Abstract
This study of 632 drug injectors enrolled in eight residential detoxification centers within the National Drug Abuse Treatment Clinical Trials Network tested three interventions to reduce drug and sex risk behaviors. Participants were randomized to: (a) a two-session, HIV/HCV counseling and education (C&E) model added to treatment as usual (TAU), (b) a one-session, therapeutic alliance (TA) intervention conducted by outpatient counselors to facilitate treatment entry plus TAU, or (c) TAU. Significant reductions in drug and sex risk behaviors occurred for all three conditions over a 6-month follow-up period. C&E participants reported significantly greater rates of attending an HIV testing appointment, but this was not associated with better risk reduction outcomes. Reporting treatment participation within 2 months after detoxification and self-efficacy to practice safer injection behavior predicted reductions in injection risk behaviors. Findings indicate that participation in detoxification was followed by significant decreases in drug injection and risk behaviors for up to 6-months; interventions added to standard treatment offered no improvement in risk behavior outcomes.
Keywords: Drug injection, Risk reduction, Treatment entry, Detoxification
Introduction
The association between HIV/AIDS and drug injection is well established. Sharing syringes and injection paraphernalia increases the risk of HIV infection and other blood borne illnesses among injection drug users (IDUs) and their sex partners [1, 2]. Through 2006, over 1,000,000 persons aged 13 years or older with AIDS were reported to the Centers for Disease Control and Prevention [3]; IDUs accounted for 31% of infected individuals with a known exposure category. In addition, IDUs play a critical role in the transmission of HIV to non-IDUs through sex risk behaviors [4, 5], including unprotected sex [6, 7], multiple sex partners [8], and sexual intercourse with other IDUs [9–11].
Transmission of Hepatitis C (HCV), primarily through sharing drug preparation and injection equipment, is also a major public health concern. Sixty percent of HCV transmission is related to drug injection and estimates of IDUs infected with HCV range from 50 to 95% [12, 13]. This high rate among IDUs, even in locations where HIV prevalence is not high, may be related to the higher transmissibility of HCV, which can be easily spread through the shared use of cottons, cookers and rinse water, even if drug users do not share needles [14, 15].
Interventions and policy proposals to prevent HIV and HCV infection among IDUs have emphasized increased access to sterile syringes and, when necessary, the use of bleach to reduce transmission [2]. Effective interventions include media campaigns [16, 17], street outreach [18–20], HIV testing and counseling [21–23], and syringe exchange programs [24, 25].
Drug treatment is also an effective intervention to reduce needle use, the risk of HIV infection [26, 27], and HIV seroconversion [28, 29]. Many IDUs enter the treatment system, but receive detoxification services only. Nearly half (45%) of the 44,169 clients served by the Target Cities project in Boston (1992–1994) were treated in detoxification centers [30]. Lundgren et al. [31] examined treatment patterns for injection drug users with multiple treatment admissions in Massachusetts from 1997 through 2001; the most common pattern (30%) was repeated admissions to detoxification only. McCusker et al. [32] found that only about one-in-four detoxification admissions led to further treatment. More than half (59%) of patients in opiate detoxification had no formal treatment for 6 months following detoxification [33]. Strategies to maximize risk reduction interventions provided in detoxification, including interventions that facilitate engagement in formal treatment following detoxification, are needed in order to reduce HIV and HCV risk among drug using patients.
This study was a multisite, randomized clinical trial conducted at residential detoxification centers participating in the National Drug Abuse Treatment Clinical Trials Network (CTN). The study was designed to intervene with IDUs at a common entry point into the treatment system to reduce HIV/HCV related injection and sex risk behaviors. Two strategies were compared, one focused directly on counseling and testing; the other focused on promoting transition to further treatment. The two individually-delivered interventions were added to treatment as usual (TAU) and compared to TAU alone: (1) an updated version of the HIV Counseling and Education (C&E) intervention model [34] and (2) a Therapeutic Alliance (TA) intervention [35] that focused on the development of a therapeutic relationship between detoxification patients and outpatient counselors to facilitate treatment continuation after detoxification. The study had three primary hypotheses related to risk behaviors: (a) C&E plus TAU would be more effective than TAU in reducing HIV/HCV injection and sex risk behaviors, as well as drug injection; (b) C&E plus TAU would be more effective than TA plus TAU in reducing HIV/HCV injection and sex risk behaviors; and (c) TA plus TAU would be more effective than TAU in reducing HIV/HCV injection risk behaviors and drug injection. Secondary hypotheses regarding treatment entry are reported elsewhere [35].
Methods
Clinical Sites
The study was conducted from November 2004 through February 2006 at eight residential detoxification centers in community treatment agencies across the U.S. participating in the CTN. Program size ranged from 16 to 100 beds with usual length of stays ranging from 1.5 to 10 days. See Table 1 for site characteristics.
Table 1.
Detoxification treatment site characteristics
| Site | Number randomized |
City population (approximate) |
Number of beds |
Usuala length of stay (days) |
Usual carea |
||
|---|---|---|---|---|---|---|---|
| HIV education/ screening |
HIV testing |
Treatment referral |
|||||
| A | 101 | 92,000 | 17 | 4–6 | Yes | Yes | Yes |
| B | 101 | 26,000 | 40 | 6 | No | No | Yes |
| C | 70 | 571,800 | 100 | 1.5 | Yes | No | Yes |
| D | 39 | 86,500 | 23 | 2–4 | Yes | Yes | Yes |
| E | 132 | 575,900 | 36 | 3–6 | Yes | No | Yes |
| F | 62 | 72,700 | 29 | 7–10 | Yes | No | Yes |
| G | 39 | 82,100 | 16 | 3–6 | Yes | No | Yes |
| H | 88 | 153,700 | 17 | 4–6 | Yes | No | Yes |
Information provided by program administrators
Participants
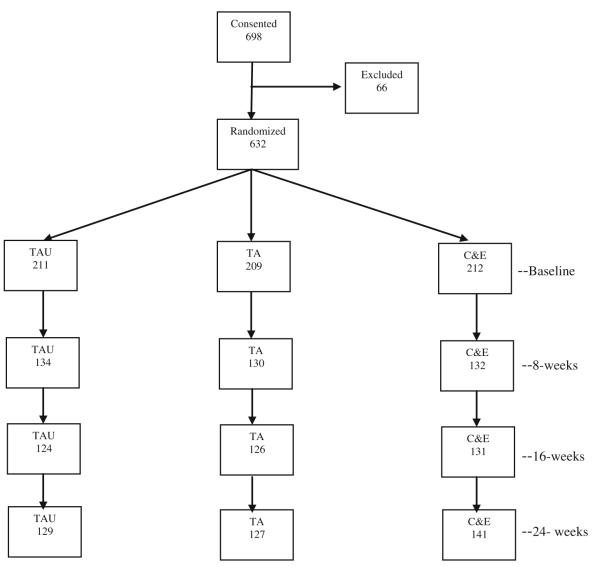
Study participants were injection drug users recruited during detoxification treatment. Eligible participants were 18 years of age or older and had been cleared by detoxification staff as sufficiently medically stable to provide consent. They demonstrated a recent history of injection drug use via self-report and signs of recent drug injection or the ability to correctly describe injection procedures. They were eligible for outpatient services and reported plans to remain in the area for the next 6 months. Potential participants were informed about the study by detoxification staff members who provided brief descriptions, including information sheets. Those who expressed interest were screened for eligibility and referred to research personnel to complete an informed consent procedure. Potential participants (N = 698) were fully assessed for eligibility following informed consent and 632 were randomized: C&E plus TAU = 212, TA plus TAU = 209, and TAU = 211. Figure 1 shows numbers of participants consented, randomized, and completing follow-up assessments for each study condition.
Fig. 1.
Consort diagram of consent, randomization, baseline assessment and follow-up rates
Procedures
Participants were randomized following the baseline assessment which took approximately 2.5 h to administer. A computer-based, centrally administered, blocked randomization scheme assigned participants to one of the three conditions, stratified by site. Whenever possible, the two experimental interventions took place the same day as the baseline assessments, or at the latest, the day of discharge from the program. Follow-up interviews were conducted at 2, 8, 16 and 24 weeks following randomization. All study procedures were reviewed and approved by a Data Safety and Monitoring Board at the National Institute on Drug Abuse. The institutional review boards at the University of Colorado Denver and the participating treatment centers approved the research protocol.
Assessment Instruments
Assessment measures included the CTN Common Assessment Battery (Addiction Severity Index-Lite, Composite International Diagnostic Interview Version 2.1-Substance Use Diagnosis, CTN Demographic Form), Urine Drug Screen test strips, and a Locator Form. Four additional measures used in these analyses are described in the following sections.
Risk Behavior Survey (RBS)
The RBS [36] administered at baseline and at all follow-up periods, is an abbreviated version of the Risk Behavior Assessment (RBA) which has demonstrated reliability and validity [37, 38]. The RBS, used widely within the CTN, assesses HIV and HCV risk behaviors in the areas of drug use and sex in the previous 30 days. It was administered through an Audio Computer Assisted Self-Interview (ACASI). Participants simultaneously read and heard questions and provided answers without an interviewer. The variables used to assess risky injection behavior in the past 30 days were composites from the RBS: (a) sum of risky injection behaviors (i.e., cumulative frequency of times sharing needles/syringes without disinfecting, sharing cookers/cottons/rinse water, and sharing the drug solution); (b) number of days injecting cocaine, heroin, speedballs (a mixture of cocaine and heroin in the same syringe), amphetamines and other opiates, capped at 30 days; and (c) frequency of injecting cocaine, heroin, speedballs, amphetamines and other opiates, capped at 10 times per drug per day and 300 times maximum. The primary outcome measure was the sum of risky injection behaviors and, for sex behaviors, always practicing safe sex in the past 30 days, defined as abstaining from sex or 100% use of condoms during vaginal or anal sex.
Timeline Follow-Back Assessment of Treatment Behavior (TFB)
The TFB, modeled after the work of Sobell and Sobell [39], was administered at the 2-week visit and all follow-up assessments to report any alcohol and drug abuse treatment received since the last study visit, including outpatient, inpatient, residential, methadone maintenance/other opiate replacement and 12-step meetings.
Self-Efficacy for Practicing Safer Injection and Sex Behaviors (SE)
Self-efficacy, or confidence, to practice safer injection and sex behaviors when faced with risky situations was collected at 2 weeks and at each follow-up assessment using a modified version of the Cooperative Agreement RBA Behaviors and Beliefs Trailer [40]. Four items (range 1–5) related to injecting drugs were summed to create injection self-efficacy (total score range 0–20). Similarly, four items (range 1–5) related to sex behaviors were summed to create a sex self-efficacy score (total score range 0–20). Higher scores on each scale indicates higher self efficacy.
Services Received Questionnaire (SRQ)
Services received during detoxification were assessed at the 2-week follow-up using the SRQ. Using a response skip pattern, participants were asked whether they had received an HIV/HCV risk assessment; if yes, they were asked about HIV testing referral, if yes, they were asked whether they had attended their testing appointment.
Interventions
Counseling and Education
The manualized C&E intervention consisted of two individual sessions; the first session included HIV pre-test counseling, and optional (although strongly encouraged) HIV testing. The second session, approximately 14 days later, included test results and post-test counseling. This model has been compared to more expensive and labor-intensive approaches (e.g., didactic education, group intervention, street outreach) and found to be as effective in reducing HIV risk behaviors [41, 42]. The 30 min pre-test counseling session provided basic information about AIDS and how to reduce the risk of HIV infection, as well as similar information about HCV. Participants rehearsed how to clean injection equipment and use a condom with an anatomical model. The use of new injection equipment was emphasized, with recommendations to use bleach only if new needles/syringes were unavailable. The content of the second, 30 min session varied according to participants’ testing status (i.e., had declined testing, positive results or negative results). The second session repeated some of the first session’s educational content, as well as rehearsal of correct bleaching and condom use. This booster session was designed to clarify participants’ understanding of risk reduction behaviors, as well as increase competence and perceived self-efficacy to practice new behaviors. Alternatives to high-risk behaviors were stressed, including drug treatment, discontinuing drug injecting, using protection during sex, avoiding sharing drug equipment or solution and reducing the number of sex partners. Basic health care advice, including the importance of partner notification and medical referral were provided to participants who tested seropositive.
Counselors provided written material at both sessions, including a study description, literature about HIV and HCV transmission and the correct way to use hygiene materials, and the names, phone numbers and addresses of social service agencies. At sites located in cities with syringe exchange programs, counselors provided information regarding locations, hours of operation and policies to encourage use of sterile syringes. The goals of C&E included education about HIV and HCV transmission, as well as learning and adopting the preventive behaviors described above.
Therapeutic Alliance
The TA intervention consisted of a single, individual session, approximately 45-min long, conducted by an outpatient counselor affiliated with each center’s outpatient treatment program. The manualized intervention focused on the development of a therapeutic alliance between client and outpatient counselor, designed to facilitate outpatient treatment entry after leaving detoxification. A strong therapeutic alliance has been shown to increase attendance [43], retention [44–47] and lead to improved outcomes [44, 48] in substance abuse treatment. The alliance facilitating intervention in the current study incorporated core elements [49]: (a) agreement about treatment tasks and roles; (b) agreement about the goals and expectations of treatment; and (c) creating a positive bond between client and counselor. These elements were combined with role induction, a treatment preparation intervention designed to educate clients about treatment that has been shown to increase treatment initiation and attendance [50–52]. The TA session included discussion of: (a) plans after detoxification; (b) life goals and possible treatment goals; (c) developing confidence in goal attainment; (d) prior treatment experiences and treatment expectations; (e) what happens in treatment; and (f) common treatment challenges and how to handle them. Throughout the session, the counselor emphasized positive feedback, clarification about treatment, mutual agreement about treatment tasks and goals and working as a team. The session ended with the opportunity for the participant to make an outpatient appointment with the counselor or to receive information about other treatment options. The primary goal of the TA intervention was to reduce HIV/HCV risk behaviors as mediated by treatment participation after detoxification. A concomitant goal was to increase treatment entry and retention post-detoxification relative to standard discharge referral. Results of treatment entry outcomes, reported by Campbell et al. [35], indicated that TA participants had significantly higher outpatient treatment entry rates than those in TAU; there were no differences among groups in outpatient treatment retention.
Treatment as Usual
The TAU intervention was the HIV/HCV risk assessment screening and referral for testing and counseling that each clinic normally used for patients in detoxification. The TAU linkage to continuing care involved referrals to treatment and assistance with arranging appointments upon the client’s request. All sites reported that standard treatment included treatment referral; seven reported HIV screening and education; two reported on site testing (see Table 1). Participants in the two experimental conditions also received TAU.
Staff Training and Certification
Intervention experts conducted a 3-day, centralized training of 50 interventionists and supervisors from the eight detoxification centers. Interventionists were trained to deliver one of the two experimental interventions. Training in each included a review of the intervention manual and videotapes of sessions conducted by experts. Trainees received didactic instruction and practiced the intervention. They were also introduced to the fidelity monitoring/supervision procedures and the fidelity rating instruments developed for the interventions. Each instrument rated interventionist performance according to the completeness of content (adherence) and quality of delivery (competence) for specified elements of the intervention. Following the centralized training, trainees were certified as interventionists via achievement of a criterion score (i.e., ratings of satisfactory or above on 80% of items) on the fidelity rating instruments for audio-taped practice sessions conducted at their treatment centers with either practice patients or role players. Forty-nine of the fifty trainees achieved certification. Staff supervisors at each site were certified as interventionists and also trained and certified as local fidelity raters by national experts.
Fidelity Monitoring
During the study, both experimental interventions were audio-taped and evaluated by local supervisors and national raters to assure intervention fidelity. Fidelity ratings review was incorporated into biweekly-to-monthly local supervision. Supervisors also held monthly teleconferences with national intervention experts. Approximately 43% (n = 159) of audio-taped sessions were randomly selected for ratings review. One of three supervisor-rated tapes was also randomly selected for co-rating by an interventionist expert. A minimum proficiency score of 80% of adherence items rated satisfactory or above was required for initial counselor certification and for ongoing delivery of the manualized interventions to insure intervention adherence. During the study, two counselors fell below satisfactory adherence criteria. Following retraining, they achieved recertification and were reinstated.
Statistical Methods
All analyses were intent-to-treat with all randomized participants included and were conducted using SAS versions 9.1 [53] and 9.2 [54] statistical software package. Variable distributions were examined for extreme values and departures from normality. In the case of non-normality, appropriate transformations (i.e., natural log) were utilized, with a back-transformation to the original scale employing Monte Carlo simulation to compute confidence intervals. Baseline comparisons between the three intervention groups on demographic and key variables were assessed using chi-square tests and analysis of variance (ANOVA). Similarly, those completing the 6-month follow-up interview were compared to those lost to follow-up on baseline values of risky injection and sex behaviors. Analyses of continuous and dichotomous outcome measures over time utilized linear and generalized linear mixed effects models, respectively, allowing for random site and subject effects, as well as estimates of changes in repeated measures in the presence of missing data, assuming those data were missing at random [54, 55]. The pattern of missing data was evaluated with chi-square tests comparing interventions at each time point.
The continuous outcome variables for risky injection behaviors were analyzed with mixed (random coefficient) models, where the best model was determined using likelihood-based procedures, such as Akaike’s Information Criterion (AIC) [56], for the mean and between-subject components of the model and specifying a compound symmetric covariance within subjects. The starting model included fixed effects for intervention, month and intervention by month, as well as random effects for site, site by intervention, site by month, site by intervention by month and subject. Random effects found to have zero variability (e.g. site by month and site by intervention by month) were removed and the model was re-run after each removal. Once all remaining random effects had positive variability (and were significant based on AIC comparisons), non-significant fixed effects were removed and the model re-run. The minimum models entertained for each outcome included site and subject as random effects, as well as intervention, month, and an intervention-by-month interaction term as fixed effects, regardless of their significance. Next, compound symmetric, first order autoregressive (AR 1) and unstructured within-subject covariance structures were compared using AIC.
Always having safe sex (i.e., abstinence or 100% condom use) in the past 30 days was compared among interventions and sites over time using generalized linear mixed models for binary outcomes and a process similar to the model-fitting procedures described above. Models specifying a random site effect would not converge; therefore, site was included as a fixed effect along with intervention and month while subject was the only random effect.
To compare outcomes by time and intervention group, estimated means (95% CIs) were obtained for each of the three continuous outcomes. These means values were adjusted for all other covariates in the models, with continuous covariate values held at zero. Odds ratios (95% CIs) were calculated to compare the odds of always practicing safe sex behaviors by month, for the three intervention groups. For both the continuous and dichotomous outcomes, if the effects of time and/or intervention were P < 0.10, specific contrasts determined which intervention(s) differed from one another and which month(s) showed significant changes in behavior. When the time effect was significant overall, the plots of the data suggested the importance of first evaluating behavior at baseline compared with the average of follow-ups, and then whether there was any difference between the follow-up time points (months 2, 4 and 6). If a difference was detected, that difference was explored through further testing (e.g., month-2 vs. month-4). This procedure, and the requirement that the overall time and intervention effects be significant before any further statistical comparisons of time or intervention differences occurred, provided some protection against multiple comparisons in the longitudinal analyses [57].
Four variables were evaluated for inclusion as covariates in each model: gender, self-reported participation in any treatment (including outpatient, residential, inpatient, methadone/opiate replacement and 12 step meetings) prior to 2-month visit, and perceived self-efficacy to practice safer injection and sex behaviors at baseline. Separately, injection (yes/no) of the following drugs in the past 30 days at baseline were evaluated for inclusion as covariates: cocaine, heroin, speedballs and injecting either amphetamines or cocaine. Finally, for each model that contained site as a random effect, we ran a reduced effects model without the random effect for site, and used the likelihood ratio test to determine whether the random effect for site was significant. Because the site effect was significant in two of the three models, and its absence in the remaining model did not affect other results, we retained the random effect for site in all three models, to account for any potentially unknown biases. In the model for always having safe sex, which included site as a main effect, we descriptively examined site differences in the percentage of participants always practicing safe sex between baseline and 6 months.
A post hoc analysis determined if differences in outcomes could be attributed to differential receipt of HIV/HCV risk assessment and testing services among the intervention groups. Differences in the proportion of participants from each intervention who reported receiving an HIV/HCV risk assessment, referral for testing, and attendance at the HIV testing appointment were assessed via χ2 tests. We created an additional variable to include in multivariate models with the following categories: (a) no HIV/HCV risk assessment, (b) HIV/HCV risk assessment only, (c) HIV/HCV risk assessment plus referral for testing and testing appointment attended, and (d) HIV/HCV risk assessment plus referral for testing and testing appointment not attended. We examined the effect of this variable, plus its interaction with time and intervention on each of the four outcomes, adjusting for the other covariates previously included in the models. All comparisons utilized a two-tailed P < .05 to define statistical significance.
Results
Study Sample
Table 2 shows age, gender, ethnicity, racial composition and previous 30 days drug use at baseline according to intervention group. Participants averaged 35.9 years (SD = 9.8) of age, 76% were male, 73% white, 8% African American, 10% multi-racial and 9% reported Latino or Hispanic ethnicity In the past 30 days, 81% reported injecting heroin, followed by other opiates (53%), cocaine (45%), speedballs (38%) and amphetamines (27%). Participants reported injecting drugs an average of 22 days and 109 times during those 30 days. Injection practices during this period included 25% reporting injection with a previously used and unbleached needle/syringe, 46% reporting sharing cotton, cooker or water and 54% reporting sharing drug solution. A total of 61% engaged in at least one of these three injection-related risk behaviors in the past 30 days, with an average of 13 risky injection behaviors reported per participant. Chi-square tests and ANOVAs comparing intervention groups at baseline found no group differences on the descriptive variables.
Table 2.
Participant characteristics at baseline by intervention group
| Characteristics | TAUa (n = 211) |
TAb (n = 209) |
C&Ec (n = 212) |
Total (n = 632) |
|---|---|---|---|---|
| Age | ||||
| Mean age (years) | 35.6 | 36.3 | 35.7 | 35.9 |
| Range | 19–62 | 19–61 | 19–65 | 19–65 |
| Gender | ||||
| Female (%) | 26.5 | 23.4 | 23.1 | 24.4 |
| Race | ||||
| White/Caucasian (%) | 73.9 | 69.4 | 77.4 | 73.4 |
| African American (%) | 9.0 | 10.1 | 5.2 | 8.1 |
| Multi-racial (%) | 9.0 | 11.5 | 8.5 | 9.7 |
| Others (%) | 8.1 | 9.0 | 8.9 | 8.8 |
| Ethnicity | ||||
| Hispanic/Latino (%) | 10.9 | 9.1 | 7.6 | 9.2 |
| Drug use past 30 days | ||||
| Heroin use (%) | 81.3 | 83.9 | 77.1 | 80.7 |
| Other opiates (%) | 54.5 | 52.3 | 52.7 | 53.2 |
| Cocaine use (%) | 48.1 | 43.8 | 44.0 | 45.3 |
| Speedball use (%) | 39.6 | 33.8 | 40.4 | 37.9 |
| Amphetamine (%) | 24.5 | 32.2 | 24.2 | 27.0 |
Treatment as usual
Therapeutic alliance
Counseling and education
Nearly one-half of the males (49%) reported vaginal sex and 21% anal sex with a member of the opposite sex (less than .5% reported anal sex with another male), while 71% of the women reported vaginal sex and 23% anal sex in the prior 30 days. Males averaged one sex partner and females two in the 30 days prior to their baseline interview. Fewer than 20% of all participants, including 17% of males and 24% of females, indicated they always used a condom during vaginal sex, while more than two-thirds reported always using a condom when having anal sex (males 68%, females 73%). Overall, 51% engaged in unsafe vaginal or anal sex practices.
Scores on both perceived self efficacy for practicing safe injection and sex behaviors averaged 15 (range 0–20). Whether or not participants entered treatment prior to the 2-month follow-up was only available for those who completed their 2-month interview (N = 396) or who responded about that time interval at a subsequent follow-up interview (N = 145); of these participants, 60% reported at least one treatment visit.
Attrition Analyses
At 6-months, 67% (N = 397) were successfully re-interviewed. Analyses revealed few differences between those interviewed and those lost to follow-up. No differences were observed on intervention assignment, gender, race, ethnicity and most risky injection and sex risk behaviors at baseline. However, successfully interviewed participants were 1.6 years older (P < .05), they injected drugs less frequently in the 30 days prior to baseline (P < .05), and they were more likely to have entered treatment at 2 months (64% vs. 51%; P < .01) than those not interviewed at follow-up. Chi-square tests supported the assumption that the pattern of missing data did not differ.
Longitudinal Analyses of Outcomes
The mixed model without covariates was determined for each of the three drug-related risk outcomes and the specified covariates of interest were evaluated separately. The final models included the covariates with P < 0.10. Prior to evaluating covariates, the model selected for the sum of risky injection behaviors in the past 30 days included intervention, month, and an intervention-by-month interaction as fixed effects; site as a between-subject random effect; and an unstructured within-subject covariance. In the covariate-adjusted model, for the 528 cases with all covariates, there were no differences among groups in changes observed across time from pre to post detoxification (F = 0.40; df = 6, 543; P = 0.8765). Based on this model of the log transformed outcome, the average sum of risky injection behaviors (transformed back to the original scale) decreased following detoxification for participants in all conditions from 26.0 (95% CI 22.4–30.9) behaviors at baseline, to 4.8 (95% CI 4.5–5.2) at 6-month follow-up. Table 3 shows mean values of risky injection behaviors and other outcome variables for all intervention groups at each time period. The decreases in risky injection behaviors were significant across time periods: baseline and follow-ups (F = 264.82; df = 1, 505; P < 0.0001), months 2 and 4 (t = 3.00; df = 377; P = 0.003); and months 2 and 6 (t = 3.86; df = 377; P = 0.0001). Reporting at least one treatment visit prior to the 2-month interview (F = 37.0; df = 1, 506; P < 0.0001) and self-efficacy to practice safer injection behavior (F = 21.16; df = 1, 489; P < 0.0001) were protective for engaging in fewer risky injection behaviors. Injecting heroin or either amphetamines/cocaine in the 30 days prior to baseline was associated with greater risky injection behaviors (F = 19.01; df = 1, 494; P < 0.0001; and F = 4.84; df = 1, 483; P = 0.028, respectively). See Table 4 for full model results.
Table 3.
Mean adjusted baseline and follow-up values of sum of risky injection behaviors, days of drug injection, frequency of drug injection, and odds ratio for always engaging in safe sex behaviors for all groups by month
| Baseline | 2-month | 4-month | 6-month | |
|---|---|---|---|---|
| Mean sum of risky injection behaviors, mean (95% CI) | ||||
| All groups (n = 528) | ||||
| Log scale | 2.2 (1.9–2.6)a | 1.4 (1.0–1.8) | 1.2 (0.8–1.6) | 1.1 (0.8–1.5) |
| Original scalea | 26.0 (22.4–30.9) | 7.5 (6.8–8.2) | 5.5 (5.1–5.9) | 4.8 (4.5–5.2) |
| C&E (n = 185) | ||||
| Log scale | 2.2 (1.8–2.6) | 1.3 (1.0–1.7) | 1.2 (0.8–1.6) | 1.1 (0.7–1.5) |
| Original scalea | 25.6 (22.1–30.2) | 7.2 (6.5–7.9) | 5.4 (5.0–5.8) | 4.7 (4.4–5.0) |
| TA (n = 173) | ||||
| Log scale | 2.2 (1.8–2.7) | 1.4 (1.1–1.8) | 1.2 (0.8–1.6) | 1.2 (0.8–1.6) |
| Original scalea | 26.2 (22.7–30.3) | 7.9 (7.2–8.7) | 5.2 (4.8–5.6) | 5.1 (4.7–5.5) |
| TAU (n = 170) | ||||
| Log scale | 2.3 (1.8–2.7) | 1.4 (1.0–1.8) | 1.3 (0.9–1.7) | 1.1 (0.7–1.5) |
| Original scalea | 26.4 (22.9–30.8) | 7.3 (6.6–8.0) | 5.8 (5.4–6.3) | 4.7 (4.4–5.0) |
| Number of days injected drugs in past 30 days, mean (95% CI) | ||||
| All groups (n = 526) | 22.0 (18.6–25.4) | 9.7 (6.2–13.1) | 9.3 (5.8–12.7) | 7.8 (4.4–11.3) |
| C&E (n = 185) | 22.3 (18.8–25.8) | 9.5 (5.8–13.3) | 8.9 (5.1–12.7) | 7.2 (3.6–10.8) |
| TA (n = 171) | 21.9 (18.4–25.4) | 10.8 (7.0–14.5) | 9.3 (5.5–13.1) | 8.3 (4.6–12.1) |
| TAU (n = 170) | 21.9 (18.3–25.4) | 8.8 (5.0–12.6) | 9.6 (5.7–13.4) | 8.0 (4.2–11.7) |
| Frequency of injecting drugs in past 30 days, mean (95% CI) | ||||
| All groups (n = 526) | 104.0 (92.4–115.6) | 30.3 (19.6–41.0) | 29.2 (18.3–40.0) | 22.5 (12.0–32.9) |
| C&E (n = 185) | 110.7 (95.7–125.6) | 31.9 (19.1–44.7) | 28.8 (15.7–42.0) | 20.5 (8.4–32.5) |
| TA (n = 171) | 100.8 (85.3–116.3) | 32.1 (19.1–45.0) | 30.8 (17.3–44.4) | 26.9 (14.3–39.5) |
| TAU (n = 170) | 100.5 (85.1–115.8) | 27.0 (14.0–39.9) | 27.8 (14.4–41.2) | 20.1 (7.7–32.4) |
| Safe sex behavior in the past 30 days, OR (95% CI) | ||||
| All groups (n = 632) | 1.00 (reference) | 1.8 (1.4–2.5) | 1.6 (1.2–2.2) | 1.7 (1.3–2.3) |
| C&E (n = 212) | 1.00 (reference) | 1.8 (1.0–3.0) | 1.7 (1.0–2.9) | 2.0 (1.2–3.3) |
| TA (n = 209) | 1.00 (reference) | 2.3 (1.3–3.9) | 1.7 (1.0–2.9) | 1.7 (1.0–2.9) |
| TAU (n = 211) | 1.00 (reference) | 1.5 (0.9–2.6) | 1.5 (0.8–2.5) | 1.5 (0.9–2.6) |
Table 4.
Model for sum of risky injection behaviors on the natural log scale, including baseline values for injecting heroin, cocaine, and speedballs in the past 30 days, perceived self-efficacy, and treatment entry before the 2-month visit (N = 528 cases included with all covariates)
| Effect | β | Standard error |
F-statistic | P |
|---|---|---|---|---|
| Intervention | ||||
| C&E | −0.00467 | 0.1126 | 0.15 | 0.8642 |
| TA | 0.07771 | 0.1162 | ||
| TAU (reference) | – | – | ||
| Months | ||||
| 0 (Baseline) | 1.1350 | 0.1204 | 92.79 | <0.0001 |
| 2 | 0.2422 | 0.1080 | ||
| 4 | 0.1410 | 0.09786 | ||
| 6 (reference) | – | – | ||
| Intervention × month interaction | ||||
| C&E, month 0 | −0.03357 | 0.1664 | 0.40 | 0.8765 |
| C&E, month 2 | −0.01564 | 0.1498 | ||
| C&E, month 4 | −0.07566 | 0.1353 | ||
| C&E, month 6 | – | – | ||
| TA, month 0 | −0.08947 | 0.1702 | ||
| TA, month 2 | 0.001875 | 0.1527 | ||
| TA, month 4 | −0.1834 | 0.1392 | ||
| TA, month 6 | – | – | ||
| TAU, month 0 | – | – | ||
| TAU, month 2 | – | – | ||
| TAU, month 4 | – | – | ||
| TAU, month 6 | – | – | ||
| Treatment entry before 2-month visit | ||||
| Yes | −0.4609 | 0.07577 | 37.00 | <0.0001 |
| No (reference) | – | – | ||
| Perceived self efficacy | −0.05417 | 0.01178 | 21.16 | <0.0001 |
| Inject heroin at baseline | ||||
| Yes | 0.4122 | 0.09455 | 19.01 | <0.0001 |
| No (reference) | – | – | ||
| Inject amphetamine or cocaine at baseline | ||||
| Yes | 0.1684 | 0.07655 | 4.84 | 0.0283 |
| No (reference) | – | – | ||
| Site random effecta | 0 | 1.00 | ||
As estimated by the likelihood ratio test, compared to a reduced model without a random effect for site
For days injected in the past 30 days, the chosen model without covariates had intervention, month, and an intervention-by-month interaction as fixed effects, site and the site by month interaction as between-subject random effects, and an unstructured within-subject covariance. In the covariate-adjusted model with 526 cases, the intervention by month interaction was not significant (Table 5). There was a significant decrease between baseline and follow-up (F = 249.36; df = 1, 26.4; P < 0.0001) for participants in all three conditions, but no difference between follow-up times. Overall, participants averaged 22.0 days injecting at baseline, 9.7 days injecting at 2-month follow-up, 9.3 days injecting at 4-month follow-up and 7.8 days injecting at 6-month follow-up (Table 3). Injecting heroin or speedballs in the 30 days prior to baseline was related to more days injecting (F = 105.64; df = 1, 418; P = 0.0001; and F = 9.21; df = 1, 498; P < 0.003, respectively) and entering treatment prior to the 2-month evaluation was associated with a decrease in days injecting (F = 14.54; df = 1, 517; P = 0.0002). Injecting cocaine in the 30 days prior to baseline (F = 2.65; df = 1, 502; P = 0.1040) and low injection self-efficacy (F = 2.90; df = 1, 501; P = 0.0891) showed trends in relationship to days injecting. When those two variables were removed, AIC increased considerably, indicating a worse model, with the remaining variables remaining significant or becoming more significant. Regardless of which covariates were included, major findings were consistent; there was a significant decrease in the number of days injecting over time following detoxification for all three conditions and no significant difference among the conditions.
Table 5.
Model for number of days injected drugs in past 30 days, including baseline values for injecting heroin, cocaine, and speedballs in the past 30 days, perceived self-efficacy, and treatment entry before the 2-month visit (N = 526 cases included with all covariates)
| Effect | β | Standard error |
F-statistic | P |
|---|---|---|---|---|
| Intervention | ||||
| C&E | −0.7903 | 1.2185 | 0.31 | 0.7337 |
| TA | 0.3452 | 1.2630 | ||
| TAU (reference) | – | – | ||
| Month | ||||
| 0 (Baseline) | 13.8864 | 1.3126 | 85.31 | <0.0001 |
| 2 | 0.7809 | 1.3128 | ||
| 4 | 1.5627 | 1.2520 | ||
| 6 (reference) | – | – | ||
| Intervention × month interaction | ||||
| C&E, month 0 | 1.1808 | 1.4633 | 0.71 | 0.6391 |
| C&E, month 2 | 1.5595 | 1.4707 | ||
| C&E, month 4 | 0.1253 | 1.3702 | ||
| C&E, month 6 | – | – | ||
| TA, month 0 | −0.3373 | 1.5093 | ||
| TA, month 2 | 1.6381 | 1.4998 | ||
| TA, month 4 | −0.5876 | 1.4142 | ||
| TA, month 6 | – | – | ||
| TAU, month 0 | – | – | ||
| TAU, month 2 | – | – | ||
| TAU, month 4 | – | – | ||
| TAU, month 6 | – | – | ||
| Treatment entry before 2-month visit | ||||
| Yes (reference) | – | – | 14.54 | 0.0002 |
| No | 2.4594 | 0.6451 | ||
| Perceived self efficacy | −0.1645 | 0.09657 | 2.90 | 0.0891 |
| Inject heroin at baseline | ||||
| Yes (reference) | – | – | 105.64 | <0.0001 |
| No | −9.0431 | 0.8798 | ||
| Inject cocaine at baseline | ||||
| Yes (reference) | – | – | 2.65 | 0.1040 |
| No | −1.1548 | 0.7090 | ||
| Inject speedballs at baseline | ||||
| Yes (reference) | – | – | 9.21 | 0.0025 |
| No | −2.2413 | 0.7387 | ||
| Site random effecta | 37.6 | <0.0001 | ||
As estimated by the likelihood ratio test, compared to a reduced model without a random effect for site
The model selected for frequency of injecting drugs in the past 30 days without covariates had intervention, month, and an intervention-by-month interaction as fixed effects, site and the site by month interaction as between-subject random effects, and an unstructured within-subject covariance. As shown in Table 6, four significant covariates entered the model—not entering treatment prior to the 2-month visit (F = 16.77; df = 1,465; P < 0.0001) and injecting cocaine (F = 7.65; df = 1, 456; P = 0.0059), heroin (F = 23.35; df = 1, 335; P < 0.0001) or speedballs (F = 9.14; df = 1, 455; P = 0.0026) in the 30 days prior to baseline. Injecting stimulants (i.e., amphetamines or cocaine) was omitted as a covariate due to high multicol-linearity with the more significant covariate, injecting cocaine in the 30 days prior to baseline. Regardless of which covariates were included, the pattern of findings remained consistent; there was a significant decrease in the overall frequency of injection from baseline to post-detoxification follow-up (F = 234.04; df = 1, 26.3; P < 0.0001) but no significant differences among the three conditions over time (F = 0.48; df = 6, 529; P = 0.8221). Participants averaged 104 times injecting at baseline (i.e., 30 days prior to detoxification), 30.3 times injecting within the last 30 days at 2-month follow-up, 29.2 times injecting at 4-month follow-up and 22.5 times injecting at 6-month follow-up after adjusting for covariates (Table 3).
Table 6.
Model for frequency of injecting drugs in past 30 days, including baseline values for injecting heroin, cocaine, and speedballs in the past 30 days, and treatment entry before the 2-month visit (N = 526 cases included with all covariates)
| Effect | β | Standard error |
F-statistic | P |
|---|---|---|---|---|
| Intervention | ||||
| C&E | 0.3735 | 5.9673 | 0.50 | 0.6082 |
| TA | 6.7826 | 6.1995 | ||
| TAU (reference) | – | – | ||
| Month | ||||
| 0 (Baseline) | 80.4101 | 8.2969 | 79.23 | <0.0001 |
| 2 | 6.8781 | 6.8458 | ||
| 4 | 7.7267 | 6.7122 | ||
| 6 (reference) | – | – | ||
| Intervention × month interaction | ||||
| C&E, month 0 | 9.7940 | 10.1378 | 0.48 | 0.8221 |
| C&E, month 2 | 4.6078 | 7.8453 | ||
| C&E, month 4 | 0.6506 | 7.6301 | ||
| C&E, month 6 | – | – | ||
| TA, month 0 | −6.5063 | 10.4064 | ||
| TA, month 2 | −1.6640 | 7.9917 | ||
| TA, month 4 | −3.7618 | 7.8625 | ||
| TA, month 6 | – | – | ||
| TAU, month 0 | – | – | ||
| TAU, month 2 | – | – | ||
| TAU, month 4 | – | – | ||
| TAU, month 6 | – | – | ||
| Treatment entry before 2-month visit | ||||
| Yes (reference) | – | – | 16.77 | <0.0001 |
| No | 16.5827 | 4.0491 | ||
| Inject heroin at baseline | ||||
| Yes (reference) | – | – | 23.35 | <0.0001 |
| No | −26.0753 | 5.3966 | ||
| Inject cocaine at baseline | ||||
| Yes (reference) | – | – | 7.65 | 0.0059 |
| No | −12.1440 | 4.3910 | ||
| Inject speedballs at baseline | ||||
| Yes (reference) | – | – | 9.14 | 0.0026 |
| No | −13.8479 | 4.5808 | ||
| Site random effecta | 14.6 | <0.0001 | ||
As estimated by the likelihood ratio test, compared to a reduced model without a random effect for site
The logistic mixed model for always practicing safe sex (abstinence or 100% condom use) in the prior 30 days, included intervention, month, an intervention-by-month interaction, and site as fixed effects with a random subject effect (Table 7). There was a main effect for intervention (F = 2.94; df = 2, 588; P = 0.0536). Further examination showed that being in either active intervention (C&E + TAU or TA + TAU) was associated with safer sex behaviors (F = 5.86; df = 1, 582; P = 0.0158); no difference was observed between the two active interventions. There was also a main effect of month. Safer sex behaviors increased from baseline to follow-up (F = 20.20; df = 1, 1735; P < 0.0001) but did not differ among the various follow-up assessments, as shown by the adjusted odds ratios in Table 3. There was no significant effect for the intervention-by-month interaction. For the active interventions, the empirical (i.e., unadjusted, non-model-based) percentage of participants always using safer sex behaviors in the past 30 days was 50.6% at baseline, 64.5% at 2-month, 60.8% at 4-month, and 62.8% at 6 month follow-ups; for the TAU group, the percentage always using safer sex behaviors in the past 30 days was 44.2% at baseline, 54.4% at 2-month, 53.5% at 4-month, and 54.8% at 6-month follow-ups. Thus, differences in safer sex between the active interventions and TAU were present at baseline as well as follow-ups. There was a main site effect (F = 5.41; df = 7, 564; P < 0.0001). Site differences among participants were present at baseline, ranging from a low of 31.6% at site D reporting always engaging in safer sex behaviors to high of 63.1% at site E. At follow-ups, all sites showed increases in reported safer sex behaviors with the greatest increase from baseline to 6 months seen in sites A and G (16.6 percentage points).
Table 7.
Model for safe sex behavior in the past 30 days including gender as a covariate with females as the referent group
| Effect | β | Standard error |
F-statistic | P |
|---|---|---|---|---|
| Intervention | ||||
| C&E | 0.5708 | 0.3344 | 2.94 | 0.0536 |
| TA | 0.3596 | 0.3407 | ||
| TAU | – | – | ||
| C&E + TA vs. TAU | 5.86 | 0.0158 | ||
| C&E vs. TA | 0.02 | 0.8927 | ||
| Month | ||||
| 0 (Baseline) | −0.4162 | 0.2687 | 6.93 | 0.0001 |
| 2 | 0.01448 | 0.2992 | ||
| 4 | −0.03435 | 0.3052 | ||
| 6 (reference) | – | – | ||
| Intervention × month interaction | ||||
| C&E, month 0 | −0.2642 | 0.3804 | 0.27 | 0.9523 |
| C&E, month 2 | −0.1249 | 0.4257 | ||
| C&E, month 4 | −0.1172 | 0.4311 | ||
| C&E, month 6 | – | – | ||
| TA, month 0 | −0.1035 | 0.3837 | ||
| TA, month 2 | 0.2815 | 0.4316 | ||
| TA, month 4 | 0.04959 | 0.4372 | ||
| TA, month 6 | – | – | ||
| TAU, month 0 | – | – | ||
| TAU, month 2 | – | – | ||
| TAU, month 4 | – | – | ||
| TAU, month 6 | – | – | ||
| Site | ||||
| A | −0.5326 | 0.2937 | 5.41 | <0.0001 |
| B | −0.7191 | 0.2985 | ||
| C | 0.2168 | 0.3259 | ||
| D | −0.9184 | 0.3949 | ||
| E | 0.4973 | 0.2824 | ||
| F | −0.5820 | 0.3388 | ||
| G | 0.4079 | 0.3921 | ||
| H (reference) | – | – | ||
| Gender | ||||
| Male | 0.6369 | 0.1981 | 10.34 | 0.0014 |
| Female (reference) | – | – | ||
There were also gender effects (F = 10.34; df = 1, 569; P = 0.0014), with the odds ratio indicating males had 1.9 (95% CI 1.3–2.8) times the odds of practicing safer sex than females.
Post hoc Analysis
There were no differences among the three intervention groups in the proportion who reported receiving an HIV/HCV risk assessment (χ2 = 0.66; df = 2; P = 0.7183) as shown in Table 8. Participants in the C&E intervention did report significantly higher rates of receiving a referral for HIV testing (73.4%) compared to TA (44.3%) and TAU (54.1%) participants (χ2 = 13.57; df = 2; P = 0.0011), as well as significantly higher rates of attending the HIV testing appointment (C&E = 65.6%; TA = 38.7%; TAU = 42.5%) (χ2 = 7.88; df = 2; P = 0.0194). However, the HIV/HCV risk assessment/referral for testing/testing attendance variable was not significant as a main effect, or as an interaction with intervention or month for any of the four outcome variables (P > 0.10).
Table 8.
Proportion of participants who received HIV/HCV risk assessment, HIV referral for testing, and attended HIV testing referral, by intervention
| Intervention | HIV/HCV risk assessment |
P | HIV referral for testinga |
P | Attended HIV testing appointmentb |
P |
|---|---|---|---|---|---|---|
| C&E | 79/212 (37.3) | 0.7183 | 58/79 (73.4) | 0.0011 | 38/58 (65.5) | 0.0194 |
| TA | 70/209 (33.5) | 31/70 (44.3) | 12/31 (38.7) | |||
| TAU | 74/211 (35.1) | 40/74 (54.1) | 17/40 (42.5) | |||
| Total | 223/632 (35.1) | 129/223 (57.9) | 67/129 (51.9) |
Limited to the 223 participants who reported that they had a risk assessment conducted
Limited to the 129 who reported having received a referral for HIV testing
Discussion
Frequent drug injection (averaging nearly four injections a day in the 30 days prior to baseline interviews), and high injection-related risk behaviors—injecting with an unclean (i.e., unbleached) used needle/syringe (25%), sharing cottons/cookers/rinse water (46%) and sharing the drug solution (54%)—characterized the IDUs in detoxification who participated in this trial. At baseline, 61% reported at least one risk behavior related to drug injection, with an average of 13 risk behaviors reported. Participants, especially women, were sexually active, yet fewer than 20% practiced safe sex. The risk behaviors reported by study participants are comparable to street-recruited IDUs [58, 59] reflecting the importance of intervening with drug injectors in detoxification settings. The opportunity for change during such “teachable moments” should be capitalized upon.
Participants in all three treatment conditions significantly reduced their injection risk behaviors, days injecting and injection frequency over time following detoxification, suggesting that standard detoxification alone was as effective as the added interventions. These results generate two questions; what accounted for significant decreases in injection risk behavior and drug injecting for participants in all conditions post-detoxification and why did the experimental conditions not improve those outcomes further? Post hoc analyses suggested that positive outcomes over time for participants in all groups were not associated with HIV/HCV risk assessment or testing services. It may be that participants’ motivation to stop or reduce drug use, implied by detoxification entry, and/or the detoxification process itself were active ingredients for all three groups. The pattern of results was similar for all outcomes; perhaps reduction in drug injection resulting from detoxification was a key mediator of decreases in risk behavior. Alternatively, in a review of psychosocial interventions for drug related risk behaviors, Gibson et al. [60] found that 9 of 15 studies had a similar pattern of results; participants in both experimental and comparison conditions demonstrated decreases in risk behavior over time. Gibson et al. [61] posited that the baseline interview, serving, in effect, as a health risk assessment, may have been an active treatment ingredient that had a positive impact on participant behavior. It is possible that the administration of the Risk Behavior Survey and other assessment instruments over a 2.5 h time period spent with an interviewer acted as an “intervention” delivered to all participants that increased both awareness of risky behavior and motivation to change.
Regarding the inability of experimental conditions to boost outcomes further, there are a number of possible explanations. Although it is possible that baseline assessments had a positive impact on participant risk behavior, they may also have had a negative impact on the interventions that followed. Interventions were usually conducted either the same day or the day following completion of baseline assessment due to the short time most participants were in detoxification; Fatigue may have negatively impacted participant engagement during the interventions.
Each active intervention may have had limitations. The C&E intervention was successful in increasing the number of participants who were referred for testing and attended the testing appointment. However, increased rates of testing did not result in reduced risk outcomes. Although our study did not obtain participant test results, other research has found that testing per se may not be associated with reduced risk behavior, unless test results are positive [62, 63]. The C&E intervention was designed for a street outreach population where it has previously been shown to be effective [41, 62]. Perhaps a more robust intervention is necessary for individuals already motivated to reduce drug use as evidenced by entry into detoxification. Meta-analyses of HIV risk reduction interventions conducted in drug abuse treatment programs have shown these interventions to be effective, particularly if they contain attitudinal arguments, educational information, behavioral skills arguments and behavioral skills training [64, 65]. Such interventions tend to be more intensive (i.e., 5 or more sessions) than the C&E.
The TA intervention was designed to promote a mediator, improving risk reduction outcomes via increases in outpatient treatment attendance. Treatment entry results reported by Campbell et al. [35] showed that the TA intervention improved outpatient treatment entry relative to TAU only, but did not improve treatment retention rates. It is possible that the “dose” of treatment participation, or the type of treatment targeted, i.e., outpatient, was insufficient to impact risk reduction and injection drug use results beyond those occurring following detoxification only. A broader measure of treatment entry did predict better outcomes. Reported participation in any type of substance abuse treatment within 2 months of leaving detoxification was associated with reduced injection risk behaviors, reduced days injecting and reduced overall frequency of injecting. This finding supports a growing literature on the effectiveness of treatment, in particular methadone maintenance, in reducing HIV-related drug risk behaviors [26], as well as HIV seroconversion [28, 29]. In a meta-analysis of drug abuse treatment as an HIV prevention approach, Sorensen and Copeland [66] concluded that while treatment reduced drug injection, there was less compelling evidence that it also reduced needle-related risk behaviors such as needle sharing. The current findings provide some indication that treatment participation within 2 months of detoxification was effective in reducing injection risk behavior, as well as injection drug use.
In light of these results, it is of interest to identify factors that facilitate treatment entry following detoxification. In addition to finding that the TA intervention improved outpatient treatment entry rates, Campbell et al. [35] found that injecting heroin and not injecting stimulants were associated with outpatient treatment entry. We previously reported that cocaine use was not only a deterrent to entering treatment, even when treatment was free [67], but that it also decreased retention [59]. In an earlier study, stimulant-only injectors were 24–25 times less likely than opiate-only injectors and those who injected both stimulants and opiates to enter treatment [68]. Interventions should be developed that specifically target engaging stimulant users in post-detoxification treatment.
Results of the current study also found that self-efficacy for safer injection practices was associated with better injection risk behavior outcomes. Self-efficacy has been associated with reduced alcohol consumption [69], reduced illicit drug use [70], entry into treatment following detoxification [71] and engaging in safer sex [40]. Although our results did not replicate findings associating self-efficacy and reduced drug use or safer sex, the positive impact of efficacy on safer injection practices in our study provides some support for interventions that focus on developing efficacy in this behavioral domain.
In terms of sex practices, findings revealed that women were more likely to have been sexually active than men at baseline. They also averaged two sex partners in the prior 30 day period compared to one for men. Less than one in five participants reported always using a condom during vaginal sex at baseline, with no significant difference between men and women. However, analysis of safer sex at follow-up revealed that women were nearly twice as likely as men to not have practiced safe sex (abstinence or always use condoms). Previous research has also noted greater sex risk behaviors among women, including unprotected anal or vaginal sex [72, 73], sex with a high risk sex partner [74, 75], sex with an IDU [76, 77] and sex with an HIV infected partner [73] or a partner of unknown HIV status [72]. In view of the increase in the sexual transmission of HIV, particularly among women [3], interventions that target sex risks continue to be a high priority.
Several limitations of this study should be considered. Although participants were randomly assigned to intervention conditions, they were not randomly selected for participation in the study. This may have resulted in a sample more willing to be involved in research and more in need of the modest monetary stipend provided for completing assessments. Generalizability of results may also be limited by the participant sample in the eight detoxification centers, although they included small and large, rural and urban programs. Overall gender and racial diversity were modest; 73% were Caucasian and 76% were male. In addition, findings were based on self-report, which may have been influenced by inaccurate recall and social desirability. Recall error was likely minimized on the primary dependent measure, the RBS, by the relatively brief 30-day time period participants were asked to recall. Moreover, prior studies have found that drug users’ self-reports have adequate validity for this type of investigation [59, 78] and the use of an ACASI to collect the primary outcomes for the study should have minimized social desirability influences. Post hoc analyses relied on a self-report measure that used a skip pattern to ask about HIV/HCV assessment, referral for testing, and attending testing. As a result of the design of this questionnaire, the number of participants with responses for referral and testing was limited. Results regarding risk assessment/testing should be interpreted with caution. Finally, we lacked complete follow-up data on approximately one-third of those who entered the study. Although baseline differences were generally minimal between participants who were followed and those who were not, those successfully followed reported injecting drugs less frequently and they were more likely to have entered treatment at 2-months.
The results of this study suggest that injection drug users benefit from participating in detoxification; it was associated with significant reductions in injection drug use, as well as reductions in drug and sex related risk behavior that were maintained for up to 6 months post detoxification. Participation in further treatment within 2 months post detoxification was also associated with significant reductions in risky injection practices, as well as the number of days injecting and the frequency of injection. The study supports the importance of access to detoxification for drug injectors followed by transition to continued treatment. These findings are meaningful in light of the severity of the sample that entered this trial, but additional steps are required if further gains are to be achieved. These include the development of brief interventions, feasible in short-term detoxification settings, which enhance risk reduction outcomes, such as health risk assessment with targeted feedback. Practical interventions that facilitate transfer to treatment after detoxification, such as that found by Campbell et al. [35], and those which promote treatment retention are also vital for the reduction of IDU and associated risk behaviors. The absence of a vaccine to prevent HIV underscores the importance of these tasks.
Acknowledgments
The authors thank the staff at the eight participating treatment centers and the patients who were involved in the study. Cooperative agreements from the National Institute on Drug Abuse supported the study design and implementation and the collection and analysis of the data: Great Lakes Node (U10 DA13710), Northern New England Node (U10 DA15831), Oregon/Hawaii Node (U10 DA13036), Rocky Mountain Node (U10 DA13716), and Pacific Northwest Node (U10 DA13714). The study also received support from the Oregon Clinical and Translational Research Institute (UL1 RR024140) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
The Counseling and Education and Therapeutic Alliance intervention training manuals can be downloaded from the CTN Dissemination Library at http://ctndisseminationlibrary.org.
Contributor Information
Robert E. Booth, Department of Psychiatry, University of Colorado, Denver, CO, USA
Barbara K. Campbell, Department of Public Health and Preventive Medicine, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA
Susan K. Mikulich-Gilbertson, Department of Psychiatry, University of Colorado, Denver, CO, USA
Carrie J. Tillotson, Oregon Clinical and Translational Research Institute, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA
Dongseok Choi, Department of Public Health and Preventive Medicine, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA.
James Robinson, Nathan S. Kline Institute for Psychiatric Research, Orangeburg, NY, USA.
Donald A. Calsyn, Alcohol and Drug Abuse Institute and Department of Psychiatry & Behavioral Sciences, University of Washington, Seattle, WA, USA
Raul N. Mandler, Clinical Trials Network, National Institute on Drug Abuse, Bethesda, MD, USA
Lindsay M. Jenkins, Recovery Centers of King County, Seattle, WA, USA
Laetitia L. Thompson, Department of Psychiatry, University of Colorado, Denver, CO, USA
Catherine L. Dempsey, Department of Psychiatry, University of Colorado, Denver, CO, USA
Michael R. Liepman, Department of Psychiatry, Michigan State University/Kalamazoo Center for Medical Studies, Kalamazoo, MI, USA
Dennis McCarty, Department of Public Health and Preventive Medicine, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA.
References
- 1.National Research Council . AIDS: sexual behavior and intravenous drug use. National Academy Press; Washington, DC: 1989. [PubMed] [Google Scholar]
- 2.National Research Council, Institute of Medicine . In: Preventing HIV transmission: the role of sterile needles and bleach. Normand J, Vlahov D, Moses LE, editors. National Academy Press; Washington, DC: 1995. [PubMed] [Google Scholar]
- 3.CDC . Centers for Disease Control and Prevention: HIV/AIDS sero-surveillance report. Vol 18. CDC; Atlanta: 2008. [Google Scholar]
- 4.Knight KR, Purcell DW, Dawson-Rose C, Halkitis PN, Gomez CA, the Seropositive Urban Injectors Study Team Sexual risk taking among HIV-positive injection drug users: contexts, characteristics, and implications for prevention. AIDS Educ Prev. 2005;17(1 suppl A):76–88. doi: 10.1521/aeap.17.2.76.58692. [DOI] [PubMed] [Google Scholar]
- 5.Kral A, Bluthenthal R, Lorvick J, Gee L, Bacchetti P, Edlin B. Sexual transmission of HIV-1 among injection drug users in San Francisco, USA: risk-factor analysis. Lancet. 2001;357(9266):1397–401. doi: 10.1016/S0140-6736(00)04562-1. [DOI] [PubMed] [Google Scholar]
- 6.Saxon AJ, Calsyn DA, Whittaker S, Freeman G. Sexual behaviors of intravenous drug users in treatment. J Acquir Immune Defic Syndr. 1991;4:938–44. [PubMed] [Google Scholar]
- 7.Watkins K, Metzger D, Woody G, McLellan A. Determinants of condom use among intravenous drug users. AIDS. 1993;7:719–23. doi: 10.1097/00002030-199305000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Bulterys M, Chao A, Dushimimana A, et al. Multiple sexual partners and mother-to-child transmission of HIV-1. AIDS. 1993;7(12):1639–45. doi: 10.1097/00002030-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Battjes R, Pickens R, Amsel Z, Brown L. Heterosexual transmission of human immunodeficiency virus among intravenous drug users. J Infect Dis. 1990;162:1007–11. doi: 10.1093/infdis/162.5.1007. [DOI] [PubMed] [Google Scholar]
- 10.Booth R, Koester S, Brewster J, Weibel W, Fritz R. Intravenous drug users and AIDS: risk behaviors. Am J Drug Alcohol Abuse. 1991;17(3):337–53. doi: 10.3109/00952999109027557. [DOI] [PubMed] [Google Scholar]
- 11.Murphy D. Heterosexual contacts of intravenous drug abusers: implications for the next spread of the AIDS epidemic. Adv Alcohol Subst Abuse. 1987;7(2):89–97. [PubMed] [Google Scholar]
- 12.Alter M. Hepatitis C virus infection in the United States. J Hepatol. 1999;31:88–91. doi: 10.1016/s0168-8278(99)80381-x. [DOI] [PubMed] [Google Scholar]
- 13.Garfein R, Doherty M, Monterroso E, Thomas D, Nelson K, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:S11–9. doi: 10.1097/00042560-199802001-00004. [DOI] [PubMed] [Google Scholar]
- 14.Coutinho R. HIV and hepatitis C among injecting drug users (editorial) Br Med J. 1998;317:424–5. doi: 10.1136/bmj.317.7156.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crofts N, Caruana S, Kerger M, Bowden S. Hepatitis C on drug injecting equipment. Br Med J. 2000;321:899. [PMC free article] [PubMed] [Google Scholar]
- 16.Bortolotti F, Stivanello A, Dall’Armi A, Rinaldi R, La Grasta F. AIDS information campaign has significantly reduced risk factors for HIV infection in Italian drug abusers. J Acquir Immune Defic Syndr. 1988;1(4):412–3. [PubMed] [Google Scholar]
- 17.Power R, Hartnoll R, Daviaud E. Drug injecting, AIDS, and risk behaviour: potential for change and intervention strategies. Br J Addict. 1988;83(6):649–54. doi: 10.1111/j.1360-0443.1988.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 18.Booth R, Wiebel W. The effectiveness of reducing needle-related risks for HIV through indigenous outreach to injection drug users. Am J Addict. 1992;1(4):277–87. [Google Scholar]
- 19.Colon H, Robles R, Freeman D, Matos T. Effects of a HIV risk reduction education program among injection drug users in Puerto Rico. Puerto Rico Health Sci J. 1993;12(1):27–34. [PubMed] [Google Scholar]
- 20.Watters J, Downing M, Case P, Lorvick J, Cheng YT, Fergusson B. AIDS prevention for intravenous drug users in the community: street-based education and risk behavior. Am J Community Psychol. 1990;18(4):587–96. doi: 10.1007/BF00938061. [DOI] [PubMed] [Google Scholar]
- 21.Calsyn DA, Saxon AJ, Freeman G. Lack of efficacy of AIDS education in reducing HIV high-risk transmission behaviors among injection drug users. Am J Public Health. 1992;82:573–5. doi: 10.2105/ajph.82.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casadonte P, Jarlais D Des, Friedman S, Rotrosen J. Psychological and behavioral impact among intravenous drug users of learning HIV test results. Int J Addict. 1990;25(4):409–26. doi: 10.3109/10826089009053168. [DOI] [PubMed] [Google Scholar]
- 23.Skidmore C, Robertson J, Roberts J. Changes in HIV risk-taking behaviour in intravenous drug users: a second follow-up. Br J Addict. 1989;84:695–6. doi: 10.1111/j.1360-0443.1989.tb03487.x. [DOI] [PubMed] [Google Scholar]
- 24.Jarlais D Des, Marmor M, Paone D, et al. HIV incidence among injecting drug users in New York City syringe-exchange programmes. Lancet. 1996;348(9033):987–91. doi: 10.1016/s0140-6736(96)02536-6. [DOI] [PubMed] [Google Scholar]
- 25.Watters J, Estilo M, Clark G. Syringe and needle exchange as HIV prevention for injection drug users. J Am Med Assoc. 1994;271:115–20. [PubMed] [Google Scholar]
- 26.Corsi KF, Kwiatkowski CF, Booth RE. Predictors of positive outcomes for out-of-treatment opiate injectors recruited into methadone maintenance through street outreach. J Drug Issues. 2002;32(3):99–1016. [Google Scholar]
- 27.Hartgers C, van den Hoek A, Krijnen P, Coutinho RA. HIV prevalence and risk behavior among injecting drug users who participate in “low-threshold” methadone programs in Amsterdam. Am J Public Health. 1992;82(4):547–51. doi: 10.2105/ajph.82.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metzger DS, Woody GE, McLellan AT, O’Brien CP, Druley P, Navaline H, et al. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. J Acquir Immune Defic Syndr. 1993;6:1049–56. [PubMed] [Google Scholar]
- 29.Moss AR, Vranizan K, Gorter R, Bacchetti P, Watters J, Osmong D. HIV seroconversion in intravenous drug users in San Francisco, 1985–1990. AIDS. 1994;8(2):223–31. doi: 10.1097/00002030-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz M, Baker G, Mulvey K, Plough A. Improving publicly funded substance abuse treatment: the value of case management. Am J Public Health. 1997;87:1659–64. doi: 10.2105/ajph.87.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundgren LM, Sullivan L, Amodeo M. How do treatment repeaters use the drug treatment system? An analysis of injection drug users in Massachusetts. J Subst Abus Treat. 2006;30:121–8. doi: 10.1016/j.jsat.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 32.McCusker J, Bigelow C, Luippold R, Zorn M, Lewis BF. Outcomes of a 21-day drug detoxification program: retention, transfer to further treatment, and HIV risk reduction. Am J Drug Alcohol Abuse. 1995;21(1):1–16. doi: 10.3109/00952999509095225. [DOI] [PubMed] [Google Scholar]
- 33.Chutuape MA, Jasinski DR, Fingerhood MI, Stitzer ML. One-, three- and six-month outcomes after brief inpatient opioid detoxification. Am J Drug Alcohol Abuse. 2001;27:19–44. doi: 10.1081/ada-100103117. [DOI] [PubMed] [Google Scholar]
- 34.Coyle S. U.S. Department of Health and Human Services: GPO; Atlanta: The NIDA HIV counseling and education intervention model: intervention manual. 1993
- 35.Campbell BK, Fuller BE, Lee ES, Tillotson C, Woelfel T, Jenkins L, et al. Facilitating outpatient treatment entry following detoxification for injection drug use: a multi-site test of three interventions. Psychol Addict Behav. 2009;23:260–70. doi: 10.1037/a0014205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NIDA . Risk behavior survey. 3rd ed Community Research Branch, National Institute on Drug Abuse; Rockville: 1993. [Google Scholar]
- 37.Dowling-Guyer S, Johnson ME, Fisher DG, Needle RH, Watters JK, Andersen MD, et al. Reliability of drug users’ self-reported HIV risk behaviors and validity of self-reported recent drug use. Assessment. 1994;1(4):383–92. [Google Scholar]
- 38.Weatherby NL, Needle R, Cesari C, Booth RE, McCoy CB, Watters JK, et al. Validity of self-reported drug use among injection drug users and crack cocaine users recruited through street outreach. Eval Program Plan. 1994;17(4):347–55. [Google Scholar]
- 39.Sobell LC, Sobell MB. Timeline followback user’s guide: a calendar method for assessing alcohol and drug use. Addiction Research Foundation; Toronto: 1996. [Google Scholar]
- 40.Booth RE, Lehman WE, Brewster JT, Sinitsyana L, Dvoryak S. Gender differences in sex risk behaviors among Ukraine injection users. J Acquir Immune Defic Syndr. 2007;46:112–7. doi: 10.1097/QAI.0b013e318141f965. [DOI] [PubMed] [Google Scholar]
- 41.Booth RE, Kwitkowski CF, Stephens RC. Effectiveness of HIV/AIDS interventions on drug use and needle risk behaviors for out-of-treatment injection drug users. J Psychoactive Drugs. 1998;30(3):269–78. doi: 10.1080/02791072.1998.10399702. [DOI] [PubMed] [Google Scholar]
- 42.Stephens RC, Kwiatkowski CF, Booth RE. The effectiveness of intervention efforts on HIV risky behaviors among injection drug users and crack smokers. In: Albrecht G, Levy J, editors. Advances in medical sociology. JAI Press; Greenwich: 2000. [Google Scholar]
- 43.Fiorentine R, Nakashima J, Anglin MD. Client engagement in drug treatment. J Subst Abus Treat. 1999;17:199–206. doi: 10.1016/s0740-5472(98)00076-2. [DOI] [PubMed] [Google Scholar]
- 44.Connors GJ, Carroll KM, DiClemente CC, Longabaugh R, Donovan DM. The therapeutic alliance and its relationship to alcoholism treatment participation and outcome. J Consult Clin Psychol. 1997;65(4):588–98. doi: 10.1037//0022-006x.65.4.588. [DOI] [PubMed] [Google Scholar]
- 45.De Weert-Van Oene GH, Schippers GM, De Jong CA, Schrijvers GJ. Retention in substance dependence treatment: the relevance of in-treatment factors. J Subst Abus Treat. 2001;20(4):253–61. doi: 10.1016/s0740-5472(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 46.Marmor M, Jarlais DC Des, Cohen H, Friedman SR, Beatrice ST, Dubin N, et al. Risk factors for infection with human immunodeficiency virus among intravenous drug abusers in New York City. AIDS. 1987;1(1):39–44. [PubMed] [Google Scholar]
- 47.Mohl PC, Martinez D, Ticknor C, Huang M, O’Brien CP. Early dropouts from psychotherapy. J Nerv Ment Dis. 1991;179:478–81. doi: 10.1097/00005053-199108000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Luborsky L, McLellan AT, Woody GE, O’Brien CP, Auerbach A. Therapist success and its determinants. Arch Gen Psychiatry. 1985;42:602–11. doi: 10.1001/archpsyc.1985.01790290084010. [DOI] [PubMed] [Google Scholar]
- 49.Bordin ES. The generalizability of the psychoanalytic concept of the working alliance. Psychother Theory Res Pract. 1979;16:252–60. [Google Scholar]
- 50.Craigie FC, Ross SM. The use of a videotape pretherapy training program to encourage treatment-seeking among alcohol detoxification patients. Behav Ther. 1980;11(2):141–7. [Google Scholar]
- 51.Stark MJ, Kane BJ. General and specific psychotherapy role induction with substance-abusing clients. Int J Addict. 1985;20(8):1135–41. doi: 10.3109/10826088509056355. [DOI] [PubMed] [Google Scholar]
- 52.Verinis JS. The effect of an orientation-to-treatment group on the retention of alcoholics in outpatient treatment. Subst Use Misuse. 1996;31(10):1423–31. doi: 10.3109/10826089609063985. [DOI] [PubMed] [Google Scholar]
- 53.SAS Institute, Inc. SAS Software Version 9.1 for Windows. SAS Institute, Inc; Cary, NC: 2004. [Google Scholar]
- 54.SAS Institute, Inc. SAS Software Version 9.2 for Windows. SAS Institute, Inc; Cary, NC: 2008. [Google Scholar]
- 55.Laird NM. Missing data in longitudinal studies. Stat Med. 1988;7:305–15. doi: 10.1002/sim.4780070131. [DOI] [PubMed] [Google Scholar]
- 56.Jones RH. Longitudinal data with serial correlation: a state-space approach. Chapman and Hall; New York: 1993. [Google Scholar]
- 57.Koch GG, Gansky SA. Statistical considerations for multiplicity in confirmatory protocols. Drug Inf J. 1996;30:523–34. [Google Scholar]
- 58.Booth RE, Corsi KF, Mikulich-Gilbertson SK. Factors associated with methadone maintenance treatment retention among street-recruited injection drug users. Drug Alcohol Depend. 2004;74:177–85. doi: 10.1016/j.drugalcdep.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Booth RE, Crowley TJ, Zhang Y. Substance abuse treatment entry, retention and effectiveness: out-of-treatment opiate injection users. Drug Alcohol Depend. 1996;42(1):11–20. doi: 10.1016/0376-8716(96)01257-4. [DOI] [PubMed] [Google Scholar]
- 60.Gibson DR, McCusker J, Chesney M. Effectiveness of psychosocial interventions in preventing HIV risk behavior in injecting drug users. AIDS. 1998;12:919–29. doi: 10.1097/00002030-199808000-00015. [DOI] [PubMed] [Google Scholar]
- 61.Gibson DR, Lovelle-Drache J, Young M, Hudes ES, Sorensen JL. Effectiveness of brief counseling in reducing HIV risk behavior in injecting drug users: final results of randomized trials of counseling with and without HIV testing. AIDS Behav. 1999;3(1):3–12. [Google Scholar]
- 62.Booth RE, Lehman WE, Dvoryak JT, Brewster JT, Sinitsyna L. Interventions with injection drug users in Ukraine. Addiction. 2009;104:1864–73. doi: 10.1111/j.1360-0443.2009.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985–1997. Am J Public Health. 1999;89(9):1397–405. doi: 10.2105/ajph.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prendergast ML, Urada D, Podus D. Meta-analysis of HIV risk-reduction interventions within drug abuse treatment programs. J Consult Clin Psychol. 2001;69:389–405. doi: 10.1037//0022-006x.69.3.389. [DOI] [PubMed] [Google Scholar]
- 65.Semaan S, Jarlais DC Des, Sogolow E, Johnson WD, Hedges WD, Ramirez G, et al. A meta-analysis of the effect of HIV prevention interventions on the sex behaviors of drug users in the United States. J Acquir Immune Defic Syndr. 2002;30:S73–93. [PubMed] [Google Scholar]
- 66.Sorenson J, Copeland A. Drug abuse treatment as an HIV prevention strategy: a review. Drug Alcohol Depend. 2000;59:17–31. doi: 10.1016/s0376-8716(99)00104-0. [DOI] [PubMed] [Google Scholar]
- 67.Booth RE, Corsi KF, Mikulich SK. Improving entry to methadone maintenance among out-of-treatment injection drug users. J Subst Abus Treat. 2003;24(4):305–11. doi: 10.1016/s0740-5472(03)00038-2. [DOI] [PubMed] [Google Scholar]
- 68.John D, Kwiatkowski CF, Booth RE. Differences among out-of-treatment drug injectors who use stimulants only, opiates only or both: implications for treatment entry. Drug Alcohol Depend. 2001;64(2):165–72. doi: 10.1016/s0376-8716(01)00120-x. [DOI] [PubMed] [Google Scholar]
- 69.Sitharthan T, Kavanagh DJ. Role of self-efficacy in predicting outcomes from a programme for controlled drinking. Drug Alcohol Depend. 1990;27:87–94. doi: 10.1016/0376-8716(91)90091-c. [DOI] [PubMed] [Google Scholar]
- 70.Reilly PM, Sees KL, Shopshire MS, Hall SM, Delucchi KL, Tusel DJ, et al. Self-efficacy and illicit opioid use in a 180-day methadone detoxification treatment. J Consult Clin Psychol. 1995;1:158–62. doi: 10.1037//0022-006x.63.1.158. [DOI] [PubMed] [Google Scholar]
- 71.Kleinman BP, Millery M, Scimeca M, Polissar NL. Predicting long-term treatment utilization among addicts entering detoxification: the contribution of help-seeking models. J Drug Issues. 2002;32(1):209–30. [Google Scholar]
- 72.Bouhnik AD, Preau M, Lert F, Peretti-Watel P, Schilz MA, Obadia Y, et al. Unsafe sex in regular partnerships among heterosexual persons living with HIV: evidence from a large representative sample of individuals attending outpatient services in France. AIDS. 2007;21:S57–62. doi: 10.1097/01.aids.0000255086.54599.23. [DOI] [PubMed] [Google Scholar]
- 73.Gollub EL, Rey D, Obadia Y, Moatti JP. Gender differences in risk behaviors among HIV? persons with an IDU history: the link between partner characteristics and women’s higher drug-sex risks. Sex Transm Dis. 1998;25:483–8. doi: 10.1097/00007435-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Lollis CM, Strothers HS, Chitwood DD, McGhee M. Sex, drugs and HIV: does methadone maintenance reduce drug use and risky sexual behavior? J Behav Sci. 2000;23:545–557. doi: 10.1023/a:1005555519831. [DOI] [PubMed] [Google Scholar]
- 75.Booth RE. Gender differences in high-risk sex behaviors among heterosexual drug injectors and crack smokers. Am J Drug Alcohol Abuse. 1995;21:419–32. doi: 10.3109/00952999509002708. [DOI] [PubMed] [Google Scholar]
- 76.Booth RE, Koester SK, Pinto F. Gender differences in sex-risk behaviors, economic livelihood, and self-concept among drug injectors and crack smokers. Am J Addict. 1995;4:313–22. [Google Scholar]
- 77.Jarlais DC Des, Friedman SR, Goldsmith D, Hopkins W. Heterosexual transmission of human immunodeficiency virus from intravenous drug users: Regular partnerships and prostitution. In: Voeller B, Reinisch JMR, Gottleib M, editors. AIDS and sex. Oxford University Press; New York: 1990. pp. 245–56. [Google Scholar]
- 78.Maisto S, McKay J, Conners G. Self-reported issues in substance abuse: state of the art and future directions. Behav Assess. 1990;121:117–34. [Google Scholar]