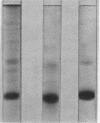
Abstract
The partial amino acid sequence of the amyloid fibril protein isolated from the small intestine of a patient with plasma cell dyscrasia and associated amyloidosis has been determined and compared with the sequence of the κ-type Bence Jones protein isolated from the urine of the same patient. Identical sequences were observed for the 27 amino-terminal residues that could be compared. The C-terminal tryptic peptide of the amyloid protein was identical with that of the Bence Jones protein. Apparent molecular weights and amino acid compositions of the Bence Jones and amyloid proteins were similar. It appears, therefore, that the predominant protein present in the amyloid deposits in this patient was an intact κ-type light polypeptide chain that was identical with the urinary Bence Jones protein.
Full text
PDF
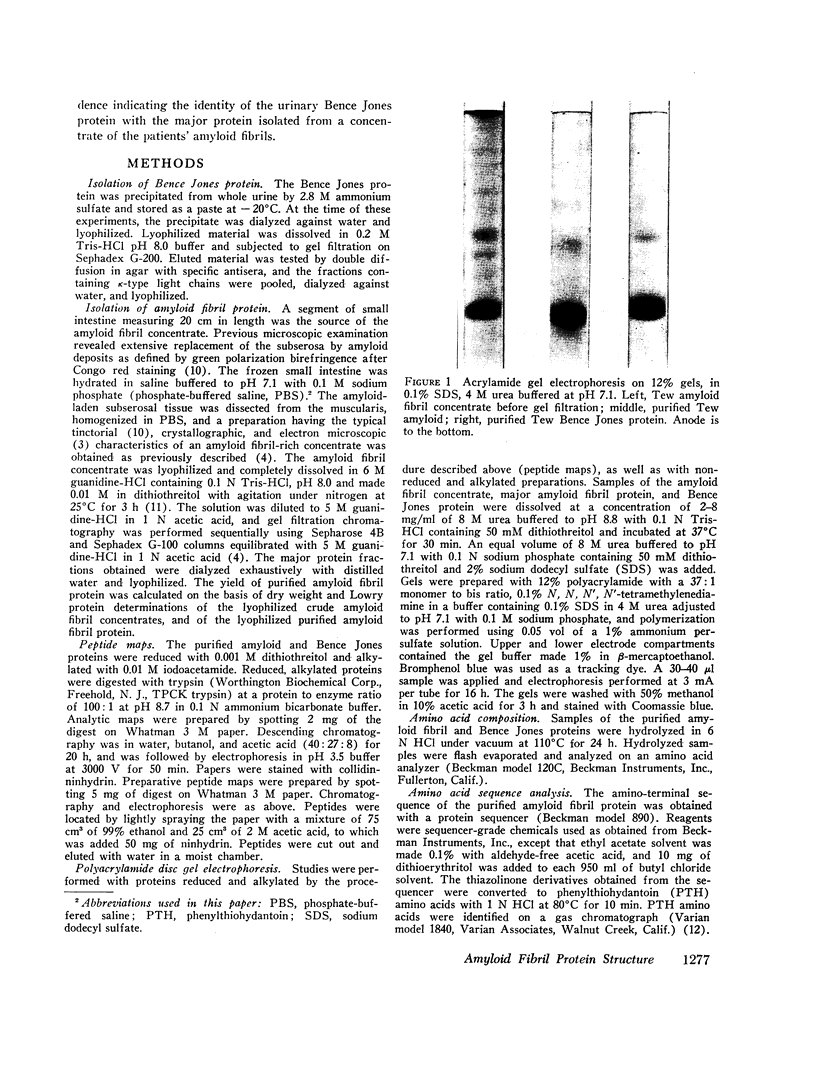
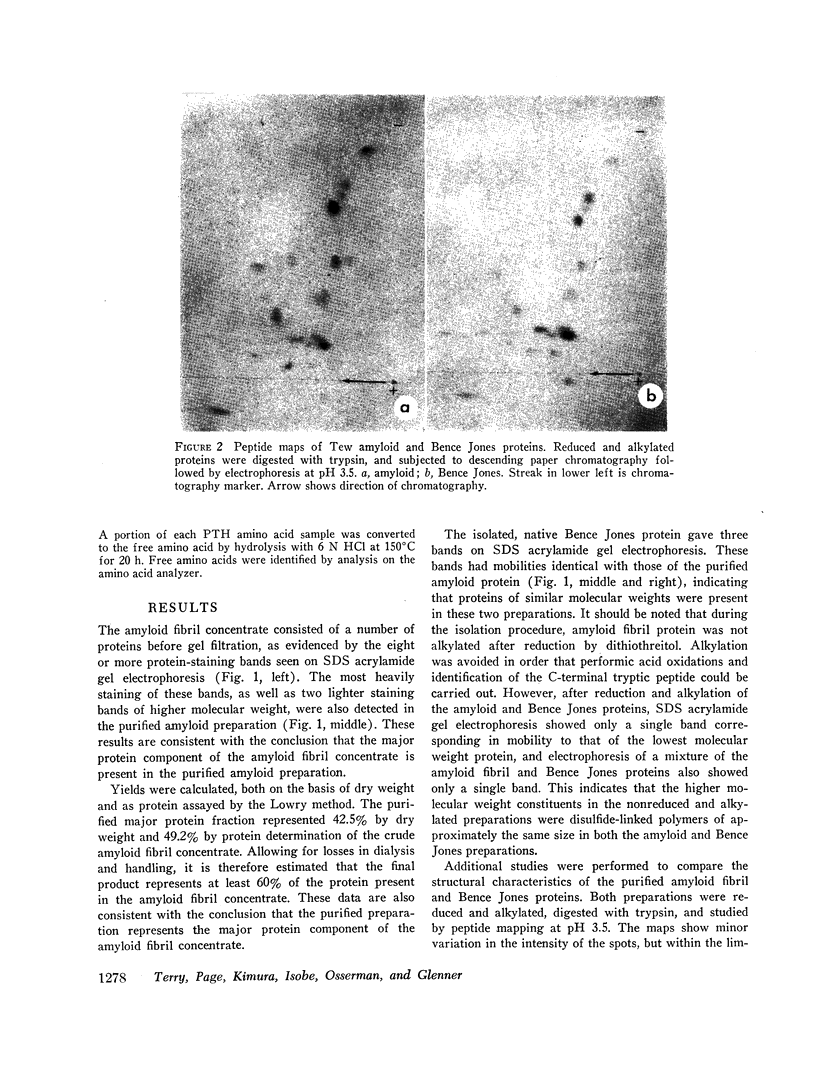
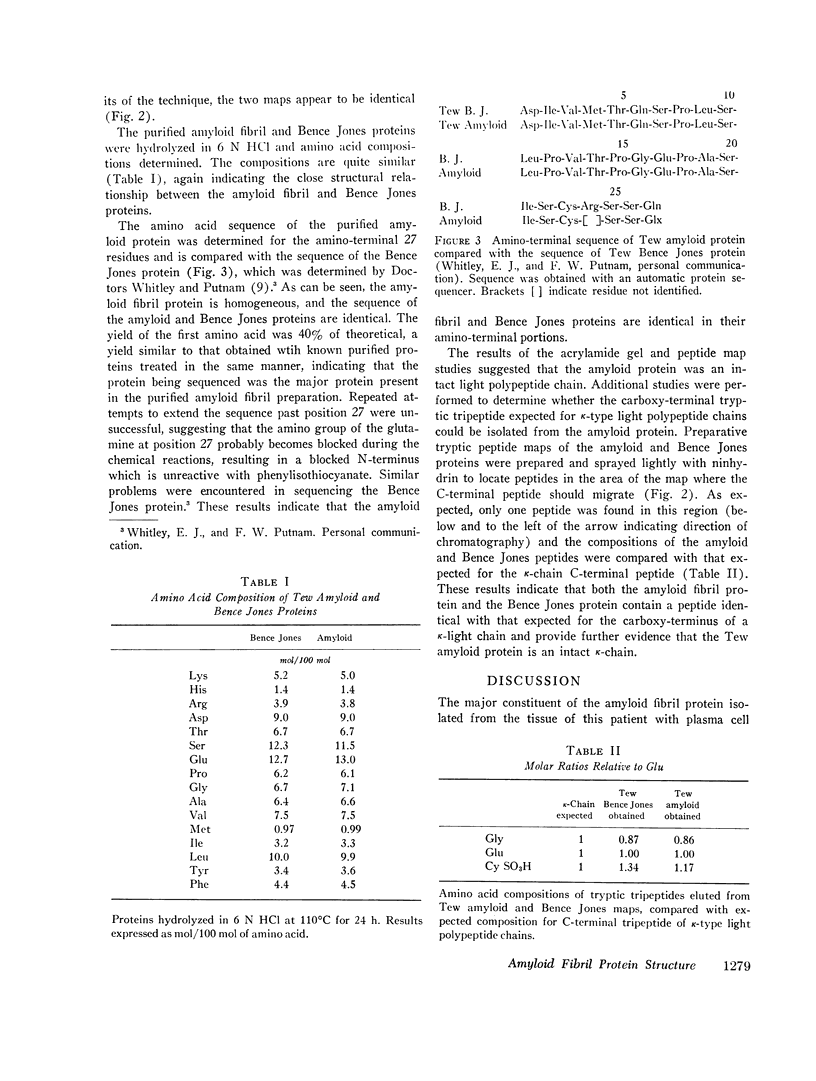


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth W. F., Willerson J. T., Waldmann T. A., Decker J. L. Primary amyloidosis. Clinical, immunochemical and immunoglobulin metabolism. Studies in fifteen patients. Am J Med. 1969 Aug;47(2):259–273. doi: 10.1016/0002-9343(69)90152-1. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Chemical classes of amyloid substance. Am J Pathol. 1971 Oct;65(1):231–252. [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hermodson M. A., Ericsson L. H. The major proteins of human and monkey amyloid substance: Common properties including unusual N-terminal amino acid sequences. FEBS Lett. 1971 Dec 1;19(2):169–173. doi: 10.1016/0014-5793(71)80506-9. [DOI] [PubMed] [Google Scholar]
- Ein D., Kimura S., Glenner G. G. An amyloid fibril protein of unknown origin: partial amino-acid sequence analysis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):498–500. doi: 10.1016/s0006-291x(72)80166-9. [DOI] [PubMed] [Google Scholar]
- Ein D., Kimura S., Terry W. D., Magnotta J., Glenner G. G. Amino acid sequence of an amyloid fibril protein of unknown origin. J Biol Chem. 1972 Sep 10;247(17):5653–5655. [PubMed] [Google Scholar]
- Franklin E. C., Pras M., Levin M., Frangione B. The partial amino acid sequence of the major low molecular weight component of two human amyloid fibrils. FEBS Lett. 1972 Apr 15;22(1):121–123. doi: 10.1016/0014-5793(72)80235-7. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Ein D., Terry W. D. The immunoglobulin origin of amyloid. Am J Med. 1972 Feb;52(2):141–147. doi: 10.1016/0002-9343(72)90063-0. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Harbaugh J., Ohma J. I., Harada M., Cuatrecasas P. An amyloid protein: the amino-terminal variable fragment of an immunoglobulin light chain. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1287–1289. doi: 10.1016/0006-291x(70)90227-5. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W., Harada M., Isersky C., Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971 Jun 11;172(3988):1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- Harada M., Isersky C., Cuatrecasas P., Page D., Bladen H. A., Eanes E. D., Keiser H. R., Glenner G. G. Human amyloid protein: chemical variability and homogeneity. J Histochem Cytochem. 1971 Jan;19(1):1–15. doi: 10.1177/19.1.1. [DOI] [PubMed] [Google Scholar]
- Kimura S., Guyer R., Terry W. D., Glenner G. G. Chemical evidence for lambda-type amyloid fibril proteins. J Immunol. 1972 Oct;109(4):891–892. [PubMed] [Google Scholar]
- MISSMAHL H. P., HARTWIG M. Polarisationsoptische Untersuchungen an der Amyloidsubstanz. Virchows Arch Pathol Anat Physiol Klin Med. 1953;324(4):489–508. doi: 10.1007/BF00954791. [DOI] [PubMed] [Google Scholar]
- OSSERMAN E. F., TAKATSUKI K., TALAL N. MULTIPLE MYELOMA I. THE PATHOGENESIS OF "AMYLOIDOSIS. Semin Hematol. 1964 Jan;1:3–85. [PubMed] [Google Scholar]
- Page D. L., Isersky C., Harada M., Glenner G. G. Immunoglobulin origin of localized nodular pulmonary amyloidosis. Res Exp Med (Berl) 1972;159(2):75–86. doi: 10.1007/BF01856034. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]
- Putnam F. W. Immunoglobulin structure: variability and homology. Science. 1969 Feb 14;163(3868):633–644. doi: 10.1126/science.163.3868.633. [DOI] [PubMed] [Google Scholar]