Abstract
Background
Diminished serum arylesterase activity, catalyzed by the high-density lipoprotein (HDL)-associated paraoxonase-1, is associated with heightened systemic oxidative stress and atherosclerosis risk. Here we sought to determine the prognostic role of serum arylesterase activity in subjects with systolic heart failure, particularly in relation to established cardiac biomarkers.
Methods and Results
We measured serum arylesterase activity in 760 subjects with impaired left ventricular systolic function (left ventricular ejection fraction [LVEF]<50%), and prospectively followed major adverse cardiac events (MACE, including death, non-fatal myocardial infarction, and stroke) for 3 years. In our study cohort (mean age 64±11 years, 74% male, median LVEF 35%, median creatinine clearance [CrCl] 96 mg/dL), mean serum arylesterase activity (98 ±25 μM/min/mL) was lower compared with healthy controls (mean 115 ±26 μM/min/mL, p<0.01) but higher compared with advanced decompensated heart failure subjects (mean 69 ±22 μM/min/mL, p<0.01). Within our cohort, there was modest correlation between serum arylesterase activity and HDL cholesterol (r=0.33, p<0.01) as well as B-type natriuretic peptide (BNP, r= -0.23, p<0.01). Lower serum arylesterase activity was a strong predictor of poorer outcomes (Hazard ratio [95%CI] 2.94 [1.54, 5.62], p<0.001). After adjusting for traditional risk factors, medication use, BNP, and CrCl, lower serum arylesterase still conferred an increased risk of MACE at 3 years (HR 2.69 [1.37-5.28], p=0.004).
Conclusions
In patients with systolic heart failure, decreased serum arylesterase activity, a measure of diminished anti-oxidant properties of HDL, predicts higher risk of incident long-term adverse cardiac event independent of established clinical and biochemical risk factors.
Keywords: chronic heart failure, lipoproteins, oxidative stress, paraoxonase
Oxidative stress plays an important role in the pathogenesis and progression of heart failure1, 2. Measures of stable oxidative byproducts, including oxidized low-density lipoproteins (LDL)1, malondialdehyde2, isoprostaines3, and urinary biopyrrins4, are increased in the setting of heart failure. Increased oxidative stress results from an imbalance between reactive oxygen, nitrogen, and halogenating species and endogenous antioxidant defense mechanisms to scavenge free radicals and their byproducts5, 6. Therefore, an imbalance between oxidative and anti-oxidative mechanisms may lead to deleterious consequences7.
Paraoxonase-1 (PON-1) is a high-density lipoprotein (HDL)-associated glycoprotein believed to play a key role in facilitating systemic anti-oxidant activities of HDL, including the remodeling of oxidized phospholipids8, 9. PON-1 is by far the most abundant of all paraoxonases within the vascular compartment. Numerous studies have shown that PON-1 serves as a primary contributor to systemic (serum) arylesterase activity (hydrolase activity on carboxylic ester bonds such as phenyl acetate)10. Serum arylesterase activity have been shown to have strong correlations with multiple systemic measures of oxidant stress, including multiple distinct fatty acid oxidation products quantified by liquid chromatography with on-line stable isotope dilution tandem mass spectrometry10. Thus, both human clinical investigations and studies employing PON-1 knockout mice11, 12 are consistent with PON-1 serving a major anti-oxidant function in vivo. Herein, we examine the potential role of HDL’s anti-oxidant activity, as monitored by serum arylesterase activity measurements of PON-1, as a predictor of adverse disease progression among patients with systolic heart failure.
Methods
Study population
The Cleveland Clinic GeneBank study is a large, prospective cohort study from 2001-2006 that established a well-characterized clinical repository with data of clinical and longitudinal outcomes comprised from consenting subjects undergoing elective diagnostic cardiac catheterization procedure not in the setting of acute coronary syndrome. All GeneBank participants gave written informed consent approved by the Cleveland Clinic Institutional Review Board. Clinical outcomes were prospectively ascertained over the ensuing 3 years for all subjects following enrollment. Major adverse cardiovascular event (MACE) was defined as all-cause mortality, non-fatal myocardial infarction, or non-fatal cerebrovascular accident following enrollment.
The present analysis included 760 consecutive subjects with stable systolic heart failure (left ventricular ejection fraction [LVEF] <50% as determined by echocardiography, radionuclide or contrast ventriculography) enrolled in GeneBank with serum samples available for analysis. An estimate of creatinine clearance (CrCl) was calculated using the Cockcroft-Gault equation. B-type natriuretic peptide (BNP), creatinine, and fasting blood glucose and lipid profiles were measured on the Abbott Architect platform (Abbott Laboratories, Abbott Park IL).
To examine the range of serum arylesterase activity using this assay, we performed a cross-sectional comparison between our cohort of stable systolic heart failure with two independent sets of subjects, all prospectively enrolled with written informed consent approved by the Cleveland Clinic Institutional Review Board. The first set is a cohort of 300 prospectively recruited, apparently healthy individuals without known cardiac diseases from a health screening program at various locations across Cleveland, Ohio. The second set is a cohort of 73 consecutive patients with advanced decompensated heart failure admitted to the heart failure intensive care unit for hemodynamically-guided therapy including intravenous diuretic therapy.
Serum arylesterase activity assay
Serum arylesterase activity was measured by UV spectrophotometry in a 96-well plate format (Spectramax 384 Plus, Molecular Devices, Sunnyvale, California) using phenyl acetate (Sigma-Aldrich, St Louis, Missouri) as substrate. Briefly, initial hydrolysis rates were determined at 270 nm in 50-fold diluted serum (final) in reaction mixtures composed of 3.4 mM phenylacetate, 9 mM Tris hydrocholoride, pH 8, and 0.9 mM calcium chloride at 24°C. An extinction coefficient (at 270 nm) of 1310 M−1•cm−1 was used for calculating units of arylesterase activity, which are expressed as the amount of phenyl acetate hydrolyzed in μM/min/mL of serum. The intra-assay and inter-assay coefficients of variation for performance of arylesterase were 1.2% and 3.9%, respectively, on 20 replicates performed on 10 different days.
Statistical analyses
We compared baseline characteristics between subjects with high versus low serum arylesterase activity levels with Student’s t-test (normally distributed) or Wilcoxon-Rank sum test (non-normally distributed) for continuous variables and chi-square test for categorical variables. One-way ANOVA was used to compare serum arylesterase activity levels between healthy, LVSD, and advanced decompensated heart failure cohorts. Spearman’s correlation was performed to determine relationship between serum arylesterase activity levels and other biochemical parameters. For each continuous variable, we investigated the log-linearity assumption of Cox models by introducing a cubic spline component. Receiver Operator Characteristic (ROC) curve analyses in the context of the time to event were performed to determine the optimal cutoff at 121 μM/min/mL, with risk of event estimated using 5-fold cross-validation by a Cox model. Kaplan–Meier analysis with log-rank test was used to compare the survival curves of the two groups (serum arylesterase activity <121 versus ≥121 μM/min/mL). Cox proportional hazards regression was used for time-to-event analysis to determine Hazard ratio (HR) and 95% confidence intervals (95%CI) for MACE. Adjustments were made for individual traditional cardiac risk factors including age, gender, systolic blood pressure, cigarette smoking, fasting cholesterol values (including low-density lipoprotein and high-density lipoprotein cholesterol levels). Additional adjustments included ischemic etiology, as well as medication use (including ACE inhibitors, beta-blockers, and statin therapy), BNP and CrCl (both logarithmic transformed) to predict incident 3-year MACE risks. In case of non log-linearity such as serum arylesterase activity, the continuous variable was transformed into a binary variable, the optimal cut-off value for dichotomization being the value minimizing the prediction error in MACE event. All analyses were performed using SAS version 8.2 (Cary, NC) and R 2.8.0 (Vienna, Austria). A p value less than 0.05 was considered statistically significant.
Results
Study population
Table 1 describes the baseline characteristics of the subjects, which is a relatively well-compensated patient cohort with median LVEF of 35%, normal mean CrCl of 100 ± 41 ml/min/1.73m2, 63% ACE inhibitor use and 39% treated with standing diuretic therapy. Serum arylesterase activity levels were normally distributed, with a mean of 98 ±25 μM/min/mL, which was lower than levels in the apparently healthy control population (mean 115 ±26 μM/min/mL, p<0.01, Figure 1), but higher than levels of hospitalized patients with advanced systolic heart failure (mean 69 ±22 μM/min/mL, p<0.01). In general, male subjects tended to have lower serum arylesterase activity levels than female subjects (97 ±25 versus 102 ±25 μM/min/mL; p<0.01). Compared to the GeneBank cohort, apparently healthy control population were younger (64 ±11 vs 42 ±14 years, p<0.01) and had less hypertension (72% vs 13%, p<0.01), and diabetes mellitus (39% vs 2%, p<0.01). In contrast, subjects with advanced decompensated heart failure were more likely to be male and had lower CrCl (68 ±35 vs 100 ±41 ml/min/1.73m2, p<0.01) and LVEF (26% vs 35%, p<0.01) than that in the GeneBank cohort.
Table 1.
Baseline Subject Characteristics
| Serum arylesterase activity | ||||
|---|---|---|---|---|
| Variable | Total | <121 μM/min/mL (n=622) | ≥121 μM/min/mL (n=138) | P-value |
| Age (years) | 64 ±11 | 64 ±11 | 64 ±11 | 0.346 |
| Male (%) | 74 | 74 | 73 | 0.800 |
| Diabetes mellitus (%) | 39 | 40 | 35 | 0.289 |
| Hypertension (%) | 72 | 71 | 76 | 0.270 |
| Ischemic etiology (%) | 68 | 67 | 73 | 0.192 |
| LDL cholesterol (mg/dL) | 94 (75, 116) | 93 (74, 113) | 108 (84, 129) | <0.001 |
| HDL cholesterol (mg/dL) | 32 (26, 38) | 31 (26, 36) | 36 (30, 45) | <0.001 |
| BNP (mg/dL) | 193 (86, 481) | 212 (94, 505) | 133 (67, 351) | <0.001 |
| LV Ejection Fraction (%u) | 35 (30, 45) | 35 (30, 45) | 40 (30, 45) | 0.110 |
| CrCl (ml/min/1.73m2) | 100 ± 41 | 100± 42 | 101±36 | 0.652 |
| Baseline Medications: | ||||
| Aspirin (%) | 73 | 72 | 80 | 0.075 |
| Statins (%) | 62 | 61 | 68 | 0.149 |
| Diuretics (%) | 39 | 40 | 38 | 0.704 |
| ACE inhibitors (%) | 63 | 64 | 61 | 0.579 |
| Beta-blockers (%) | 67 | 67 | 67 | 0.983 |
Values expressed in mean ± standard deviation or median (interquartile range). Abbreviations: LV = left ventricle; LDL = low-density lipoprotein; HDL = high-density lipoprotein; BNP = B-type natriuretic peptide; CrCl = creatinine clearance; ACE = angiotensin converting enzyme
Figure 1.
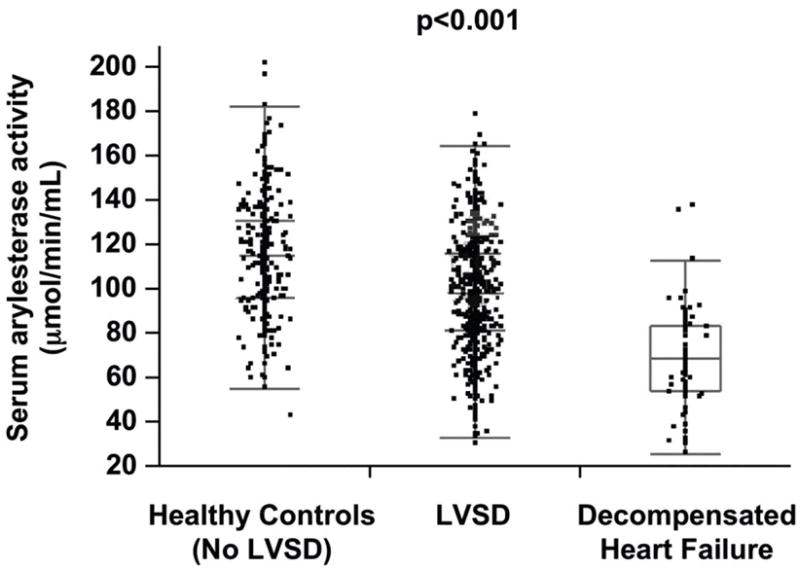
Comparison of serum arylesterase activity between healthy controls, patients with stable systolic heart failure, and hospitalized patients with advanced decompensated heart failure. P-value for one-way ANOVA comparison across the three subgroups.
Serum arylesterase activity, fasting lipid profile, and cardiac biomarkers
There was modest correlation between serum arylesterase activity and HDL cholesterol (r=0.33, p<0.001), LDL cholesterol (r=0.20, p<0.001), and triglyceride (r=0.18, p<0.001) levels. Lower serum arylesterase activity levels were associated with higher plasma BNP levels (r= -0.23, p<0.01; Kruskal-Wallis test across BNP quartile groups, p<0.001). In contrast, weaker correlations were observed between serum arylesterase activity and LVEF (r= 0.12, p<0.01). As a result, subjects with lower serum arylesterase activity at baseline demonstrated greater abnormalities in fasting lipid profiles as well as higher plasma BNP levels compared to that of subjects with higher serum arylesterase activity (Table 1).
Serum arylesterase levels and major adverse cardiac outcomes
A total of 134 events (non-fatal MI, stroke, or death) were recorded within the 3-year period of follow-up. As continuous variables, lower serum arylesterase activity was associated with poorer long-term outcomes (HR for lowering serum arylesterase activity per standard deviation decrease: 1.32 [95%CI 1.12 – 1.54], p<0.001). This was observed in the setting of both ischemic (HR 1.28 [95%CI 1.05 – 1.56], p=0.013) and non-ischemic (HR for lowering serum arylesterase activity: 1.52 [95%CI 1.11 – 2.04], p=0.008) heart failure. When stratified according to serum arylesterase activity quartiles, subjects within the lowest three quartiles (<116 μM/min/mL) demonstrated increased risk compared with the highest quartile (HR 1.98 [1.23-3.18], p=0.005). In Kaplan-Meier analysis, serum arylesterase activity levels below the ROC-determined optimal cut-off level of 121 μM/min/mL were associated with increased risk for development of MACE at 3 years (HR 2.94 [95%CI 1.54-5.62], p=0.003, Figure 2). After adjusting for traditional risk factors, medication use, BNP, and CrCl, serum arylesterase levels below this cut-off value still maintained a 2.7-fold increased risk in the development of future MACE (HR 2.69 [95%CI 1.37-5.28], p=0.004, Table 2). To test whether associations differed by heart failure etiology, we included an interaction term in our Cox models between serum arylesterase activity and ischemic/non-ischemic etiology. This was no significant interaction, suggesting that a higher serum arylesterase activity maintains a 3-fold increased risk in the development of future MACE regardless in both groups (Hazard Ratio 3.03 [95% CI 1.35-6.79], p<0.001).
Figure 2.
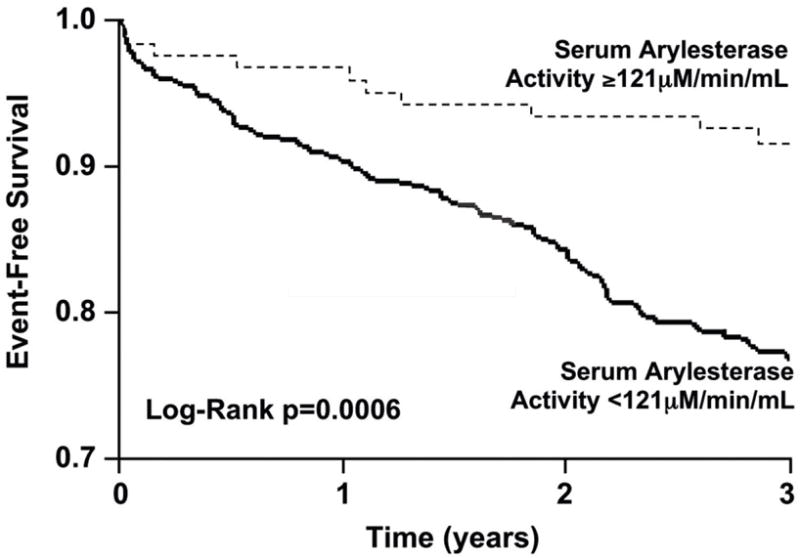
Kaplan-Meier analysis for long-term major adverse cardiac events. P-value for Log-rank test.
Table 2.
Cox Proportional Hazard Analysis for Diminished Serum Arylesterase Activity on Major Adverse Cardiac Events at 3 Years.
| Analysis | Hazard Ratio (95% confidence interval) | p-value |
|---|---|---|
| Univariate Analysis * | 2.94 (1.54-5.62) | 0.001 |
| Multivariate Analysis * | ||
| Model 1 (Traditional risk factors + ischemic etiology )** | 3.14 (1.57-6.30) | 0.001 |
| Model 2 (Traditional risk factors + medications + CrCl + BNP) *** | 2.69 (1.37-5.28) | 0.004 |
Serum arylesterase activity levels were dichotomized at 121 μM/min/mL.
Traditional risk factors include age, gender, systolic blood pressure, diabetes mellitus, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol smoking, ischemic etiology
Medications use including ACE inhibitor use, beta-blocker use, and statin use. CrCl and BNP were log-transformed.
Discussion
We report for the first time there is a notable reduction in HDL-associated anti-oxidant PON-1 activity (as monitored by serum arylesterase activity) in stable patients with impaired LV systolic function, as well as those admitted with advanced decompensated systolic heart failure, when compared with healthy controls. We further demonstrated a robust association between low serum arylesterase activities and poor long-term prognosis independent of ischemic etiology and traditional cardiac risk factors in patients with impaired LV systolic function undergoing cardiac evaluation. These observations are in parallel with reports of the prognostic value of other oxidative stress markers in patients with heart failure, but were performed on a large scale using a high-throughput platform. Our finding imply a potential importance of the HDL-associated protein PON-1, which has established links with multiple measures of systemic oxidant stress, as an important protective pathway that is diminished in the setting of cardiac dysfunction.
Human studies of oxidative stress in heart failure have focused on end-products rather than mediators of (or in this case protectors against) the oxidative process. The potential contribution of low HDL and low apolipoprotein A1 levels to increased risk of incident heart failure has been demonstrated in large epidemiology studies13, 14, although there has been some dispute noting that coronary ischemia may be an important confounder15. These findings are supported by animal studies of human apolipoprotein A1 gene transfer that prevents the development of diabetic cardiomyopathy16. In patients with established heart failure, the role of low HDL and/or low apolipoprotein A1 in predicting heart failure events as well as mortality has been demonstrated17-20, even in those with non-ischemic etiologies21. While most have speculated the primary contribution of HDL’s favorable effects is via its reverse cholesterol transport properties, other anti-inflammatory, anti-apoptotic, and anti-thrombotic effects of HDL can also play important roles as evidenced by with its inverse relationship with inflammatory mediators22, 23.
PON-1 activity monitored via serum arylesterase activity is a readily detectable and quantifiable process in human serum. Much of the HDL attributed anti-oxidant effects reported are now believed to be mediated by PON-1, which in the plasma compartment exists essentially only as an HDL-associated protein owing to the high binding affinity of PON-1 to HDL and the ability of HDL to stabilize PON-1 activity. Low levels of serum arylesterase activity are associated with increased systemic measures of oxidative stress, heightened cardiovascular disease risk13, and increased disease severity in other end-organ dysfunction16, 17. Instead of indirectly measuring byproducts of oxidative stress, which is often unstable and tedious, quantifying serum arylesterase activity levels facilitates a direct measurement of the anti-oxidative process that has been previously described in protecting against organophosphate toxicity24 and reducing atherosclerotic cardiovascular risk in both humans10, 25 and animal models12. The broad overlap between serum arylesterase activity levels in normal controls versus those with underlying systolic heart failure may preclude the potential for its use as a diagnostic marker for the presence of cardiac dysfunction or heart failure. However, the ability of arylesterase levels to predict long-term outcomes in patients with heart failure is intriguing, especially when the strength of risk prediction for serum arylesterase activity appears robust, even following adjustments for BNP and other cardiometabolic risk factors and renal function. The strong prognostic value of serum arylesterase activity is not linear, as the lowest three quartiles of subjects had poorer outcomes compared to the highest quartile. This finding may imply that there exists a “threshold” of anti-oxidant activity needed. The cutoff level of activity suggested by ROC curve analyses for defining a high-risk cohort suggests a broad systolic heart failure population may be identified as being at risk using this biomarker. Of note, the present results also suggest that those with relatively preserved (high) serum arylesterase activity may also be reassured that they are in a relatively lower risk category for long-term adverse cardiac events.
The precise pathophysiologic mechanism of PON-1 activity in human heart failure has not been previously examined. The expression and variability of systemic arylesterase activity over the natural history of heart failure is largely unknown, although it is conceivable that reduced serum arylesterase activity can adversely contribute to disease progression in a number of ways. The best described mechanism is the association between PON-1 activity and protection against lipoprotein oxidation, thereby antagonizing progression of atherosclerotic coronary artery disease, a major cause of heart failure in Western societies. Another potential protective mechanism for arylesterase activity may include the possibility for limiting microvascular dysfunction as a result of endothelial dysfunction26, 27. Indeed, lower serum paraoxonase/arylesterase activity has been reported in patients with cardiac syndrome X28, and genetic polymorphisms favoring preserved paraoxonase/arylesterase are protective of microvascular diseases in patients with diabetes mellitus29. Regardless, the possibility that a specific anti-oxidative pathway, such as catalyzed by PON-1, modulates systemic oxidative stress in protecting heart failure progression warrants further mechanistic investigations.
Study limitations
Since serum arylesterase levels were only measured at a single time-point, we were unable to examine the variability and prognostic value of level changes over time or the impact of different therapeutic strategies (such as ACE inhibitors or beta-blockers) on serum arylesterase activity. The heterogeneity of LV ejection fraction determination (either by echocardiography, contrast ventriculography, or radionuclide ventriculography) may arise because not all patients in the GeneBank study had simultaneous echocardiographic evaluation at the time of blood sample collection. Even though the traditional “cut-off” of LVEF for determining systolic heart failure is ≤40%, our results were essentially identical when only analyzed within the subgroup of patients defined by this criterion. The original GeneBank protocol also did not include prospective collection of heart failure outcomes including heart failure-related hospitalizations, cardiac transplantation, or mechanical circulatory assist devices. Selection bias may also be present for those undergoing cardiac catheterization for evaluation and management of heart failure at a tertiary care setting despite being a common practice to rule out underlying ischemia (as we observed a relatively large majority of patients had ischemic etiology), and for those potentially earlier in the disease course (evident from comparison with advanced decompensated heart failure cohort). The relative risks might be smaller if heart failure-specific covariates and endpoints are applied. Nevertheless, the large sample size of our patient population together with careful phenotypic evaluation at the time of enrollment provided a well-characterized population of stable patients with impaired LV systolic function. Further understanding of such anti-oxidative pathways is warranted, and may provide opportunities to modulate or enhance such pathways in the setting of systolic heart failure.
Conclusions
In patients with systolic heart failure, diminished activity levels of the anti-oxidant HDL-associated enzyme PON-1, as monitored by serum arylesterase levels, predict shorter time to develop incident long-term adverse cardiac events independent of established clinical and biochemical risk factors. These intriguing results provide further support for a role for oxidative processes in the disease progression of heart failure and for a potential anti-oxidant compensatory role of HDL. Further investigations on the relationship of serum arylesterase activity and heart failure are warranted, as are studies aimed at modulating PON-1 activity as a means of potentially protecting the failing heart from disease progression.
Acknowledgments
Sources of Funding
This research was supported by National Institutes of Health grants 1P01 HL098055-01, P01 HL076491-055328, P01 HL087018-020001, 1R01 DK080732-01A1, P50 HL077107-050004, and 1RO1 HL103931-01, and the Cleveland Clinic Clinical Research Unit of the Cleveland Clinic/Case Western Reserve University CTSA 1UL1RR024989. Sample collections were supported in part by previous grant funding from American Society of Echocardiography, Heart Failure Society of America. Supplies and funding for performance of fasting lipid profiles, blood glucose, creatinine, and BNP were provided by Abbott Laboratories Inc.
Footnotes
Disclosures
Dr. Tang reports having received research grant support from Abbott Laboratories, Inc, and serves as consultant for Medtronic Inc and St Jude Medical. Dr. Wu, Ms. Mann, Mr. Pepoy, Mr. Shrestha, and Mr. Borowski report no relationships to disclose. Dr. Hazen reports being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Hazen reports having been paid as a consultant for the following companies: Abbott, AstraZeneca Pharmaceuticals LP, BG Medicine, Inc., Merck & Co., Inc., Pfizer Inc., Cleveland Heart Lab, Inc., Esperion, Liposcience, and Takeda. Dr. Hazen reports receiving research funds from Abbott, Esperion, Liposcience, and Cleveland Heart Lab Inc. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics and the companies shown below: Cleveland Heart Lab, Inc., Abbott Laboratories, Inc., Biosite Incorporated, Frantz Biomarkers, LLC, and Siemens.
References
- 1.George J, Wexler D, Roth A, Barak T, Sheps D, Keren G. Usefulness of anti-oxidized LDL antibody determination for assessment of clinical control in patients with heart failure. Eur J Heart Fail. 2006;8:58–62. doi: 10.1016/j.ejheart.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Diaz-Velez CR, Garcia-Castineiras S, Mendoza-Ramos E, Hernandez-Lopez E. Increased malondialdehyde in peripheral blood of patients with congestive heart failure. Am Heart J. 1996;131:146–152. doi: 10.1016/s0002-8703(96)90063-0. [DOI] [PubMed] [Google Scholar]
- 3.Cracowski JL, Tremel F, Marpeau C, Baguet JP, Stanke-Labesque F, Mallion JM, Bessard G. Increased formation of F(2)-isoprostanes in patients with severe heart failure. Heart. 2000;84:439–440. doi: 10.1136/heart.84.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hokamaki J, Kawano H, Yoshimura M, Soejima H, Miyamoto S, Kajiwara I, Kojima S, Sakamoto T, Sugiyama S, Hirai N, Shimomura H, Nagayoshi Y, Tsujita K, Shioji I, Sasaki S, Ogawa H. Urinary biopyrrins levels are elevated in relation to severity of heart failure. J Am Coll Cardiol. 2004;43:1880–1885. doi: 10.1016/j.jacc.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Dieterich S, Bieligk U, Beulich K, Hasenfuss G, Prestle J. Gene expression of antioxidative enzymes in the human heart: increased expression of catalase in the end-stage failing heart. Circulation. 2000;101:33–39. doi: 10.1161/01.cir.101.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Sam F, Kerstetter DL, Pimental DR, Mulukutla S, Tabaee A, Bristow MR, Colucci WS, Sawyer DB. Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. J Card Fail. 2005;11:473–480. doi: 10.1016/j.cardfail.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Ungvari Z, Gupte SA, Recchia FA, Batkai S, Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol. 2005;3:221–229. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James RW, Deakin SP. The importance of high-density lipoproteins for paraoxonase-1 secretion, stability, and activity. Free Radic Biol Med. 2004;37:1986–1994. doi: 10.1016/j.freeradbiomed.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblat M, Aviram M. Paraoxonases role in the prevention of cardiovascular diseases. Biofactors. 2009;35:98–104. doi: 10.1002/biof.16. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozenberg O, Shih DM, Aviram M. Human serum paraoxonase 1 decreases macrophage cholesterol biosynthesis: possible role for its phospholipase-A2-like activity and lysophosphatidylcholine formation. Arterioscler Thromb Vasc Biol. 2003;23:461–467. doi: 10.1161/01.ATV.0000060462.35946.B3. [DOI] [PubMed] [Google Scholar]
- 12.Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 13.Velagaleti RS, Massaro J, Vasan RS, Robins SJ, Kannel WB, Levy D. Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation. 2009;120:2345–2351. doi: 10.1161/CIRCULATIONAHA.109.830984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karadag MK, Akbulut M. Low HDL levels as the most common metabolic syndrome risk factor in heart failure. Int Heart J. 2009;50:571–580. doi: 10.1536/ihj.50.571. [DOI] [PubMed] [Google Scholar]
- 15.Dhingra R, Sesso HD, Kenchaiah S, Gaziano JM. Differential effects of lipids on the risk of heart failure and coronary heart disease: the Physicians’ Health Study. Am Heart J. 2008;155:869–875. doi: 10.1016/j.ahj.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Van Linthout S, Spillmann F, Riad A, Trimpert C, Lievens J, Meloni M, Escher F, Filenberg E, Demir O, Li J, Shakibaei M, Schimke I, Staudt A, Felix SB, Schultheiss HP, De Geest B, Tschope C. Human apolipoprotein A-I gene transfer reduces the development of experimental diabetic cardiomyopathy. Circulation. 2008;117:1563–1573. doi: 10.1161/CIRCULATIONAHA.107.710830. [DOI] [PubMed] [Google Scholar]
- 17.Holme I, Strandberg TE, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Larsen ML, Lindahl C, Pedersen TR. Congestive heart failure is associated with lipoprotein components in statin-treated patients with coronary heart disease Insights from the Incremental Decrease in End points Through Aggressive Lipid Lowering Trial (IDEAL) Atherosclerosis. 2009;205:522–527. doi: 10.1016/j.atherosclerosis.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Freitas HF, Barbosa EA, Rosa FH, Lima AC, Mansur AJ. Association of HDL cholesterol and triglycerides with mortality in patients with heart failure. Braz J Med Biol Res. 2009;42:420–425. doi: 10.1590/s0100-879x2009000500004. [DOI] [PubMed] [Google Scholar]
- 19.Wedel H, McMurray JJ, Lindberg M, Wikstrand J, Cleland JG, Cornel JH, Dunselman P, Hjalmarson A, Kjekshus J, Komajda M, Kuusi T, Vanhaecke J, Waagstein F. Predictors of fatal and non-fatal outcomes in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA): incremental value of apolipoprotein A-1, high-sensitivity C-reactive peptide and N-terminal pro B-type natriuretic peptide. Eur J Heart Fail. 2009;11:281–291. doi: 10.1093/eurjhf/hfn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehra MR, Uber PA, Lavie CJ, Milani RV, Park MH, Ventura HO. High-density lipoprotein cholesterol levels and prognosis in advanced heart failure. J Heart Lung Transplant. 2009;28:876–880. doi: 10.1016/j.healun.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Iwaoka M, Obata JE, Abe M, Nakamura T, Kitta Y, Kodama Y, Kawabata K, Takano H, Fujioka D, Saito Y, Kobayashi T, Hasebe H, Kugiyama K. Association of low serum levels of apolipoprotein A-I with adverse outcomes in patients with nonischemic heart failure. J Card Fail. 2007;13:247–253. doi: 10.1016/j.cardfail.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Sampietro T, Neglia D, Bionda A, Dal Pino B, Bigazzi F, Puntoni M, Startari U, Morales A, Minichilli F, Bianchi F, L’Abbate A. Inflammatory markers and serum lipids in idiopathic dilated cardiomyopathy. Am J Cardiol. 2005;96:1718–1720. doi: 10.1016/j.amjcard.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 23.Sezgin N, Sezgin AT, Gullu H, Karabulut A, Barutcu I, Topal E, Yalcintas D, Temel I. Decreased serum lipoprotein levels as a guide for clinical severity in patients with idiopathic dilated cardiomyopathy. Tohoku J Exp Med. 2005;206:219–224. doi: 10.1620/tjem.206.219. [DOI] [PubMed] [Google Scholar]
- 24.Camps J, Marsillach J, Joven J. The paraoxonases: role in human diseases and methodological difficulties in measurement. Crit Rev Clin Lab Sci. 2009;46:83–106. doi: 10.1080/10408360802610878. [DOI] [PubMed] [Google Scholar]
- 25.La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1988;43:227–229. [PMC free article] [PubMed] [Google Scholar]
- 26.den Uil CA, Klijn E, Lagrand WK, Brugts JJ, Ince C, Spronk PE, Simoons ML. The microcirculation in health and critical disease. Prog Cardiovasc Dis. 2008;51:161–170. doi: 10.1016/j.pcad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. The cardiac microvasculature in hypertension, cardiac hypertrophy and diastolic heart failure. Curr Vasc Pharmacol. 2008;6:292–300. doi: 10.2174/157016108785909779. [DOI] [PubMed] [Google Scholar]
- 28.Gur M, Yildiz A, Demirbag R, Yilmaz R, Aslan M, Ozdogru I, Erel O. Paraoxonase and arylesterase activities in patients with cardiac syndrome X, and their relationship with oxidative stress markers. Coron Artery Dis. 2007;18:89–95. doi: 10.1097/MCA.0b013e32801104e8. [DOI] [PubMed] [Google Scholar]
- 29.Hofer SE, Bennetts B, Chan AK, Holloway B, Karschimkus C, Jenkins AJ, Silink M, Donaghue KC. Association between PON 1 polymorphisms, PON activity and diabetes complications. J Diabetes Complications. 2006;20:322–328. doi: 10.1016/j.jdiacomp.2005.08.008. [DOI] [PubMed] [Google Scholar]


