Abstract
Purpose
The vitamin E analog γ-tocotrienol (GT3) is a powerful radioprotector. GT3 reduces post-radiation vascular peroxynitrite production, an effect dependent on inhibition of hydroxy-methyl-glutaryl coenzyme A (HMG-CoA) reductase. HMG-CoA reductase inhibitors mediate their pleiotropic effects via eNOS that requires the co-factor tetrahydrobiopterin (BH4). This study investigated the effects of radiation on BH4 bioavailability and of GT3 on BH4 metabolism.
Methods and Materials
Mice were exposed to 8.5 Gy total body irradiation (TBI). Lung BH4 and total biopterin concentrations were measured 0, 3.5, 7, 14 and 21 days after TBI using differential oxidation followed by HPLC. The effect of exogenous GT3 and BH4 treatment on post-radiation vascular oxidative stress and bone marrow colony-forming units (BM-CFU) were assessed in vivo. The effect of GT3 on endothelial cell apoptosis and endothelial expression of GTP cyclohydrolase 1 (GTPCH), GTPCH regulatory protein (GFRP), GFRP transcription, GFRP protein levels, and GFRP-CTPCH protein binding were determined in vitro.
Results
Compared to baseline levels, lung BH4 concentrations decreased by 24% at 3.5 days after TBI, an effect that was reversed by GT3. At 14 and 21 days after TBI, compensatory increases in BH4 (58% and 80%, respectively) were observed. Relative to vehicle-treated controls, both GT3 and BH4 supplementation reduced post-irradiation vascular peroxynitrite production at 3.5 days (by 66% and 33%, respectively), and BH4 resulted in a 68% increase in BM-CFU. GT3 ameliorated endothelial cell apoptosis and reduced endothelial GFRP protein levels and GFRP-GTPCH binding by decreasing transcription of the GFRP gene.
Conclusions
BH4 bioavailability is reduced in the early post-radiation phase. Exogenous administration of BH4 reduces post-irradiation vascular oxidative stress. GT3 potently reduces the expression of GFRP, one of the key regulatory proteins in the BH4 pathway, and may thus exert some of its beneficial effects on post-radiation free-radical production partly by counteracting the decrease in BH4.
Keywords: radiation, oxidative stress, nitric oxide synthase, tetrahydrobiopterin, vitamin E, endothelial cells
Introduction
Vitamin E analogs, collectively called tocols, have been subject to active investigation for many years as radiation protectors, both in patients undergoing radiation therapy and in the context of possible radiation accidents or terrorism scenarios. Moreover, tocols, alone or in combination with pentoxifylline, have been used in the actual treatment of various radiation-induced delayed normal tissue sequelae (1, 2). The exact mechanisms, however, by which tocols exert their radioprotective and/or anti-fibrotic effects, remain unclear.
Tocols protect from the effects of ionizing radiation mainly by virtue of their antioxidant properties. However, the radioprotective properties of tocols vary considerably despite the fact that comparative studies show that the antioxidant effects of tocols are rather similar (3, 4). Consequently, these effects alone cannot explain the substantial differences among tocols in terms of radioprotective efficacy (5, 6).
Among the 8 naturally occurring vitamin E analogs, γ-tocotrienol (GT3) is particularly potent as a radioprotector. GT3 reduces hematopoietic and gastrointestinal tissue injury, vascular oxidative/nitrosative stress, and reduces post-TBI lethality with a dose reduction factor in excess of 1.3 at non-toxic doses (7-9). In fact, GT3 appears to be one of the most, if not the most, effective radioprophylactic natural compound to described to date (6). An important difference among the tocols that could help explain this finding relates to the ability of GT3 to inhibit 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, similar to the drug class statins (10, 11). Compared to α-tocopherol, the HMG-CoA reductase inhibitory activity of GT3 is 30-fold greater (12), GT3 accumulates in endothelial cells to levels that are 25-95-fold greater (13), and GT3 is 10-fold more effective in reducing adhesion molecule expression (14).
HMG-CoA reductase inhibitors inhibit the conversion of HMG-CoA to mevalonic acid, the rate-limiting step in cholesterol biosynthesis. In addition, by decreasing other mevalonate pathway intermediates, HMG-CoA reductase inhibitors also reduce the isoprenylation of small GTP-binding proteins (eg, Rho, Ras, Rac), resulting in effects that are unrelated to lipid-lowering. These pleiotropic effects regulate a vast array of metabolic and physiological processes, including cell proliferation, apoptosis, immune function, inflammation, coagulation, and fibrinolysis. The mechanism underlying most, if not all, pleiotropic effects s related to increased expression and enhanced activity of eNOS (15).
Under conditions of increased oxidative stress, such as after radiation exposure, endothelial nitric oxide synthase (eNOS) may switch from producing nitric oxide (NO) to instead becoming an important source of superoxide and peroxynitrite, a process termed enzymatic “uncoupling”. Inadequate availability of the redox-sensitive NOS cofactor 5,6,7,8-tetrahydrobiopterin (BH4), as a result of rapid oxidation of BH4 to 7,8-dihydrobiopterin (BH2), is believed to be an important cause of eNOS uncoupling. Hence, BH4 insufficiency plays an important role in the pathogenesis of several conditions characterized by increased oxidative stress and endothelial dysfunction, for example, diabetes, hypertension, and arteriosclerosis (16-18).
We here confirm the hypothesis that exposure to ionizing radiation indeed reduces BH4 availability in the early post-irradiation phase in vivo, and that “restoring” post-irradiation BH4 availability via exogenous administration reduces radiation-induced vascular oxidative stress. We also provide in vitro evidence to support the notion that the radioprotective properties of GT3 may depend on modulation of BH4 metabolism, specifically by demonstrating that GT3 affects the expression of GTP cyclohydrolase 1 (GTPCH) feedback regulatory protein (GFRP), a key negative regulator of BH4 synthesis.
Methods and materials
Animals
The experimental protocol was reviewed and approved by the Central Arkansas Veterans Healthcare System (CAVHS) Institutional Animal Care and Use Committee (IACUC). All experiments were carried out in random bred male CD2F1 mice (Harlan Sprague Dawley, Indianapolis, IN) that were 6-7 weeks of age at the initiation of the experiments. Animals were housed in conventional cages under standardized conditions with controlled temperature and humidity, and a 12-12 hour day-night light cycle. Animals had free access to water and chow (Harlan Teklad laboratory diet 7012, Purina Mills, St. Louis, MO).
A total of 60 mice was used to study the effect of radiation exposure on BH4 availability and to determine the effect of GT3 treatment on radiation-induced changes in BH4. Mice were randomly assigned to treatment with control vehicle and GT3. Twenty-four hours before irradiation mice received either a single dose of GT3 (400 mg/kg) or the excipient alone via by subcutaneous (s.c.) injection. At 0, 3.5, 7, 14 and 21 days after total body irradiation (TBI), mice were euthanized and lung tissue were collected, and aortic tissue was collected for vascular measurement of peroxynitrite/oxidative stress. Lung samples were snap frozen in liquid nitrogen and stored at −80°C until analysis.
To study whether enhancing BH4 levels can reduce post-irradiation peroxynitrite production, an experiment was performed in which BH4 was supplemented. Mice were randomly assigned to receive vehicle, BH4 (10mg/kg) or tetrahydroneopterin (NH4) (10mg/kg) per gavage twice daily (n=8). NH4 has similar antioxidant properties as BH4, but lacks nitric oxide synthase (NOS) cofactor function. Treatment was started 2 days before TBI and continued until 4 days after TBI. Mice were euthanized at day 0 and at day 4 after TBI. Aortas were collected to assess vascular peroxynitrite levels.
Irradiation and dosimetry
TBI and dosimetry was performed as described before (7). Un-anesthetized mice were exposed to a single dose of 8.5 Gy whole body irradiation in a Shepherd Mark I, model 25 Cs-137 irradiator (J.L. Shepherd & Associates, San Fernando, CA). Previous experiments with CD2F1 mice have shown that 8.5 Gy TBI induces pronounced intestinal and hematopoietic radiation injury, but no or minimal lethality with sufficient survival at 21 days. During irradiation, the animals were held in well ventilated custom-made Plexiglas restrainers on a turntable rotating at 5 revolutions per minute. Irradiation of endothelial cell cultures for apoptosis detection was performed in the same irradiator, using doses of 0 Gy, 5 Gy, and 10 Gy. The average dose rate was 1.35 Gy per minute and was corrected for decay every day.
BH4 measurement
BH4 determinations were performed by HPLC after oxidation with iodine in acid and base as described before (19), by methods modified from Fukushima & Nixon (20). The lung was chosen as “organ of interest” because of its extraordinarily rich microvasculature, i.e., because it has more endothelial cells per unit tissue mass than any other organ and because GT3 concentrates particularly in endothelial cells.
Briefly, lung tissue was homogenized by an Ultra Turrax micro-homogenizer (IKA Stauffen, Germany) in distilled water containing 5 mM dithiothreitol, centrifuged at 13000 g for 10 minutes at 4 °C. Twenty μl of a 1:1 (v/v) mixture of HCl (0.1 M) and iodine (0.1 M in 0.25 M KI), or NaOH (0.1 M) and iodine (0.1 M in 0.25 M KI), was added to 100 μl of the supernatant, mixed, and incubated for 60 minutes in the dark. Subsequently, 20 μl 0.1 M HCl was added to the alkaline solution. Insoluble material was removed from both incubations by centrifugation (5 min, 13 000 g), followed by addition of 20 μl of freshly prepared ascorbic acid (0.1M in water). The incubation mixtures were then analyzed on an Agilent 1200 HPLC System (Agilent, Vienna, Austria). Twenty μl of the final mixture was injected onto a Nucleosil 10 SA column (Machery Nagl, Düren, Germany), isocratically eluted with 100 mM potassium phosphate buffer (pH 3.0) at a flow rate of 1.5 ml/min and a temperature of 35°C. Biopterin was detected by fluorescence (Jasco FP 920, Jasco, Tokyo, Japan), excitation 350 nm, emission 440 nm with a detection limit of 1 nMol/l. Amounts of tetrahydrobiopterin were calculated from the difference of the oxidation in acid and base, respectively. Values were related to the protein content of extracts measured by the Biorad Bradford assay (Biorad, Hercules, CA).
Vascular peroxynitrite production
The effect of GT3, BH4, and NH4 on vascular peroxynitrite production was measured using dihydrorhodamine 123 incubation as described before (7). Briefly, the abdominal aorta was dissected with as little peri-vascular tissue as possible. After collection, the aortas were incubated with 10 μM dihydrorhodamine 123 (DHR123) (Axxora, San Diego, CA) in EGM-2 medium (Lonza, Walkersville, MA) for 90 minutes at 37 °C in the dark. Subsequently, aortas were washed twice with PBS and homogenized in buffer (PBS, 0.1 % Tween-20, 0.1 % SDS) using a Polytron PT 6100 homogenizer (Kinematica Inc., Bohemia, NY). Samples were centrifuges for 5 minutes at 2000 rpm and supernatant was collected to determine fluorescence (485/515 ) using a Synergy HT multi plate reader (BioTek Instruments, Winooski, VT).
Protein concentration in the supernatant was measured using a modified Bradford reaction (Coomassie Plus Protein Assay, Thermo Scientific, Rockford, IL). Fluorescence was expressed per mg protein.
Bone marrow colony forming units (BM-CFUs)
Bone marrow cells collected 24 h post-irradiation were used to compare the effect of treatment with vehicle, BH4, or NH4 (n=6) on hematopoietic toxicity. Bone marrow cells were isolated from two femurs and suspended in MethoCult methylcellulose medium (Stem Cell Technologies, Vancouver, BC, Canada) at a concentration of 107 cells/ml. Cell-medium suspension (1.1 ml) was dispensed in 35 mm cell culture dishes and cell colonies (BM-CFUs) were counted after 10 days after fixation with formalin and eosin staining. For each mouse, the average number of colonies in 3 separate cell culture dishes was determined and considered a single value for statistical purposes.
In vitro assessment of the effect of GT3 on endothelial cell apoptosis
FLICA (Fluorochrome Inhibitor of Caspases) Apoptosis detection kit (Immunochemistry Technologies; MN, USA) was used to determine caspase activation in irradiated HUVEC cells pretreated with GT3 as recommended by the manufacturer. Briefly, endothelial cells (passage 5) were treated either with GT3 (10 μM) or same amount of vehicle (absolute ethanol with 10% DMSO) for 24 h before exposure to 0, 5, and 10 Gy. Twenty-four hours after irradiation, cells were harvested by trypsinization, resuspended in 300 μl culture media containing 10 μl 30X FLICA solution and incubated for 1 h at 37 °C in CO2. Cells were washed 2 times with 2 ml of wash buffer, resuspended in 400 μl of wash buffer containing 10% fixative, and analyzed by flow cytometry (LSR II, BD Biosciences, San Jose, CA).
For fluorescent microscopy analyses, 5×104 cells were seeded on glass cover slips in 30 mm tissue culture Petri dishes 24 h before irradiation. Twenty-four hours after irradiation, the media were removed and replaced with 300 μl fresh media containing 10 μl 30X FLICA solution and the cells were incubated for 1 h at 37 °C, followed by replacement of the FLICA-containing media with media containing 1.5 μl Hoechst 33258 for 5 min. The cells were then washed 2 times with 2 ml 1x washing buffer and examined under a fluorescence microscope.
In vitro assessment of the effect of GT3 on BH4 bioavailability
Cell culture and reagents
Unless otherwise specified, chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Human umbilical vein endothelial cells (HUVECs) were obtained from Lonza (Walkersville, MD) and cell cultures were performed as described previously (21). HUVECs were cultured in EGM-2 medium with growth supplements (Lonza). Passage 4-6 cells were used in all experiments. Cells were plated on 4 or 10 cm Petri dishes. Confluent cells were treated with vehicle or 10 μM GT3 (Yasoo Health Inc. Johnson City, YN) or 10 μM atorvastatin. In order to determine whether the effects of GT3 and atorvastatin depend on inhibition of HMG-CoA reductase, cells were pretreated with 500 μM mevalonate for 30 minutes. The drug concentrations used in these experiments have been shown to be non-toxic by us and others. All experiments were performed at least 3 times.
Real time PCR analysis
Total RNA was isolated using Ultraspec reagent (Biotex Laboratories, Houston, Texas) and cDNA was generated with the cDNA Archive Kit (Applied Biosystems, Foster, CA). Gene expression levels were measured with TaqMan real-time quantitative PCR with the following pre-designed primer/probe sets: GTPCH, Hs00609198_m1; GFRP, Hs00193360_m1 and S27, Hs01378332_g1 (Applied Biosystems). PCR amplification and detection were carried out on an ABI Prism 7000 Sequence Detection Sytem (Applied Biosystems) according to the manufacturer’s instructions. Each reaction was performed in triplicate. GTPCH and GFRP expression was normalized for S27 expression
Nuclear run-on assay
Cells were collected and lysed in a buffer containing 1 M Tris-HCl, 100 mM CaCl2, 1 M MgCl2 and 0.5% NP-40 using a Dounce homogenizer. Nuclei were resuspended in glycerol buffer (50 mM Tris-HCl, 40% glycerol, 5 mM MgCl2) and stored in liquid nitrogen until further use. For the nuclear run on, the suspension of nuclei was incubated with an equal amount of transcription buffer (5 mM Tris, 2.5 mM MgCl2, 150 mM KCl, 2.5 mM ATP, 2.5 mM GTP, 2.5 mM CTP, 2.5 mM biotin-16-UTP, 2.5 mM DTT, 400 U/ml RNAsin) for 45 minutes. After stopping the reaction, RNA was isolated using Ultraspec RNA reagent (Biotex laboratories) and biotinylated RNA was purified using streptavidin particle beads (Sigma-Aldrich). Biotinylated RNA was used for reverse transcriptase cDNA synthesis and subsequent real time PCR for GFRP.
Western blot
Total protein of the treated HUVEC cells was isolated using the Nuclear Extract Kit (Active Motif, Carlsbad, CA). Equal amounts of protein were loaded and run on NuPAGE Novex Bis-Tris Mini gels (Invitrogen, Carlsbad, CA). After blotting to PVDF membranes (Invitrogen), membranes were blocked for 60 minutes at room temperature and incubated overnight at 4°C in buffer (TBS with 0.1% Tween 20, 5% milk power) containing 1:1000 diluted Santa Cruz antibodies against GFRP (sc-22697), GTPCH (sc-48510) or β-actin (sc-10731) and if necessary a relevant blocking peptide (Santa Cruz Biotechnology, sc-22687 P, sc-48510 P). Detection of the primary antibodies was performed using an HRP-conjugated rabbit anti-goat secondary antibody (Santa Cruz Biotechnology) diluted 1:2500. Immunoreactive bands were visualized with chemiluminescent substrate (Pierce, Rockford, IL). The western blot films were scanned in a Lexmark X73 (Lexmark International, Lexington, KY) scanner. The images were not enhanced or altered with any software. Densitometric analysis was performed using Quantity One software (Bio-Rad, Hercules, CA).
Immunoprecipitation
Cells were lysed in ice-cold modified RIPA lysis Buffer (Upstate, Temecular, CA) (1x RIPA lysis buffer: 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 μg/ml leuleptin, 1 μg/ml pepstatin, 1 μg/ml aprotinin). To reduce non-specific binding the lysate was pre-cleared by overnight incubation with protein G agarose beads (Millipore, Billerica, MA). After removal of the agarose beads by centrifugation, lysate (500 μg total protein) was incubated with 2 μg of GTPCH1 monoclonal antibody (sc-48510, Santa Cruz Biotechnology Inc, Santa Cruz, CA) overnight at 4°C on a rotating platform. Immunocomplexes were captured using protein G-agarose beads (Millipore, Billerica, MA) and overnight incubation. Beads were washed extensively with ice cold PBS and resuspended in 2x Laemmli sample buffer (100mM Tris-HCl, 2%SDS, 20% glycerol, 0.2% bromophenol blue and 5% β-mercapto-ethanol). The suspension was boiled for 5 minutes to dissociate the immunocomplexes from the agarose beads. The amount of bound GFRP was measured using western blotting.
Results
Exposure to 8.5 Gy TBI induced some signs of radiation sickness (i.e. weight loss, lethargy) starting at approximately 7 days after radiation exposure. However, in accordance with results from previous experiments, no radiation-induced lethality was observed in this study. Although not assessed objectively in this study, consistent with what has been reported previously (7-9), treatment with GT3 attenuated the subjective occurrence of signs of radiation sickness. Previous experiments with CD2F1 mice receiving 8.5 Gy TBI according to the same irradiation protocol as applied in the current experiments have shown that 8.5 Gy TBI induces pronounced intestinal and hematopoietic injury together with sufficient 21 day survival. Exposure to 8.5 Gy TBI induces a slight reduction in intestinal crypt cells, a decrease in intestinal mucosal surface area, intestinal bacterial translocation, as well as hematopoietic injury with peripheral blood pancytopenia.
Lung BH4 concentrations
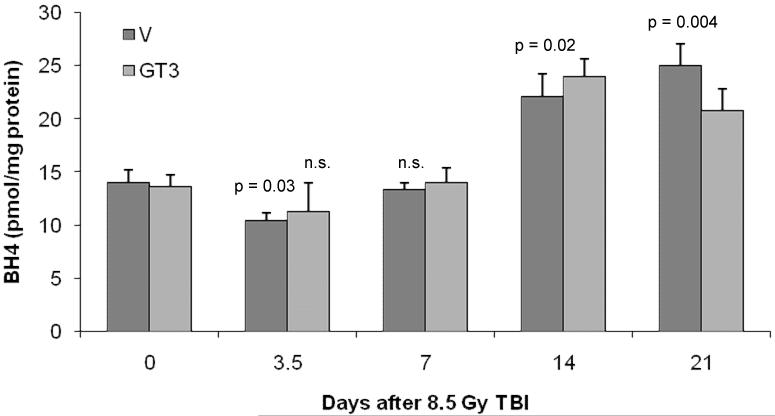
Lung BH4 concentrations were significantly decreased on day 3.5 after TBI (p = 0.03), but returned to baseline levels at day 7 (Figure 1). GT3 treatment prevented the decrease in lung BH4 on day 3.5 after TBI. At day 14 and 21 after irradiation a compensatory increase in BH4 concentrations was observed (p = 0.02 and p = 0.004 respectively).
Figure 1.
BH4 levels in lung at various times after exposure to TBI.
Lung BH4 levels were reduced at day 3.5 after 8.5 TBI. At day 14 and 21 post-TBI an increase in BH4 levels was observed. GT3 treatment prevents a significant decrease in BH4 levels at day 5.5 after TBI.
Mean + standard error of the mean (sem), N=8
Effect of GT3 on vascular peroxynitrite production and endothelial cell apoptosis
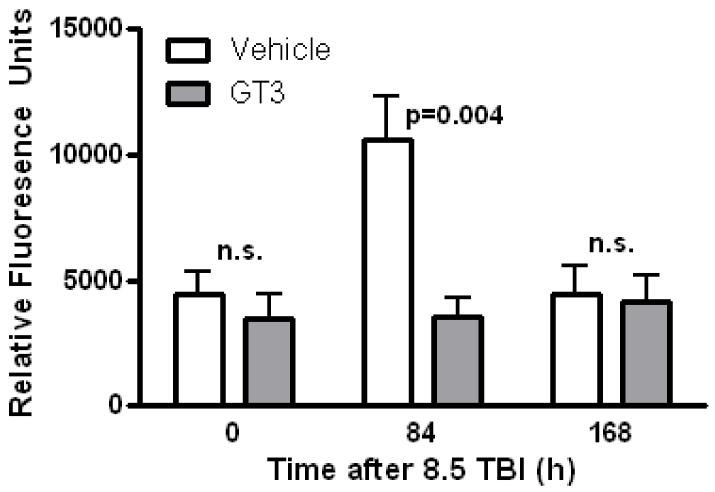
On day 0, before radiation exposure, no differences in vascular peroxynitrite production were observed between vehicle treated and GT3 treated animals. GT3 treatment was associated with a marked reduction in vascular peroxynitrite production on day 3.5 post-TBI (p= 0.004), whereas, the levels had returned to baseline by day 7 (Figure 2).
Figure 2.
Effect of GT3 on vascular oxidative stress.
Treatment with GT3 significantly reduced post-irradiation peroxynitrite production in the aorta 3.5 days after exposure to 8.5 Gy.
Mean + sem, N =8
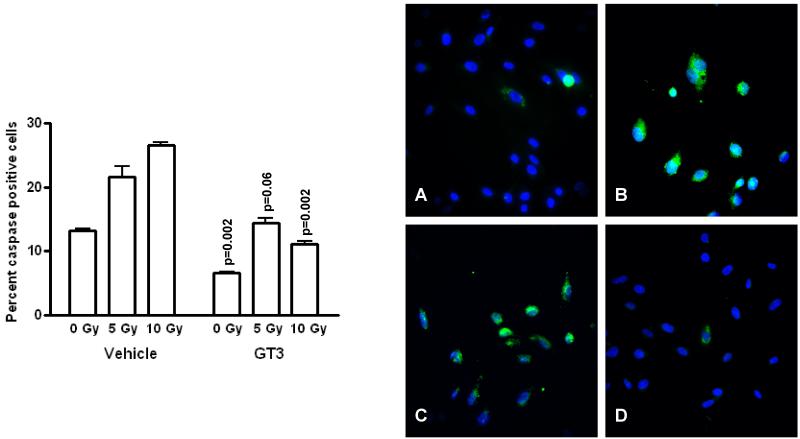
As shown in Figure 3, there was a significant radiation dose-dependent increase in caspase activity in non-GT3 treated cells (p=0.0004). GT3 treatment was associated with a significantly lower levels of apoptosis at baseline and after exposure to 10 Gy (p=0.002 for both), whereas, the difference after 5 Gy almost reached the level of significance (p=0.06).
Figure 3.
Effect of GT3 on endothelial cell apoptosis.
Left panel: Exposure to 5 Gy and 10 Gy irradiation was associated with a highly significant radiation-dose dependent increase in poly-caspase activation as assessed by flow cytometric analysis (p=0.0004). Treatment with GT3 significantly reduced poly-caspase activation. P-values above each bar indicate the significance level of the difference between GT3 treated cells and the corresponding non-GT3 treated group.
Right panel: Representative immunofluorescence images showing sham-irradiated endothelial cells (A); cells exposed to 10 Gy (B); vehicle treated cells exposed to 10 Gy (C); and GT3 treated cells exposed to 10 Gy (D). All images obtained 24 h after (sham-) irradiation. Cells stained with FLICA (green, polycaspase) and Hoechst 33258 (blue, cell nuclei). 20X magnification.
Effect of BH4, and NH4 on hematopoietic toxicity and vascular oxidative stress
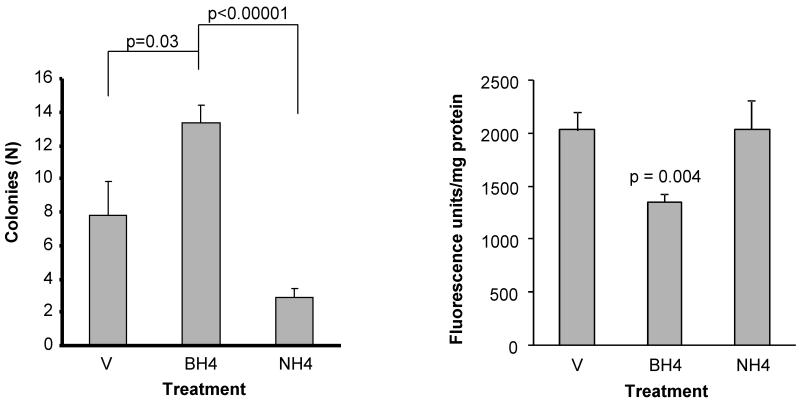
Treatment with BH4, or NH4 did not elicit signs of toxicity. BH4 supplementation was associated with a significantly greater number of colonies (BM-CFUs) 11 days after exposure to 8.5 Gy than in vehicle-treated mice (p=0.03) or NH4 treated mice (p<0.00001), suggesting that BH4s function as a cofactor for NOS is critically important (Figure 4, left panel).
Figure 4.
Effect of BH4 and NH4 on post-TBI hematopoietic toxicity and vascular peroxynitrite production.
Left panel: Bone marrow colony-forming units (BM-CFUs) 10 days after irradiation. Bone marrow was removed 24 h after exposure to 8.5 Gy TBI and cultured for 10 days (see Methods). BH4 supplementation was associated with a significantly greater number of colonies than in vehicle-treated mice (p=0.03) or NH4 treated mice (p<0.00001), demonstrating the importance of BH4’s function as a cofactor for NOS. Mean ± sem, N=6.
Right panel: Treatment with BH4 reduces post-irradiation peroxynitrite production in the aorta. NH4 does not affect post-irradiation peroxynitrite production, indicating that BH4 exerts its effect by acting as a NOS cofactor and not by acting as a free radical scavenger. Mean ± sem, N =8
BH4 supplementation also markedly reduced vascular peroxynitrite production on day 3.5 post-TBI (p= 0.004), while NH4 had no effect (Figure 4, right panel).
Effect of GT3 on BH4 bioavailability in vitro
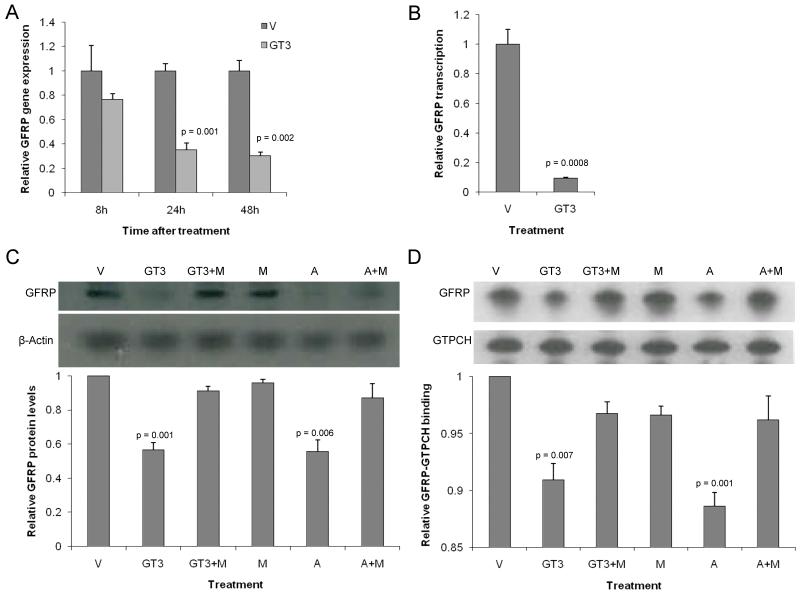
GT3 did not affect the expression of GTPCH. On the other hand, GT3 treatment significantly reduced the expression of GFRP (24h p=0.001, 48h p=0.002) (Figure 3a). Nuclear run-on assays showed that GT3 reduced GFRP expression by causing a reduction in GFRP gene transcription (p=0.0008) (Figure 3b). At 24 hours after GT3 treatment GFRP protein levels as well as GFRP-GTPCH protein binding were reduced (Figure 3c, Figure 3d). The fact that statin treatment influenced GFRP protein levels and GFRP-protein binding in a similar way as GT3 and that mevalonate co-administration reversed the effects of both GT3 and statin, strongly indicates that GT3 modulates GFRP levels and GFRP-CTPCH protein binding through inhibition of HMG-CoA reductase.
Discussion
This study demonstrates that BH4 bioavailability is reduced during the early post-TBI phase and that exogenous supplementation of BH4 reduces post-TBI vascular oxidative stress by a mechanism that relates to its eNOS cofactor function. Our study also suggests that at least some of GT3’s radioprophylactic properties may be related to increased generation of BH4 through the de novo synthesis pathway by a mechanism that involves suppression of the regulatory protein, GFRP.
Radiation-induced endothelial dysfunction is believed to play an important role in the pathogenesis of both early and delayed radiation injury (22-25). Radiation exposure increases vascular oxidative stress and induces various functional and morphological changes in endothelial cells, such as loss of thromboresistance, increased permeability, and apoptosis. Endothelial nitric oxide synthase (eNOS) is considered to be one of the key regulatory enzymes of endothelial function. Changes in eNOS function are believed to be involved in the development of radiation-induced endothelial dysfunction. eNOS dependent endothelial dysfunction may not only result in inadequate production of the regulatory molecule NO, but also to significant production of the highly reactive oxygen radical O2− by eNOS in certain situations, referred to as eNOS “uncoupling”. In the uncoupled state, eNOS produces O2− at the expense of NO.
One of the main causes of eNOS uncoupling is inadequate availability of the eNOS cofactor BH4. BH4 is highly redox sensitive and its availability may be reduced under conditions of oxidative stress due to rapid oxidation of BH4 to BH2. Insufficient BH4 availability and consequential eNOS uncoupling has been shown to play important roles in the pathogenesis of endothelial dysfunction during various conditions characterized by increased oxidative stress, such as hypercholesterolemia, diabetes, and hypertension (16-18, 26, 27). Until now, little was known about the effects of radiation exposure on the availability of BH4, the possible importance of BH4 dependent eNOS uncoupling in radiation-induced endothelial dysfunction, and the extent to which radioprotective agents regulate BH4.
Our current data show that radiation exposure induces a reduction in BH4 availability during the early post-irradiation phase. The occurrence and relevance of radiation-induced, BH4 dependent eNOS uncoupling, is supported by the observation that supplementation with GT3 as well as BH4 reduces vascular peroxynitrite production during the early post-TBI phase. In contrast, supplementation with NH4, a compound with similar anti-oxidant properties as BH4 but no NOS cofactor function, does not reduce peroxynitrite production, indicating that BH4 exerts its effect by acting as a NOS cofactor and not by acting as a free radical scavenger.
The beneficial effects of GT3 on post-irradiation vascular peroxynitrite production depend on inhibition of HMG-CoA reductase by GT3. Statins, well known inhibitors of HMG-CoA reductase, have been reported to regulate eNOS function and BH4 availability, thereby reducing eNOS-uncoupling and oxidative stress. The main underlying mechanism for this effect of statins is upregulation of the expression of GTPCH, a key enzyme in BH4 synthesis (28). Whether GT3 may attenuate post-irradiation vascular peroxynitrite production by modulating BH4 metabolism has, to our knowledge, not been investigated previously. Moreover, because the mechanism by which GT3 inhibits HMG-CoA reductase differs from that of statins (29), it is interesting to speculate that there may be synergy between statins and GT3 in terms of radioprotective efficacy, just as there is for cholesterol lowering (30).
BH4 is synthesized in endothelial cells by de novo synthesis from GTP or by a salvage pathway that converts BH2 to BH4. The rate limiting enzyme in de novo BH4 production is GTPCH. GTPCH activity is regulated on multiple levels. Transcriptional and posttranslational changes, like phosphorylation at serine 81, are known to regulate GTPCH activity (31, 32). Protein-protein interaction is another important regulatory mechanism. In this context, GFRP provides an important negative feedback mechanism for BH4 production (33-35). The binding of GFRP to GTPCH enables end-product feedback inhibition by BH4. Conversely, phenylalanine can stimulate GTPCH enzymatic activity via GFRP.
Unlike statins, GT3 does not affect the expression of GTPCH1. On the other hand, GT3 induces a reduction in GFRP protein levels by reducing GFRP gene transcription. Moreover, GT3 not only reduces general cellular GFRP levels, but also reduces GFRP-GTPCH1 protein binding. These effects appear to depend on inhibition of HMG-CoA reductase by GT3. Further research is needed to determine whether GT3 can prevent the post-irradiation decline in BH4 availability by suppressing GFRP production in vivo.
Restoration of BH4 supplies or preventing BH4 shortage appears to be a novel, promising, and interesting approach to ameliorate radiation injury. Since the effects of BH4 supplementation are very unpredictable due the high extracellular concentrations compared to the desired intracellular levels, it seems appropriate to instead focus on strategies that could increase intracellular BH4 without high concentration biopterin supplementation.
In conclusion, exposure to TBI reduces the availability of the eNOS cofactor BH4 during the early post-irradiation phase. Post-irradiation free radical production can be attenuated by increasing BH4 availability. The radioprotective vitamin E analogue, GT3, regulates the expression of GFRP and may thus exert its radioprotective effects partly through regulation of BH4 availability. Clearly, further research is warranted, for example, to explore the effects of GT3 on BH4 metabolism and BH4 related radioprotection in vivo; to investigate whether the findings in the present study also apply to localized and/or fractionated irradiation as used clinically; whether synergy exists between the effects of GT3 and statins in terms of protection, mitigation, or treatment of radiation-induced normal tissue injury; and to what extent similar mechanisms apply to other antioxidants and vitamin E analogues with HMG-Co reductase inhibitory activities, such as δ-tocotrienol (29).
Figure 5.
Effect of GT3 on GFRP in unirradiated endothelial cells (HUVEC).
- Real-time quantitative PCR analysis of RNA from HUVECs treated with vehicle or GT3 (10 μM) for 8, 12 or 24 hours. GT3 reduces the expression of GFRP at 24 and 48 hours after treatment.
- Nuclear run-on assay for GFRP at 24 hours after treatment with GT3 (10 μM). GT3 reduces the transcription of GFRP.
- Western blot analysis of protein from HUVECs treated with either vehicle, GT3 (10 μM), atorvastatin (10 μM), mevalonate (500 μM) or with both mevalonate and GT3 or mevalonate and atorvastatin. Both GT3 and atorvastatin reduce GFRP protein levels, effects that are reversed by pre-treatment with mevalonate.
-
Immunoprecipitation assay for GFRP-CTPCH protein binding. HUVECs were treated with either vehicle, GT3 (10 μM), atorvastatin (10 μM), mevalonate (500 μM) or with both mevalonate and GT3 or mevalonate and atorvastatin. Both GT3 and atorvastatin reduce GFRP-GTPCH protein binding, effects that are reversed by pre-treatment with mevalonate.All panels show mean +sem of 3 replicatesV: vehicle; GT3: γ-tocotrienol; M: mevalonate; A: atorvastatin.
Acknowledgements
Measurement of BH4 concentrations by Ernst R. Werner, PhD, Biozentrum der Medizinischen Universitaet, Innsbruck, Austria, is gratefully acknowledged. Dr. Berbée is enrolled in the PhD program of the Department of Radiation Oncology (Maastro), GROW research institute, University of Maastricht, The Netherlands.
Financial support: National Institutes of Health/National Cancer Institute (grant CA83719 to MH-J), the Department of Defense/Defense Threat Reduction Agency (grant HDTRA1-07-C-0028 to MH-J and H.10027-07-AR-R to KSK), and the Veterans Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Delanian S, Porcher R, Balla-Mekias S, et al. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol. 2003;21:2545–2550. doi: 10.1200/JCO.2003.06.064. [DOI] [PubMed] [Google Scholar]
- 2.Boerma M, Roberto KA, Hauer-Jensen M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys. 2008;72:170–177. doi: 10.1016/j.ijrobp.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suarna C, Hood RL, Dean RT, et al. Comparative antioxidant activity of tocotrienols and other natural lipid-soluble antioxidants in a homogeneous system, and in rat and human lipoproteins. Biochim Biophys Acta. 1993;1166:163–170. doi: 10.1016/0005-2760(93)90092-n. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida Y, Niki E, Noguchi N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects. Chem Phys Lipids. 2003;123:63–75. doi: 10.1016/s0009-3084(02)00164-0. [DOI] [PubMed] [Google Scholar]
- 5.Kumar KS, Srinivasan V, Toles R, et al. Nutritional approaches to radioprotection: Vitamin E. Mil Med. 2002;167:57–59. [PubMed] [Google Scholar]
- 6.Kumar KS, Ghosh SP, Hauer-Jensen M. Gamma-tocotrienol: potential as a countermeasure against radiological threat. In: Watson RR, Preedy VR, editors. Tocotrienols: vitamin E beyond tocopherols. CRC Press; Boca Raton, FL: 2009. pp. 379–398. [Google Scholar]
- 7.Berbee M, Fu Q, Boerma M, et al. Gamma-tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh SP, Kulkarni S, Hieber K, et al. Gamma-tocotrienol, a tocol antioxidant as a potent radiation countermeasure. Int J Radiat Biol. 2009;85:598–606. doi: 10.1080/09553000902985128. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni S, Ghosh SP, Satyamitra M, et al. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat Res. 2010;173:738–747. doi: 10.1667/RR1824.1. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AA, Burger WC, Peterson DM, et al. The structure of an inhibitor of cholesterol biosynthesis isolated from barley. J Biol Chem. 1986;261:10544–10550. [PubMed] [Google Scholar]
- 11.Parker RA, Pearce BC, Clark RW, et al. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1993;268:11230–11238. [PubMed] [Google Scholar]
- 12.Pearce BC, Parker RA, Deason ME, et al. Hypocholesterolemic activity of synthetic and natural tocotrienols. J Med Chem. 1992;35:3595–3606. doi: 10.1021/jm00098a002. [DOI] [PubMed] [Google Scholar]
- 13.Naito Y, Shimozawa M, Kuroda M, et al. Tocotrienols reduce 25-hydroxycholesterol-induced monocyte-endothelial cell interaction by inhibiting the surface expression of adhesion molecules. Atherosclerosis. 2005;180:19–25. doi: 10.1016/j.atherosclerosis.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Theriault A, Chao JT, Gapor A. Tocotrienol is the most effective vitamin E for reducing endothelial expression of adhesion molecules and adhesion to monocytes. Atherosclerosis. 2002;160:21–30. doi: 10.1016/s0021-9150(01)00540-8. [DOI] [PubMed] [Google Scholar]
- 15.Laufs U, La Fata V, Plutzky J, et al. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 16.Pannirselvam M, Simon V, Verma S, et al. Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br J Pharmacol. 2003;140:701–706. doi: 10.1038/sj.bjp.0705476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cosentino F, Hurlimann D, Delli GC, et al. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart. 2008;94:487–492. doi: 10.1136/hrt.2007.122184. [DOI] [PubMed] [Google Scholar]
- 18.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller R, Unbehaun A, Schellenberg B, et al. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276:40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 20.Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. 1980;102:176–188. doi: 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- 21.Boerma M, Burton GR, Wang J, et al. Comparative expression profiling in primary and immortalized endothelial cells: changes in gene expression in response to hydroxy methylglutaryl-coenzyme A reductase inhibition. Blood Coagul Fibrinolysis. 2006;17:173–180. doi: 10.1097/01.mbc.0000220237.99843.a1. [DOI] [PubMed] [Google Scholar]
- 22.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 23.Maj JG, Paris F, Haimovitz-Friedman A, et al. Microvascular function regulates intestinal crypt response to radiation. Cancer Res. 2003;63:4338–4341. [PubMed] [Google Scholar]
- 24.Hopewell JW, Calvo W, Jaenke R, et al. Microvasculature and radiation damage. Recent Results Cancer Res. 1993;130:1–16. doi: 10.1007/978-3-642-84892-6_1. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Boerma M, Fu Q, et al. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007;13:3047–3055. doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alp NJ, Mussa S, Khoo J, et al. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosentino F, Patton S, d’Uscio LV, et al. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenzel P, Daiber A, Oelze M, et al. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis. 2008;198:65–76. doi: 10.1016/j.atherosclerosis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song BL, DeBose-Boyd RA. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase stimulated by delta- and gamma-tocotrienols. J Biol Chem. 2006;281:25054–25061. doi: 10.1074/jbc.M605575200. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi AA, Sami SA, Salser WA, et al. Synergistic effect of tocotrienol-rich fraction (TRF(25)) of rice bran and lovastatin on lipid parameters in hypercholesterolemic humans. J Nutr Biochem. 2001;12:318–329. doi: 10.1016/s0955-2863(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 31.Kalivendi S, Hatakeyama K, Whitsett J, et al. Changes in tetrahydrobiopterin levels in endothelial cells and adult cardiomyocytes induced by LPS and hydrogen peroxide--a role for GFRP? Free Radic Biol Med. 2005;38:481–491. doi: 10.1016/j.freeradbiomed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Widder JD, Chen W, Li L, et al. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ Res. 2007;101:830–838. doi: 10.1161/CIRCRESAHA.107.153809. [DOI] [PubMed] [Google Scholar]
- 33.Maita N, Hatakeyama K, Okada K, et al. Structural basis of biopterin-induced inhibition of GTP cyclohydrolase I by GFRP, its feedback regulatory protein. J Biol Chem. 2004;279:51534–51540. doi: 10.1074/jbc.M409440200. [DOI] [PubMed] [Google Scholar]
- 34.Gesierich A, Niroomand F, Tiefenbacher CP. Role of human GTP cyclohydrolase I and its regulatory protein in tetrahydrobiopterin metabolism. Basic Res Cardiol. 2003;98:69–75. doi: 10.1007/s00395-003-0394-y. [DOI] [PubMed] [Google Scholar]
- 35.Ishii M, Shimizu S, Wajima T, et al. Reduction of GTP cyclohydrolase I feedback regulating protein expression by hydrogen peroxide in vascular endothelial cells. J Pharmacol Sci. 2005;97:299–302. doi: 10.1254/jphs.sc0040146. [DOI] [PubMed] [Google Scholar]