induced by long-term maternal and social deprivation are generally greater the earlier the mother’s removal and the longer and the more complete the deprivation of stimuli. Since the peer-reared infants experience a lower degree of sensory, social, and motor deprivation than the isolation-reared infants, peer-rearing is considered a less intense form of social deprivation than isolation-rearing. Thus, when peer-reared infants are compared to isolation-reared and mother-reared infants, the assumption is made that these three groups of infants differ in the “quantity” of early social experience and that, as a result, the peer-reared infants should exhibit behavioral and physiological parameters that are intermediate between those of mother-reared and isolation-reared infants. In reality, both the quantity and the quality of early experience of each group of infants differ in multiple and complex ways from those of the other two groups. For example, it is almost impossible to assess whether peer-reared monkeys differ from mother-reared ones because they lack a mother, because they extensively interact with their human caretakers, because they drink formula instead of monkey milk, or because they have the opportunity to interact with peers much earlier and more extensively than the mother-reared monkeys.
The fact that the maternal and social deprivation paradigm combines and confounds different forms of deprivation (e.g., motor, sensory, and social) and stress (physical and psychosocial) makes it difficult to assess the specific mechanisms and pathways through which early adverse experience alters the course of normal biobehavioral development. Primate research conducted with maternal and social deprivation paradigms has undoubtedly contributed some general principles of the relationship between early stress and development such as: (1) early experiences that deviate greatly from the norm are those most likely to result in long-term effects, (2) the most dramatic long-term effects are observed after stressful challenges (i.e., not at baseline), and (3) there may be considerable interindividual variability in the extent to which early experiences affect biobehavioral development (Suomi 1991). We believe, however, that research with primate models can and should, not only contribute general principles, but also elucidate the specific mechanisms and pathways through which early stress affects development in humans. In this regard, however, the maternal and social deprivation paradigm has already shown its limitations.
Since the type of environmental deprivation experienced by isolation-reared and peer-reared monkeys is rarely, if ever, experienced by primates or humans under natural conditions, the ecological validity of such paradigms is limited. The functioning of stress-sensitive physiological systems is also typically impaired by these drastic alterations of early development and it cannot be expected that these alterations have adaptive consequences, i.e. result in later resilience to stress. Since we believe that the relationship between early stress exposure and later resilience is a natural phenomenon, rather than an artifact of experimental manipulations, this relationship should be investigated using models of early experience that are within the norm for a particular species, i.e. models with greater ecological validity than the maternal and social deprivation paradigms.
3.1.2. Long-term effects of short mother-infant separations in macaques
An alternate approach to isolate-rearing and peer-rearing models of early life stress has been to expose infant macaques to short mother-infant separation paradigms. Although macaque mother-infant separation protocols have varied across laboratories, in general, infants are separated from their mothers for brief periods ranging from a few days to a few weeks during the infant’s first months of life (Caine and Reite, 1983; Hinde and Spencer-Booth, 1971; Hinde et al 1978; Suomi et al 1983). In some cases, the infant is removed from its group and housed alone in a cage (e.g., Suomi et al 1983), while in others the mother is removed (e.g., Caine and Reite, 1983), or both mothers and infants are removed, separated, and individually housed (e.g., Hinde et al 1978). When sufficient detail is available from published reports, it is included in the findings from the mother-infant separation paradigms reviewed below.
The earliest studies of the long-term effects of short mother-infant separation in macaques were independently conducted in Hinde’s and Harlow’s laboratories in the early 1970s. In one study of rhesus monkeys, Spencer-Booth and Hinde (1971) separated infants from their mothers for 6 or 13 days at the age of 21–32 weeks. Infant activity and performance in a variety of tests were assessed when infants were 12 and 30 months old and compared to those of infants that had never been separated from their mothers. There were few or no behavioral differences in infants’ interactions with their mothers or in their tendency to approach novel objects in the home cage. When tested in a novel environment, however, the previously separated infants showed significantly greater disturbance of behavior, lower exploration, and lower manipulation of novel objects than controls. Such differences were evident at both 12 and 30 months of age, although they were less marked when the animals were older. Hinde et al (1978) subsequently reported some other effects of a brief maternal separation of 13 days on subsequent mother-infant interactions 5 months after the separation, and Stevenson-Hinde et al (1980) reported some differences in subjectively assessed personality measures in infants previously separated from their mothers.
In Harlow’s laboratory, the long-term effects of brief separations from the mother were investigated by Suomi et al (1983). In this study, four infants were subjected to repeated 4-day maternal separations conducted between the 3rd and 9th month of life. The separated infants spent more time in contact with their mothers following reunion, although the relative role played by mother and infant in maintaining contact was not assessed. Separated infants and non-separated control infants showed few or no differences in response to permanent separation from their mothers, which occurred at the age of 43 weeks, and during the subsequent 7 months of peer housing. When separated infants and controls were exposed to their mothers, 7 months after permanent separation, previously separated infants showed less interest in interacting with them than did non-separated control infants. Thus, although the long-term effects of early separations seemed to disappear in some situations (e.g., peer housing), they were apparent in others (e.g., in the presence of mothers). The mechanisms underlying the long-term effects of peer or maternal separations remained unclear.
Studies of the long-term effects of brief maternal separations that lasted 10 days in duration were also conducted with pigtail macaques by Reite and colleagues. Caine et al (1983) reported that 6 pigtail infants who experienced a 10-day maternal separation at 4–7 months of age did not differ in dominance rank but were rated as less sociable than controls at the age of 40.7 months. Capitanio and Reite (1984) reported that such previously separated infants had fewer social contact preferences, and Capitanio et al (1986) reported that they showed more disturbance behavior and longer latency to retrieve food (but no difference in cortisol levels) in a novel environment than controls when tested 2.5–4.9 years after the separation experience. Finally, Laudenslager et al (1985) found that when individuals previously separated from their mothers or peers for 10–14 days in infancy were later tested at 4–7 years of age, they showed a suppression of the in vitro lymphocyte proliferation in response to B cell and T cell mitogens, suggesting that the early separation experience resulted in altered immune function. In reviewing these studies, Reite (1987) argued that possible alterations in maternal behavior following early separation and reunion (as is known to be the case in rodents; e.g. Liu et al 1997) may have been the mechanism underlying the developmental effects of early separation on infant behavior and physiology. Due to lack of further research using this paradigm in macaques, the mechanisms underlying the developmental effects of brief separation experiences have remained unclear and the question of whether these effects should be viewed as the direct consequence of an acute stressful experience or as mediated by subsequent alterations in maternal behavior or maternal physiology (e.g., cortisol in mother’s milk) remains unanswered.
More recently, however, some of these questions have been addressed by studies of New World monkeys such as marmosets and squirrel monkeys, which have used repeated mother-infant separations as a model for studying the relationship between early stress and development. Some of these studies have not only examined how exposure to early stress results in increased vulnerability to stress later in life, but also how early life stress exposure may enhance later resilience under certain circumstances.
3.1.3. Long-term effects of short parent-infant separations in common marmosets
The parental separation paradigm in common marmosets was first employed in the early 2000s, and therefore is comparatively new amongst the various primate models considered herein. This paradigm is notable for involving both parents, the only such primate separation paradigm to do so. Common marmoset social ecology is characterized by monogamous breeding pairs, twin births, and intensive biparental care of young (Pryce 1996). Infants are in constant (i.e., 24 hours a day) contact with their parents throughout the first 2–3 weeks of life (Ingram 1977; Pryce 1993).
To examine the long-term consequences of early stressful experience, Pryce and colleagues exposed marmoset infants to daily parental separations on postnatal days 2–28 (Dettling et al 2002a; Dettling et al 2002b; Law et al 2009; Pryce et al 2004a; Pryce et al 2004b; Pryce et al 2002). During each separation session, marmoset monkeys are socially isolated in a plastic mouse cage which is placed in an isolation chamber with 4 W light (Dettling et al 2002b). Marmoset infants have total auditory, olfactory, and visual deprivation of their families and other monkeys during this time. Each infant is exposed to a total of 9 hours of social deprivation each week, with each session being variable in duration (30–120 minutes per session) and time of day, a feature implemented to increase stressor unpredictability. The parental separation procedure is carried out consecutively on each twin pair. Control infants are briefly handled on their parents’ backs daily during postnatal days 2–28. After postnatal day 28, infants typically remain undisturbed in their family groups.
Infants exposed to repeated parental separations have 12% lower body weights than control infants at the conclusion of this repeated separation protocol. The observed body weight loss is likely due to impaired homeostasis, reduction in time available for nursing, and/or reduction in milk quality/quantity from stressed moms (Dettling et al 2002b). These separation sessions, therefore, interfere with both the physiological and psychological needs of marmoset infants.
During the first year of life, marmosets exposed to repeated parental separations exhibited increased basal urinary NE levels, increased basal urinary dopamine levels, and increased systolic blood pressure in the absence of a significant change in heart rate compared to non-separated controls (Pryce et al 2004a; Pryce et al 2004b). Previously separated marmosets also perform fewer operant responses on a neuropsychological test, indicative of anhedonia (Pryce et al 2004b). Several domains of cognitive functioning are likewise impaired in this parental separation paradigm. For example, previously separated marmosets exhibit impaired behavioral inhibition on an object retrieval/detour task as well as impaired reversal learning on a two-way discrimination task (Pryce et al 2004a).
Studies of emotional and cognitive functioning in previously separated marmosets have recently been extended to include postmortem neuroanatomical and neurobiological analyses. Brains were collected when adolescent monkeys were 48 weeks of age. In the first paper from this series, genes implicated in synaptic function and plasticity were examined in the hippocampus. Previously separated marmosets exhibited a reduction in hippocampal GAP-43 mRNA and serotonin 1A receptor mRNA, findings similar to those reported for patients with stress-related mood disorders (Law et al 2009). Although previously separated monkeys did not differ from control monkeys on hippocampal volumes (Law et al 2009), evidence from a follow-up study indicates that repeated parental separations during the first month of life induce mild reductions in MR and GR gene expression in the hippocampus (in CA 1–2 subfield). These effects were found to be specific to this neuroanatomical region, as no differences in MR or GR gene expression were observed in the prefrontal cortex, other cortical areas, or the hypothalamus (Arabadzisz et al 2010). These findings are consistent with human data indicating that individuals with major depression likewise exhibit mild to moderate reductions in hippocampal MR and GR gene expression.
In summary, the marmoset model of parental separation has yielded important behavioral and neurobiological findings relevant to stress vulnerability. Perhaps due to its relatively recent conception, data are not yet available from this model of parental separation beyond one year of age (i.e., marmoset adolescence). There is a great deal of evidence for long-term effects of early experience in rodents, but surprisingly few studies have examined the enduring effects of early experiences in the same cohorts of adult monkeys or humans (Schaffer 2000). Given the growing interest in early experience effects in biological psychiatry, longitudinal studies of these behavioral, neuroendocrine, neuroanatomical, and molecular measures are critically needed in the marmoset parental separation paradigm to verify (or refute) findings from rodent research in animals with a life span of greater than 2–3 years.
In the common marmoset, as well as in the isolate-rearing and peer-rearing, paradigms, the types of early parental manipulations were all “non-biological events” (Dettling et al 2002b), and as such, were of limited ecological validity. Moreover, in each of these paradigms, key features of early stress exposure were shown to act together to undermine the development of resilience and produce stress vulnerable phenotypes. We now turn our attention to another parental separation model involving squirrel monkeys. This laboratory model is unique in that it is employed in late infancy when free-living infants often face the challenge of coping with intermittent separations from their mothers while foraging in the forest canopy. Not coincidentally, this is the only parental separation paradigm that produces stress resilience, and likely does so because it is employed during a developmental period when the key features of early stress exposure do not overwhelm the infant’s coping capacity as discussed in greater detail below.
3.1.4. Long-term effects of short mother-infant separations in squirrel monkeys
In this maternal separation model, called “stress inoculation”, squirrel monkey infants are initially raised in undisturbed natal groups of 3–5 mother-infant pairs. At ~ 17 weeks of age, when free-living young monkeys are becoming nutritionally independent (Boinski and Fragaszy 1989), natal groups are randomized to one of two postnatal treatment conditions. In one condition, monkeys are exposed to a 10-week stress inoculation protocol that consists of weekly 1-hour sessions of separation from the mother and natal group (Parker et al 2004). The separation session occurs in an elevated wire-mesh cage in a colony room different from the one in which the infant lives. During the separation, the infant is surrounded by monkeys housed in adjacent cages. Infants are able to vocally communicate with their mother and natal group which are housed in a colony room on the same hallway. Control monkeys remain in their home cages throughout the same developmental period. Separation protocols for different monkey cohorts have varied slightly over the years, with separations being fewer in frequency but of greater duration (i.e., 4–6 total separation sessions, each lasting 4–6 hours in duration) (Levine and Mody 2003) or beginning earlier in life and occurring with diminished frequency for longer periods (postnatal week 13, every other week for 5 hours) (Lyons et al 1999), or with the mother accompanying the infant during each separation session (Parker et al 2006). These protocols have generally produced the same developmental outcomes, and will be considered together below.
Short repeated social separations in squirrel monkeys evoke species-typical distress peep-calls, locomotor agitation, and acute elevations in plasma cortisol levels. Recovery of baseline measures is achieved after each social reunion (Coe et al 1983; Hennessy 1986; Stanton and Levine 1985). Unlike previous primate paradigms, the stress inoculation protocol provides repeated opportunities for emotion regulation, while not overwhelming the juvenile monkey’s capacity for coping with stress. The protocol is administered weekly or biweekly to allow sufficiently long intervals for recovery at an age when squirrel monkeys are developing physical, emotional, and psychosocial independence.
Outcome studies of squirrel monkeys exposed to this paradigm have shown that stress inoculated monkeys better regulate negative emotional arousal and exhibit diminished HPA axis activation in response to subsequent acute stressors. For example, at 9 months of age, stress inoculated vs. non-inoculated monkeys were tested with their mothers for 30 minutes on five consecutive days in a novel environment that contained an assortment of familiar and unfamiliar foods and toys (Parker et al 2004). The novel environment test revealed that stress inoculated vs. non-inoculated monkeys were less anxious as inferred by decreased maternal clinging, increased object exploration, and increased food consumption. Stress inoculated monkeys also more effectively used their mothers as a secure base from which to explore. Stress inoculated compared to non-inoculated monkeys also showed smaller increases in plasma cortisol and ACTH.
These experimental results indicate that stress inoculated monkeys more readily self-regulate negative emotional arousal, engage in more exploration, and exhibit diminished stress-induced HPA axis activation compared to non-inoculated monkeys. This conclusion agrees with findings from independent studies of two additional cohorts of stress inoculated monkeys. In one cohort, stress inoculated monkeys responded to the removal of mothers at weaning with fewer distress calls, more time spent in proximity to peers, and smaller increases in plasma cortisol levels compared to non-inoculated monkeys (Lyons et al 1999). In the other cohort, stress inoculated monkeys exhibited fewer distress calls, diminished adrenocortical activation, and smaller increases in CSF levels of MHPG compared to non-inoculated monkeys at 2, and again at 3, years of age (Levine and Mody 2003). Finally, stress inoculated monkeys exhibited enhanced sensitivity to glucocorticoid feedback in early adulthood compared to non-inoculated monkeys (Lyons et al 2000b). These observed stress inoculation-induced differences in cortisol suppression of the CRF-stimulated ACTH response (Lyons et al 2000b), CSF MHPG levels (Levine and Mody 2003), and plasma ACTH levels (Parker et al 2004) suggest that brain mechanisms above the pituitary enhance the stress inoculated monkey’s ability to diminish HPA axis activation in response to subsequent stressful life events.
Stress inoculated monkeys have also been evaluated for postnatal treatment differences in prefrontal-dependent cognitive response inhibition as juveniles (Parker et al 2005). Inhibitory control of the reaching response in marmosets is impaired in the marmoset monkey model reviewed above (Pryce et al 2004a) and in squirrel monkeys treated with cortisol according to a protocol that simulates a chronic HPA axis stress response (Lyons et al 2000a). The stress inoculated and non-inoculated monkeys performed equally well on all trials except for those that required inhibitory control of the straight-reaching response. Performance improved gradually over repeated test days, but at peak levels of performance all stress inoculated monkeys successfully completed all response inhibition trials whereas fewer than half of the non-inoculated monkeys achieved similar levels of success. These findings suggest that stress inoculation may have directly altered the neural substrates involved in cognitive function, or indirectly influenced cognitive performance by primarily changing emotion regulation. The available data do not help to distinguish between these two possibilities. It should nevertheless be noted that the rearing protocol was initiated during a period of increasing offspring independence. Far from exceeding the young monkeys’ coping abilities, the mildly stressful separation experiences which constituted the stress inoculation protocol may have provided important opportunities for stress inoculated monkeys to develop the capacity for enhanced emotion regulation. Non-inoculated monkeys, which had fewer emotional challenges, failed to adequately develop this capacity. Thus, when non-inoculated monkeys were faced with the demanding cognitive test, they engaged in more impulsive and perseverative behavior than stress inoculated monkeys, who were better equipped to deal with such challenges. Magnified across repeated life challenges, the cumulative consequences of how inoculated and non-inoculated monkeys respond to stress beginning in infancy may be profound.
Stress inoculation-induced resilience in monkeys resembles the effects of early intermittent “postnatal handling” in rats exposed to repeated, brief maternal separations. When studied as adults, “handled” rats display diminished emotionality, increased exploration, improved learning, and diminished HPA axis activation compared to rats raised in standard undisturbed laboratory conditions (Fernandez-Teruel et al 2002; Levine 1957; Levine 2000; Meaney et al 1996). In rats, these outcomes appear to reflect the effects of maternal behavior directed toward pups that are briefly separated and then returned to the nest (Denenberg 1999; Smotherman and Bell 1980). Importantly, the neuroendocrine indications of resilience observed in rats exposed to intermittent separations can be replicated in non-manipulated offspring that naturally receive high levels of maternal care (Liu et al 1997).
To determine whether resilience in monkeys exposed to early intermittent separations is maternally mediated, maternal behavior and subsequent neuroendocrine measures of resilience were examined in monkeys randomized to three postnatal conditions (Parker et al 2006). In one condition, each monkey was separated from its mother and the natal group for 10 weekly 1-hr sessions. In the second condition, each monkey and its mother were removed together as a pair and separated from the natal group for 10 weekly 1-hr sessions. In the third treatment condition, non-separated monkeys remained undisturbed in their natal groups. Neither separation condition induced long-lasting changes in maternal behavior (Parker et al 2006). Moreover, the transient changes observed in maternal behavior did not correspond with differences in the development of arousal regulation and resilience. Monkeys exposed along with their mother to intermittent separations received less maternal care in the home cage, as mothers direct their attention toward re-integration with the group. Yet both intermittent separation conditions induced neuroendocrine indications of resilience compared to control monkeys not exposed to intermittent separations. Specifically, monkeys separated alone or separated along with their mother exhibited diminished cortisol responses to a subsequent novel environment stress test compared to monkeys not exposed to either separation condition (Parker et al 2006). These and related findings suggest that arousal regulation and resilience correspond more closely to prior stress exposure than to separation-induced changes in maternal care. These findings also suggest that the mechanisms involved in fostering stress resilience may differ considerably between rodents and primates. However, it should be noted that recent investigations of developmental plasticity and early life stress in rats suggest that the rodent story of stress resilience may be more complicated than previously thought (Macri and Wurbel 2006).
Very little is known about the neuroanatomical basis of stress resilience. However, because prefrontal corticolimbic brain circuits play a role in cognitive control of behavior in humans and monkeys, regulate the HPA axis stress response in various species, and have been implicated in emotional resilience in humans (Diorio et al 1993; Garavan et al 1999; Herman et al 2003; Kern et al 2008; Konishi et al 1998; Milad et al 2007; Miller 2000; Ochsner and Gross 2005; Sullivan and Gratton 2002; Urry et al 2006), stress inoculated and non-inoculated squirrel monkeys were tested for differences in prefrontal cortical and/or hippocampal structural volumes at 3 years of age (Katz et al 2009). Early life stress inoculation promoted the growth of larger prefrontal cortical gray but not white matter volumes. This effect was primarily driven by differences in ventromedial prefrontal cortex, as dorsolateral prefrontal cortical volumes did not differ between stress inoculated and non-inoculated monkeys. These larger ventromedial prefrontal volumes do not reflect increased cortical thickness but instead represent surface area expansion (Katz et al 2009).
Similar to findings from the marmoset model reviewed above, exposure to early life stress inoculation did not affect hippocampal volumes. These data are in keeping with evidence that mildly stressful early experiences promote the development of larger prefrontal cortical volumes without affecting hippocampal volumes as shown for 5 year old adult male and female squirrel monkeys exposed to slightly different postnatal conditions (Lyons et al 2002; Lyons et al 2001). Experiential effects on brain structure are most potent when the neural circuit is maturing and before it is stabilized (Knudsen 2004). Hippocampal growth and development occurs primarily in utero (Eckenhoff and Rakic 1991; Khazipov et al 2001; Rakic and Nowakowski 1981), and so it is perhaps not surprising that structural stress inoculation effects are most evident in prefrontal cortex, the growth and development of which extends across childhood into early adulthood (Giedd et al 1999; Rakic 1995).
The absence of stress inoculation effects on hippocampal structure, however, does not preclude the existence of postnatal treatment-related differences in hippocampal molecular biology. Recent evidence from rodents (Kaffman and Meaney 2007) suggests that stress resilience may be mediated by increased hippocampal glucocorticoid receptor (GR) expression due to diminished methylation of a cytosine residue in the GR promoter. It is not known whether early life stress inoculation increases hippocampal GR expression in adulthood, protects against stress-induced GR downregulation, and whether changes in GR promoter methylation similar to those observed in stress resilient rodents underlie these effects in monkeys. Follow-up studies are required to address these important questions to better understand the molecular mechanisms underlying the etiology and maintenance of stress resilience in monkeys.
Similar to many of the primate models reviewed above, very little is known about the scope, extent, or enduring effects of early life stress inoculation in squirrel monkeys. For instance, it is not known whether early stress inoculation effects persist across the lifespan, as the latest assessments of stress inoculated monkeys occurred three years after completion of postnatal treatment protocols (Katz et al 2009; Levine and Mody 2003). The persistence of stress inoculation effects, therefore, has only been documented across 14% of the squirrel monkey lifespan. Longitudinal studies, which rigorously evaluate the enduring consequences of early life stress inoculation on behavioral, neuroendocrine, neuroanatomical, and molecular measures throughout adulthood, are critically needed to address this gap in knowledge.
In concluding our review of the various Parental Separation Models, we believe that the balance of evidence suggests that primate studies employing short parent-infant separations are more ecologically valid and therefore more promising models for research on stress vulnerability and resilience than those involving extended social deprivation. Even though wild primates may not experience mother-infant separations for the duration of those used in some laboratory paradigms, a short mother-infant separation is a naturally occurring event that is likely to occur in the lifetime of every primate and for which individuals are likely to have pre-programmed adaptive coping responses. In the next section we review the Maternal Behavior Models, which are likewise well positioned to provide critical insights into the role early experiences play in shaping developmental outcomes in humans.
3.2. Maternal behavior models
Maternal behavior models were developed in an attempt to address several limitations of the Parental Separation Models. First, Parental Separation Models, in their extreme forms, consider, and often confound, not only the effects of lack of social experience (the outcome of the maternal separation) but also the stressful effects of the process through which the deprivation of social experience occurs (e.g., permanent loss of the mother). Second, Parental Separation Models, with the exception of the squirrel monkey Stress Inoculation paradigm, typically have little ecological validity and therefore do not model challenging experiences the infant would face (and survive) under free-living conditions. Research employing Maternal Behavior Models has developed along two independent lines: one in which the quality of maternal behavior is experimentally manipulated through alterations in the mother’s ecological environment (e.g., foraging demand paradigms), and the other in which researchers take advantage of naturally occurring variation in maternal behavior. These latter paradigms include variation in maternal style, and maternal abuse.
3.2.1. Experimental alterations of maternal behavior: Foraging paradigms
Rosenblum and collaborators developed a primate model of early stress and development in which they investigated the developmental effects of experimental alterations of accessibility and amount of food in laboratory-housed bonnet macaque mother-infant dyads (Rosenblum and Paully 1984; Andrews and Rosenblum 1991). It was hypothesized that when food was abundant and easy to obtain mothers would encourage early independence, whereas when food was scarce and effort was required to obtain it, this could jeopardize offspring survival and hence mothers would become more protective. Finally, unpredictable changes in food availability and accessibility should pose stressful and conflicting demands on the mother and impair her ability to interact effectively with her infant.
In the early studies in which this model was developed, several mother-infant pairs were exposed to different foraging treatments: Low Foraging Demand (LFD), High Foraging Demand (HFD), and Variable Foraging Demand (VFD). In the LFD condition animals had access to ad libitum food, and such food could be retrieved without effort. In the HFD condition, animals had access to 6 times less food than the LFD animals. Finally, in the VFD condition, animals were exposed to a 2-week alternation of HFD and LFD. The treatment period began when infant ages ranged from 4 to 17 weeks and lasted 14 weeks. Shortly after the foraging treatments, mothers and infants were tested for 1 hour in a novel room for 4 consecutive days. Mother-infant pairs in all three experimental conditions spent more time in contact in the novel room but the increase was most marked for the VFD pairs. VFD infants also showed less object exploration and play. It was not clear, however, whether differences in mother-infant interactions were the result of differences in maternal behavior or infant activity, or both.
At the age of 2.5–3.5 years, after the infants had been permanently separated from their mothers and housed in peer groups, they were tested in a novel environment and the VFD individuals were reported as being less sociable and less assertive/dominants than the LFD individuals (Andrews and Rosenblum 1994). In subsequent studies, VFD individuals exhibited a number of differences from LFD or HFD individuals in terms of physiological variables or responses to pharmacological manipulations, suggesting hyperreactivity of the HPA axis and blunted noradrenergic responsivity. In one study, VFD and LFD juveniles showed differential responses to treatment with two anxiety-provoking drugs: a noradrenergic agent (yohimbine) and a serotonergic agent (mCPP). Relative to the LFD juveniles, VFD animals were hyperresponsive to yohimbine but hyporesponsive to mCPP (Rosenblum et al 1994). VFD juveniles also exhibited higher CSF concentrations of CRF, SOM, 5-HIAA, and HVA and lower CSF concentrations of cortisol, whereas HFD and LFD juveniles did not differ in these variables (Coplan et al 1996; 1998). However, because at the time of the study the VFD juveniles were 2 years old whereas the HFD and LFD juveniles were 4 years old, it could not be unequivocally excluded that age and not the early foraging treatment was responsible for the observed differences. Nor was it clear whether some of the observed differences reflected altered acute HPA axis responses to capture versus chronic alterations of the HPA axis activity. Finally, Smith et al. (1997) treated VFD and LFD juveniles with the alpha2-adrenoceptor agonist clonidine and found that individuals with higher baseline CSF levels of CRF showed less growth hormone secretion in response to the challenge, a finding that was interpreted as consistent with blunting of noradrenergic responsivity in these animals. As with the other models reviewed above, there has been some variability in when the application of the VFD paradigm is initiated. When VFD is initiated when infants are approximately 10–12 weeks of age, it induces enduring increases in CSF levels of CRF (Coplan et al 1996). However, if the VFD paradigm is initiated at around 18–20 weeks of age, experimental monkeys have lower CSF levels of CRF than control animals as adults (Mathew et al 2002). Whether or not these neuroendocrine changes are accompanied by more resilient behavioral indices (e.g., diminished anxiety) is unknown, but merits further investigation.
In the above reviewed studies of bonnet macaques, the developmental effects of the VFD treatment on behavior and neuroendocrine function were assumed to result from alterations in maternal behavior. As a result of the environmental manipulation, VFD mothers presumably became more anxious, erratic, dismissive, less responsive to their infants’ signals, and less likely to engage in the “intense compensatory patterns typical of normal mothers following periods of dyadic disturbance” (Coplan et al 1995; 1996; 1998). The empirical evidence that maternal behavior was affected by the foraging treatment, however, was weak. For example, the VFD mothers did not show significant differences in foraging between the HD and LD conditions (Andrews and Rosenblum 1991; Rosenblum & Paully 1984), and foraging only occurred less than 10% of the time in both conditions (Andrews and Rosenblum 1991). There were also few or no differences in mother-infant interactions between VFD and LFD animals. VFD and LFD pairs did not differ in the percentage of contacts broken by mothers, contacts made by mothers, and maternal rejections (Andrews and Rosenblum 1991). Thus, the causes of long-term behavioral and physiological alterations in VFD animals remain unclear.
Foraging paradigms have also been used in research on early stress and development in squirrel monkeys. Much of this work has focused on the immediate effects of foraging stress on maternal and infant behavior and physiology (Champoux et al 2001; Lyons et al 1998), and is therefore beyond the scope of this review. A few studies, however, have examined long-term effects of foraging paradigms and will be considered here. In these studies, squirrel monkey mothers were exposed to either high foraging demand (HFD) or low demand (LFD) foraging paradigms in which they had to search for food buried in foraging boards (HFD: 120% by body weight; LFD: 600% by body weight). These studies were conducted when infants were between 10–21 weeks of age. As reviewed previously, this developmental period corresponds with increasing physiological and psychological independence of offspring. Unlike the marmoset model of parental neglect, this paradigm does not induce infant weight loss (Lyons et al 1998). Nine years after completion of these early life conditions, HFD monkeys showed significantly diminished restraint stress-induced HPA axis activation, and faster recovery to baseline neuroendocrine values than LFD monkeys (Parker et al 2006). Thus, although the work with macaques did not reveal whether the manipulation of early experience achieved through the variable foraging paradigm resulted in behavioral or neuroendocrine signs of stress resilience, a study of squirrel monkeys suggests that this may be the case. If it could be demonstrated that the variable foraging manipulation reliably alters maternal behavior, and that changes in maternal behavior are responsible for the observed developmental outcomes, then this paradigm would represent an ecologically valid and promising model with which to investigate the development of stress vulnerability and resilience. Of course, since the goal of this model is to experimentally cause variation in maternal behavior within the normal range for a species, the usefulness of this model depends on the extent to which we understand how naturally occurring variation in maternal behavior affects the development of responsiveness to stress.
3.2.2. Naturally occurring variation in maternal style
Half a century of studies of mother-infant interactions conducted in the field or in captive but socially complex environments have shown that there are striking individual differences in maternal behavior. In Old World monkeys, mothers show marked individual differences in maternal style along the two orthogonal dimensions of maternal Protectiveness and Rejection (e.g., Fairbanks 1996; Hinde and Spencer-Booth 1971; Maestripieri 1998a; Schino et al 1995; Tanaka 1989). The dimension of maternal Protectiveness includes variation in the degree to which the mother physically restrains infant exploration, initiates proximity and contact, and provides nurturing behaviors such as grooming. The dimension of maternal Rejection includes the degree to which the mother limits the timing and duration of suckling, carrying, and contact. These dimensions combine to make four parenting style types: mothers who are high on both dimensions are classified as Controlling; mothers low on both dimensions are classified as Laissez-Faire; mothers high on one dimension and low on the other are classified as Protective or Rejecting. Longitudinal studies have demonstrated that parenting style along these dimensions tends to be consistent over time and across infants of the same mother (Berman 1990; Fairbanks 1989; Maestripieri et al 1999).
Several studies of macaques and vervet monkeys have examined variation in offspring independence from the mother and their tendency to explore the environment or respond to challenges at various ages in relation to exposure to variable levels of maternal protectiveness and maternal rejection experienced in early infancy. An early study by Simpson (1985) showed that exposure to high levels of maternal rejection in the first few months of life was associated with reduced infant’s exploration at the end of the first year. Subsequent studies, however, showed that infants reared by highly rejecting (or less responsive mothers) mothers generally develop independence at an earlier age (e.g., spend more time out of contact with their mothers, explore the environment more, and play more with their peers) than infants reared by mothers with low rejection levels (Bardi and Huffman 2006; Simpson et al 1989; Simpson & Datta 1990; Simpson and Simpson 1985). In contrast, infants reared by more protective mothers appear to be delayed in the acquisition of their independence and are relatively fearful and cautious when faced with challenging situations (Fairbanks and McGuire 1988; 1993). Although these findings may be the result of inherited temperamental similarities between mothers and offspring, similar findings were also obtained in studies in which maternal protectiveness was experimentally enhanced through manipulations of the environment (Fairbanks and McGuire 1987; Vochteloo et al 1993). For example, Fairbanks and McGuire (1987) showed that following the introduction of new males, vervet monkey mothers became more protective of their infants, presumably because of the increased risk of infanticide, or male aggression, or simply the social instability resulting from this manipulation. Increased maternal protectiveness affected the development of the offspring, as these offspring showed increased anxiety/fearfulness and lower tendency to explore when tested for responsiveness to novelty months or years later (Fairbanks and McGuire 1987; 1988).
The issue of whether these maternal effects on offspring reactivity to the environment also persist into later stages of development and adulthood has been addressed by a few studies of vervet monkeys and macaques. In vervet monkeys, juveniles who were exposed to greater maternal protectiveness in infancy had a higher latency to enter a new enclosure and to approach novel food containers (Fairbanks and McGuire 1988; 1993), whereas adolescent males reared by highly rejecting mothers were more willing to approach and challenge a strange adult male (Fairbanks 1996). Schino et al (2001) found no significant association between variation in maternal protectiveness or rejection early in life and the offspring’s behavior several years later in Japanese macaques. However, they did report a relationship between early maternal rejection and offspring responsiveness to stressful situations. Specifically, individuals that were rejected more by their mothers early in life were less likely to respond with submissive signals or with avoidance to an approach from another individual and exhibited lower rates of scratching in the 5-min period following the receipt of aggression. Finally, Maestripieri et al (2006b) showed that rhesus macaques that were rejected more by their mothers in the first 6 months of life engaged more in solitary play and greater avoidance of other individuals in the second year. In this study, the association between maternal behavior and offspring behaviors later in life was also reported in females that were cross-fostered at birth and reared by unrelated adult females, thus excluding the possibility of inherited temperamental similarities between mothers and offspring.
Developmental differences in reactivity to novel stimuli or responsiveness to other individuals are likely to be accompanied by differences in neurochemical and neuroendocrine substrates regulating emotional and social processes. Neuropeptides and hormones of the HPA axis such CRF, ACTH, and cortisol, along with the brain monoamine neurotransmitters norepinephrine, serotonin, and dopamine would be likely candidates, as these substances play an important role in the regulation of emotional and behavioral processes and their concentrations can be relatively stable over long periods of time. Very few and preliminary data are available on the relationship between variable maternal care and offspring hormonal profiles (e.g., Bardi et al 2005), although some relevant data have been provided by recent human studies (e.g., Gunnar 2003; Hane and Fox 2006).
Maestripieri et al (2006a; 2006b) reported that offspring reared by mothers with higher levels of maternal rejection exhibited lower CSF levels of 5-HIAA, MHPG, and HVA in the first 3 years of life than offspring reared by mothers with lower levels of rejection. This difference was observed in both non-fostered and cross-fostered infants, suggesting that exposure to variable parenting style early in life interacts with genetically inherited propensities in determining CSF monoamine metabolite levels (Rogers et al. 2004). Furthermore, CSF MHPG levels in the second year of life were negatively correlated with solitary play and avoidance of other individuals, while CSF 5-HIAA levels were negatively correlated with scratching rates, suggesting that individuals with low CSF 5-HIAA had higher anxiety (Maestripieri et al 1992). In contrast, variation in maternal protectiveness early in life did not predict later variation in CSF monoamine metabolite levels or offspring behavior (Maestripieri et al 2006a). Taken together, the studies by Maestripieri et al (2006a; 2006b) suggest that exposure to maternal rejection early in life may affect the development of different neural circuits underlying emotion regulation, ranging from fear to anxiety to impulse control.
It is very likely that the effects of variable maternal protectiveness and rejection on behavioral and neuroendocrine development result from different mechanisms. Maternal rejection is a physically and psychologically stressful experience for a primate infant. Mothers often reject their infants by hitting them or biting them, which appears to cause pain and distress in the infant. Being denied bodily contact and access to nipple, even in the absence of physical punishment, also causes significant distress in infants, which is expressed in loud screams and geckers, and temper tantrums (Maestripieri 2002). Frequently rejected infants also show behavioral signs of depression. Therefore, exposure of different rates of maternal rejection early in life is directly comparable to exposure to a stressor with different levels of intensity. In rhesus monkeys, the average infants begin being rejected in the 3rd or 4th week of life at the rate of 1 episode every 2 hours, or less (Maestripieri 1998b). The rate of rejection gradually increases as infants grow older and peaks at 6 months of age, when mothers resume their mating activities. Some infants do not experience rejection at all, however, while others are rejected at the rate of 3–4 episodes per hour, or higher, as early as in their first week of life (Maestripieri 1998b). When the behavioral and physiological effects of rejection are considered together, they support the resilience model of stress development, which assumes a J-shaped relationship between early stress intensity and the development of stress vulnerability and rejection. In fact, the data reviewed above suggest that infants that experience little or no rejection become fearful and behaviorally inhibited later in life, whereas those exposed to extremely high rates of rejection become highly anxious and impulsive. Although the relevant neuroendocrine data are still lacking, the behavior of infants exposed to moderate levels of rejection in the first few months of life suggests that they show adaptive responses to challenges and resilience to stress later on.
Unlike rejection, maternal protectiveness in itself is not stressful and exposure to different levels of protectiveness is not expected to directly affect the development of stress-sensitive physiological systems such as the HPA axis or the serotonergic system. Different levels of maternal protectiveness, however, provide infants with different opportunities to independently explore the environment and develop behavioral and physiological coping strategies to deal with stress. Again, the data reviewed above suggest that moderate levels of protectiveness may provide infants with opportunities to master environmental challenges with their mothers’ supervision and support, and therefore foster resilience, while too little or too much protectiveness may hamper the developing individual’s ability to effectively cope with challenges. More behavioral and physiological data are needed to demonstrate the developmental effects of variation in maternal protectiveness, as well as to investigate the interactive effects of simultaneous variation in protectiveness and rejection.
That variation in maternal style, at least in the maternal rejection dimension, has long-lasting consequences for development is also demonstrated by intergenerational effects. Maestripieri et al (2007) found significant similarities in maternal rejection rates between mothers and daughters for both nonfostered and cross-fostered rhesus females (see also Berman 1990), suggesting that the daughters’ behavior was affected by exposure to their mothers’ rejection in their first 6 months of life. Both nonfostered and cross-fostered rhesus females reared by mothers with high rates of maternal rejection had significantly lower CSF concentrations of 5-HIAA in their first 3 years of life than females reared by mothers with lower (below the median) rates of maternal rejection, and that low CSF 5-HIAA was associated with high rejection rates when the daughters produced and reared their first offspring (Maestripieri et al 2006a; 2007). Long-term changes in the offspring serotonergic system and concomitant changes in anxiety and impulsivity resulting from early exposure to variable maternal rejection may be responsible not only for the intergenerational transmission of maternal rejection rates, but also for the transmission of infant abuse.
3.2.3. Naturally occurring maternal abuse
Abusive parenting represents one extreme of the range of variation in maternal behavior in primates. Among rhesus macaques and other cercopithecine monkeys living in large captive groups, 5–10% of all infants born in a given year are physically abused by their mothers (Maestripieri and Carroll 1998a; 1998b; Maestripieri et al 1997). In rhesus macaques, abusive mothers may drag their infants by their tail or leg, or throw them in the air. Abuse bouts last only a few seconds, and the rest of the time abusive mothers show competent patterns of maternal behavior. Unlike humans, in which child physical maltreatment is often accompanied by neglect, in rhesus macaques abuse and neglect do not occur together and are displayed by individuals with different characteristics (e.g. neglectful mothers tend to be very young or very old, whereas abuse occurs regardless of the mother’s age). Abuse is most frequent in the first month of infant life and rare or nonexistent after the third month, when infants are more independent from their mothers (Maestripieri 1998b). Rhesus mothers can give birth once a year, and abusive mothers generally maltreat all of their infants with similar rates and patterns of behavior (Maestripieri et al 1999). The contributions of infant behavior to the occurrence of abuse are negligible, whereas abusive behavior appears to be a stable maternal trait that is transmitted across generations, from mothers to daughters. As a result, it is concentrated in particular families and absent in others (Maestripieri and Carroll 1998b). Cross-fostering experiments demonstrated that early experience plays an important role in the intergenerational transmission of infant abuse (Maestripieri 2005a). Approximately half of cross-fostered and non-cross-fostered females abused early in life exhibit abusive parenting with their first-born offspring (Maestripieri 2005a), and those who do so have lower CSF concentrations of 5-HIAA than those who don’t (Maestripieri et al 2006a; 2007).
The natural occurrence of infant abuse in monkeys provides the opportunity to study the developmental consequences of an early adverse experience that is within the range of experience of the species and shares many similarities with child maltreatment. Although monkey infants who survive early maternal abuse appear to be far less behaviorally and physically traumatized than socially deprived monkeys, abuse perpetrated by the infant’s biological mother probably entails a psychological trauma with profound negative consequences. Moreover, even if abuse is limited to the first months of infant life, continuous coexistence with the abusive mother and observation of abuse being repeated with younger siblings could contribute to reinforce and perpetuate the traumatic effects of abuse into adulthood.
Observations of social and behavioral development have suggested that abused infants may be delayed in the acquisition of independence from their mothers and in the development of peer relations in the first year of life (Maestripieri and Carroll 1998c). In rhesus macaques, the stressful (both physically and psychologically) experience of being abused early in life results in both acute and long-term alterations in HPA axis function. In recent studies, 10 abused and 10 control infants were studied during their first 6 months of life (McCormack et al. 2009; under review). Basal morning levels of cortisol were measured at 1, 3 and 6 months of age, and ACTH and cortisol responses to stress were measured in month 6. In addition, infants were genotyped for the serotonin transporter (SERT) gene and individuals carrying one or two copies of the short allele of this gene were compared to those carrying two copies of the long allele. During the first month, when physical abuse rates were the highest, abused infants had elevated basal morning cortisol levels compared to controls and showed greater distress responses to handling. In this month, in addition to a main effect of abuse on basal cortisol levels, there was also a significant interaction between early experience and SERT genotype such that the effects of abuse on basal cortisol levels were especially strong in infants carrying the short SERT allele. After the first month, abused infants’ basal HPA axis function recovered to levels similar to controls. Despite the normalization of basal activity, there were group by sex effects on the HPA axis stress response in month 6, such that abused males showed significantly higher ACTH stress responses than control males when exposed to novelty stress in the absence of the mother. The higher ACTH stress responses were associated with higher levels of anxious behaviors at that age. Thus, abused exhibited both increased HPA axis activity and increased emotional reactivity in the period of time while abuse occurred and for a few months following abuse.
In a larger study comparing the development of 21 abused and 21 nonabused rhesus monkey infants, the infants who were physically abused by their mothers in the first 3 months of life showed significantly different responses to CRF challenges performed at 6-month intervals during their first 3 years of life when compared to nonabused infants (Sanchez et al 2010). Specifically, the administration of exogenous CRF resulted in a greater increase in plasma cortisol concentrations in abused than in nonabused infants, both males and females, when these were 6, 12, 18, 24, 30 and 36 months old. The abused infants also showed a blunted plasma ACTH response to CRF, but this difference was observed only at 6 months of age. The blunted ACTH response observed in the abused monkey infants may be due to rapid negative feedback glucocorticoid inhibition, which normally inhibits the secretion of ACTH when there is a rapid increase in circulating cortisol, or to a dysfunction of the mechanisms that regulate the anterior pituitary’s response to hypothalamic CRF. Finally, a subset of abused females, but not males, also showed greater plasma cortisol responses to stress (a 20-min novel environment test) than nonabused females during their first 3 years of life (Maestripieri 2005b). Taken together, these results suggest that early maternal abuse results in greater adrenocortical, and possibly also pituitary, responsiveness to challenges later in life. The greater cortisol levels (basal, and in response to stress and to CRF) observed in the abused subjects are consistent with the results of many animal and human studies showing that individuals who are exposed to traumatic stress early in life exhibit later HPA hyperreactivity to challenges (see Gunnar and Quevedo 2007, for a review). Blunted ACTH responses to a CRF challenge have also been observed in abused children (De Bellis et al 1994; Kaufman et al 1997) as well as in adults suffering from clinical depression (e.g. Holsboer et al 1987).
All of the above reviewed research on the developmental effects of maternal abuse in macaques has been conducted with infants exposed to relatively low rates of abuse, whose life was not in jeopardy and which, in many cases, suffered only minor bruises and scratches that did not require an intervention. Although maternal abuse invariably begins in the 1st or 2nd week of life, peaks in frequency later in the first month, and gradually decreases until it ends by the end of the 3rd month (Maestripieri 1998b), there are stable differences among abusive mothers in the rate with which they exhibit abuse. Essentially, abuse that occurs at a rate of 1 or more episodes per hour in the 1st or 2nd week of life can potentially jeopardize the infant’s life (some rhesus monkey mothers that exhibit such high rates of abuse end up killing all of their infants, year after year; Maestripieri & Carroll 1998b), whereas abuse rates lower than 1 episode per hour are associated with good chances of infant survival. Since no data are available on the behavioral and neuroendocrine development of infants exposed to very different levels of abuse, the effects of variation in the intensity of this stressor on the development of stress vulnerability vs. resilience are not well understood. The data reviewed above concerning infants exposed to low/moderate levels of abuse suggest that these infants exhibit increased vulnerability to stress later in life, rather than resilience. This effect, however, may be the result of the high rates of maternal rejection that typically accompany maternal abuse, regardless of its frequency, rather than the result of abuse alone. Further research is needed to examine the effects of different rates of abuse on development as well as to disentangle the effects of high maternal rejection and abuse when they co-occur.
4. Summary and translational implications of primate models
Research conducted with primates during the last 4–5 decades has made important contributions to the understanding of the effects of early experience on the development of stress vulnerability and resilience. Observations of mother-infant interactions and early infant development in monkeys provided the foundations upon which attachment theory was formulated. Attachment theory predicts that exposure to variable maternal responsiveness and behavior early in life results in differences in personality and reactivity to the environment later on (Bowlby 1969). Primate studies of infants that were separated from their mothers and raised under conditions of social deprivation confirmed that a minimal amount of social experience is required for normal social development. Such studies, however, did not clarify whether it was the absence of a primary caregiver or the complete lack of social experience that produced subsequent dysregulation of behavior, emotion, and stress-sensitive physiological systems. The recognition that there is variation in early interactions with caregivers and that this variation has important consequences for infant development is a critical insight that has redirected the focus of primate studies from deprivation of early experience to altering the quality of early social experiences.
Although primate research has increasingly acknowledged the important role played by the quality of early experience in the development of stress vulnerability and resilience, primate research must further explore the role played by the frequency, duration, and timing of early stress exposure. Knowing the frequency, duration, and timing with which specific social experiences occur during development may be as important as knowing the quality of those experiences in explaining differences in developmental outcomes. The more recent primate models presented here lend themselves to expanding research into studies that take intensity and timing effects into account while documenting the contribution made by the quality of social experience.
The challenge to primate researchers interested in modeling early stress and development is to find experimental paradigms that reproduce events and circumstances within the normal range of experience of their species. We believe that primate paradigms that are based on naturally occurring phenomena, or at least that reproduce in the laboratory phenomena that are within the range of experience of a species, hold great promise as models for research in this area. Future research with primates will also benefit from the incorporation of the notion that a particular early stressful experience may not necessarily result in the same outcome in different individuals or circumstances, and that there may be multiple pathways to the same developmental outcome (Cicchetti 1993). In primates, just as in humans, stress-related pathologies such as anxiety and depression, as well as resilience to such stress-related disorders, are unlikely to be the direct consequence of a single adverse experience. Rather, they are likely to be multiply determined and dependent on the balance of risk and protective factors, including individual or genetic factors (e.g., temperament, genetic polymorphisms, gender) and environmental or social factors (e.g., social support, dominance rank, maternal style). Primate models that combine key features of early stress exposure (e.g., the type, duration, frequency, ecological validity, sensory modality, and developmental timing), and consider them in the context of risk and protective factors are in their infancy, but hold extraordinary promise for understanding the vast variability in developmental outcomes observed in human populations. Moreover, a better understanding of the etiology and psychobiology of stress vulnerability and resilience is of great clinical significance because such knowledge could subsequently be used to prevent stress-related psychiatric disorders by enhancing resistance to psychosocial adversity.
Table 1.
Primate models of early life stress and the features of the early stressful experiences.
| Human Phenomenon | Animal Model | Species |
|---|---|---|
| Parental Separation Models (parents absent) | ||
| Parental Loss/Rearing in Extremely Deprived Environment | Isolate/Surrogate-Rearing | Rhesus monkeys and other Old World monkeys and apes |
| Parental Loss/Rearing with Minimal Adult Contact | Peer-Rearing | Rhesus monkeys |
| Non-Permanent Disruption of the Parent-Child Bond | Repeated Parental Separations | Old World (e.g. rhesus monkeys) and New World (e.g. marmosets and squirrel monkeys) monkeys |
Acknowledgments
This was work was supported in part by Public Health Service Grants MH47573 and MH77884. We thank Serena Tanaka for library research and for creating Figure 1.
Figure 1.
Conceptual diagram identifying stressor features, stressor perception, modulating risk and protective factors, and developmental outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Anderson CO, Mason WA. Early experience and complexity of social organization in groups of young rhesus monkeys. J Comp Physiol Psychol. 1974;87:681–690. [Google Scholar]
- Anderson CO, Mason WA. Competitive social strategies in groups of deprived and experienced rhesus monkeys. Dev Psychobiol. 1978;11:289–299. doi: 10.1002/dev.420110402. [DOI] [PubMed] [Google Scholar]
- Andrews MW, Rosenblum LA. Attachment in monkey infants raised in variable- and low-demand environments. Child Dev. 1991;62:686–693. [PubMed] [Google Scholar]
- Andrews MW, Rosenblum LA. The development of affiliative and agonistic social patterns in differentially reared monkeys. Child Dev. 1994;65:1398–1404. doi: 10.1111/j.1467-8624.1994.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Arabadzisz D, Diaz-Heijtz R, Knuesel I, Weber E, Pilloud S, Dettling AC, et al. Primate early life stress leads to long-term mild hippocampal decreases in corticosteroid receptor expression. Biol Psychiatry. 2010;67:1106–1109. doi: 10.1016/j.biopsych.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Bardi M, Bode AE, Ramirez SM, Brent LY. Maternal care and the development of the stress response. Am J Primatol. 2005;66:263–278. doi: 10.1002/ajp.20143. [DOI] [PubMed] [Google Scholar]
- Bardi M, Huffman MA. Maternal behavior and maternal stress are associated with infant behavioral development in macaques. Dev Psychobiol. 2006;48:1–9. doi: 10.1002/dev.20111. [DOI] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, Lindell SG, Kasckow JW, Suomi SJ, Goldman D, Higley JD, Heilig M. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci USA. 2009;106:14593–14598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, Zhou Z, Schwandt ML, Lindell SG, McKee M, Becker ML, Kling MA, Gold PW, Higley JD, Heilig M, Suomi SJ, Goldman D. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2008;65:934–44. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence LHPA-axis response to stress in infant macaques. Biol Psychiatry. 2004a;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Higley JD. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004b;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Bebbington P, Der G, MacCarthy B, Wykes T, Brugha T, Sturt P, Potter J. Stress incubation and the onset of affective disorders. Br J Psychiatry. 1993;162:358–362. doi: 10.1192/bjp.162.3.358. [DOI] [PubMed] [Google Scholar]
- Berman CM. Intergenerational transmission of maternal rejection rates among free-ranging rhesus monkeys. Anim Behav. 1990;39:329–337. [Google Scholar]
- Boinski S, Fragaszy DM. The ontogeny of foraging in squirrel monkeys, Saimiri oerstedi. Anim Behav. 1989;37:415–428. [Google Scholar]
- Bowlby J. Attachment and Loss. I. Attachment. New York: Basic Books; 1969. [Google Scholar]
- Bowman RE, Wolf RC. Plasma 17-Ohcs response of the infant rhesus monkey to a noninjurious, noxious stimulus. Proc Soc Exp Biol Med. 1965;119:133–135. doi: 10.3181/00379727-119-30118. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Chesterman E. Life events, social support, and cardiovascular reactivity in adolescence. J Dev Behav Pediatr. 1990;11:105–111. [PubMed] [Google Scholar]
- Brown GW, Harris TO, Hepworth C, Robinson R. Clinical and psychosocial origins of chronic depressive episodes. II. A patient enquiry. Br J Psychiatry. 1994;165:457–465. doi: 10.1192/bjp.165.4.457. [DOI] [PubMed] [Google Scholar]
- Caine NG, Earle H, Reite M. Personality traits of adolescent pig-tailed monkeys (Macaca nemestrina): an analysis of social rank and early separation experience. Am J Primatol. 1983;4:253–260. doi: 10.1002/ajp.1350040304. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Rasmussen KLR, Snyder DS, Laudenslager M, Reite M. Long-term follow-up of previously separated pigtail macaques: group and individual differences in response to novel situations. J Child Psychol Psychiatry. 1986;27:531–538. doi: 10.1111/j.1469-7610.1986.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Reite M. The roles of early separation experience and prior familiarity in the social relations of pigtail macaques: a descriptive multivariate study. Primates. 1984;25:475–484. [Google Scholar]
- Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29:777–784. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- Chamove AS, Rosenblum LA, Harlow HF. Monkeys (Macaca mulatta) raised with only peers: A pilot study. Anim Behav. 1973;21:316–325. doi: 10.1016/s0003-3472(73)80073-9. [DOI] [PubMed] [Google Scholar]
- Champoux M, Coe CL, Schanberg SM, Kuhn CM, Suomi SJ. Hormonal effects of early rearing conditions in the infant rhesus monkey. Am J Primatol. 1989;19:111–117. doi: 10.1002/ajp.1350190204. [DOI] [PubMed] [Google Scholar]
- Champoux M, Hwang L, Lang O, Levine S. Feeding demand conditions and plasma cortisol in socially-housed squirrel monkey mother-infant dyads. Psychoneuroendocrinology. 2001;26:461–477. doi: 10.1016/s0306-4530(01)00006-3. [DOI] [PubMed] [Google Scholar]
- Champoux M, Metz B, Suomi SJ. Behavior of nursery/peer-reared and mother-reared rhesus monkeys from birth through 2 years of age. Primates. 1991;32:509–514. [Google Scholar]
- Cicchetti D. Developmental psychopathology: Reactions, reflections, projections. Dev Review. 1993;13:471–502. [Google Scholar]
- Clarke AS. Social rearing effects on HPA activity over early development and in response to stress in rhesus monkeys. Dev Psychobiol. 1993;26:433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- Coe CL, Glass JC, Wiener SG, Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8:401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci USA. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Rosenblum LA, Friedman S, Bassoff TB, Gorman JM. Behavioral effects of oral yohimbine in differentially reared nonhuman primates. Neuropsychopharmacology. 1992;6:31–37. [PubMed] [Google Scholar]
- Coplan JD, Rosenblum LA, Gorman JM. Primate models of anxiety. Longitudinal perspectives. Psychiatr Clin North Amer. 1995;18:727–743. [PubMed] [Google Scholar]
- Coplan JD, Trost RC, Owens MJ, Cooper TB, Gorman JM, Nemeroff CB, Rosenblum LA. Cerebrospinal fluid concentrations of somatostatin and biogenic amines in grown primates reared by mothers exposed to manipulated foraging conditions. Arch Gen Psychiatry. 1998;55:473–477. doi: 10.1001/archpsyc.55.5.473. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, Trickett P, Putnam FW. Adrenal axis dysregulation in sexually abused girls. J Clin Endocr Metab. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Commentary: is maternal stimulation the mediator of the handling effect in infancy? Dev Psychobiol. 1999;34:1–3. doi: 10.1002/(sici)1098-2302(199901)34:1<1::aid-dev2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacol Biochem Behav. 2002a;73:259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Repeated parental deprivation in the infant common marmoset (Callithrix jacchus, Primates) and analysis of its effects on early development. Biol Psychiatry. 2002b;52:1037–1046. doi: 10.1016/s0006-3223(02)01460-9. [DOI] [PubMed] [Google Scholar]
- Dienske H, de Jonge G. A comparison of development and deprivation in non- human primates and man. Journal of Human Evolution. 1982;11 (6):511–516. [Google Scholar]
- Dienstbier RA. Arousal and physiological toughness: implications for mental and physical health. Psychol Rev. 1989;96:84–100. doi: 10.1037/0033-295x.96.1.84. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenhoff MF, Rakic P. A quantitative analysis of synaptogenesis in the molecular layer of the dentate gyrus in the rhesus monkey. Dev Brain Res. 1991;64:129–135. doi: 10.1016/0165-3806(91)90216-6. [DOI] [PubMed] [Google Scholar]
- Edge MD, Ramel W, Drabant EM, Kuo JR, Parker KJ, Gross JJ. For better or worse? Stress inoculation effects for implicit but not explicit anxiety. Depress Anxiety. 2009;26:831–837. doi: 10.1002/da.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck H. Stress, disease, and personality: The ‘inoculation effect’. In: Cooper CL, editor. Stress Research: Issues for the eighties. Chichester, England: John Wiley and Sons, Ltd; 1983. pp. 121–146. [Google Scholar]
- Fairbanks LA. Early experience and cross-generational continuity of mother-infant contact in vervet monkeys. Dev Psychobiol. 1989;22:669–681. doi: 10.1002/dev.420220703. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA. Individual differences in maternal styles: causes and consequences for mothers and offspring. Adv Study Behav. 1996;25:579–611. [Google Scholar]
- Fairbanks LA, McGuire MT. Mother-infant relationships in vervet monkeys: response to new adult males. Int J Primatol. 1987;8:351–366. [Google Scholar]
- Fairbanks LA, McGuire MT. Long-term effects of early mothering behavior on responsiveness to the environment in vervet monkeys. Dev Psychobiol. 1988;21:711–724. doi: 10.1002/dev.420210708. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, McGuire MT. Maternal protectiveness and response to the unfamiliar in vervet monkeys. Am J Primatol. 1993;30:119–129. doi: 10.1002/ajp.1350300204. [DOI] [PubMed] [Google Scholar]
- Fergus S, Zimmerman MA. Adolescent resilience: a framework for understanding healthy development in the face of risk. Annu Rev Public Health. 2005;26:399–419. doi: 10.1146/annurev.publhealth.26.021304.144357. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teruel A, Gimenez-Llort L, Escorihuela RM, Gil L, Aguilar R, Steimer T, Tobena A. Early-life handling stimulation and environmental enrichment: are some of their effects mediated by similar neural mechanisms? Pharmacol Biochem Behav. 2002;73:233–245. doi: 10.1016/s0091-3057(02)00787-6. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Greenough WT. Cerebellar plasticity: Modification of Purkinje cell structure by differential rearing in monkeys. Science. 1979;206:227–229. doi: 10.1126/science.113873. [DOI] [PubMed] [Google Scholar]
- Forest KB. The interplay of childhood stress and adult life events on women’s symptoms of depression. Dissert Abstr s Intern. 1991;51:3237. [Google Scholar]
- Frank E, Anderson B, Reynolds CF, 3rd, Ritenour A, Kupfer DJ. Life events and the research diagnostic criteria endogenous subtype. A confirmation of the distinction using the Bedford College methods. Arch Gen Psychiatry. 1994;51:519–524. doi: 10.1001/archpsyc.1994.03950070011005. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmezy N, Masten AS, Tellegen A. The study of stress and competence in children: a building block for developmental psychopathology. Child Dev. 1984;55:97–111. [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Hof PR, McKinney WT, Morrison JH. Quantitative analysis of tuberoinfundibular tyrosine hydroxylase- and corticotropin-releasing factor-immunoreactive neurons in monkeys raised with differential rearing conditions. Exp Neurol. 1993a;120:95–105. doi: 10.1006/exnr.1993.1043. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Hof PR, McKinney WT, Morrison JH. The noradrenergic innervation density of the monkey paraventricular nucleus is not altered by early social deprivation. Neurosci Lett. 1993b;158:130–134. doi: 10.1016/0304-3940(93)90246-h. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Integrating neuroscience and psychological approaches in the study of early experiences. Ann NY Acad Sci. 2003;1008:238–247. doi: 10.1196/annals.1301.024. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Ann Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Haglund ME, Nestadt PS, Cooper NS, Southwick SM, Charney DS. Psychobiological mechanisms of resilience: Relevance to prevention and treatment of stress-related psychopathology. Dev Psychopathol. 2007;19:889–920. doi: 10.1017/S0954579407000430. [DOI] [PubMed] [Google Scholar]
- Hane AA, Fox NA. Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psych Science. 2006;117:550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Harlow HF. Affectional response in the infant monkey. Science. 1959;130:421–432. doi: 10.1126/science.130.3373.421. [DOI] [PubMed] [Google Scholar]
- Harlow HF. Age-mate or peer affectional system. Adv Study Behav. 1969;2:333–383. [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Multiple, brief maternal separations in the squirrel monkey: Changes in hormonal and behavioral responsiveness. Physiol Behav. 1986;36:245–250. doi: 10.1016/0031-9384(86)90011-9. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Higley JD. Aggression. In: Maestripieri D, editor. Primate Psychology. Cambridge: Harvard University Press; 2003. pp. 17–40. [Google Scholar]
- Higley JD, Hopkins WD, Thompson WW, Byrne EA, Hirsch RM, Suomi SJ. Peers as primary attachment sources in yearling rhesus monkeys (Macaca mulatta) Dev Psychol. 1992a;28:1163–1171. [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology. 1991;103:551–556. doi: 10.1007/BF02244258. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry. 1992b;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Higley JD, King ST, Hasert MF, Champoux M, Suomi SJ, Linnoila M. Stability of interindividual differences in serotonin function and its relationship to aggressive wounding and competent social behavior in rhesus macaque females. Neuropsychopharmacology. 1996a;14:67–76. doi: 10.1016/S0893-133X(96)80060-1. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996b;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- Hinde RA, Spencer-Booth Y. Towards understanding individual differences in rhesus mother-infant interaction. Anim Behav. 1971;19:165–173. [Google Scholar]
- Hinde RA, Leighton-Shapiro ME, McGinnis L. Effects of various types of separation experience on rhesus monkeys five months later. J Child Psychol Psychiatry. 1978;19:199–211. doi: 10.1111/j.1469-7610.1978.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Holmes FB. Experimental study of the fears of young children. In: Jersild AT, Holmes FB, editors. Children’s fears, Vol. 20. Child development monographs. New York: Teacher’s college, Columbia University; 1935. pp. 167–296. [Google Scholar]
- Holsboer F, Gerkin A, Stalla G, Muller A. Blunted aldosterone and ACTH release after human RH administration in depressed patients. Am J Psychiatry. 1987;144:229–231. doi: 10.1176/ajp.144.2.229. [DOI] [PubMed] [Google Scholar]
- Huether G, Doering S, Ruger U, Ruther E, Schussler G. The stress-reaction process and the adaptive modification and reorganization of neuronal networks. Psychiatry Res. 1999;87:83–95. doi: 10.1016/s0165-1781(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Ingram JC. Interactions between parents and infants, and the development of independence in the common marmoset (Callithrix jacchus) Anim Behav. 1977;25:811–827. [Google Scholar]
- Insel TR. Oxytocin and the neurobiology of attachment. Behav Brain Sci. 1992;15:515–516. doi: 10.1017/S0140525X00069818. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA. Social behavior and gender in biomedical investigations using monkeys: studies in atherogenesis. Lab Anim Sci. 1991;41:334–343. [PubMed] [Google Scholar]
- Katz M, Liu C, Schaer M, Parker KJ, Ottet MC, Epps A, et al. Prefrontal plasticity and stress inoculation-induced resilience. Dev Neurosci. 2009;31:293–299. doi: 10.1159/000216540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, Wells W, Ryan ND. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biol Psychiatry. 1997;42:669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Esclapez M, Caillard O, Bernard C, Khalilov I, Tyzio R, et al. Early development of neuronal activity in the primate hippocampus in utero. J Neurosci. 2001;21:9770–9781. doi: 10.1523/JNEUROSCI.21-24-09770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshaba DM, Maddi SR. Early experiences in hardiness development. Cons Psychol J: Pract Res. 1999;51:106–116. [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Kraemer GW. A psychobiological theory of attachment. Behav Brain Sci. 1992;15:493–541. doi: 10.1017/S0140525X00069752. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, Ebert MH, Lake CR, McKinney WT. Cerebrospinal fluid measures of neurotransmitter changes associated with pharmacological alteration of the despair response to social separation in rhesus monkeys. Psychiatry Res. 1983;11:303–315. doi: 10.1016/0165-1781(84)90004-0. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, Ebert MH, Lake CR, McKinney WT. Hypersensitivity to d-amphetamine several years after early social deprivation in rhesus monkeys. Psychopharmacology. 1984;82:266–271. doi: 10.1007/BF00427788. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, Ebert MH, Schmidt DE, McKinney WT. A longitudinal study of the effect of different social rearing conditions on cerebrospinal fluid norepinephrine and biogenic amine metabolites in rhesus monkeys. Neuropsychopharmacology. 1989;2:175–189. doi: 10.1016/0893-133x(89)90021-3. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, McKinney WT. Interactions of pharmacological agents which alter biogenic amine metabolism and depression. An analysis of contributing factors within a primate model of depression. J Affect Dis. 1979;1:33–54. doi: 10.1016/0165-0327(79)90023-5. [DOI] [PubMed] [Google Scholar]
- Laudenslager M, Capitanio JP, Reite M. Possible effects of early separation experiences on subsequent immune function in adult macaque monkeys. Am J Psychiatry. 1985;142:862–864. doi: 10.1176/ajp.142.7.862. [DOI] [PubMed] [Google Scholar]
- Law AJ, Pei Q, Walker M, Gordon-Andrews H, Weickert CS, Feldon J, et al. Early parental deprivation in the marmoset monkey produces long-term changes in hippocampal expression of genes involved in synaptic plasticity and implicated in mood disorder. Neuropsychopharmacology. 2009;34:1381–1394. doi: 10.1038/npp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic- pituitary-adrenal axis. Eur J Pharmacol. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic--pituitary--adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S, Mody T. The long-term psychobiological consequences of intermittent postnatal separation in the squirrel monkey. Neurosci Biobehav Rev. 2003;27:83–89. doi: 10.1016/s0149-7634(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Levine S, Wiener SG. Psychoendocrine aspects of mother-infant relationships in nonhuman primates. Psychoneuroendocrinology. 1988;13:143–154. doi: 10.1016/0306-4530(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Levine S, Weiner S, Coe C. The psychoneuroendocrinology of stress: A psychobiological perspective. In: Brush FR, Levine S, editors. Psychoneuroendocrinology. New York: Academic Press; 1989. pp. 341–377. [Google Scholar]
- Lewis MH, Gluck JP, Beauchamp AJ, Keresztury MF, Mailman RB. Long-term effects of early social isolation in Macaca mulatta: changes in dopamine receptor function following apomorphine challenge. Brain Res. 1990;513:67–73. doi: 10.1016/0006-8993(90)91089-y. [DOI] [PubMed] [Google Scholar]
- Lindell SG, Schwandt ML, Sun H, Sparenborg JD, Björk K, Kasckow JW, Sommer WH, Goldman D, Higley JD, Suomi SJ, Heilig M, Barr CS. Functional NPY variation as a factor in stress resilience and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2010;6:423–431. doi: 10.1001/archgenpsychiatry.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Afarian H, Schatzberg AF, Sawyer-Glover A, Moseley ME. Experience-dependent asymmetric variation in primate prefrontal morphology. Behav Brain Res. 2002;136:51–59. doi: 10.1016/s0166-4328(02)00100-6. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Kim S, Schatzberg AF, Levine S. Postnatal foraging demands alter adrenocortical activity and psychosocial development. Dev Psychobiol. 1998;32:285–291. [PubMed] [Google Scholar]
- Lyons DM, Lopez JM, Yang C, Schatzberg AF. Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. J Neurosci. 2000a;20:7816–7821. doi: 10.1523/JNEUROSCI.20-20-07816.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Martel FL, Levine S, Risch NJ, Schatzberg AF. Postnatal experiences and genetic effects on squirrel monkey social affinities and emotional distress. Horm Behav. 1999;36:266–275. doi: 10.1006/hbeh.1999.1547. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Mobley BW, Nickerson JT, Schatzberg AF. Early environmental regulation of glucocorticoid feedback sensitivity in young adult monkeys. J Neuroendocrinol. 2000b;12:723–728. doi: 10.1046/j.1365-2826.2000.00505.x. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF. Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry. 2001;58:1145–1151. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. J Trauma Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- Macri S, Wurbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav. 2006;50:667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Social and demographic influences on mothering style in pigtail macaques. Ethology. 1998a;104:379–385. [Google Scholar]
- Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Anim Behav. 1998b;55:1–11. doi: 10.1006/anbe.1997.0578. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Parent-offspring conflict in primates. Int J Primatol. 2002;23:923– 951. [Google Scholar]
- Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc Natl Acad Sci USA. 2005a;102:9726–9729. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D. Effects of early experience on female behavioural and reproductive development in rhesus macaques. Proc R Soc Lond B. 2005b;272:1243–1248. doi: 10.1098/rspb.2005.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Carroll KA. Child abuse and neglect: Usefulness of the animal data. Psych Bull. 1998a;123:211–223. doi: 10.1037/0033-2909.123.3.211. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Carroll KA. Risk factors for infant abuse and neglect in group-living rhesus monkeys. Psych Science. 1998b;9:143–145. [Google Scholar]
- Maestripieri D, Carroll KA. Behavioral and environmental correlates of infant abuse in group-living pigtail macaques. Inf Behav Devel. 1998c;21:603–612. [Google Scholar]
- Maestripieri D, Lindell SG, Ayala A, Gold PW, Higley JD. Neurobiological characteristics of rhesus macaque abusive mothers and their relation to social and maternal behavior. Neurosci Biobehav Rev. 2005;29:51–57. doi: 10.1016/j.neubiorev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack K, Sanchez MM. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques. Behav Neurosci. 2006a;120:1017–1024. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Lindell SG, Higley JD. Intergenerational transmission of maternal behavior in rhesus monkeys and its underlying mechanisms. Dev Psychobiol. 2007;49:165–171. doi: 10.1002/dev.20200. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, McCormack K, Lindell SG, Higley JD, Sanchez MM. Influence of parenting style on the offspring’s behavior and CSF monoamine metabolites levels in crossfostered and noncrossfostered female rhesus macaques. Behav Brain Res. 2006b;175:90–95. doi: 10.1016/j.bbr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Schino G, Aureli F, Troisi A. A modest proposal: Displacement activities as an indicator of emotions in primates. Anim Behav. 1992;44:967–979. [Google Scholar]
- Maestripieri D, Tomaszycki M, Carroll KA. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Dev Psychobiol. 1999;34:29–35. [PubMed] [Google Scholar]
- Maestripieri D, Wallen K. Nonhuman primate models of developmental psychopathology: problems and prospects. In: Cicchetti D, Walker E, editors. Neurodevelopmental Mechanisms in Psychopathology. Cambridge: Cambridge University Press; 2003. pp. 187–214. [Google Scholar]
- Maestripieri D, Wallen K, Carroll KA. Infant abuse runs in families of group-living pigtail macaques. Child Ab Negl. 1997;21:465–471. doi: 10.1016/s0145-2134(97)00006-9. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Spicer DM, Lewis MH, Gluck JP, Cork LC. Social deprivation of infant rhesus monkeys alters the chemoarchitecture of the brain: I. Subcortical regions. J Neurosci. 1991;11:3344–3358. doi: 10.1523/JNEUROSCI.11-11-03344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Coplan JD, Smith EL, Scharf BA, Owens MJ, Nemeroff CB, et al. Cerebrospinal fluid concentrations of biogenic amines and corticotropin-releasing factor in adolescent non-human primates as a function of the timing of adverse early rearing. Stress. 2002;5:185–193. doi: 10.1080/1025389021000010521. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K, Grand AP, LaPrairie J, Maestripieri D, Sanchez MM. The effects of maternal maltreatment on the development of HPA axis function in infant rhesus monkeys (under review) [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McKinney WT, Bunney WE. Animal model of depression. Arch Gen Psychiatry. 1969;21:240–248. doi: 10.1001/archpsyc.1969.01740200112015. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Bowman RE. Rearing experience, stress, and adrenocorticosteroids in the rhesus monkey. Physiol Behav. 1972;8:339–343. doi: 10.1016/0031-9384(72)90382-4. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59– 65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller NE. A perspective on the effects of stress and coping on disease and health. In: Levine S, Ursin H, editors. Coping and Health. New York: Plenum; 1980. pp. 323–354. [Google Scholar]
- Mineka S, Suomi SJ. Social separation in monkeys. Psych Bull. 1978;85:1376– 1400. [PubMed] [Google Scholar]
- Morrison JH, Hof PR, Janssen W, Bassett JL, Foote SL, Kraemer GW, McKinney WT. Quantitative neuroanatomic analyses of cerebral cortex in rhesus monkeys from different rearing conditions. Proc Soc Neurosci. 1990;16:789. [Google Scholar]
- Newman TK, Syalgailo Y, Barr CS, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine Oxidase A Gene polymorphism and infant rearing experience interact to influence aggression and injuries in rhesus monkeys (Macaca mulatta) Biol Psychiatry. 2005;57:167–72. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Newman TK, Parker CC, Suomi SJ, Goldman D, Barr CS, Higley JD. DRD1 5′UTR variation, sex and early infant stress influence ethanol consumption in rhesus macaque. Genes Brain Behav. 2009;8:626–630. doi: 10.1111/j.1601-183X.2009.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Bloom FE. Child development and neuroscience. Child Dev. 1997;68:970–987. doi: 10.1111/j.1467-8624.1997.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Novak MA, Harlow HF. Social recovery of monkeys isolated for the first year of life: I. Rehabilitation and therapy. Dev Psychol. 1975;11:453–465. [Google Scholar]
- O’Leary VE. Strength in the face of adversity: Individual and social thriving. J Soc Issues. 1998;54:425–446. [Google Scholar]
- O’Leary VE, Ickovics JR. Resilience and thriving in response to challenge: an opportunity for a paradigm shift in women’s health. Women’s Health. 1995;1:121–142. [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci USA. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Winslow JT, Davis M. Rearing experience differentially affects somatic and cardiac startle responses in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2002;116:378–386. doi: 10.1037//0735-7044.116.3.378. [DOI] [PubMed] [Google Scholar]
- Paykel ES. Contribution of life events to causation of psychiatric illness. Psychol Med. 1978;8:245–253. doi: 10.1017/s003329170001429x. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Pryce CR. The regulation of maternal behaviour in marmosets and tamarins. Behav Proc. 1993;30:201–224. doi: 10.1016/0376-6357(93)90133-C. [DOI] [PubMed] [Google Scholar]
- Pryce CR. Socialization, hormones, and the regulation of maternal behavior in nonhuman simian primates. Adv Study Behav. 1996;25:423–473. [Google Scholar]
- Pryce CR, Dettling A, Spengler M, Spaete C, Feldon J. Evidence for altered monoamine activity and emotional and cognitive disturbance in marmoset monkeys exposed to early life stress. Ann NY Acad Sci. 2004a;1032:245–249. doi: 10.1196/annals.1314.030. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol Psychiatry. 2004b;56:72–79. doi: 10.1016/j.biopsych.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Palme R, Feldon J. Development of pituitary-adrenal endocrine function in the marmoset monkey: infant hypercortisolism is the norm. J Clin Endocrinol Metab. 2002;87:691–699. doi: 10.1210/jcem.87.2.8244. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Rakic P. The development of the frontal lobe. A view from the rear of the brain. Adv Neurol. 1995;66:1–6. discussion 6–8. [PubMed] [Google Scholar]
- Rakic P, Nowakowski RS. The time of origin of neurons in the hippocampal region of the rhesus monkey. J Comp Neurol. 1981;196:99–128. doi: 10.1002/cne.901960109. [DOI] [PubMed] [Google Scholar]
- Reite M. Maternal separation in monkey infants: A model of depression. In: Hanin I, Usdin E, editors. Animal Models in Psychiatry and Neurology. New York: Pergamon Press; 1977. pp. 127–139. [Google Scholar]
- Reite M. Infant abuse and neglect: Lessons from the primate laboratory. Child Ab Negl. 1987;11:347–355. doi: 10.1016/0145-2134(87)90008-1. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psych Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- Rogers J, Martin LJ, Comuzzie AG, Mann JJ, Manuck SB, Leland M, Kaplan JR. Genetics of monoamine metabolites in baboons: overlapping sets of genes influence levels of 5-hydroxyindolacetic acid, 3-hydroxy-4-methoxyphenylglycol, and homovanillic acid. Biol Psychiatry. 2004;55:739–744. doi: 10.1016/j.biopsych.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Coplan JD, Friedman S, Bassoff T, Gorman JM, Andrews MW. Adverse early experiences affect noradrenergic and serotonergic functioning in adult primates. Biol Psychiatry. 1994;35:221–227. doi: 10.1016/0006-3223(94)91252-1. [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Forger C, Noland S, Trost RC, Coplan JD. Response of adolescent bonnet macaques to an acute fear stimulus as a function of early rearing conditions. Dev Psychobiol. 2001;39:40–45. doi: 10.1002/dev.1026. [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Paully GS. The effects of varying environmental demands on maternal and infant behavior. Child Dev. 1984;55:305–314. [PubMed] [Google Scholar]
- Rutter M. Resilience in the face of adversity. Protective factors and resistance to psychiatric disorder. Br J Psychiatry. 1985;147:598–611. doi: 10.1192/bjp.147.6.598. [DOI] [PubMed] [Google Scholar]
- Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987;57:316–331. doi: 10.1111/j.1939-0025.1987.tb03541.x. [DOI] [PubMed] [Google Scholar]
- Rutter M. Resilience: some conceptual considerations. J Adolesc Health. 1993;14:626–631. 690–626. doi: 10.1016/1054-139x(93)90196-v. [DOI] [PubMed] [Google Scholar]
- Sackett GP, Bowman RE, Meyer JS, Tripp RL, Grady SA. Adrenocortical and behavioral responses by differentially raised rhesus monkeys. Physiol Psychol. 1973;1:209–212. [Google Scholar]
- Sackett GP, Holm RA, Ruppenthal GP. Social isolation rearing: species differences in behavior of macaque monkeys. Dev Psychol. 1976;12:283–288. [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, McCormack K, Grand AP, Fulks R, Graff A, Maestripieri D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Dev Psychopathol. 2010;22:45–53. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer HR. The early experience assumption: Past, present, and future. Int J Behav Devel. 2000;24:5–14. [Google Scholar]
- Schapiro S, Geller E, Eiduson S. Neonatal adrenal cortical response to stress and vasopressin. Proc Soc Exp Biol Med. 1962;109:937–941. doi: 10.3181/00379727-109-27384. [DOI] [PubMed] [Google Scholar]
- Schino G, D’Amato FR, Troisi A. Mother-infant relationships in Japanese macaques: Sources of interindividual variation. Anim Behav. 1995;49:151–158. [Google Scholar]
- Schino G, Speranza L, Troisi A. Early maternal rejection and later social anxiety in juvenile and adult Japanese macaques. Dev Psychobiol. 2001;38:186–190. doi: 10.1002/dev.1012. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci. 2003;21:125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Sjoberg RL, Chisholm KL, Higley JD, Suomi SJ, Heilig M, Barr CS. Gene-environment interactions and response to social intrusion in male and female rhesus macaques. Biol Psychiatry. 2010;67:323–330. doi: 10.1016/j.biopsych.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seery MD. Challenge or threat? Resilience and vulnerability to potential stress in humans. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2011.03.003. (under review) [DOI] [PubMed] [Google Scholar]
- Seligman ME, Rosellini RA, Kozak MJ. Learned helplessness in the rat: time course, immunization, and reversibility. J Comp Physiol Psychol. 1975;88:542–547. doi: 10.1037/h0076431. [DOI] [PubMed] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. Am J Primatol. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, Higley JD. Maternal absence and stability of individual differences in CSF5-HIAA concentrations in rhesus monkey infants. Am J Psychiatry. 2005;162:1658–1664. doi: 10.1176/appi.ajp.162.9.1658. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Ginsberg SD, Hof PR, Foote SL, Young WG, Kraemer GW, McKinney WT, Morrison JH. Effects of social deprivation in prepubescent rhesus monkeys: immunohistochemical analysis of the neurofilament protein triplet in the hippocampal formation. Brain Res. 1993;619:299–305. doi: 10.1016/0006-8993(93)91624-2. [DOI] [PubMed] [Google Scholar]
- Simpson MJA. Effects of early experience on the behaviour of yearling rhesus monkeys (Macaca mulatta) in the presence of a strange object: classification and correlation approaches. Primates. 1985;26:57–72. [Google Scholar]
- Simpson MJA, Datta SB. Predicting infant enterprise from early relationships in rhesus macaques. Behaviour. 1990;116:42–63. [Google Scholar]
- Simpson AE, Simpson MJA. Short-term consequences of different breeding histories for captive rhesus macaque mothers and young. Behav Ecol Sociobiol. 1985;18:83–89. [Google Scholar]
- Simpson MJA, Gore MA, Janus M, Rayment FDG. Prior experience of risk and individual differences in enterprise shown by rhesus monkey infants in the second half of their first year. Primates. 1989;30:493–509. [Google Scholar]
- Smith ELP, Coplan JD, Trost RC, Scharf BA, Rosenblum LA. Neurobiological alterations in adult nonhuman primates exposed to unpredictable early rearing. Ann NY Acad Sci. 1997;821:545–548. doi: 10.1111/j.1749-6632.1997.tb48326.x. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW. Maternal mediation of early experience. In: Bell RW, Smotherman WP, editors. Maternal influences and early behavior. New York: Spectrum; 1980. pp. 201–210. [Google Scholar]
- Spencer-Booth Y, Hinde RA. Effects of brief separations from mothers during infancy on behaviour of rhesus monkeys 6–24 months later. J Child Psychol Psychiatry. 1971;12:157–172. doi: 10.1111/j.1469-7610.1971.tb01079.x. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early life stress induces long-term morphologic changes in primate brain. Arch Gen Psychiatry. 2009;66:658–65. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey M, Dearden R, Pill R, Robinson D. Hospitals, children and their families: The report of a pilot study. London: Routledge and Kegan Paul; 1970. [Google Scholar]
- Stanton M, Levine S. Brief separation elevates cortisol in mother and infant squirrel monkeys. Physiol Behav. 1985;34:1007–1008. doi: 10.1016/0031-9384(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Stevens HE, Leckman JF, Coplan JD, Suomi SJ. Risk and resilience: early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48:114–127. doi: 10.1097/CHI.0b013e318193064c. [DOI] [PubMed] [Google Scholar]
- Stevenson-Hinde J, Stillwell-Barnes R, Zunz M. Subjective assessment of rhesus monkeys over four successive years. Primates. 1980;21:66–82. [Google Scholar]
- Struble RG, Riesen AH. Changes in cortical dendritic branching subsequent to partial social isolation in stumptailed monkeys. Dev Psychobiol. 1978;11:479–486. doi: 10.1002/dev.420110511. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Harlow HF. Social rehabilitation of isolate-reared monkeys. Developmental Psychology. 1972;6:487–496. [Google Scholar]
- Suomi SJ. Ciba Foundation Symposium 156 “The childhood environment and adult disease”. Wiley; Chichester: 1991. Early stress and adult emotional reactivity in rhesus monkeys; pp. 171–188. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: evidence from primate studies. Br Med Bull. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Mineka S, DeLizio RD. Short- and long-term effects of repetitive mother-infant separations on social development in rhesus monkeys. Dev Psychol. 1983;19:770–786. [Google Scholar]
- Tanaka I. Variability in the development of mother-infant relationships among free-ranging Japanese macaques. Primates. 1989;30:477–491. [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vochteloo JD, Timmermans PJA, Duijghuisen JAH, Vossen JMH. Effects of reducing the mother’s radius of action on the development of mother-infant relationships in longtailed macaques. Anim Behav. 1993;45:603–612. [Google Scholar]