Abstract
Background
Elevated circulating levels of soluble lectin-like oxidized low-density lipoprotein receptor-1 (sLOX-1) have been observed in obese persons and are reduced by weight loss. However, it is not known if combining caloric restriction (CR) with exercise training is better in reducing sLOX-1 levels than CR alone.
Objective
We examined whether the addition of aerobic exercise to a weight loss intervention differentially affects sLOX-1 levels in 61 abdominally obese postmenopausal women randomly assigned to a CR only (n=22), CR + moderate-intensity exercise (n=22), or CR + vigorous-intensity exercise (n=17) intervention for 20 weeks. The caloric deficit was ~2,800 kcal/week for all groups.
Results
The intervention groups were similar at baseline with respect to body weight, body composition, lipids, and blood pressure. However, plasma sLOX-1 levels were higher in the CR only group (99.90 ± 8.23 pg/ml) compared to both the CR + moderate-intensity exercise (69.39 ± 8.23 pg/ml, p=0.01) and CR + vigorous-intensity exercise (72.83 ± 9.36 pg/ml, p=0.03) groups. All three interventions significantly reduced body weight (~14%), body fat, and waist and hip circumferences to a similar degree. These changes were accompanied by a 23% reduction in sLOX-1 levels overall (−19.00 ± 30.08 pg/ml, p<0.0001), which did not differ among intervention groups (p=0.13). Changes in body weight, body fat, and VO2 max were not correlated with changes in sLOX-1 levels. In multiple regression analyses in all women combined, baseline sLOX-1 levels (β = − 0.70 ± 0.06, p<0.0001), age (β = 0.92 ± 0.43, p=0.03) and baseline BMI (β = 1.88 ± 0.66, p=0.006) were independent predictors of the change in sLOX-1 with weight loss.
Conclusions
Weight loss interventions of equal energy deficit have similar effects on sLOX-1 levels in overweight and obese postmenopausal women, with the addition of aerobic exercise having no added benefit when performed in conjunction with CR.
Keywords: obesity, weight loss, caloric restriction, aerobic exercise, soluble receptor
Introduction
The lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is the major receptor for oxidized LDL in endothelial cells and plays a critical role in the initiation and progression of atherosclerosis. LOX-1 is expressed in endothelial cells, smooth muscle cells, macrophages, and adipoctytes.(1;2) In vascular cells, LOX-1 expression is upregulated by various pro-inflammatory, pro-oxidant, vasoactive, and hemodynamic stimuli, and the increased expression of LOX-1 likely contributes to the pathogenesis of hypertension, hyperlipidemia, diabetes, myocardial ischemia, and atherosclerosis.(2;3) In adipocytes, LOX-1 enhances oxidized LDL and fatty acid uptake and increases cholesterol content, which, in excess, could contribute to the development of obesity and insulin resistance.(1) Indeed, LOX-1 expression is increased 2-fold in the adipose tissue of obese mice compared with lean controls.(1)
LOX-1 can be cleaved from the cell surface by serine proteases and released in a soluble form, and the expression of membrane-bound LOX-1 precedes the release of soluble LOX-1 (sLOX-1).(4) Thus, plasma sLOX-1 levels may be a surrogate marker of cell-surface LOX-1 expression. Increased sLOX-1 levels are associated with cardiovascular disease, oxidative stress, and diabetes.(5-7) In addition, in diabetic patients lower sLOX-1 levels are found after glucose-lowering therapy and are associated with anti-hypertensive medication use.(7) These data suggest that sLOX-1 levels may be reduced after lifestyle interventions in persons with cardiovascular disease risk factors. We recently showed that sLOX-1 levels are positively associated with body weight, body mass index (BMI), percent body fat, and trunk fat in postmenopausal women.(8) Nomata et al. later reported that weight reduction via diet and exercise decreases sLOX-1 levels in overweight middle-aged men.(9) However, it is not clear whether the reduction in sLOX-1 was due to the diet or the exercise program. Thus, the main objective of this study was to determine whether the type of weight loss intervention (i.e. caloric restriction [CR] alone vs. CR combined with moderate- or vigorous-intensity aerobic exercise) differentially affects changes in sLOX-1 levels among women with similar amounts of total weight loss. A secondary objective was to determine if the baseline obesity status was associated with changes in sLOX-1.
Methods and Procedures
Study Participants and Screening
Women in this study were participants in a randomized controlled trial designed to compare the effects of various weight loss interventions on abdominal visceral fat. The results from the main trial have been reported previously.(10) Women from the Piedmont Triad area of NC were recruited and enrolled in the study if the following inclusion criteria were met: 1) overweight or obese, defined as BMI = 25-40 kg/m2 and waist circumference > 88 cm; 2) 50-70 yrs and at least 1 yr without menses; 3) non-smoker; 4) not on hormone replacement therapy; 5) sedentary, defined as <15 min of exercise, 2 times/week in the past 6 months; and 6) weight stable (< 5% weight change) for at least 6 months before enrollment. Participants were excluded if they had untreated hypertension (blood pressure > 160/90 mmHg), triglycerides > 400 mg/dl, insulin-dependent or uncontrolled diabetes (fasting glucose > 140 mg/dl), active cancer, liver, renal or hematological disease, cognitive dysfunction (Mini-Mental State Examination score < 25), or exercise-induced ischemia.(11) Participants were also excluded if they were currently taking any medication known to affect body weight, except for statins, oral hypoglycemic agents, and thyroid medications. This study was approved by the Wake Forest University Institutional Review Board and all women provided informed consent to participate in the study. The present analysis is based on 61 women with sLOX-1 levels measured before and after weight loss.
Study Design
Eligible participants were randomly assigned to either a CR alone (CR only), CR plus moderate-intensity aerobic exercise (CR + moderate-intensity) or CR plus vigorous-intensity exercise (CR + vigorous-intensity) intervention for 20 weeks. For each intervention group, the calorie deficit was adjusted to ~2800 kcal/week (~400 kcal/day). The deficit for the CR only group resulted totally from a reduction in dietary intake, whereas deficits for the CR + moderate-intensity and CR + vigorous-intensity groups resulted from reductions in dietary intake and increases in energy expenditure. The CR only group was asked not to alter their physical activity habits during the study.
Dietary Intervention
The goal of the CR intervention was to elicit a similar energy deficit and total weight loss among the 3 groups. Throughout the 20-week intervention period, all meals were provided by the Wake Forest University General Clinic Research Center (GCRC) metabolic kitchen staff. These meals were prepared individually after the participants chose from a hypocaloric menu designed by a Registered Dietitian. Participants purchased and prepared their own breakfast meal, in consultation with the GCRC dietitian, while lunch, dinner, and snacks were prepared by the GCRC staff. There were no restrictions on the consumption of non-caloric, non-caffeinated beverages. All participants were allowed 2 free days per month, during which they were asked to report all dietary intake. In addition, all women were provided with a daily calcium supplement (1000 mg/day). They picked up their food 3 times/week and we asked to keep a log of all foods consumed. The records were monitored weekly by the registered dietitian to assess compliance. As reported previously, the average daily calorie intake recorded by all women was ~100% of the provided calorie level.(10)
Exercise Intervention
The goal of the aerobic exercise intervention was to examine the effects of exercise intensity while holding exercise energy expenditure constant at ~700 kcal/week. Participants walked on a treadmill 3 days/week under the supervision of an exercise physiologist. Treadmill speed and grade were adjusted to ensure that each woman exercised at the prescribed intensity: 45-50% of heart rate reserve (HRR) for the CR + moderate-intensity group and 70-75% of HRR for the CR + vigorous-intensity group, where HRR is the maximal heart rate obtained from the maximal oxygen consumption (VO2 max) test minus the resting heart rate. Heart rate was assessed during each exercise session with the use of heart rate monitors (Polar Electro Inc., Lake Success, NY) and recorded in a log book to monitor compliance. The duration of exercise progressed from 20 to 25 min during the first week to 55 min by the end of week 6 in the CR + moderate-intensity group. In the CR + vigorous-intensity group the exercise duration progressed from 10 to 15 min during the first week to 30 min by the end of week 6. As reported previously, the exercise compliance was ≥ 90% for both exercise groups.(10)
Plasma sLOX-1 Levels
Fasting blood samples were drawn in an EDTA-treated vacutainer between 7:00 a.m. and 9:00 a.m. in the morning after an overnight fast. Plasma was separated via centrifugation and stored at −80°C until analyses. Plasma sLOX-1 levels were measured by a sandwich chemiluminescent assay using two different human LOX-1-specific monoclonal antibodies and a recombinant human LOX-1 extracellular domain as an assay standard. This procedure was modified from the previously described sandwich assay.(5) Monoclonal antibodies directed to human LOX-1 were established by standard hybridoma techniques after immunizing mice with a recombinant protein corresponding to the extracellular domain of human LOX-1. Intra-assay and inter-assay coefficients of variation were 1.8-6.4% and 4.4-10.7%, respectively.
Body Composition and Fat Distribution
BMI was calculated from measured height and weight. Fat mass, lean mass, and percent body fat were measured by dual energy x-ray absorptiometry (Hologic Delphi QDR, Bedford, MA). Waist (minimal circumference) and hip (maximal gluteal protuberance) were measured in triplicate, and waist-to-hip ratio was calculated. Abdominal visceral and subcutaneous fat areas were measured by computed tomography as previously described.(10)
Maximal Oxygen Consumption
VO2 max was measured on a motor-driven treadmill (Medical Graphics Corporation, Minneapolis, MN) using a ramp protocol during a progressive exercise test to voluntary exhaustion.(10) A valid VO2 max was obtained when at least two of the following criteria were achieved: 1) < 200 ml/min change in VO2 max with increasing work rate, 2) heart rate > 90% of age-predicted maximal heart rate, and 3) respiratory exchange ratio ≥ 1.10. If a valid VO2 max test was not achieved, then the test was repeated.
Other Laboratory and Clinical Measurements
Blood samples were collected in the morning after an overnight fast for the measurement of plasma cholesterol and triglycerides using standardized procedures. The values reported are the average of samples collected on two separate occasions both pre- and post-intervention. Blood pressure was measured in the right arm, using a conventional mercury sphygmomanometer and the appropriately sized cuff, with the participant in a seated position after having rested quietly for 10-15 minutes. The values reported are the average of two repeated measures.
Statistical Analysis
All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC). Group differences for baseline and change values were analyzed with the use of one-way analysis of variance (ANOVA). Paired t-tests were used to examine changes with weight loss within groups. Pearson correlation coefficients were used to examine the relationships between sLOX-1 levels and body weight, body composition, body fat distribution, and other cardiovascular disease risk factors. Multiple regression analyses were used to determine the independent predictors of the change in sLOX-1 levels in all women combined after adjusting for the baseline sLOX-1 level, age, race, intervention group, and change in body weight. To examine the effect of baseline obesity status, we further adjusted the multiple regression models for BMI at baseline, both as a continuous and categorical variable. We also replaced BMI with waist circumference to adjust for baseline abdominal obesity. A p-value ≤ 0.05 was considered statistically significant.
Results
Baseline Characteristics
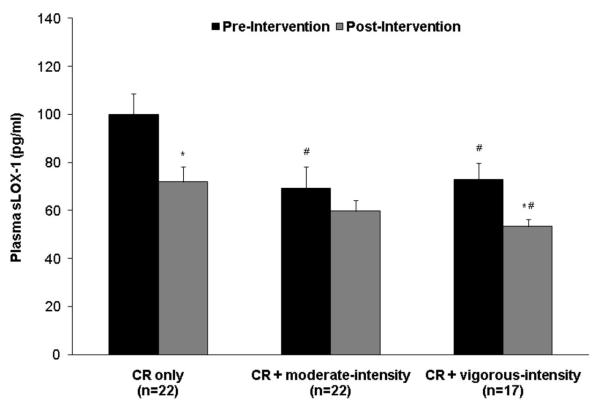
On average, the women in this study were middle-aged (58.1 ± 5.3 yrs), obese (BMI = 33.2 ± 3.8 kg/m2; body fat = 42.0 ± 3.4%), normolipidemic (total cholesterol = 197 ± 36 mg/dl; LDL cholesterol = 119 ± 30 mg/dl; HDL cholesterol = 49 ± 11 mg/dl; triglycerides = 142 ± 65 mg/dl), prehypertensive (systolic blood pressure = 129 ± 18 mmHg; diastolic blood pressure = 76 ± 8 mmHg), and predominantly Caucasian (62%). They were also largely unfit, with an average VO2 max of 20.6 ± 3.0 ml/kg/min. The CR only, CR + moderate-intensity, and CR + vigorous-intensity groups were similar at baseline with respect to age, body weight, body composition, body fat distribution, lipid levels, and blood pressure (Table 1). Baseline plasma sLOX-1 levels averaged 81.35 ± 40.47 pg/ml across all 3 groups (range: 28.18 pg/ml to 226.34 pg/ml), and as shown in Figure 1, the CR only group had higher levels (99.90 ± 8.23 pg/ml) compared to both the CR + moderate-intensity (69.39 ± 8.23 pg/ml, p=0.01) and CR + vigorous-intensity (72.83 ± 9.36 pg/ml, p=0.03) groups.
Table 1.
Baseline characteristics of study participants by intervention group
| Characteristic | CR only (n=22) |
CR + moderate intensity (n=22) |
CR + vigorous-intensity (n=17) |
|---|---|---|---|
| Age | 58.1 ± 1.2 | 58.3 ± 1.2 | 57.2 ± 1.3 |
| Weight, kg | 90.2 ± 10.6 | 87.3 ± 9.5 | 92.2 ± 13.0 |
| BMI, kg/m2 | 33.3 ± 4.1 | 32.9 ± 3.5 | 33.6 ± 3.9 |
| Total fat mass, kg | 38.7 ± 7.0 | 37.7 ± 5.6 | 40.9 ± 8.1 |
| Trunk fat, kg | 19.0 ± 4.0 | 18.5 ± 3.1 | 19.4 ± 4.4 |
| Lean mass, kg | 54.3 ± 5.0 | 51.9 ± 5.5 | 54.0 ± 5.6 |
| Total body fat, % | 41.4 ± 3.9 | 42.0 ± 3.1 | 42.8 ± 3.2 |
| Abdominal VAT, cm3 | 2332 ± 889 | 2348 ± 901 | 2390 ± 844 |
| Abdominal SAT, cm3 | 5772 ± 1595 | 5963 ± 3132 | 6011 ± 2017 |
| Waist circumference, cm | 100.0 ± 8.5 | 98.7 ± 7.9 | 99.3 ± 9.3 |
| Hip circumference, cm | 117.3 ± 8.6 | 118.5 ± 9.5 | 119.5 ± 9.5 |
| Waist-to-hip ratio | 0.85 ± 0.07 | 0.84 ± 0.09 | 0.83 ± 0.06 |
| VO2 max, ml/kg/min | 20.7 ± 3.0 | 21.3 ± 2.8 | 19.6 ± 3.3 |
| Total cholesterol, mg/dl | 198 ± 34 | 186 ± 38 | 210 ± 32 |
| LDL cholesterol, mg/dl | 121 ± 30 | 109 ± 29 | 131 ± 30 |
| HDL cholesterol, mg/dl | 49 ± 13 | 49 ± 10 | 51 ± 10 |
| Triglycerides, mg/dl | 143 ± 86 | 143 ± 48 | 138 ± 55 |
| Systolic BP, mmHg | 128 ± 16 | 126 ± 19 | 134 ± 19 |
| Diastolic BP, mmHg | 74 ± 8 | 77 ± 7 | 76 ± 9 |
Table values are mean ± SD. No significant differences among groups were observed for any variable. Abbreviations: BMI, body mass index; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; VO2 max, maximal oxygen consumption; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BP, blood pressure
Figure 1.
Plasma sLOX-1 levels before and after weight loss by intervention group. #Significantly different from the CR only group, p<0.05. *Significantly different from the baseline level, p<0.05. There was no difference in the change in sLOX-1 levels among intervention groups by ANOVA (p=0.13).
Changes with Weight Loss
The effects of the weight loss interventions on body composition and cardiovascular disease risk factors have been reported previously.(10) In the present analysis, the CR only, CR + moderate-intensity, and CR + vigorous-intensity groups had similar reductions in body weight, body composition, body fat distribution, lipid levels, and blood pressure, as shown in Table 2. On average, the women lost 12.3 ± 4.5 kg (14 ± 5%) body weight, and reduced their total body fat by 3.8 ± 2.3%, their abdominal subcutaneous and visceral fat by 1195 ± 888 cm3 and 605 ± 321 cm3, respectively, and their waist and hip circumferences by 9.7 ± 4.3 cm and 9.2 ± 4.7 cm, respectively (all p<0.0001). As expected, VO2 max increased significantly in the CR + moderate-intensity (2.8 ± 1.8 ml/kg/min, p<0.001) and the CR + vigorous-intensity (4.0 ± 2.7 ml/kg/min, p<0.0001) groups, but not in the CR only group (1.3 ± 2.9 ml/kg/min, p=0.06).
Table 2.
Changes in body composition, body fat distribution, and VO2 max with weight loss by intervention group
| Characteristics | CR only (n=22) |
CR + moderate- intensity(n=22) |
CR + vigorous- intensity(n=17) |
P-value# |
|---|---|---|---|---|
| Δ Weight (kg) | −11.7 ± 3.9* | −12.8 ± 4.3* | −12.4 ± 5.5* | 0.72 |
| Δ BMI (kg/m2) | −3.8 ± 1.3* | −4.3 ± 1.7* | −4.0 ± 1.6* | 0.58 |
| Δ Total fat mass (kg) | −7.4 ± 2.8* | −8.3 ± 3.2* | −8.4 ± 4.3* | 0.57 |
| Δ Total lean mass (kg) | −4.1 ± 2.0* | −3.7 ± 1.7* | −3.4 ± 1.6* | 0.53 |
| Δ Total body fat (%) | −3.3 ± 2.0* | −4.3 ± 2.4* | −3.9 ± 2.4* | 0.33 |
| Δ Abdominal VAT (cm3) | −591 ± 338* | −622 ± 327* | −603 ± 308* | 0.95 |
| Δ Abdominal SAT (cm3) | −986 ± 920* | −1423 ± 658* | −1183 ± 1061 | 0.27 |
| Δ Waist circumference (cm) | −9.4 ± 3.7* | −9.5 ± 4.5* | −10.2 ± 4.9* | 0.83 |
| Δ Hip circumference (cm) | −7.9 ± 4.9* | −10.4 ± 4.9* | −9.5 ± 3.9* | 0.21 |
| Δ Waist-to-hip ratio | −0.02 ± 0.04* | −0.01 ± 0.05 | −0.02 ± 0.04 | 0.55 |
| Δ VO2 max (ml/kg/min) | 1.3 ± 2.9 | 2.8 ± 1.8* | 4.0 ± 2.7* | 0.007 |
| Δ Total Cholesterol, mg/dl | −13 ± 26* | 0 ± 27 | 0 ± 21 | 0.17 |
| Δ LDL Cholesterol, mg/dl | −6 ± 21 | 3 ± 20 | 4 ± 21 | 0.23 |
| Δ HDL Cholesterol, mg/dl | −0 ± 4 | 3 ± 7* | 2 ± 5 | 0.09 |
| Δ Triglycerides, mg/dl | −29 ± 63* | −31 ± 44* | −23 ± 41* | 0.87 |
| Δ Systolic BP, mmHg | −4 ± 16 | −10 ± 19 | −11 ± 15* | 0.47 |
| Δ Diastolic BP, mmHg | −3 ± 8 | −9 ± 10* | −7 ± 7* | 0.10 |
Table values are means ± SD.
Significant change with intervention, p<:0.05.
Based on one-way ANOVA across intervention groups
Overall, plasma sLOX-1 levels decreased by 23% (−19.00 ± 30.08 pg/ml, p<0.0001) in response to weight loss, and these changes were similar among the groups (p=0.13). As shown in Figure 1, sLOX-1 levels decreased significantly in the CR only (−27.97 ± 29.17 pg/ml, p=0.0002) and CR + vigorous-intensity (−19.48 ± 23.87 pg/ml, p=0.004) groups, with smaller changes in the CR + moderate-intensity group (−9.67 ± 33.52 pg/ml, p=0.13).
Correlation Analyses
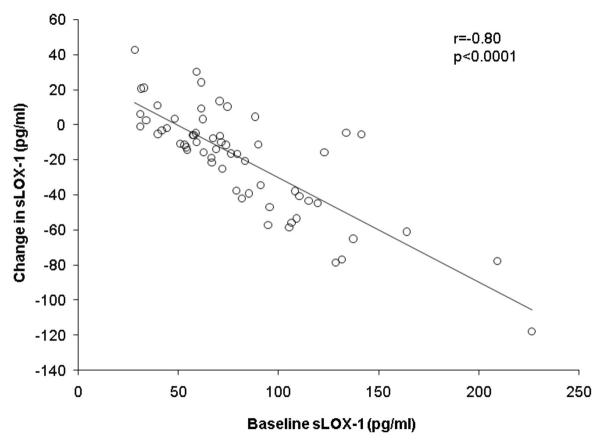
In all women combined, higher sLOX-1 levels at baseline were associated with a higher BMI (r=0.26, p=0.04), but were not related to body weight, body composition, or body fat distribution. Plasma sLOX-1 levels were also unrelated to age, VO2 max, lipid levels, or blood pressure at baseline. As shown in Figure 2, women with the highest sLOX-1 levels at baseline had the greatest reductions in sLOX-1 with weight loss (r=-0.80, p<0.0001). However, there were no significant correlations between changes in sLOX-1 levels and changes in body weight, body composition, or body fat distribution. There was also no significant association between changes in VO2 max and changes in sLOX-1 with weight loss (r=−0.06, p=0.66).
Figure 2.
Association of baseline sLOX-1 levels with changes in sLOX-1 levels in all women combined based on Pearson correlation analyses.
Regression Analyses
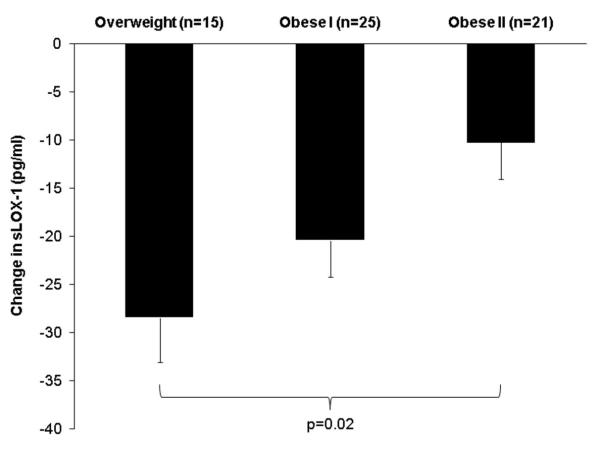
Table 3 shows the results from the multiple regression analyses. When age, race, intervention group, baseline sLOX-1, and change in body weight were included in the model, the only independent predictors of the change in sLOX-1 levels were baseline sLOX-1 (p<0.0001), age (p=0.04), and race (p=0.03), such that women with lower baseline sLOX-1 levels, older women, and African American women had smaller reductions in sLOX-1 levels with weight loss. Inclusion of baseline BMI in the model showed that baseline sLOX-1 level (p<0.0001) and age (p=0.03), but not race (p=0.28), remained independently associated with changes in sLOX-1 levels. BMI was also an independent predictor of the change in sLOX-1 levels, with a higher baseline BMI associated with smaller reductions in sLOX-1 with weight loss (p=0.006). In addition, we found that changes in sLOX-1 levels with weight loss differed significantly (p=0.04) among women who were categorized as overweight (BMI = 25.0-29.9 kg/m2, n=15), obese class I (BMI = 30.0-34.9 kg/m2, n=25), or obese class II (BMI = 35.0-39.9 kg/m2, n=21). After adjusting for baseline sLOX-1, age, race, intervention group, and change in body weight, BMI category remained an independent predictor of the change in sLOX-1 levels (p=0.02), with overweight women having the greatest reduction in sLOX-1 (adjusted mean ± SE = −28.45 ± 4.64 pg/ml, p<0.0001), followed by obese class I (−20.41± 3.80 pg/ml, p<0.0001), and then obese class II (−10.23 ± 3.86 pg/ml, p=0.01), as shown in Figure 3. On the other hand, baseline waist circumference was not predictive of changes in sLOX-1 with weight loss (p=0.11).
Table 3.
Independent predictors of the change in sLOX-1 levels with weight loss
| Predictor | Regression coefficient | SE | P for predictor |
|---|---|---|---|
| Baseline sLOX-1 | −0.70 | 0.09 | 0.007 |
| Age | 0.92 | 0.43 | 0.03 |
| BMI | 1.88 | 0.66 | 0.02 |
Multiple linear regression was used to model changes in sLOX-1, with the baseline sLOX-1 level, age, race, intervention group, change in body weight, and baseline BMI included as covariates. Variables shown are only those that showed a statistically significant independent association with change in sLOX-1.
Figure 3.
Adjusted mean ± SE of the change in sLOX-1 levels with weight loss by BMI category based on multiple regression analysis adjusted for baseline sLOX-1, age, race, intervention group, and change in body weight.
Discussion
Soluble LOX-1 is an emerging risk factor with potential relevance in cardiovascular and metabolic diseases. Accumulating evidence indicates that sLOX-1 levels are increased in obese women and can be reduced with weight loss in overweight men.(8;9) This study sought to clarify the role of weight loss interventions by determining the effects of CR, with and without aerobic exercise training, on circulating sLOX-1 levels in abdominally obese postmenopausal women. Our results not only confirm that sLOX-1 levels are reduced after weight loss, but extend these findings to women. More importantly, our study indicates that the magnitude of change in sLOX-1 levels is not different among weight loss interventions of equal energy deficit, regardless of whether the weight loss is induced by CR alone or in conjunction with aerobic exercise.
We previously reported that sLOX-1 levels were higher in obese postmenopausal women compared to lean and overweight women.(8) In addition, sLOX-1 levels were positively correlated with body weight, BMI, percent body fat, and trunk fat. Similarly, Nomata et al. found that in obese men higher sLOX-1 levels were associated with a higher body weight and BMI.(9) In the present study, we also found a positive association with BMI, but sLOX-1 levels were not correlated with body weight or any measure of body composition or body fat distribution. In addition, although weight loss resulted in significant decreases in sLOX-1 levels, changes in sLOX-1 were not related to changes in body weight or body fat in our study population.
One of the novel findings of this study was that the addition of either moderate- or vigorous-intensity aerobic exercise training had no added benefit in reducing sLOX-1 levels when performed in conjunction with CR. This observation is supported by the lack of an association between changes in sLOX-1 and improvements in VO2 max with weight loss. The similar changes in sLOX-1 levels among groups may have been due to the fact that the weight loss interventions provided equal caloric deficits, and therefore equal amounts of weight loss. Another novel finding was that age and BMI at baseline were significant predictors of the change in sLOX-1 levels with weight loss. Most notably, with increasing levels of obesity, the reduction in sLOX-1 was attenuated 2- to 3-fold compared to overweight women. These findings are consistent with the idea that with both aging and obesity, the normal physiological responses to a weight loss intervention may become impaired.
LOX-1 is expressed in endothelial cells, smooth muscle cells, macrophages, and adipocytes.(1;2) At the cell surface LOX-1 can be cleaved at the membrane proximal extracellular domain by serine proteases and released into the circulation as sLOX-1.(4;12) While elevated levels of sLOX-1 in the blood may reflect increased expression of membrane-bound proteins and disease activity, only a few studies have actually examined its relationship with cardiovascular disease and its associated risk factors. Hayashida et al. was the first to report on circulating sLOX-1 levels in humans, demonstrating that serum levels were significantly increased in acute coronary syndromes.(5) Other studies have reported that sLOX-1 levels are higher in diabetics and coronary artery disease (CAD) patients than healthy controls.(7;13;14) Interestingly, circulating sLOX-1 levels were positively correlated with hemoglobin A1c and advanced glycation endproducts in diabetics, while in CAD patients sLOX-1 levels increased with increasing numbers of diseased vessels and higher levels of inflammation and oxidative stress.(6;7;13) As described above, we and others have shown that higher sLOX-1 levels are also associated with a higher BMI and greater body weight and body fat.(8;9) Thus, these findings support a relationship between sLOX-1 and inflammatory and metabolic diseases, and highlight its potential as an important cardiovascular disease risk factor in various populations.
Several studies show that in vascular cells LOX-1 expression is induced in vitro by various pro-inflammatory, pro-oxidant and vasoactive stimuli, such as oxidized LDL, angiotensin II, and C-reactive protein, and is upregulated in vivo by hyperlipidemia, hypertension, and diabetes.(2) These data strongly support a pathophysiological role for LOX-1 in the development of cardiovascular disease. On the other hand, one study by Chui et al. found that treatment with the anti-diabetic drug rosiglitazone significantly increased LOX-1 expression in mouse 3T3-L1 adipocytes.(1) In addition, they reported that LOX-1 mRNA levels in white adipose tissue are higher in ob/ob mice compared to lean mice and are further increased by ciglitazone treatment. The authors suggested that adipose tissue could help to inhibit the formation of atherosclerotic lesions by removing oxidized LDL from the circulation. Alternatively, adipose tissue LOX-1 expression may be essential for the uptake of cholesterol required for normal cellular function; however, in excess, it could contribute to the development of obesity and insulin resistance. Taken together, these data suggest that the pathophysiological role of LOX-1 may be cell-specific and influenced by the presence of other risk factors. More studies are needed to better characterize the factors that regulate its expression in adipose and non-adipose tissues.
Little is known about the regulators of sLOX-1 expression. Our data show that weight loss can reduce sLOX-1 levels in overweight and obese women. In fact, 20 weeks of CR with and without aerobic exercise elicited a 14% reduction in body weight and a 23% reduction in sLOX-1 levels. Nomata et al. recently reported the effects of a 12-week weight reduction intervention on serum sLOX-1 levels in overweight middle-aged men.(9) They found that sLOX-1 levels decreased 72% with a 10% reduction in body weight. While gender differences likely influence the magnitude of change in sLOX-1 levels with weight loss, these data indicate that clinically significant weight reduction is effective at lowering sLOX-1 levels in both men and women. In addition, although we did not find a correlation between reductions in body weight and sLOX-1 levels as was previously reported in men,(9) both our results and the results of Nomata et al. suggest that changes in sLOX-1 with weight loss are associated with obesity-related factors. Mitsuoka et al. demonstrated that the inflammatory cytokine interleukin-18 (IL-18) stimulates the release of sLOX-1 in cultured cells, and IL-18 infusion significantly increases sLOX-1 levels in mice.(15) Data from other studies indicate that diabetes-related factors, including glucose and advanced glycation endproducts stimulate the release of sLOX-1 in endothelial cells.(7;14) In addition, sLOX-1 levels are reduced by 13% in diabetics after 6 months of glucose-lowering therapy.(7) The reduction in sLOX-1 correlated with improvements in hemoglobin A1c levels and advanced glycation endproducts. Use of anti-hypertensive medications was also associated with lower sLOX-1 levels in these patients.(7) Taken together, it appears that sLOX-1 levels are increased by pathological stimuli and can be reduced by various lifestyle therapies. Given that obesity is associated with a number of cardiometabolic risk factors, it is possible that improvements in inflammatory status, glucose homeostasis, and blood pressure may contribute to reductions in sLOX-1 levels in overweight and obese men and women.
In conclusion, we found that weight loss interventions of equal energy deficit have similar effects on sLOX-1 levels in overweight and obese postmenopausal women, regardless of whether they involve aerobic exercise training. The present study has several strengths including the highly controlled dietary intervention, the well-standardized exercise program, and the randomization of abdominally obese postmenopausal women, a population at high risk for cardiovascular disease. However, there are a few limitations to point out: 1) the use of a controlled diet may limit the applicability of the results to the general population; 2) the relatively short duration of the study limits our ability to predict the effect of long-term weight loss on changes in sLOX-1 levels; and 3) we cannot determine the exact source of the circulating sLOX-1 (i.e. vascular or adipose tissue). Future studies will need to determine whether increased expression of sLOX-1 is a cause or a consequence of obesity and its related metabolic abnormalities. More importantly, it will be necessary to identify the processes that regulate LOX-1 cleavage from the cell surface. Although its physiological role remains to be elucidated, sLOX-1 is emerging as a cardiovascular and metabolic risk factor, which highlights the need to identify interventions that reduce circulating levels of this receptor. In this regard, the main findings of the present study indicate that weight loss is a potential lifestyle therapy that warrants further investigation.
Acknowledgements
We are grateful to the study coordinators, dietitians, exercise physiologists, nurses and other research staff of the Wake Forest University General Clinical Research Center and Geriatric Research Center for their assistance in the conduct of this study. We would also like to thank all the women who volunteered to participate in this study. This study was supported by NIH grants R01-AG/DK20583, the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332), and the Wake Forest University General Clinical Research Center (M01-RR07122).
Grant Support: This study was supported by NIH grants R01-AG/DK20583, the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332), and the Wake Forest University General Clinical Research Center (M01-RR07122).
Footnotes
Disclosures: None
References
- 1.Chui PC, Guan HP, Lehrke M, Lazar MA. PPARgamma regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J Clin Invest. 2005;115:2244–2256. doi: 10.1172/JCI24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Nagase M, Ando K, Nagase T, Kaname S, Sawamura T, Fujita T. Redox-sensitive regulation of lox-1 gene expression in vascular endothelium. Biochem Biophys Res Commun. 2001;281:720–725. doi: 10.1006/bbrc.2001.4374. [DOI] [PubMed] [Google Scholar]
- 4.Murase T, Kume N, Kataoka H, Minami M, Sawamura T, Masaki T, Kita T. Identification of soluble forms of lectin-like oxidized LDL receptor-1. Arterioscler Thromb Vasc Biol. 2000;20:715–720. doi: 10.1161/01.atv.20.3.715. [DOI] [PubMed] [Google Scholar]
- 5.Hayashida K, Kume N, Murase T, Minami M, Nakagawa D, Inada T, Tanaka M, Ueda A, Kominami G, Kambara H, Kimura T, Kita T. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: a novel marker for early diagnosis. Circulation. 2005;112:812–818. doi: 10.1161/CIRCULATIONAHA.104.468397. [DOI] [PubMed] [Google Scholar]
- 6.Kamezaki F, Yamashita K, Tasaki H, Kume N, Mitsuoka H, Kita T, Adachi T, Otsuji Y. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 correlates with oxidative stress markers in stable coronary artery disease. Int J Cardiol. 2008;134:285–287. doi: 10.1016/j.ijcard.2007.12.069. [DOI] [PubMed] [Google Scholar]
- 7.Tan KC, Shiu SW, Wong Y, Leng L, Bucala R. Soluble lectin-like oxidized low density lipoprotein receptor-1 in type 2 diabetes mellitus. J Lipid Res. 2008;49:1438–1444. doi: 10.1194/jlr.M700551-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Brinkley TE, Kume N, Mitsuoka H, Phares DA, Hagberg JM. Elevated soluble lectin-like oxidized LDL receptor-1 (sLOX-1) levels in obese postmenopausal women. Obesity (Silver Spring) 2008;16:1454–1456. doi: 10.1038/oby.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomata Y, Kume N, Sasai H, Katayama Y, Nakata Y, Okura T, Tanaka K. Weight reduction can decrease circulating soluble lectin-like oxidized low-density lipoprotein receptor-1 levels in overweight middle-aged men. Metabolism. 2009;58:1209–1214. doi: 10.1016/j.metabol.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Nicklas BJ, Wang X, You T, Lyles MF, Demons J, Easter L, Berry MJ, Lenchik L, Carr JJ. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009;89:1043–1052. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Sports Medicine . ACSM’s Guidelines for Exercise Testing and Prescription. 6th edn Lippincott Williams & Wilkins; Baltimore: 2000. [Google Scholar]
- 12.Kume N, Kita T. Roles of lectin-like oxidized LDL receptor-1 and its soluble forms in atherogenesis. Curr Opin Lipidol. 2001;12:419–423. doi: 10.1097/00041433-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Lubrano V, Del Turco S, Nicolini G, Di Cecco P, Basta G. Circulating levels of lectin-like oxidized low-density lipoprotein receptor-1 are associated with inflammatory markers. Lipids. 2008;43:945–950. doi: 10.1007/s11745-008-3227-9. [DOI] [PubMed] [Google Scholar]
- 14.Shiu SW, Tan KC, Wong Y, Leng L, Bucala R. Glycoxidized LDL increases lectin-like oxidized low density lipoprotein receptor-1 in diabetes mellitus. Atherosclerosis. 2008;203:522–527. doi: 10.1016/j.atherosclerosis.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Mitsuoka H, Kume N, Hayashida K, Inui-Hayashiada A, Aramaki Y, Toyohara M, Jinnai T, Nishi E, Kita T. Interleukin 18 stimulates release of soluble lectin-like oxidized LDL receptor-1 (sLOX-1) Atherosclerosis. 2008;202:176–182. doi: 10.1016/j.atherosclerosis.2008.04.002. [DOI] [PubMed] [Google Scholar]