Abstract
Background
The workup of patients with suspected subarachnoid haemorrhage (SAH) presenting late is complicated by loss of diagnostic sensitivity of CT brain imaging and cerebrospinal fluid (CSF) bilirubin levels.
Methods
A prospective, longitudinal study on CSF ferritin levels in SAH.
Results
Serial CSF samples from 14 aneurysmal SAH cases requiring extraventricular drainage (EVD) were collected. The control group consisted of 44 patients presenting with headaches suspicious of SAH. In 9 cases a traumatic spinal tap occurred. CSF ferritin levels were significantly higher following SAH compared to controls (p<0.0001). The upper reference range of CSF ferritin is 12 ng/mL and there was no significant difference between a traumatic (mean 9.0 ng/mL) or normal spinal tap (3.9 ng/mL, p=0.59). CSF ferritin levels increased following the SAH from an average of 65 ng/mL (day 1) to 1750 ng/mL (day 11, p<0.01). Both the Fisher and Columbia CT score significantly correlated with CSF ferritin levels.
Conclusion
CSF ferritin levels increase after a SAH and may potentially provide additional diagnostic information in patients with suspected SAH who present late to clinic.
Keywords: ferritin, bilirubin, biomarker, subarachnoid haemorrhage, cerebrospinal fluid
1 Introduction
The diagnostic workup of patients with a suspected subarachnoid haemorrhage (SAH) is complicated if patients present late to clinic. A late presentation could be due to logistic problems or very subtle initial symptoms. A time dependent loss of sensitivity is well established for the routine diagnostic tests, CT brain scan and cerebrospinal fluid (CSF) spectrophotometry [1, 2]. It is not always straight forward to distinguish patients with a common headache from those in whom headaches may be related to a potentially life threatening disease such as SAH. The question arising is whether further investigations should be considered if the CT brain scan is normal and the cerebrospinal fluid (CSF) shows no trace of bilirubin.
A candidate biomarker to be tested in this situation is CSF ferritin. Following a SAH erythrocytes haemolyse, haemoglobin is degraded and iron is released into the CSF. Ferritin is the main extra–cellular transporter for iron and produced intrathecally following a bleed [3]. In two patients who suffered from a SAH we observed in longitudinal CSF samples that ferritin levels increased as bilirubin disappeared. We suggested that CSF ferritin levels may be a useful additional diagnostic test for the work up of patients with suspected SAH who present late to clinic [4].
This prospective longitudinal study is a follow up on our previous case report [4]. Here we tested whether there was a significant increase of CSF ferritin levels over time following a SAH. Next, we investigated whether the profile of CSF ferritin levels would be related to the amount of blood released during SAH.
2 Methods
Patients
The study was approved by the local Ethics Committees of the University of Pittsburgh Medical Centre and the joint Ethics Committee of the National Hospital for Neurology and Neurosurgery (NHNN) and the Institute of Neurology (ION), London. Written informed consent was obtained from all patients or if this was impossible by written assent from the next of kin. All patients suffered from an angiography confirmed aneurysmal SAH which required insertion of an extraventricular drain (EVD) for management of acute hydrocephalus. The first CSF sample was collected at least 8 hours after insertion of the EVD. Samples were collected until EVD removal.
The control group consisted of patients presenting with headaches suspicious of SAH. All control patients underwent CT brain imaging and lumbar puncture. The lumbar puncture was defined as a normal tap if there were no CSF pigments and all other CSF parameters (glucose, lactate, total protein, cell count) were in the normal range. The lumbar puncture was defined as a traumatic tap if spectroscopy showed presence of oxyhaemoglobin but not of bilirubin. The CSF samples were coded and pseudo–anonymised in accordance with the Medical Research Council Guidelines on the Ethical Use of Biological Specimen Collections in Clinical Research.
CSF analysis
CSF was centrifuged and the supernatant stored at −80°C until analysis. CSF levels of ferritin were analysed using a previously described ELISA technique [3] with the analyst being blinded to the clinical details. The upper normal range of 12 ng/mL was determined in a large reference population (N=388) [3]. CSF spectroscopy was performed as recommended [5].
Statistical analysis
Statistical analysis was performed using SAS software (version 9.2). Subgroup analyses were performed on dichotomised data using the World Federation of Neurological Surgeons (WFNS) [6] grading where a poor grade SAH was rated as grade IV to V and a good grade SAH as grade I to III. Because we hypothesised the concentration of the iron binding protein ferritin to be related to either the volume or distribution of blood further subgroup analyses were performed using the Fisher [7], Columbia rating scales [8] and the presence of an IVH. We further investigated whether the profile of CSF ferritin levels was related to the outcome (survival). All longitudinal analyses were performed using general linear models. Independent variables were compared using the non-parametric two-sample exact Wilcoxon rank-sum test. The linear relationship between continuous variables was evaluated using the Spearman correlation coefficient. Multiple correlations were corrected using the Bonferroni method. Two–tailed tests were used throughout and p values of <0.05 were accepted as significant.
3 Results
The baseline data is shown in Table 1. Seven patients (50%) had an admission GCS above 13/15 and seven were of poor grade (WFNS grade IV and V). Two patients underwent surgical clipping of their aneurysm. Four of the poor grade (57%) and two (29%) of the good grade SAH patients died. None of the patients suffered from ventriculitis or shunt infection.
Table 1.
Patient characteristics
| SAH | Control patients | ||
|---|---|---|---|
| Characteristic | patients | Normal tap |
Traumatic tap |
| Age, years | 56 (52–65) | 36 (29–49) | 31 (24–50) |
| Sex, F/M | 5/9 | 21/14 | 5/4 |
| Time from onset | 3 (1–3) | 5 (2–13) | 7 (3–11) |
| GCS score on admission |
13 (7–14) | 15 (15–15) | 15 (8–15) |
| WFNS grade I–III | 7 (50%) | — | — |
| WFNS grade IV–V | 7 (50%) | — | — |
| CT brain imaging | |||
| Fisher I | 5 (36%) | — | — |
| Fisher II | 1(7%) | — | — |
| Fisher III | — | — | — |
| Fisher IV | 8 | (57%) | — |
| Columbia 0 | 4 (29%) | — | — |
| Columbia I | 2 (14%) | — | — |
| Columbia II | 2 (14%) | — | — |
| Columbia III | — | — | — |
| Columbia IV | 6 (43%) | — | — |
| IVH | 8 (57%) | — | — |
| Clipping | 2 (14%) | — | — |
| Coiling | 12 (86%) | — | — |
Data are median (interquartile range) or number (percentage).
In the control group 9/44 (21%) of the lumbar punctures a traumatic tap occurred (Table 1). The diagnoses were tension type headaches in 20/44 (46%) and migraine in 8/44 (18%). From the remaining patients four developed headaches on the background of a previous aneurismal SAH 1, 2, 6 and 10–years ago. In two patients a coincidental aneurysm was detected (bilateral carotides, ACOM) without evidence for a bleed. Four patients presented with seizures (one due to a brain tumour). In the remainder the headaches were due to: benign intracranial hypertension (n=1), systemic lupus erythomatosus (n=1), coital headaches (n=1), viral labyrinthitis (n=1), viral meningitis (n=1), cluster headache (n=1).
CSF ferritin in control patients
The averaged CSF ferritin level in the control group with a clean lumbar puncture was 3.9±1.8 ng/mL. These levels did not differ statistically to those found following a traumatic tap (9.0±10.5 ng/mL, p=0.59). In a traumatic tap the CSF ferritin levels were not correlated to the optical density measured for oxyhaemoglobin.
High CSF ferritin levels in the patient cohort
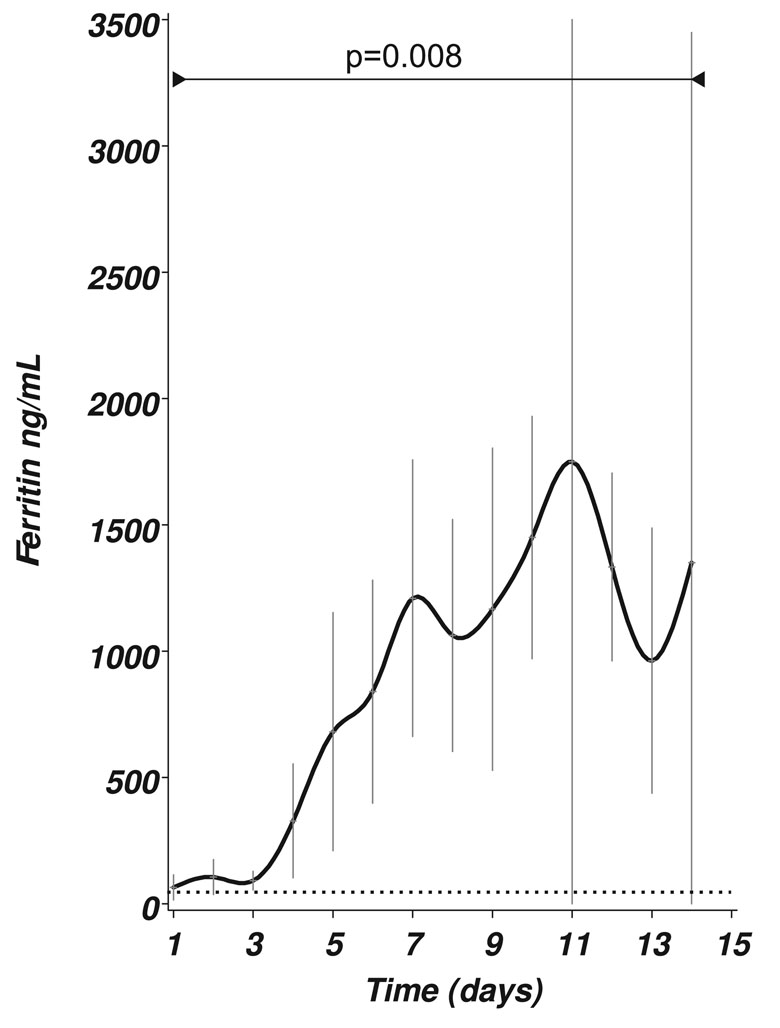
CSF ferritin levels were significantly higher in patients with SAH if compared to control patients either with a clean or traumatic tap (F=12.21, p<0.0001, Figure 1A). In fact, all (100%) of the CSF ferritin levels in the patient cohort were above the published cutoff of 12 ng/mL [3] (horizontal dotted line in Figure 1A).
Figure 1.
Figure 1: (A) CSF ferritin levels [ng/mL] in SAH patients over a 14 day period. There was a significant change of the mean (±SEM) levels over time (F=2.41, p=0.008). The horizontal dotted line indicates the upper reference limit of 12 ng/mL.
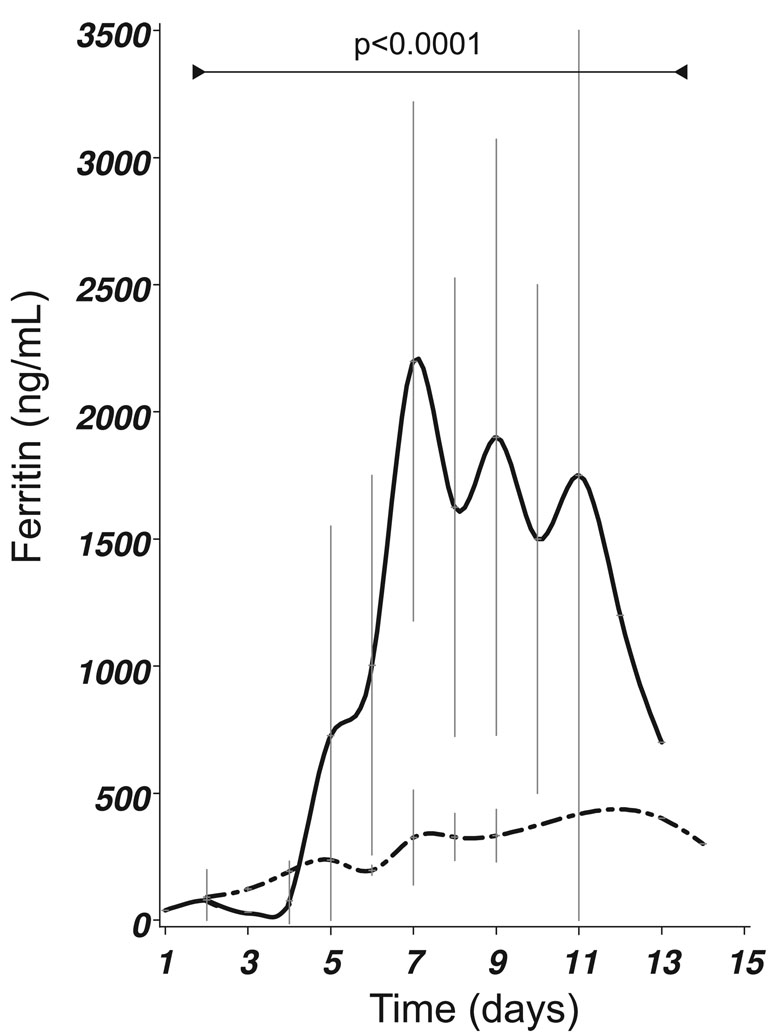
Figure 1: (B) Presence of IVH (closed line) was related to significantly higher CSF ferritin levels (F22,46 =3.63, p<0.0001) compared to any other form of blood distribution following SAH (dashed–dotted line). All patients shown in this graph underwent coiling of their aneurysm. The mean±SEM is shown.
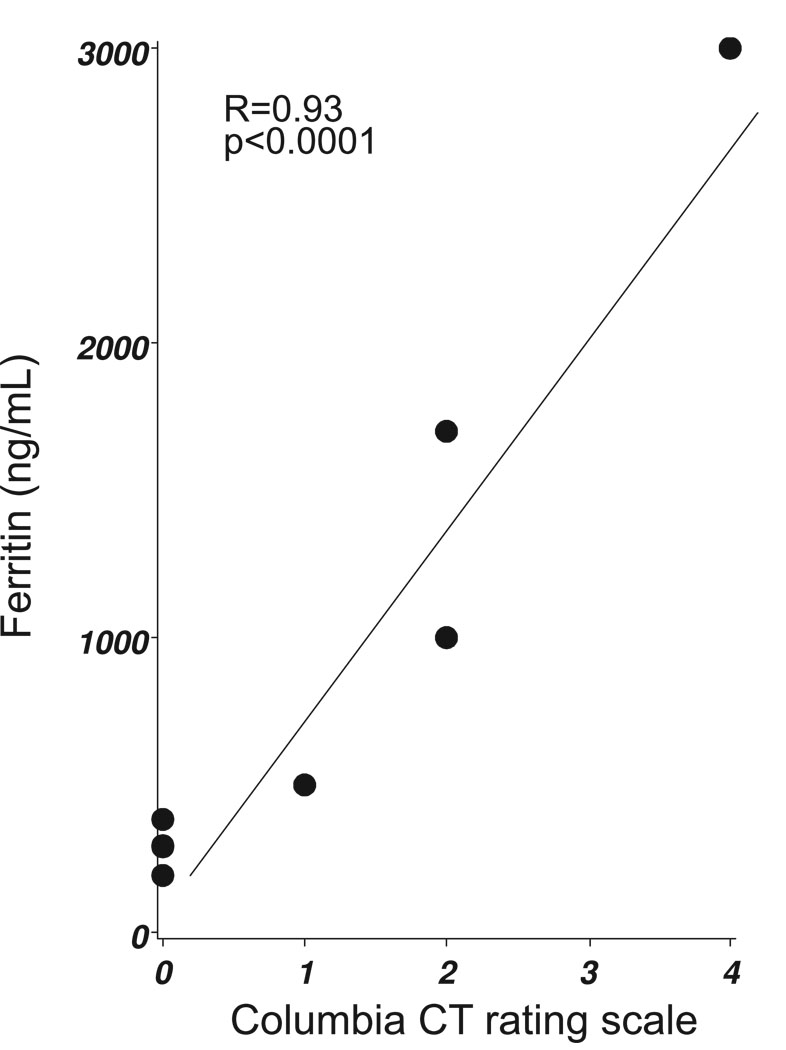
Figure 1: (C) The Columbia CT rating scale score correlated with CSF ferritin levels on day 9 (Spearman’s R=0.93, p<0.0001).
The longitudinal CSF ferritin profile in the patient cohort
Figure 1A shows that there was a significant increase of CSF ferritin levels with time (F=2.41, p=0.008). CSF ferritin levels peaked between day 7 (mean 1209 ng/mL) to 11 (mean 1750 ng/mL).
The distribution and volume of blood on the brain CT was related to CSF ferritin levels
In patients who underwent embolisation, the presence of an intraventricular haemorrhage (IVH) was followed by a significantly more rapid and higher increase of CSF ferritin levels compared to any other blood distribution without IVH (F=3.64, p<0.0001). The separation between the two curves became significant on day 6 (p=0.05), day 7 (p<0.0001), day 8 (p<0.0001), day 9 (p=0.01), day 10 (p<0.01) and day 11 (p=0.01, Figure 1B). Significance remained when correcting for repeated measurements (p=0.03). In contrast, for both the Fisher (F=2.83, p<0.001) and Columbia CT scale (F=3.12, p<0.0001) significance was lost after correcting for repeated measurements.
A correlation matrix investigating the relationship between CSF ferritin levels and the grading on the Fisher and Columbia scales revealed significant correlations between days six to nine (Table 2). The strongest correlation was found for day nine, but samples were on this day were only available from 7 patients (Figure 1C). For days 10 and 11 there were too few data points to allow for a correlation analysis.
Table 2.
Fisher and Columbia CT brain scan rating scores correlated with CSF ferritin level at days 6–9 after SAH
| Day | Fisher | Columbia |
|---|---|---|
| 6 | 0.77 | 0.81 |
| 7 | 0.66 | 0.71 |
| 8 | 0.77 | 0.83 |
| 9 | 0.91 | 0.93 |
P <.05;
P <.01;
P <.001.
Because both an IVH and surgery may be regarded as secondary events causing an increase of CSF ferritin levels we also analysed the longitudinal profile excluding these two conditions. In patients who underwent coiling and had no IVH CSF averaged ferritin levels rose significantly; 90 ng/mL (day 2), 122 ng/mL (day 3), 194 ng/mL (day 4), 235 ng/mL (day 5), 203 (day 6), 324 ng/mL (day 7), 327 ng/mL (day 8), 332 ng/mL (day 9), 400 ng/mL (day 13), 300 ng/mL (day 14).
The profile of CSF ferritin levels was not related to clinical data
There was no difference in the longitudinal CSF ferritin profile between patients presenting with a poor WFNS grade SAH when compared to those with a good WFNS grade SAH (F=1.52, p=0.096). Likewise, the trend for CSF ferritin levels to rise quicker in patients who died (F=1.74, p=0.04), was lost after correcting for repeated measurements (p=0.92).
4 Discussion
The main finding of this study was that following SAH CSF ferritin levels were significantly higher compared to control patients with either a clean or a traumatic spinal tap. Importantly, in this patient cohort following SAH CSF ferritin levels increased to an average of 1,750 ng/mL on day 11. This is over two magnitudes above the published upper reference value of 12 ng/mL [3] and the values found in our control patients. This finding is in line with previous data from one study describing average concentrations of 1237.9 ng/mL between days 8 and 10 [9].
In addition, our data suggests that the presence of blood in the ventricles was responsible for a substantial upregulation of CSF ferritin levels. We speculate that given the high physiological turnover and distribution of CSF, any access to ventricular CSF will result in stimulation of microglia as toxic free iron comes into contact with a large surface area of the CNS. In response to this the expression of ferritin is upregulated in the microglia. In contrast, with a more localized bleed free iron may only come into contact with a limited number of microglia. This hypothesis is supported by the longitudinal data showing not only that in patients who had no IVH, the CSF ferritin levels remained below 500 ng/mL before they started to decrease, but also that the slope of increasing CSF ferritin levels was gentle compared to the steep slope seen following a IVH. This interpretation is further supported by the strong correlations found between both the Fisher [7] and Columbia [8] CT rating scales and CSF ferritin levels at their peak. In the study of Suzuki et al. the investigation of this relationship was not possible because only patients with a Fisher grade III were recruited [9].
We were not able to show a correlation between the longitudinal profile of CSF ferritin levels and the WFNS grade on admission. This is consistent with the findings of Suzuki et al. who also found similar CSF ferritin levels in SAH patients with a WFNS of I–III when compared to those with a WFNS of IV–V [9]. The survival outcome was independent of the longitudinal profile of CSF ferritin levels. Again, this finding confirms the results by Suzuki et al. [9]. As CSF ferritin levels in SAH can rise to extremely high values one might be tempted to use CSF ferritin to aid the differential diagnosis of a bleed due to an aneurysmal SAH compared to a traumatic SAH. In fact, because any intracerebral or subarachnoid blood will trigger the biochemical cascade leading to CSF ferritin upregulation, we do not consider this to be reliable and would not use CSF ferritin levels for diagnostic guidance in this context.
There are important limitations to our study. Firstly, a pre–analytical problem is that a ventricular to lumbar gradient (V:L) of CSF ferritin concentrations is likely. Reiber has convincingly shown the presence of such a gradient for other CSF proteins. The V:L CSF gradient was found to be 3.5:1 for S100B, 2:1 for NSE, 1.5:1 for tau protein, 1:2.5 for cystatin C (gamma trace) and 1:11 for beta-trace protein [10]. Given the magnitude of our finding this is unlikely to be a major shortcoming. Secondly, the sample size is small, again the magnitude of the finding on CSF ferritin levels is such that increasing numbers further is unlikely to change the significance of the results. Additionally the present results are consistent with previously published data [9]. However, in a single case with an aneurysmal SAH we found a CSF ferritin level of 28 ng/mL (twice above cutoff) 61 days after the bleed [4]. Thus further study investigating CSF ferritin levels in patients with suspected SAH presenting late to clinic would benefit from including substantially more patients. Thirdly, the longitudinal collection of CSF samples was limited to the time patients required EVD drainage. Therefore the present data does not permit estimating the time interval following a SAH when CSF ferritin levels may still be of diagnostic value. For the same reason the present data does not allow a reliable estimation of sensitivity/specificity for CSF ferritin levels in the diagnostic work up of patients with suspected SAH who present late to clinic. Finally, ferritin levels are known to rise in a number of conditions, particularly following ventriculitis and shunt infections [3]. Therefore, as with other paraclinical tests it will be important to interpret the results of CSF ferritin levels in the context of a careful medical history and clinical examination. The value of measuring CSF ferritin levels in the diagnostic work–up of patients with a suspected SAH has been questioned in three studies analysing CSF in the first days following the bleed [11, 12, 13]. Our and previous longitudinal data [9] suggest that the value of measuring CSF ferritin levels in a suspected SAH may be when the CSF sample is taken after a considerable delay following a suspected bleed rather then at day one, when CSF spectrophotometry is a very sensitive test [2, 14]. In patients who are investigated after a delay of more then two weeks, chances are that the CT brain scan will be normal [15] and CSF bilirubin will have been washed out, resulting in normal CSF spectrophotometry [4]. In these patients an additional analysis of CSF ferritin levels may potentially provide important additional diagnostic information.
Acknowledgments
Acknowledgements and Funding
The authors have nothing to declare. This work was undertaken at University College London Hospitals and partially funded by the Department of Health’s National Institute for Health Research Centres funding scheme. CSF samples at the University of Pittsburgh Medical Center were collected in conjunction with an NIH funded project RO1NR0433.
The data were collected and research conducted while Dr. Kerr was employed at the University of Pittsburgh. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29–36. doi: 10.1056/NEJM200001063420106. [DOI] [PubMed] [Google Scholar]
- 2.Petzold A, Sharpe LT, Keir G. Spectrophotometry for cerebrospinal fluid pigment analysis. Neurocrit Care. 2006;4:153–162. doi: 10.1385/NCC:4:2:153. [DOI] [PubMed] [Google Scholar]
- 3.Keir G, Tasdemir N, Thompson EJ. Cerebrospinal-fluid ferritin in brain necrosis - evidence for local synthesis. Clin Chim Acta. 1993;216:153–166. doi: 10.1016/0009-8981(93)90148-w. [DOI] [PubMed] [Google Scholar]
- 4.Petzold A, Worthington V, Pritchard C, Appleby I, Kitchen N, Smith M. The longitudinal profile of bilirubin and ferritin in the cerebrospinal fluid following a subarachnoid hemorrhage: diagnostic implications. Neurocritical Care. 2009 doi: 10.1007/s12028-009-9244-6. [in print] [DOI] [PubMed] [Google Scholar]
- 5.Cruickshank A, Auld P, Beetham R, Burrows G, Egner W, Holbrook I, Keir G, Lewis E, Patel D, Watson I, White P. Revised national guidelines for analysis of cerebrospinal fluid for bilirubin in suspected subarachnoid haemorrhage. Ann Clin Biochem. 2008;45:238–244. doi: 10.1258/acb.2008.007257. [DOI] [PubMed] [Google Scholar]
- 6.Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry. 1988;51:1457. doi: 10.1136/jnnp.51.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Claassen J, Bernardini GL, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke. 2001;32:2012–2020. doi: 10.1161/hs0901.095677. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Muramatsu M, Tanaka K, Fujiwara H, Kojima T, Taki W. Cerebrospinal fluid ferritin in chronic hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurol. 2006;253:1170–1176. doi: 10.1007/s00415-006-0184-1. [DOI] [PubMed] [Google Scholar]
- 10.Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta. 2001;310:173–186. doi: 10.1016/s0009-8981(01)00573-3. [DOI] [PubMed] [Google Scholar]
- 11.Watson ID, Beetham R, Fahie-Wilson MN, Holbrook IB, O’Connell DM. What is the role of cerebrospinal fluid ferritin in the diagnosis of subarachnoid haemorrhage in computed tomography-negative patients? Ann Clin Biochem. 2008;45:189–192. doi: 10.1258/acb.2007.007043. [DOI] [PubMed] [Google Scholar]
- 12.Page KB, Howell SJ, Smith CM, Dabbs DJ, Malia RG, Porter NR, Thickett KJ, Wilkinson GM. Bilirubin, ferritin, D-dimers and erythrophages in the cerebrospinal fluid of patients with suspected subarachnoid haemorrhage but negative computed tomography scans. J Clin Pathol. 1994;47:986–989. doi: 10.1136/jcp.47.11.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connell DM, Watson ID. Definitive angiographic detection of subarachnoid haemorrhage compared with laboratory assessment of intracranial bleed in CT-negative patients. Ann Clin Biochem. 2003;40:269–273. doi: 10.1258/000456303321610592. [DOI] [PubMed] [Google Scholar]
- 14.UK NEQAS. National guidelines for analysis of cerebrospinal fluid for bilirubin in suspected subarachnoid haemorrhage. Ann Clin Biochem. 2003;40:481-408. doi: 10.1258/000456303322326399. [DOI] [PubMed] [Google Scholar]
- 15.van Gijn J, van Dongen KJ. The time course of aneurysmal haemorrhage on computed tomograms. Neuroradiology. 1982;23:153-106. doi: 10.1007/BF00347559. [DOI] [PubMed] [Google Scholar]