Abstract
Cranberry juice is used routinely, especially among women and the elderly, to prevent and treat urinary tract infections. These individuals are likely to be taking medications concomitantly with cranberry juice, leading to concern about potential drug-dietary substance interactions, particularly in the intestine, which, along with the liver, is rich in expression of the prominent drug metabolizing enzyme, cytochrome P450 3A (CYP3A). Using a systematic in vitro-in vivo approach, a cranberry juice product was identified recently that elicited a pharmacokinetic interaction with the CYP3A probe substrate midazolam in 16 healthy volunteers. Relative to water, a cranberry juice inhibited intestinal first-pass midazolam metabolism. In vitro studies were initiated to identify potential enteric CYP3A inhibitors from cranberry via a bioactivity-directed fractionation approach involving dried whole cranberry [Vaccinium macrocarpon Ait. (Ericaceae)], midazolam, and human intestinal microsomes (HIM). Three triterpenes (maslinic acid, corosolic acid, and ursolic acid) were isolated. The inhibitory potency (IC50) of maslinic acid, corosolic acid, and ursolic acid was 7.4, 8.8, and <10 μM, respectively, using HIM as the enzyme source and was 2.8, 4.3, and <10 μM, respectively, using recombinant CYP3A4 as the enzyme source. These in vitro inhibitory potencies, which are within the range of those reported for two CYP3A inhibitory components in grapefruit juice, suggest that these triterpenes may have contributed to the midazolam-cranberry juice interaction observed in the clinical study.
Keywords: cranberry, Vaccinium macrocarpon (Ericaceae), maslinic acid, corosolic acid, ursolic acid, cytochrome P450 3A
Introduction
Dangerous interactions between medications arise frequently from one drug impairing the elimination of another, most commonly via inhibition of one or more cytochrome P450 (CYP) enzymes. Consequently, regulatory agencies recommend thorough characterization of CYP inhibition properties of new chemical entities, at least during preclinical development [1]. In contrast, CYP inhibition potential of most herbal remedies/dietary supplements is not evaluated prior to marketing, since such products are not regulated in the same manner as drugs, even though they consist of mixtures of diverse chemical entities [2].
Grapefruit juice (Citrus paradisi, Macf.) is an extensively studied dietary substance shown to inhibit enteric (i.e., gut) CYP3A in a mechanism-based, irreversible (so-called “suicide substrate”) manner [2-4]. Increased drug systemic exposure can lead to untoward effects. Consequently, package inserts of several drug products contain cautionary statements regarding concomitant grapefruit juice intake. Furanocoumarins, including bergamottin and 6′,7′-dihydrobergamottin, have been identified as major candidate CYP3A inhibitors using various human-derived in vitro systems [2, 4]. A clinical study involving a “furanocoumarin-free” grapefruit juice and the model CYP3A substrate, felodipine, established furanocoumarins, in aggregate, as key mediators of the felodipine-grapefruit juice interaction [5]. These observations may be extended to other drugs that undergo extensive first-pass metabolism by enteric CYP3A, including some calcium channel antagonists (e.g., felodipine, verapamil), HMG CoA reductase inhibitors (e.g., simvastatin, lovastatin), and immunosuppressants (e.g., cyclosporine, tacrolimus).
Protocols, regulations, or even general strategies to investigate interactions between drugs and dietary substances are in nascent stages of development [2]. Recognition of this knowledge gap prompted our research group to investigate potential drug-diet interactions prospectively. Inhibition of enteric CYP3A-mediated metabolism has been a primary focus, as dietary substances enter the body by the oral route and are most likely to alter drug absorption/elimination processes in the intestine [2]. Moreover, dietary substances used as herbal remedies were selected, since patients often mix folkloric and prescription medications, often unbeknownst to their physicians and/or pharmacists [6, 7]. Finally, we have approached drug-diet interactions from the vantage points of both natural products and drug metabolism sciences, melding bioactivity-directed fractionation with in vitro, and ultimately, in vivo CYP phenotyping methods. This merger of disciplines should allow identification of causative ingredients, as well as underlying mechanisms, that contribute to clinically relevant drug-dietary substance interactions in a time-efficient manner.
To test the aforementioned strategy, a natural products/drug metabolism approach was used to investigate five different cranberry juices as inhibitors of intestinal CYP3A activity [8]. Cranberry juice is used commonly, and often prophylactically, for urinary tract infections (UTIs), particularly by women and the aged. This folkloric treatment of UTIs has been ascribed to proanthocyanidins with A-type linkages [9], which have been shown to inhibit adhesion of bacterial fimbriae to uroepithelial cells (reviewed in [10]). In addition, some data have emerged regarding benefits of cranberry products to mitigate some cancers and vascular and dental diseases [11-13], likely stimulating cranberry product consumption. Yet, only a few drug-cranberry juice interaction studies have been reported, of which results were inconclusive. For example, in rats, cranberry juice was as effective as grapefruit juice in enhancing systemic exposure of the CYP3A substrate nifedipine [14]. Alternatively, a clinical study involving a different CYP3A substrate (cyclosporine) and cranberry juice indicated no interaction [15]. Similarly, a separate clinical study reported that cranberry juice had no effect on the pharmacokinetics of the CYP3A probe substrate midazolam [16]. Although caveats to these studies were discussed previously [8], until a year ago, the literature suggested that cranberry juice has a drug interaction liability for rats, but not humans, thus having no clinical concerns. While these inconsistencies may demonstrate a lack of a drug-cranberry juice interaction, an alternative explanation, as described and demonstrated [8], is that inherent variability in chemical constituents in study materials could lead to disparate results. Similarly, inconsistency in study materials has been cited as a reason why studies of clinical benefits of cranberry have been inconclusive [17]. Indeed, as reported by our research group [8], a commercial product was identified that showed a significant interaction with midazolam via both in vitro assays and a proof-of-concept clinical study.
Inhibition of enteric CYP3A by some cranberry juices suggests a potential for pharmacokinetic interactions with medications that undergo extensive intestinal first-pass metabolism by CYP3A. To identify potential intestinal CYP3A inhibitory constituents from cranberry in vitro, dried whole cranberries [Vaccinium macrocarpon (Ericaceae)] were carried through a bioactivity-directed fractionation approach involving midazolam and human intestinal microsomes (HIM). Three compounds were characterized, and IC50 values were determined using HIM and recombinant CYP3A4.
Materials and Methods
Materials and Instrumentation
HPLC-grade solvents were purchased from Burdick & Jackson (Muskegon, MI, USA). HIM were prepared previously from the jejunal portion of a donor small intestine [18]. Baculovirus-insect cell-expressed CYP3A4 (supplemented with cDNA-expressed reductase) and 1′-hydroxymidazolam were purchased from BD Biosciences (San Jose, CA). Midazolam, alprazolam, ketoconazole (>99% pure), NADPH, and formic acid were purchased from Sigma-Aldrich (St. Louis, MO). All NMR experiments were performed in CDCl3 with TMS as an internal standard on a Varian Unity INOVA-500 instrument using a 5 mm broad-band inverse probe with z-gradient. HRMS spectra were acquired on an Agilent 6220 TOF mass spectrometer with a dual APCI/ESI ionization source. Samples (dissolved in CH2Cl2) were injected directly into the mass spectrometer at a flow rate of 0.6 mL/min; a two component internal reference standard was used to achieve <5 ppm accuracy. Flash chromatography was accomplished with a CombiFlash Companion flash chromatography system (Teledyne-Isco; Lincoln, NE, USA), and reverse phase-HPLC was carried out on Varian Prostar HPLC systems (Walnut Creek, CA, USA), both as described in detail previously [19]. The evaporative light scattering detector (ELSD) used for comparisons between purchased and isolated maslinic acid was a Varian 380-LC.
Plant material
Whole, dried cranberries [Vaccinium macrocarpon Ait (Ericaceae)] were acquired by Botanical Liaisons, LLC of Boulder, Colorado, U.S.A. on November 6, 2004 and identified by Trish Flaster. A voucher specimen (JW2006) was archived at Botanical Liaisons, LLC. A high resolution digital image of the voucher (NCU 577707) was deposited in the Herbarium of the University of North Carolina at Chapel Hill.
Extraction, Isolation and Characterization
Whole, dried cranberries were ground to a powder using a MicroHammerMill IV milling machine (Glen Mills, Clifton, NJ) and processed in a manner similar to a previous study with cranberry juices [8]. Briefly, the powder (817 g) was stirred in MeOH at rt (5 L, 60 h) and then filtered. The solvent was evaporated under reduced pressure to generate a MeOH extract, which was partitioned between MeOH:H2O:hexane (9:1:10) to generate the hexane and aqueous MeOH fractions. The aqueous MeOH fraction was concentrated under reduced pressure and partitioned with CHCl3:MeOH:H2O (4:1:5) to generate the CHCl3 fraction and the aqueous fraction 1. The aqueous fraction 1 was partitioned with n-BuOH:H2O (1:1) to generate the n-BuOH fraction and the aqueous fraction 2.
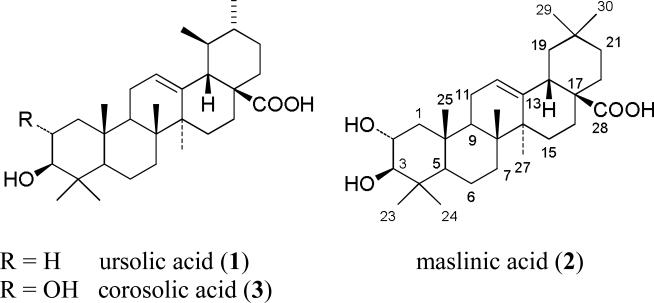
The hexane fraction (7.83 g) was dried onto Celite and subjected to column chromatography over silica gel 60 (~500 g; 70-230 mesh, Merck, Darmstadt, Germany) via a stepwise gradient that initiated with 100% hexane to 100% CHCl3 to 40% MeOH in CHCl3. Over 50 fractions were generated that were combined into 17 pools (labeled 1 through 17) based on similarity on TLC (silica gel 60 F254 plates, 0.25 mm thickness) visualized with vanillin-sulfuric acid in EtOH. Pool 13 (335.6 mg) was a single compound (purity described below) and was characterized as ursolic acid (3β-hydroxyurs-12-en-28-oic acid; 1; Figure 1) by a suite of NMR experiments including 1H, 13C, COSY, HSQC, and HMBC; these data were in excellent agreement with the literature [20]. Furthermore, 1 (C30H48O3) was subjected to HRMS, where an ion for [M-H]- and corresponding to C30H47O3 was observed (m/z = 455.3544 vs 455.3525; observed vs calculated). Pools 14 and 15 were combined (233 mg) and separated further with the flash chromatography system on silica gel (4 g) using a gradient from hexane to ethyl acetate. New fractions were combined into six pools (labeled A-F) based on the aforementioned TLC protocol. Pool D (15 mg) was dissolved in DMSO and separated further by reverse phase preparatory HPLC with a Synergi Max-RP 80 C12 column (4 μm, 250 × 21.2 mm; Phenomenex, Torrance, CA, USA) using an isocratic system of 88:12 MeOH:H2O (19 mL/min) to isolate another aliquot of 1 (1.8 mg). In total, 337.4 mg of 1 were isolated, and purity was >99% based on analytical HPLC with a diol column (YMC-Pack Diol-120NP, 5 μm, 250 × 4.6 mm; Waters; Milford, MA, USA) using a gradient from 40:60 to 80:20 ethyl acetate:hexane over 30 min at a flow rate of 1 mL/min.
Figure 1.
Structures of the CYP3A inhibitory compounds isolated from cranberry.
The chloroform fraction (6.44 g) was dried onto Celite and subjected to column chromatography over silica gel 60 (~400 g) with a stepwise gradient of 100% CHCl3 up to 30% MeOH in CHCl3. Fractions were analyzed by TLC and combined into 10 pools (labeled 1 through 10). Pools 6 and 7 were combined (224 mg) and separated further with the flash chromatography system on silica gel (12 g) using a gradient from hexane to ethyl acetate. Based on TLC profiles, seven new pools were generated (labeled A through G). Pools B, C, and D were combined (134 mg), dissolved in DMSO, and separated further by reverse phase preparatory HPLC (YMC ODS-A 5 μm, 250 × 20 mm) using an isocratic system of CH3CN:H2O (60:40) (10 mL/min); 100% MeOH was eluted at the end to wash out any residue. This purification was carried out in two separate batches, generating four fractions on the initial separation (labeled 1-1 through 1-4) and two fractions on the second separation (labeled 2-1 and 2-2). Fractions 1-3, 1-4, and 2-2 were combined (50 mg), dissolved in DMSO, and purified further by reverse phase preparatory HPLC using the aforementioned C12 column via an isocratic system of 77:23 MeOH:H2O (19 mL/min) to isolate maslinic acid (2α,3β-dihydroxyolean-12-en-28-oic acid; 2) (6.6 mg) and corosolic acid (2α,3β-dihydroxyurs-12-en-28-oic acid; 3) (21.1 mg). Each compound was analyzed by HPLC using a Synergi Max-RP 80 C12 column (4 μm, 250 × 4.6 mm) via an isocratic solvent system of 80:20 MeOH:H2O (1 mL/min) and was >90% and >99% pure, respectively. Both compounds were characterized using the aforementioned suite of NMR experiments; data were in excellent agreement with the literature [21-23]. These data were supported by HRMS measurements. Compound 2 (C30H48O4) yielded an ion for [M-H]-, corresponding to C30H47O4 (m/z = 471.3493 vs 471.3474; observed vs calculated); compound 3 (C30H48O4) yielded an ion for [M-H]-, corresponding to C30H47O4 (m/z = 471.3491 vs 471.3474, observed vs calculated). Since a limited amount of compound 2 was isolated, two different HPLC conditions were used to verify the identity of 2 with a purchased reference standard of maslinic acid (lot 184071-6; Cayman Chemical, Ann Arbor, MI. USA). One analysis was accomplished on a C18 column (YMC ODS-A 5 μm, 150 × 4.6 mm) using an isocratic solvent system of CH3CN:H2O (60:40) at a flow rate of 1.0 mL/min. Another analysis was conducted via the aforementioned C12 column using an isocratic solvent system of MeOH:H2O (80:20) at a flow rate of 1.0 mL/min. Peaks were detected via PDA at 203 nm and via ELSD. Isolated maslinic acid (2) and the reference standard had the same retention times in both systems and co-eluted when mixed together; Figures 4a and 4b (Supporting Information) display these data as observed via ELSD under both conditions.
Testing of Cranberry Fractions for Inhibition of Intestinal CYP3A Activity
Fractions from whole cranberries were tested as inhibitors of intestinal CYP3A activity in vitro using midazolam and HIM as described [8]. The final concentration of each component in incubation mixtures was as follows: midazolam, 4 μM; HIM, 0.05 mg/mL; cranberry fraction, 0.05-50 μg/mL; potassium phosphate buffer, 0.1 M (pH 7.4), containing magnesium chloride (3.3 mM); and NADPH, 1 mM. The CYP3A inhibitor, ketoconazole (1 μM), was used as a positive control in place of cranberry fraction. Vehicle control incubation mixtures contained 1% MeOH in place of cranberry fraction or ketoconazole. Incubation mixtures were analyzed for 1′-hydroxymidazolam by HPLC/MS-MS as described [24].
Determination of IC50 for Triterpenes using HIM and Recombinant CYP3A4
Reference maslinic acid and isolated corosolic acid were reconstituted with MeOH to yield a working concentration of 10 mM. Ursolic acid was reconstituted with MeOH to yield a working concentration of 5 mM. Incubations with HIM (0.05 mg/mL) or recombinant CYP3A4 (rCYP3A4) (10 pmol/mL) were prepared as described above, only cranberry fraction was replaced with triterpene (1-80 μM); vehicle control incubation mixtures contained 1% MeOH in place of triterpene or ketoconazole.
Data analysis
Initial estimates of apparent IC50 values were derived from linear regression of velocity vs. natural logarithm of triterpene concentration data. Apparent IC50 values were determined by fitting either of the following equations with untransformed data using WinNonlin (v5.2.1, Pharsight, Mountain View, CA):
| (Eq. 1) |
| (Eq. 2) |
where S denotes concentration of substrate (midazolam); v0 and v denote velocity of 1′-hydroxymidazolam formation in the absence and presence of triterpene, respectively; and h denotes the Hill coefficient. The best-fit equation was assessed from visual inspection of observed vs predicted data, randomness of residuals, Akaike information criteria, and standard errors of parameter estimates.
Statistical Analysis
Data are presented as means ± standard deviations of triplicate determinations unless indicated otherwise. IC50 values are presented as estimates ± standard error of the estimates.
Results and Discussion
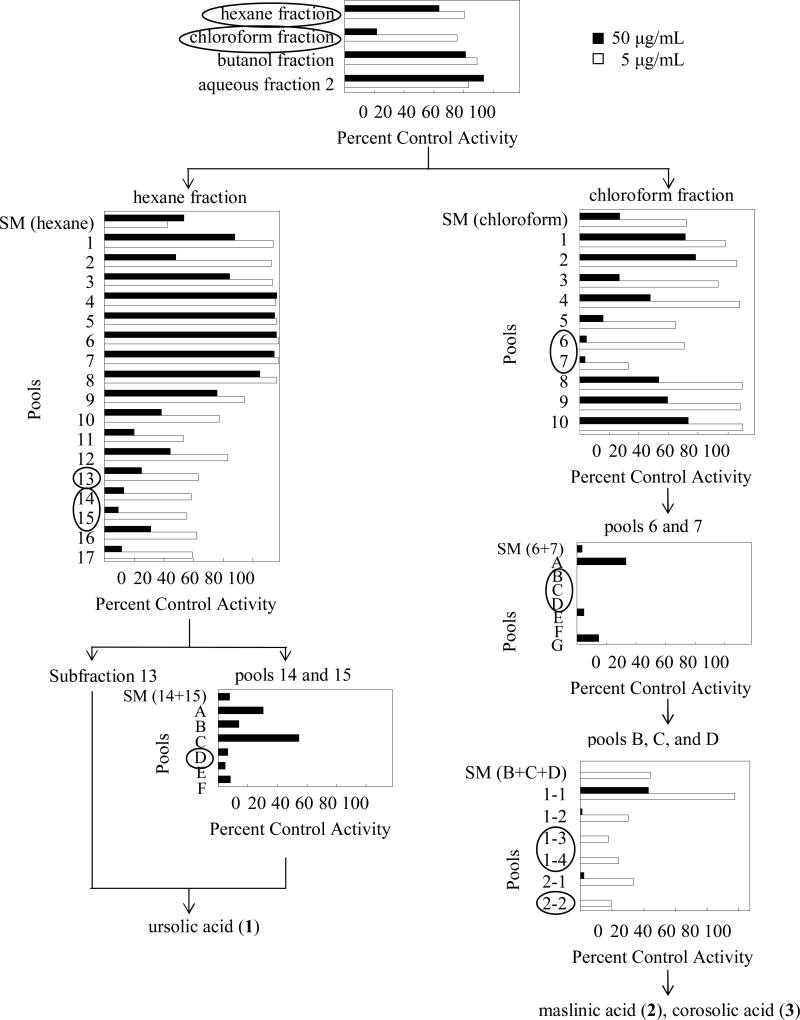
Previously, an in vitro-in vivo approach involving HIM, the CYP3A probe substrate midazolam, and five cranberry juice products identified a cranberry juice that inhibited enteric CYP3A activity in human volunteers, increasing midazolam AUC by 30% relative to water (p<0.001) [8]. These observations spurred bioactivity-directed fractionation of cranberries, using an in vitro bioassay, to identify candidate CYP3A inhibitory constituents. Dried whole cranberries, rather than the clinical test juice, were utilized for several pragmatic reasons. First, it was possible to acquire large amounts of cranberries and to deposit an herbarium voucher specimen, thereby tracing the study materials back to the site of growth and harvest; as has been noted, herbarium vouchers often are missing from investigations of herbal medicines [25]. Moreover, cranberry juices are supplemented routinely with juices of other fruits, added sugars, and preservatives [8], any of which could confound interpretation of results. Finally, extracts and fractions of cranberries could be evaluated at 50 and 5 μg/mL, concentrations consistent with other natural product investigations. In short, the study was designed to mimic as closely as possible bioactivity-directed fractionation studies used commonly for discovery of new anticancer and/or antibiotic leads, but via monitoring of inhibition of CYP3A activity in vitro.
Of the four initial fractions generated from whole cranberry, the hexane- and chloroform-soluble fractions were the most potent, inhibiting intestinal CYP3A activity by approximately 40% and 80%, respectively, at 50 μg/mL (Figure 2). The hexane-soluble fraction, which was the most potent in the previous study of cranberry juices [8], was separated to generate 17 subfractions. Some of the more polar subfractions, particularly 13-15, were the most potent, inhibiting activity by ≥70% at 50 μg/mL (Figure 2). Subfraction 13 consisted of one major compound by HPLC that was characterized as ursolic acid (1; Figure 1) via a suite of NMR experiments. Fractions 14 and 15 were purified further, yielding six pools (labeled A-F; Figure 2), and another aliquot of 1 was isolated from pool D. Other inhibitory pools (i.e., pools E and F) were examined as well (data not shown), and 1 was presumed to be the major constituent, likely responsible for the observed in vitro CYP3A inhibitory properties.
Figure 2.
Bioactivity-directed fractionation of cranberry, and inhibitory effects on intestinal CYP3A activity in vitro, as measured by midazolam 1′-hydroxylation. Circled fractions were carried forward in purification processes. Bars denote means of duplicate incubations using HIM as the enzyme source. Ketoconazole (1 μM) was used as a positive control for CYP3A inhibition and inhibited activity by >90%.
The parent chloroform-soluble fraction was separated in a similar manner to generate 10 subfractions (labeled 1-10; Figure 2). Subfractions 6 and 7 were the most potent (>90% inhibition at 50 μg/mL) and were purified further to yield seven subfractions (labeled A-G) whose inhibition at 50 μg/mL was below the limit of quantification. Two rounds of preparatory HPLC using both C18 and then C12 columns yielded two major compounds from subfractions 1-3, 1-4, and 2-2 (Figure 2), which were characterized as maslinic acid (2) and corosolic acid (3) using a suite of NMR experiments (Figure 1). Since a limited amount of 2 was isolated, a standard was purchased for the in vitro assays; isolated and purchased compounds were identical, as characterized by HPLC (Supporting Information; Figures 4a and 4b).
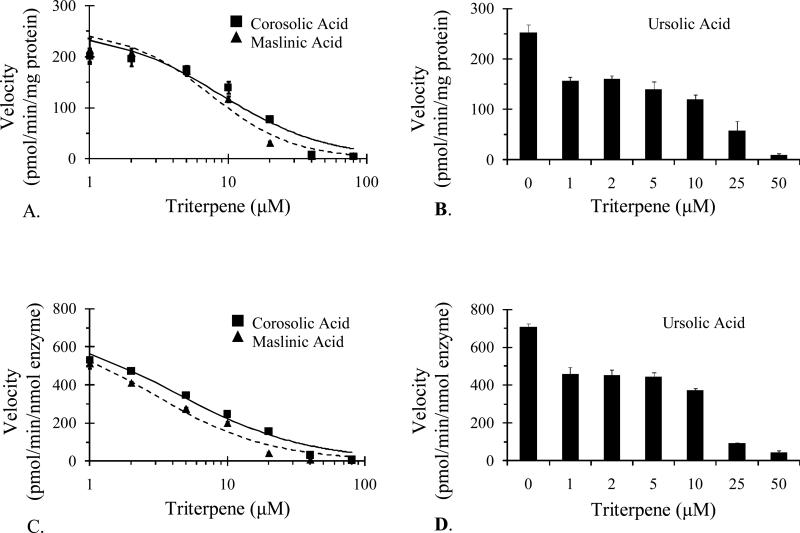
Inhibitory potencies for compounds 1-3 were evaluated in vitro using HIM and rCYP3A4. Compounds 2 and 3 were approximately equipotent. With HIM, IC50 values were 7.4 and 8.8 μM, respectively; with rCYP3A4, values were 2.8 and 4.3 μM, respectively (Table 1 and Figure 3). A robust estimate of IC50 for 1 was not recoverable with either enzyme source, most likely due to the sharp decrease in extent of inhibition between 0 and 1 μM (Figure 3). As such, data are displayed as bar graphs; by visual inspection, the IC50 was approximated at 10 μM for both HIM and rCYP3A4 (Table 1).
Table 1.
IC50 values* (μM) for triterpenes using human intestinal microsomes (HIM) or recombinant cytochrome P450 3A4 (rCYP3A4) as the enzyme source. Ketoconazole (1 μM) was used as a positive control for CYP3A inhibition and inhibited activity by >90%.
| Compound | HIM | rCYP3A4 |
|---|---|---|
| Ursolic acid (1) | <10 | <10 |
| Maslinic acid (2) | 7.4 ± 0.5 | 2.8 ± 0.2 |
| Corosolic acid (3) | 8.8 ± 0.9 | 4.3 ± 0.3 |
Values denote means ± SEs of parameter estimates.
Figure 3.
IC50 curves for ursolic acid (1), maslinic acid (2), and corosolic acid (3) in HIM (A and B) and in rCYP3A4 (C and D). Symbols/bars and error bars denote means and SDs, respectively, of triplicate incubations.
In summary, a bioactivity-directed fractionation study of cranberries led to isolation of three triterpenes (1-3), all of which inhibited enteric CYP3A activity in vitro. The appreciable potencies for 1-3 toward enteric CYP3A activity suggest that they could have contributed to the midazolam-cranberry juice interaction observed in healthy volunteers [8], although their relative concentration and solubility in various products could preclude the generality of this observation. Regardless, as isolated compounds, their IC50 values are in the range of, or somewhat higher, than IC50 or Ki values determined previously for bergamottin (6->100 μM) and 6′,7′-dihydroxybergamottin (<1-5 μM), two furanocoumarins and CYP3A inhibitors in grapefruit juice [26, 27], supportive of a bioactivity-directed fractionation approach for the identification of CYP3A inhibitors. Studies are underway to determine inhibition potency of 1-3 in Caco-2 cell monolayers modified to express CYP3A4 [28] and to measure their concentration in commercial products; NAPRALERT indicates that at least two of these compounds (1 and 2) have been reported in either cranberry or related species of Vaccinium.
Supplementary Material
Acknowledgements
This research was supported by the National Institutes of Health/National Institute for General Medical Sciences via grant R01 GM077482. EK was supported via a fellowship from the Ministry of Public Administration and Security in the Republic of Korea. MFP dedicates this article to Dr. David P. Paine.
References
- 1.Huang SM, Strong JM, Zhang L, Reynolds KS, Nallani S, Temple R, Abraham S, Habet SA, Baweja RK, Burckart GJ, Chung S, Colangelo P, Frucht D, Green MD, Hepp P, Karnaukhova E, Ko HS, Lee JI, Marroum PJ, Norden JM, Qiu W, Rahman A, Sobel S, Stifano T, Thummel K, Wei XX, Yasuda S, Zheng JH, Zhao H, Lesko LJ. New era in drug interaction evaluation: US Food and Drug Administration update on CYP enzymes, transporters, and the guidance process. J Clin Pharmacol. 2008;48:662–670. doi: 10.1177/0091270007312153. [DOI] [PubMed] [Google Scholar]
- 2.Paine MF, Oberlies NH. Clinical relevance of the small intestine as an organ of drug elimination: drug-fruit juice interactions. Expert Opin Drug Metab Toxicol. 2007;3:67–80. doi: 10.1517/17425255.3.1.67. [DOI] [PubMed] [Google Scholar]
- 3.Lown KS, Bailey DG, Fontana RJ, Janardan SK, Adair CH, Fortlage LA, Brown MB, Guo W, Watkins PB. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP 3A protein expression. J Clin Invest. 1997;99:2545–2553. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertens-Talcott SU, Zadezensky I, De Castro WV, Derendorf H, Butterweck V. Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol. 2006;46:1390–1416. doi: 10.1177/0091270006294277. [DOI] [PubMed] [Google Scholar]
- 5.Paine MF, Widmer WW, Hart HL, Pusek SN, Beavers KL, Criss AB, Brown SS, Thomas BF, Watkins PB. A furanocoumarin-free grapefruit juice establishes furanocoumarins as the mediators of the grapefruit juice-felodipine interaction. Am J Clin Nutr. 2006;83:1097–1105. doi: 10.1093/ajcn/83.5.1097. [DOI] [PubMed] [Google Scholar]
- 6.Gardiner P, Graham R, Legedza ATR, Ahn AC, Eisenberg DM, Phillips RS. Factors associated with herbal therapy use by adults in the United States. Altern Ther Health Med. 2007;13:22–29. [PubMed] [Google Scholar]
- 7.Gardiner P, Graham RE, Legedza ATR, Eisenberg DM, Phillips RS. Factors associated with dietary supplement use among prescription medication users. Arch Intern Med. 2006;166:1968–1974. doi: 10.1001/archinte.166.18.1968. [DOI] [PubMed] [Google Scholar]
- 8.Ngo N, Yan Z, Graf TN, Carrizosa DR, Kashuba AD, Dees EC, Oberlies NH, Paine MF. Identification of a cranberry juice product that inhibits enteric CYP3A-mediated first-pass metabolism in humans. Drug Metab Dispos. 2009;37:514–522. doi: 10.1124/dmd.108.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foo LY, Lu YR, Howell AB, Vorsa N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod. 2000;63:1225–1228. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 10.Howell AB. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol Nutr Food Res. 2007;51:732–737. doi: 10.1002/mnfr.200700038. [DOI] [PubMed] [Google Scholar]
- 11.Neto CC, Amoroso JW, Liberty AM. Anticancer activities of cranberry phytochemicals: An update. Mol Nutr Food Res. 2008;52:S18–S27. doi: 10.1002/mnfr.200700433. [DOI] [PubMed] [Google Scholar]
- 12.Neto CC. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007;51:652–664. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- 13.Bodet C, Grenier D, Chandad F, Ofek I, Steinberg D, Weiss EI. Potential oral health benefits of cranberry. Crit Rev Food Sci Nutr. 2008;48:672–680. doi: 10.1080/10408390701636211. [DOI] [PubMed] [Google Scholar]
- 14.Uesawa Y, Mohri K. Effects of cranberry juice on nifedipine pharmacokinetics in rats. J Pharm Pharmacol. 2006;58:1067–1072. doi: 10.1211/jpp.58.8.0007. [DOI] [PubMed] [Google Scholar]
- 15.Grenier J, Fradette C, Morelli G, Merritt GJ, Vranderick M, Ducharme MP. Pomelo juice, but not cranberry juice, affects the pharmacokinetics of cyclosporine in humans. Clin Pharmacol Ther. 2006;79:255–262. doi: 10.1016/j.clpt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Lilja JJ, Backman JT, Neuvonen PJ. Effects of daily ingestion of cranberry juice on the pharmacokinetics of warfarin, tizanidine, and midazolam--probes of CYP2C9, CYP1A2, and CYP3A4. Clin Pharmacol Ther. 2007;81:833–839. doi: 10.1038/sj.clpt.6100149. [DOI] [PubMed] [Google Scholar]
- 17.Raz R, Chazan B, Dan M. Cranberry juice and urinary tract infection. Clin Infect Dis. 2004;38:1413–1419. doi: 10.1086/386328. [DOI] [PubMed] [Google Scholar]
- 18.Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos. 2006;34:880–886. doi: 10.1124/dmd.105.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graf TN, Wani MC, Agarwal R, Kroll DJ, Oberlies NH. Gram-scale purification of flavonolignan diastereoisomers from Silybum marianum (milk thistle) extract in support of preclinical in vivo studies for prostate cancer chemoprevention. Planta Med. 2007;73:1495–1501. doi: 10.1055/s-2007-990239. [DOI] [PubMed] [Google Scholar]
- 20.Alves JS, de Castro JCM, Freire MO, da-Cunha EVL, Barbosa JM, de Silva MS. Complete assignment of the 1H and 13C NMR spectra of four triterpenes of the ursane, artane, lupane and friedelane groups. Magn Reson Chem. 2000;38:201–206. [Google Scholar]
- 21.Zucaro YL, Compagnone RS, Hess SC, Delle Monache F. 6 beta-hydroxymaslinic acid, a triterpene from Vochysia ferruginea. J Braz Chem Soc. 2000;11:241–244. [Google Scholar]
- 22.Bilia AR, Palme E, Catalano S, Flamini G, Morelli I. New triterpenoid saponins from the roots of Potentilla tormentilla. J Nat Prod. 1994;57:333–338. [Google Scholar]
- 23.Seo S, Tomita Y, Tori K. 13C NMR Spectra of Urs-12-Enes and application to structural assignments of components of Isodon japonicus Hara tissue cultures. Tetrahedron Lett. 1975:7–10. [Google Scholar]
- 24.Wang MZ, Wu JQ, Bridges AS, Zeldin DC, Kornbluth S, Tidwell RR, Hall JE, Paine MF. Human enteric microsomal CYP4F enzymes O-demethylate the antiparasitic prodrug pafuramidine. Drug Metab Dispos. 2007;35:2067–2075. doi: 10.1124/dmd.107.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett BC, Balick MJ. Phytomedicine 101: plant taxonomy for preclinical and clinical medicinal plant researchers. J Soc Integr Oncol. 2008;6:150–157. [PubMed] [Google Scholar]
- 26.Greenblatt DJ, von Moltke LL, Harmatz JS, Chen G, Weemhoff JL, Jen C, Kelley CJ, LeDuc BW, Zinny MA. Time course of recovery of cytochrome p450 3A function after single doses of grapefruit juice. Clin Pharmacol Ther. 2003;74:121–129. doi: 10.1016/S0009-9236(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 27.Paine MF, Criss AB, Watkins PB. Two major grapefruit juice components differ in intestinal CYP3A4 inhibition kinetic and binding properties. Drug Metab Dispos. 2004;32:1146–1153. doi: 10.1124/dmd.104.000547. [DOI] [PubMed] [Google Scholar]
- 28.Paine MF, Criss AB, Watkins PB. Two major grapefruit juice components differ in time to onset of intestinal CYP3A4 inhibition. J Pharmacol Exp Ther. 2005;312:1151–1160. doi: 10.1124/jpet.104.076836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.