Abstract
Bladder cancer metastasis is virtually incurable with current platinum-based chemotherapy. We used the novel COXEN informatic approach for in silico drug discovery and identified NSC-637993 and NSC-645809 (C1311), both imidazoacridinones, as agents with high-predicted activity in human bladder cancer. Because even highly effective monotherapy is unlikely to cure most patients with metastasis and NSC-645809 is undergoing clinical trials in other tumor types, we sought to develop the basis for use of C1311 in rational combination with other agents in bladder cancer. Here, we demonstrate in 40 human bladder cancer cells that the in vitro cytotoxicity profile for C1311 correlates with that of NSC-637993 and compares favorably to that of standard of care chemotherapeutics. Using genome-wide patterns of synthetic lethality of C1311 with open reading frame knockouts in budding yeast, we determined that combining C1311 with a taxane could provide mechanistically rational combinations. To determine the preclinical relevance of these yeast findings, we evaluated C1311 singly and in doublet combination with paclitaxel in human bladder cancer in the in vivo hollow fiber assay and observed efficacy. By applying COXEN to gene expression data from 40 bladder cancer cell lines and 30 human tumors with associated clinical response data to platinum-based chemotherapy, we provide evidence that signatures of C1311 sensitivity exist within nonresponders to this regimen. Coupling COXEN and yeast chemigenomics provides rational combinations with C1311 and tumor genomic signatures that can be used to select bladder cancer patients for clinical trials with this agent.
Introduction
Bladder cancer is common and costly [1]. Nearly 30% of patients present with muscle invasive bladder tumors at diagnosis, and approximately 50% of these patients develop distant recurrence and require systemic chemotherapy [2]. With standard platinum combination therapy (commonly cisplatin or carboplatin and gemcitabine, GC), a median survival of only 13 months can be achieved in patients with advanced disease, with modest response rates reported for second line agents for treatment failures [3].
We have recently reported an informatics approach termed COXEN, for coexpression extrapolation, that uses cell line transcriptional signatures and associated in vitro sensitivity to therapeutic compounds to predict sensitivity of independent cell line panels and patient responses to such agents [4]. The novel aspect of this approach is its ability to select from sensitivity biomarker genes derived from cell lines a subset that maintain concordant expression in a second cohort of cell lines or human tumor samples. Importantly, this analysis is done a priori, without knowledge of the pattern of sensitivity or clinical response is in the second set. Originally reported as predictive of the outcomes of separate clinical studies in 84 patients [5], recently this algorithm has been used successfully to stratify clinical outcomes nearly 500 patients with diverse tumor types [6].
One COXEN application we have reported is in drug discovery, using publicly available data for 45,545 compounds from the US NCI Developmental Therapeutics Program's screen of 60 cell lines from nine different tumor histologies (NCI-60) [7]. Because bladder cancer cell lines were not part of the NCI-60, we used COXEN to predict which of the 45,000 drugs would be highly active in human bladder cancer. Top hits from this analysis included NSC-637993 and NSC-645809 (C1311), two imidazoacridinone class compounds. Evaluation of NSC-637993 on a panel of 40 bladder cancer cell lines indicated that more than 60% exhibited 50% growth inhibition at the micromolar level or better [5].
The imidazoacridinones are a promising new class of compounds for human cancer [8] and are thought to function through several mechanisms. Work with C1311 suggests that its mechanisms of action may include DNA intercalation [9], as well as inhibition of topoisomerase II [10] and the FLT3 tyrosine kinase [11]. Recent studies additionally suggest that the mechanism of inhibition of topoisomerase II may be due to C1311 interfering with ATP binding to the enzyme, perhaps in a fashion analogous to its inhibition of FLT3 [12]. Given our in vitro results with NSC-637993 in bladder cancer cells and promising results obtained for C1311 in early clinical trials in other tumor types [13,14], we decided to perform a preclinical evaluation for these two related molecules in bladder cancer with the intent to pave the way for future clinical trials with these agents.
Because essentially no cures are observed in the setting of second-line therapy for metastatic disease treated with single agents [3], we applied yeast chemical genetics methods to define and then validate in human bladder cancer, rational combination therapy with C1311. In addition, given our success with COXEN-based gene expression signatures in predicting chemotherapeutic outcomes, we also provide evidence that, among patients who fail first-line platinum chemotherapy for metastatic bladder cancer, there exists a cohort that exhibits transcriptional signatures suggestive of response to C1311.
Materials and Methods
Cell Culture, In Vitro, and In Vivo Drug Sensitivity
All human bladder cancer cells (BLA-40 panel), culture conditions, and our protocol for assay of drug sensitivity have been reported previously [5,15]. IC50 values (concentrations capable of inducing 50% inhibition of cellular growth) were calculated for the 40 cell lines using an improved Spline-fitting approach in the statistics suite, R (www.R-project.org). In vivo sensitivity studies used the hollow fiber assay (HFA), reported before [16], and in Supplementary Methods. The significance of growth inhibition in HFA results was tested by single-sample t tests against the hypothesis that there was no inhibition, in PRISM (GraphPad Software, La Jolla, CA), with two-sided P values reported.
Competitive Yeast Growth Experiments
In Saccharomyces cerevisiae, mutant strains with knockouts of all non-lethal open reading frames (ORFs; ∼4600) are available that are tagged with two unique oligonucleotide “barcodes” that are flagged by universal polymerase chain reaction primers for detection through microarrays, as detailed before [17]. For competitive growth experiments, the collection of homozygous diploid mutant cells (EUROSCARF; Institute of Molecular Biosciences, Frankfurt, Germany) were grown on YPD agar containing G418, pooled and frozen in 0.23-ml aliquots at OD = 21.5. For YPD growth, cells were diluted to 6.17 x 10 E7 cells/ml and grown to saturation (five generations). Cultures were sequentially diluted to 6.17 x 10 E7 cells/ml for consecutive growth experiments (10, 15, and 20 generations). C1311 stocks were maintained at 100 µM in DMSO, and cells were treated with 0, 1, or 5 µl in YPD plus 1% DMSO. Benomyl treatment was at 15 µg/ml in YPD plus 1% DMSO. Genomic DNA was recovered using MasterPure yeast DNA purification kit (EPICENTRE Biotechnologies, Madison, WI) and hybridized to Affymetrix Yeast TAG4.0 microarrays (Affymetrix Inc, Santa Clara, CA) as per the manufacturer's instructions.
Analysis of Yeast TAG Array and Synthetic Lethal Data
The Affymetrix Yeast TAG4.0 array data were analyzed using the software developed by the Giaever laboratory, which normalizes, quality filters, and background adjusts data as detailed in the Supplementary Methods and previous publication [18]. The Yeast TAG4.0 drug data as well as synthetic lethal data were binarized, assigning a 1 to synthetic lethal query-target pairs and 0 to all other ORF pairs. Combining the drug and synthetic lethal data resulted in a binary matrix with 1521 rows of yeast query genes and 6 drug treatments (4 C1311 and 2 benomyl) and 2804 columns of yeast target genes. We note that the original size of each drug binary vector was 6431 (i.e., the number of yeast deletion strains interrogated on the array) and reduced to 2804 after being projected onto the set of available yeast target genes. These data were clustered in two dimensions (i.e., cluster both rows and columns) with a cosine distance metric to this 1521 x 2804 binary matrix using the clustergram function in MATLAB Version 7.9.0 (MathWorks, Natick, MA). Lists of yeast strains with reduced fitness for benomyl and C1311 were examined for statistically significant enrichment of gene ontology terms by GO::TermFinder [19] using default settings.
Drug Sensitivity Correlation Analyses
We calculated Spearman correlations between C1311 and NSC-637993 compound data, paired distributions for C1311 and NSC-637993 were compared using the Wilcoxon matched-pairs test, in MATLAB and PRISM, respectively. For correlation analysis of the ∼4600 developmental therapeutics program drugs [20] to C1311 across the NCI-60, we calculated Spearman correlation coefficients of cell line IC50 values for C1311 to all other drugs, calculated Benjamini-Hochberg-corrected P values, and used Kernel-Smoothing function to plot the distribution of correlation coefficients, all in MATLAB.
Training and Testing COXEN-Based Classifier
Gene expression profiling of the BLA-40 bladder cancer panel [GEO:GSE5845] [15] and that of the NCI-60 panel [GEO: GSE5720] [21] and in vitro testing data for the compounds [22] were used. For detailed methods on interplatform prediction of C1311 sensitivity and biomarker selection, see Supplementary Methods.
Results
Cytotoxicity of Imidazoacridinones on Human Bladder Cancer Cells In Vitro and In Vivo
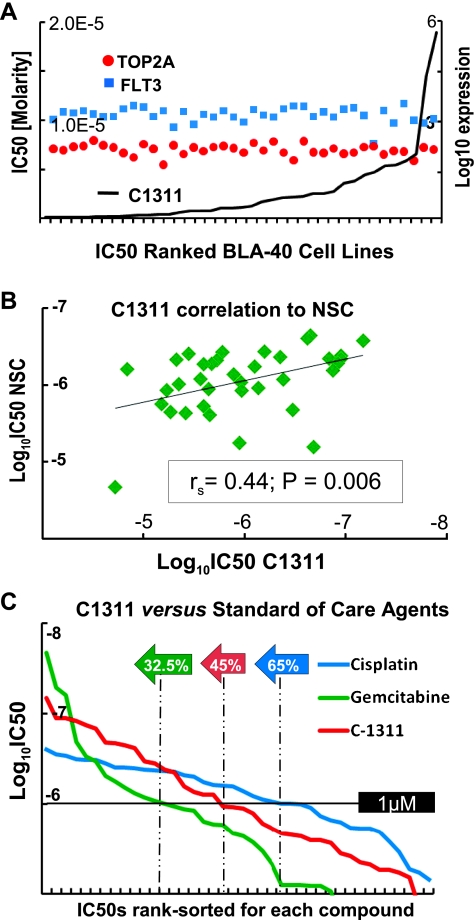
Having discovered that NSC-637993 exhibits activity in many bladder cancer cell lines [5], we were interested whether the related imidazoacridinone, C1311, might also have activity in bladder cancer. We generated dose-response curves for C1311 in our 40 bladder cancer cell lines (BLA-40) [15] across a concentration range of five logs, estimated IC50 concentrations, and compared them to IC50 values for NSC-637993 for the same panel. We observed robust activity of C1311 in these cells, with IC50 values uncorrelated to the expression of TOP2A (rs = -0.11, P = .52) and FLT3 (rs = -0.26, P = .11), putative targets of C1311 (Figure 1A; for complete data, see Table W1 and Figure W1).
Figure 1.
C1311 and NSC exhibit similar, favorable in vitro activities, comparable to standard-of-care agents. (A) IC50 values for C1311 were determined by Spline regression for the BLA-40 cell line panel, plotted ranked ordered from left to right, then each corresponding cell line's expression of TOP2A and FLT3 expression (Affymetrix probes 201291_s_at and 206674_at, both log10 values for visualization in scale). (B) Scatter plot of NSC compound (ordinate) and C1311 (abscissa) IC50 values across the BLA-40 panel, nonparametric Spearman correlation, and P value. (C) Comparison of C1311 and standard-ofcare drugs by IC50 across the BLA-40 panel. IC50 values C1311, cisplatin, and gemcitabine were rank ordered for the 40 cell lines for each drug and plotted in ascending order on the log-scale y axis. The green, pink, and blue arrows indicate the percentage of the BLA-40 cell lines that exhibit IC50 values below the 1-µM range, demonstrating that C1311 exhibits similar activity to agents currently in clinical use.
Given the structural and functional similarity of C1311 to NSC-637993, we wished to determine whether IC50 values for NSC-637993 and C1311 were correlated. We found that the IC50 values of the two drugs were significantly correlated (Figure 1B, rs = 0.44, P = .006), whereas there was no significant difference between the paired distributions of IC50 values for C1311 and NSC-637993 (P = .42) across these cells. This is consistent with data for the NC-I60 panel of cells, for which a similar correlation was also observed (rs = 0.51, P < .01), as well as a trend toward superiority of C1311 over NSC-637993 (P <.01;not shown). In addition, we observed that C1311 compares favorably with cisplatin and gemcitabine, the standard-of-care drugs for bladder cancer (Figure 1C).
We next tested these compounds in vivo using HFA [16]. On the basis of our in vitro characterization of sensitivity to C1311 and NSC-637993 (Table W1) as well as evaluation of cell lines for compatibility with the HFA, we selected one cell line exhibiting a low IC50 (T24T, sensitive), two cell lines with intermediate IC50 values (FL3 and UMUC1), and one cell line with a high IC50 (KK47, resistant) to C1311, as indicated in Table 1 for HFA studies. Mice were treated four times daily at 20 mg/kg by intraperitoneal injection, with animals killed at 96 hours for quantitative measurement of cell growth. Compared with untreated animals, we found that, in three of four cell lines tested, there was a significant inhibition of growth (all P ≤ 0.02), the exception being the resistant KK47 cell line (Table 1). Similar results were shown testing NSC-637993 in this assay (Table W2A).
Table 1.
HFA Results for C1311.
| Cell Line | C1311 Status* | Log10 IC50 | SQ† | P‡ | IP§ | P‡ | Overall | Overall P‡ |
| T24T | Sensitive | -6.62 | 65.5 | <.0001 | 71.0 | .0006 | 68.3 | <.0001 |
| FL3 | Intermediate | -5.45 | 78.6 | .014 | 58.3 | <.0001 | 68.4 | .0002 |
| UMUC1 | Intermediate | -5.35 | 97.7 | .067 | 60.5 | <.0001 | 79.1 | .02 |
| KK47 | Resistant | -4.84 | 80.1 | <.0001 | 101.8 | .57 | 90.9 | .10 |
Relative sensitivity to C1311 of indicated cell line. Of cell lines adaptable to the hollow fiber assay, four cell types of varying in vitro sensitivities were selected for validation in vivo.
Average percentage of control growth across four replicates at the subcutaneous implantation site.
Two-tailed P value for single-sample t test against the hypothesis that the inhibition was 0%.
Overage percentage of control growth across four replicates at the intraperitoneal implantation site.
Chemigenomic Profiling in Yeast Suggests a Mode of Action for C1311
Given the wide variety of potential targets reported for C1311 and the imidazoacridinone class [8] as well as the need to better characterize C1311's mechanism for potential rational drug combinations [23], we embarked on an unbiased, new strategy using budding yeast to begin to characterize C1311's mechanism. Recent advances in yeast genetics enable high-throughput screening of yeast ORF deletion strains for those that are sensitized to compounds or for synthetic lethal relationships between two deletion mutants [24]. Formally, the analyses are comparable; a compound may be effective because it inactivates a gene product and is therefore similar to deletion of the ORF. Such analyses have even yielded promising results for inference of conserved cellular pathways perturbed by drugs by comparing the pattern of strains sensitized to a drug to genome-wide genetic synthetic lethal relationships [25].
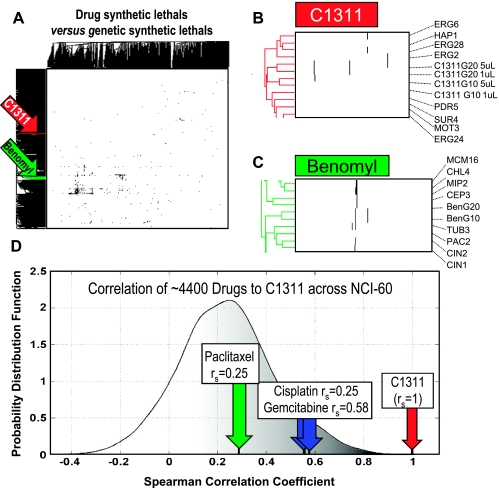
We grew the pooled yeast deletion mutants in the presence of two increasing concentrations of C1311 and profiled the strains remaining after 10 and 20 generations of growth, compared with control-treated pools, using Yeast TAG4.0 microarrays. For the lower concentration of C1311, we found that 27 and 32 yeast strains showed significantly reduced fitness when assayed after 10 and 20 generations. As expected, at higher a concentration, we found more strains displaying reduced fitness, 32 and 49, after 10 and 20 generations of growth, respectively. We also found 12 (62.5-fold over mean expected by random chance) and 15 (42.5-fold over mean expected by random chance) strains in common when comparing the low-and high-concentration data at 10 and 20 generations, respectively, both P « .0001. The highly nonrandom concordance in reduced fitness strains identified by separate growth replicates treated at two different drug concentrations illustrates the significant reproducibility of the results. The nonredundant union of the strains that displayed reduced fitness at both concentrations and generations yielded 91 strains (Table W3A).
Combining our C1311 data with the 25,540 synthetic lethal interactions identified in yeast (as of November 24, 2009 [17]) resulted in a binary matrix with 1521 rows of yeast query genes. To cluster these data in an interpretable way and allow comparison of the pattern of strains sensitized to C1311 to genome-wide synthetic lethal interactions, as has identified pathways targeted by drugs before [25], we used two-dimensional hierarchical clustering, as shown in Figure 2, A to C. The C1311 replicates (two concentrations after 10 or 20 generations of competitive growth) cluster immediately next to each other into a region of the clustergram enriched with ORFs involved with membrane lipid biogenesis (erg2 erg6 erg24 and erg28; Figure 2B). We interpreted this as suggesting a function for C1311 in perturbing cellular lipid biosynthesis or membrane function. Also consistent with this finding, using gene ontology to evaluate the 91 C1311 reduced fitness strains (Table W3A), we found a highly significant enrichment of gene ontology terms related to several lipid biogenesis pathways (Table 2A).
Figure 2.
Chemigenomic analysis of C1311 and paclitaxel between yeast and human cells. (A) Two-way hierarchical cluster of a 1521 x 2804 binary matrix where a black pixel represents either a synthetic lethal interaction between two yeast ORFs or reduced fitness between a drug and a yeast deletion mutant, or a white pixel represents all other two-ORF or drug-ORF pairs. We highlight the C1311 (red) and benomyl (green) clusters. (B) We see that C1311 is in a relatively sparse area of the matrix, and the four treatments with C1311 (two concentrations after 10 or 20 generations of competitive growth) cluster immediately next to each other and among several deletion strains involved with membrane lipid biogenesis, implicating this pathway in the function of C1311. (C) Enlarged inset view of the benomyl cluster from (A) showing benomyl treatments for 10 and 20 generations and neighboring deletion strains, enriched for microtubule and spindle components, as would be expected for this microtubule poison. (D) IC50 patterns across 60 cell lines for ∼4400 drugs from the 60 cell lines of NCI-60 screen [20] were correlated to that of C1311 and the probability distribution function of the coefficients was plotted. Paclitaxel, cisplatin, and gemcitabine exhibited the indicated correlation coefficients.
Table 2.
Gene Ontology Term Enrichment among C1311-(A) and Benomyl-(B) Sensitized Yeast Strains.
| GOID* | GO_Term* | Cluster Frequency | Background Frequency | P† |
| (A) | ||||
| 6629 | Lipid metabolic process | 19/90 genes, 21.1% | 259/7166 background, 3.6% | 1.17E-07 |
| 44255 | Cellular lipid metabolic process | 18/90 genes, 20.0% | 254/7166 background, 3.5% | 6.51E-07 |
| 8610 | Lipid biosynthetic process | 12/90 genes, 13.3% | 140/7166 background, 2.0% | 5.66E-05 |
| 9987 | Cellular process | 81/90 genes, 90.0% | 4819/7166 background, 67.2% | .00015 |
| 16126 | Sterol biosynthetic process | 6/90 genes, 6.7% | 33/7166 background, 0.5% | .00111 |
| 6694 | Steroid biosynthetic process | 6/90 genes, 6.7% | 33/7166 background, 0.5% | .00111 |
| 9058 | Biosynthetic process | 46/90 genes, 51.1% | 2011/7166 background, 28.1% | .00112 |
| 44249 | Cellular biosynthetic process | 45/90 genes, 50.0% | 1994/7166 background, 27.8% | .00239 |
| 16129 | Phytosteroid biosynthetic process | 5/90 genes, 5.6% | 26/7166 background, 0.4% | .0059 |
| 6696 | Ergosterol biosynthetic process | 5/90 genes, 5.6% | 26/7166 background, 0.4% | .0059 |
| 16125 | Sterol metabolic process | 6/90 genes, 6.7% | 46/7166 background, 0.6% | .0082 |
| 6631 | Fatty acid metabolic process | 6/90 genes, 6.7% | 46/7166 background, 0.6% | .0082 |
| 8202 | Steroid metabolic process | 6/90 genes, 6.7% | 46/7166 background, 0.6% | .0082 |
| 16128 | Phytosteroid metabolic process | 5/90 genes, 5.6% | 28/7166 background, 0.4% | .00864 |
| 8204 | Ergosterol metabolic process | 5/90 genes, 5.6% | 28/7166 background, 0.4% | .00864 |
| (B) | ||||
| 43486 | Histone exchange | 5/33 genes, 15.2% | 10/7166 genes, 0.1% | 4.52E-08 |
| 7021 | Tubulin complex assembly | 4/33 genes, 12.1% | 5/7166 genes, 0.1% | 2.24E-07 |
| 43044 | ATP-dependent chromatin remodeling | 5/33 genes, 15.2% | 29/7166 genes, 0.4% | 2.00E-05 |
| 7023 | Post-chaperonin tubulin folding pathway | 3/33 genes, 9.1% | 5/7166 genes, 0.1% | .00011 |
| 34728 | Nucleosome organization | 5/33 genes, 15.2% | 51/7166 genes, 0.7% | .00037 |
| 43933 | Macromolecular complex subunit organization | 11/33 genes, 33.3% | 470/7166 genes, 6.6% | .00054 |
| 34621 | Cellular macromolecular complex subunit organization | 10/33 genes, 30.3% | 392/7166 genes, 5.5% | .00077 |
| 6338 | Chromatin remodeling | 5/33 genes, 15.2% | 76/7166 genes, 1.1% | .00268 |
| 6457 | Protein folding | 5/33 genes, 15.2% | 86/7166 genes, 1.2% | .00488 |
GOID and GO Terms from the Gene Ontology Consortium, www.geneontology.org, identified by GO::TermFinder.
Bonneferoni-corrected P value for enrichment of indicated term among yeast ORF knockout strains identified as exhibiting reduced fitness on growth with C1311 or benomyl (cluster frequency) to the background frequency of such GO terms in the genome. All GOIDs presented were associated with an FDR approximating 0%.
Chemigenomic Profiling in Yeast Identifies Known Taxane Targets
New anticancer agents are tried first in the setting of primary treatment failure, meaning a potential future trial would use C1311 alone or a C1311-based combination regime after failure of GC [26]. Paclitaxel, a taxane, has shown substantial activity both alone and in combination therapies for bladder cancer [27] and is the leading second-line agent in practice today. Hence, a doublet combination of C1311 with paclitaxel would seem appealing.
Evaluation of paclitaxel in the chemigenomic assay would provide two significant insights. First, it would validate our chemigenomic approach because the molecular target of taxanes is known, supporting the premise that deletion strains sensitized to C1311 (Table W3A) identify its true mode of action. Second, if comparison of such unbiased evaluation of taxanes mode of action with that of C1311 reveals little overlap, such information would support combination treatment with these two drugs [23]. Here we use benomyl, an antitubulin drug that inhibits β-tubulin-like paclitaxel because the latter does not bind yeast β-tubulin because of the slight differences in the sequences of the proteins between yeast and humans [28].
Using a sublethal concentration of benomyl to treat pooled yeast deletion mutants and assaying by the same microarray platform after 10 or 20 generations of competitive growth, we found 32 and 16 significantly reduced strains (a union resulting in 34 strains; Table W3B). These data cluster immediately beside each other in a region of the clustergram that is highly enriched in genes that function in the mitotic spindle and immediately beside the tub3 tubulin deletion mutant (Figure 2C). These findings provide “proof-of-principle” because the pattern of synthetic sensitivities caused by inhibiting microtubule function with benomyl is most similar to inactivating tubulin with through deletion mutation, confirming that our approach may define modes of action of drugs. Also supporting this finding, when we used GO::TermFinder, the 34 benomyl-sensitized strains showed significant enrichment of terms relating to tubulin complex formation, among others (Table 2B).
Nonoverlapping Pathways and Sensitivities for C1311 and the Taxane Benomyl
To examine whether the strains identified as synthetic lethal to C1311 and benomyl (Table W3) significantly overlapped, we used the χ2 test, finding no significant overlap (two strains, P = .14, Yates corrected). Given that the strains that showed reduced fitness to the drugs were essentially mutually exclusive, these findings are consistent with C1311 and benomyl having distinct modes of action in yeast. However, to provide additional support for the strategy of combining imidazoacridinones with taxanes, we availed ourselves to published data encompassing ∼4400 drugs tested across the aforementioned NCI-60 cell line panel [20]. Such data afford the opportunity to test correlation of each drug's pattern of IC50 values across the 60 cell lines to that of C1311, allowing objective comparisons of patterns of each drug to C1311 but also relative comparisons among a large number of diverse bioactive compounds. Consistent with their targeting disparate cellular pathways, we found that paclitaxel was nonsignificantly correlated to C1311 at a level of rs = 0.25 (P = .11), essentially indistinguishable from the average correlation of rs = 0.24 (P = .27) across all drugs. Interestingly, even the standard-of-care, GC doublet drugs, cisplatin, and gemcitabine were more significantly correlated to C1311 than paclitaxel (rs = 0.56, P < .0001, rs = 0.58, P < .0001, respectively). Figure 2D shows these findings graphically, plotted against the ranked distribution of correlations of the ∼4400 drugs. Taken together, these findings from yeast and human cells suggested preclinical evaluation of a paclitaxel C1311 doublet therapy, which we undertook below.
In Vivo Evaluation of Imidazoacridinone-Taxane Combination Therapy in Human Bladder Cancer
For C1311 or NSC-637993 plus paclitaxel doublet HFA experiments, we used our previously reported in vitro IC50 data for paclitaxel in the BLA-40 panel [15] to select cell lines evincing several informative combinations of paclitaxel versus C1311 sensitivities as indicated in Table 3. We used a cell line with low IC50 values to both the imidazoacridinones and paclitaxel (UMUC6), two cell lines with intermediate IC50 values to both (T24 and 5637), and KK47 that had high IC50 values to both drug classes. Animals were treated by intraperitoneal injection four times with C1311 or NSC (20 mg/kg) plus paclitaxel (15 mg/kg) and sacrificed at 96 hours. Comparing untreated and doubly treated animals, we found that combinations were effective against all cell lines (all P < .01), including KK47, which was resistant to imidazoacridinone monotherapy (Table 3), with similar findings for combinations with NSC-637993 and paclitaxel (Table W2B). These findings suggest that despite the significant differences between in vitro screening and quantitative evaluation of the pharmacologic capacity of the drugs to reach subcutaneous and intraperitoneal compartments in vivo, C1311 (and NSC) remain highly effective, whereas addition of paclitaxel expands the spectrum of cell lines inhibited significantly by treatment.
Table 3.
HFA Results for C1311 and Paclitaxel.
| Tx | Cell Line | C1311 Status* | Log10 IC50 | Paclitaxel Status* | Log10 IC50 | SQ† | P‡ | IP§ | P‡ | Overall | Overall P‡ |
| C1311 + Paclitaxel | UMUC6 | Sensitive | -6.92 | Sensitive | -8.99 | 66.1 | .0003 | 51.2 | <.0001 | 58.7 | <.0001 |
| HTB9 | Intermediate | -5.64 | Sensitive | -8.93 | 40.5 | .065 | 74.0 | .0024 | 57.2 | .0047 | |
| T24 | Intermediate | -5.66 | Intermediate | -7.36 | 76.9 | .002 | 55.7 | <.0001 | 66.3 | <.0001 | |
| KK47 | Resistant | -4.84 | Resistant | >-7 | 70.9 | <.0001 | 59.4 | .0002 | 65.1 | <.0001 |
Relative responsiveness to C1311 or paclitaxel of indicated cell line. Of cell lines adaptable to the hollow fiber assay, four cell types of varying in vitro combinations of sensitivities to both drugs were selected for validation in vivo. We have reported in vitro sensitivities to paclitaxel across the BLA-40 panel before [15].
Average percentage of control growth across four replicates at the subcutaneous implantation site.
Two-tailed P value for single-sample t test against the hypothesis that the inhibition was 0%.
Average percentage of control growth across four replicates at the intraperitoneal implantation site.
Development of a Predictive Biomarker Model of C1311 Sensitivity
Taken together, the promising in vitro and in vivo results for C1311 suggest the possibility of testing it in a future clinical trial, a setting where significant efficiencies may achieved by biomarker-guarded selection of patients most likely to respond to therapy [29]. In particular, if gene expression signatures suggestive of sensitivity to C1311 were present in patients who did not respond to cisplatin-based therapy, such data would further support its evaluation. We examined this question using the COXEN algorithm, which develops, based on drug sensitivity and gene expression profiling in cell lines, gene expression model (GEM) predictors of drug response in patients [4].
To derive a gene expression signature of C1311 sensitivity, we began by selecting candidate sensitivity biomarkers by rank-based correlation (rs = 0.4) of C1311 IC50 values across the NCI-60 panel, finding that 219/22283 Affymetrix HG-U133A microarray probes meet this criterion (false discovery rate = 0.1 by random permutation testing, for probe information and correlation coefficients, see Table W4). Evaluation of the potential functional associations of these 219 probe sets using the Ingenuity Pathway Analysis program identified the glycerophospholipid metabolism pathway as the most significantly enriched, supporting in cancer cells our observations associating C1311 with lipid biogenesis in budding yeast. Linoleic and arachidonic acid metabolic pathways were also high scoring pathways (Table 4).
Table 4.
Canonical Pathways Enriched in C1311-Correlated Microarray Probes.
| Ingenuity Canonical Pathways* | P† | Ratio | Molecules |
| Glycerophospholipid metabolism | .004 | 3.11E-02 | PPAP2B, PLA2R1, CHKA, AGPAT1, PGS1, PCYT1A |
| N-glycan biosynthesis | .028 | 3.23E-02 | B4GALT4, ST6GAL1, MAN1C1 |
| Riboflavin metabolism | .028 | 3.64E-02 | ACP5, ACP1 |
| Arachidonic acid metabolism | .045 | 1.76E-02 | CYP2F1, CYP1A1, PLA2R1, PLOD1 |
| Linoleic acid metabolism | .048 | 2.42E-02 | CYP2F1, CYP1A1, PLA2R1 |
| Sphingolipid metabolism | .059 | 2.68E-02 | NAGA, PPAP2B, ASAH1 |
| AMPK signaling | .100 | 2.41E-02 | MTOR, ACACB, STRADA, PPM1D |
| Glycerolipid metabolism | .104 | 1.92E-02 | NAGA, PPAP2B, AGPAT1 |
| EIF2 signaling | .107 | 3E-02 | EIF2C2, EIF2AK2, GSK3B |
Ingenuity Pathway Analysis (IPA), Version 8, www.ingenuity.com.
P values from IPA are Benjamini-Hochberg corrected.
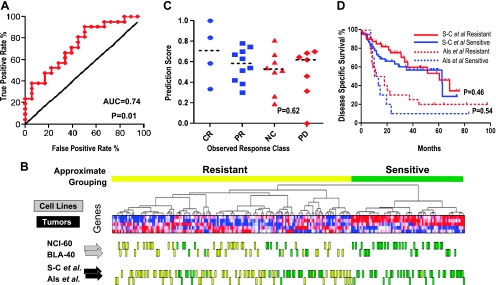
We then applied a critical aspect of the COXEN algorithm to uncover which of the 219 probe sets were concordantly expressed across three data sets (NCI-60, BLA-40, and a published bladder tumor data set [30]) and then derive a GEM predicting C1311 sensitivity from them. The human tumor data set was included so that the model could be applied on human tumor data sets, as we have reported before [6]. We systematically examined subsets of the 219 C1311-associated probes that maintained concordant expression between the three data sets as described in Supplementary Methods 1, for performance in predicting sensitivity of BLA-40 cells based on the similarity of their gene expression to the NCI-60 cells used for training. The maximally performing subset of five probes (Table W4) exhibited highly concordant expression between all three of the aforementioned data sets and was implemented in a weighted k nearest-neighbor (weighted kNN) classifier in our final GEM [31]. This strategy assigned a prediction for each BLA-40 test sample, based on the correlation of it its expression of the five probes, to sensitive and resistant groups of NCI-60 cells. The GEM exhibited significant ability to predict the sensitivity of the BLA-40 from the NCI-60 (P = .01; Figure 3A). For full details on the development of GEM, please see Supplementary Methods 1.
Figure 3.
Prediction of C1311 sensitivity between cell line panels and in human tumors. (A) COXEN analysis was used to develop a set of probe sets associated with sensitivity to C1311 in the NCI-60 cell line panel and concordantly expressed between the NCI-60 panel, the BLA-40 panel, and Sanchez-Carbayo et al. tumor gene expression data sets. Then, a nearest neighbor-based classification approach used to classify the BLA-40 cell line panel based on the NCI-60 panel, and the ROC curve was plotted for the classes assigned (sensitive or resistant) to test its ability to discriminate (area under the curve = 0.74, 95% CI = 0.59-0.90, P = .01). (B) Clustering of multiple data sets by C1311 sensitivity genes. A two-dimensional hierarchical cluster of NCI-60, BLA-40, Sanchez-Carbayo et al., and Als et al. data sets across 28 three-way concordant probe sets. Individual NCI-60 (actual) and BLA-40 (predicted) cells are indicated by boxes, showing resistance in yellow and sensitivity in green. Individual Sanchez-Carbayo et al. and Als et al. (both also predicted) tumor data sets are also indicated as for the BLA-40 cells. The clustergram illustrates how the COXEN methodology may select concordant biomarkers between platforms such that such gene expression patterns allow visualization or computational prediction of interpretable relationships between diverse biologic systems. (C) C1311 prediction values were dot-scatter plotted for response classes from the Als et al. data set, including CR (complete responder), PR (partial responder), NC (no change), and PD (progressive disease), finding no significant difference by nonparametric analysis of variance. (D) Kaplan-Meier analysis of survival by C1311 prediction indicates no systematic association between C1311 prediction class and survival in either study.
Evaluation of the C1311 GEM in Patients Undergoing Platinum-Based Chemotherapy
To test whether the GEM-identified signatures of sensitivity among patients showed resistance to cisplatin-based therapy, we tested it on the microarray data for a cohort of 30 patients, published by Als et al. [32], where the response to standard cisplatin-based chemotherapy was known. We also used it to evaluate another reported microarray study by Sanchez-Carbayo et al. [30] to examine the association of the GEM's predictions with other clinicopathologic characteristics. Both studies profiled histologically verified, fresh-frozen primary tumor tissues (biopsies and surgical resection specimens, respectively) on the Affymetrix HG-U133A platform. Figure 3B demonstrates through hierarchical clustering how the two cell lines and two human tumor data sets are capable of being clustered together in an interpretable fashion across the five COXEN-selected C1311 sensitivity GEM genes.
Using the weighted kNN classifier to classify the human tumors, we found in the chemotherapy study of Als et al. that distributions of our C1311 prediction scores showed no differences among the study's observed chemotherapy response groups (P = .62; Figure 3C), suggesting that predictions were not reflective of general drug resistance and supportive of the idea that candidates for C1311 treatment exist in non-responders to standard-of-care drugs. In addition, in both studies of Sanchez-Carbayo et al. and Als et al., we observed no difference in C1311 sensitivity prediction scores based on patient age, sex, tumor grade, or stage (data not shown). Furthermore, we did not observe significant associations between our C1311 predictions and survival in either data set (P = .46 and P = .54, respectively; Figure 3D). These findings again suggest that the C1311 predictions are also not associated with general phenotypes, like aggressiveness of tumors, and are independent of traditional pathologic factors and outcome, an important requirement for molecular assays to have clinical utility [6].
Discussion
Efficient drug development relies on the identification of candidate compounds, their preclinical validation in model systems, and translation in clinical trials. The availability of data from high-throughput technologies such as drug screens on cell panels [7] or publicly available gene expression profiling [33] provides building blocks that can be synthesized with informatic tools such as COXEN to provide an integrated pipeline for drug development, an example of which we report herein. We previously reported the discovery of NSC-637993 as a promising candidate compound for bladder cancer through the COXEN algorithm; the first imidazoacridinone class drug to be so evaluated for bladder cancer [5]. Among the top hits was a related imidazoacridinone, C1311, with favorable activity, toxicity, and tolerability profiles [13,14,34]. Not surprisingly, we observed a correlation of C1311 to NSC-637993 responsiveness, as well as a similar range of IC50 values on bladder cell lines. Interestingly, our chemigenomic screen using budding yeast suggested that C1311's mechanism may involve lipid biosynthesis pathways, a novel observation adding to the large number of potential targets postulated for the drug in recent reports [9,11,35–37]. This unexpected finding was nonetheless supported in mammalian cells by our observed enrichment of probes for genes involved in glycerophospholipid metabolism among those correlated to C1311 IC50 values across the NCI-60 cell line panel. Our group is currently using these data to attempt to identify the target and sensitizing agents for C1311.
The observation of essentially mutually exclusive patterns of synthetic lethality between benomyl and C1311 provided a testable therapeutic combination because of the similarity between benomyl and the approved chemotherapeutic agent, paclitaxel [28]. Paclitaxel is especially useful because it has complementary toxicities to those of C1311 and has been used as monotherapy [15] in patients with advanced bladder cancer that have failed platinum agents [3]. Supporting our observations of different classes of yeast knockouts conferring sensitivity to the two drugs, correlation of sensitivity patterns of C1311 across the NCI-60 cell lines to 4463 drugs [20] found that C1311 and paclitaxel were not correlated and even less correlated than C1311 to cisplatin or gemcitabine. Also supportive of this concept is a previous report of activity of C1311 in advanced breast cancer failures that included taxane failures [37]. We performed an in vivo evaluation for both C1311 and NSC-637993 using the National Institutes of Health/National Cancer Institute-developed HFA and found that both agents were effective alone in most bladder cancer cells. Importantly, combining these with paclitaxel in cells found to be resistant to imidazoacridinone monotherapy, such as KK47, led to significant inhibition. Taken together, these data suggest that clinical evaluation of C1311 with or without paclitaxel in the setting of cisplatin-based treatment failures is warranted.
To optimize patient selection for bladder cancer clinical trials with C1311, a biomarker for sensitivity to this drug is needed. In particular, retrospective examination of such a biomarker or prediction model on gene expression data from patients who have already been treated with platinum regimens would provide an indication whether there is cross resistance to C1311. Because COXEN-based classifiers have been shown to be predictive of outcome in nearly 500 patients from nine clinical trials [5,6], we used this technology to develop a GEM predicting response to C1311. We found that COXEN predictions did not differ significantly based on patient age, sex, tumor grade, or stage in two different data sets of patients [30,32]. Making predictions on the study of Als et al., which includes standard cisplatin-based therapy response and survival outcome data, we found that predictions did not differ significantly between patients evincing complete response, partial response, no change, or progressive disease. An important limitation of these findings is that, without data from an actual trial, it is not possible to assign a cutoff value for prediction scores that definitively identifies a responder or nonresponder to C1311. However, these findings do suggest that our C1311 predictions were not simply an index of tumor aggressivity or general drug resistance. Taken together, they provide the rational framework for developing a future (biomarker selected or correlated) clinical trial of C1311 in the clinical setting of cisplatin-based treatment failures.
In summary, we demonstrate that combining COXEN and yeast chemigenomics allows formulation of rational drug combinations of novel with established agents. Specifically, given the favorable characteristics of C1311, clinical evaluation of this agent alone or in combination with paclitaxel, for patients with metastatic bladder cancer that have failed first-line platinum therapy seems indicated.
Supplementary Methods
Hollow Fiber Assay
National Institutes of Health and University of Virginia ACUC guidelines were strictly observed. The National Cancer Institute HFA, developed by Hollingshead et al. [1] was performed as described to evaluate the in vivo activity of these two imidazoacridinone drugs and in combination with other chemotherapeutics in two physiologic compartments of the mouse, subcutaneous (SC) and intraperitoneal (IP). We used an in vitro control incubation to verify cell viability, sterility, and drug activity. Treated animals were compared to untreated controls with compounds administered daily on days 1 to 4 by IP injection. The compounds were administered once daily on day 1 to 4 by IP injection. Individual mouse body weights were recorded daily, and treatments would have been discontinued if an individual mouse body weight decreased ≥3 g or if other signs of toxicity/distress were evident, which did not occur. Imidazoacridinones were given at 20 mg/kg daily x 4, whereas paclitaxel was added at 15 mg/kg daily x 4. All mice were sacrificed on day 5, fibers were removed, and viable cell mass was quantified by the “stable end point” MTT dye conversion assay. Values presented are averages across four treated mice.
Analysis of Yeast TAG Array and Synthetic Lethal Data
The Affymetrix Yeast TAG4.0 array data were analyzed using software developed by the Giaever laboratory (http://chemogenomics.stanford.edu/supplements/04tag/), which quantile normalizes up and down tag intensities separately, applies quality filters, estimates and subtracts background from the treatment and control intensities, calculates log2 ratio of treatment over control enrichment, and identifies yeast strains that display significantly reduced fitness in a group of drug-treated replicates compared to control replicates. The method is detailed in the software documentation and companion publication [2]. The Yeast TAG4.0 drug data were converted to binary data as discussed in the Results section. Similarly, we converted the synthetic lethal data to binary data by assigning a 1 to synthetic lethal query-target pairs and 0 to all other ORF pairs. Combining the drug and synthetic lethal data resulted in a binary matrix with 1521 rows of yeast query genes and six drug treatments (four C1311 and two benomyl) and 2804 columns of yeast target genes. We note that the original size of each drug binary vector was 6431 (i.e., the number of yeast deletion strains interrogated on the array) and reduced to 2804 after being projected onto the set of available yeast target genes. We generated Figure 2, A to C, by applying two-way hierarchical clustering (i.e., cluster both rows and columns) with a cosine distance metric to this 1521 x 2804 binary matrix using the clustergram function in MATLAB Version 7.9.0 (The Mathworks, Natick, MA). Lists of yeast strains with reduced fitness for benomyl and C1311 were examined for statistically significant enrichment of gene ontology terms by GO::TermFinder [19] using default settings.
Development and Testing of a GEM Predictive of C1311 Sensitivity
Data sets Used:
All data sets used are Affymetrix HG-U133A and publicly available.
The BLA-40 data set is available as GSE5845 at NCBI GEO (www.ncbi.nlm.nih.gov/geo).
The NCI-60 cell line data set is GSE5720, also on NCBI GEO.
The Sanchez-Carbayo et al. data set is available as supplementary data online with the referenced manuscript http://jco.ascopubs. org/cgi/content/full/24/5/778 [3]
In all cases, authors' processed data were downloaded and used, log-transformed (if not already) and z-scored for standardization for inter-data set comparison.
Biomarker Discovery
The Spearman rank correlations of expression of each of the 22283 Affymetrix probes on the U133A platform across the NCI-60 cell line panels to the C1311 IC50 values for these cells were first calculated in Matlab (The Mathworks). To identify an appropriate cutoff point for these correlation values, we conducted random permutation testing to estimate the false discovery rate [5,6] at various cutoff values. We carried out 100 random permutation tests and recorded how many probes exhibited correlation values greater than the various cutoff points tested. Specifically, we examined absolute correlation values from 0.0 to 0.5 by 0.01 intervals, as shown:
By comparing the number of probes identified on average from the random permutation tests versus the number identified in the actual data across the range of absolute correlation values mentioned, we chose to accept a 10% false discovery rate rate, which represented a threshold correlation coefficient (ρ) of 0.40. These methods identified 219 probes that exhibited a significant correlation to C1311.
Development and Testing of the GEM
To help further refine these 219 probes and uncover subsets maintaining concordant expression between the two cell lines and human tumor data sets, we next used an application of the cross-correlation step of the COXEN algorithm, however, adapted to multiple data sets. Three cross-correlation comparisons were made, namely 1) NCI-60 to BLA-40, 2) NCI-60 to S-C et al., and 3) BLA-40 to S-C et al. To select three-way concordant probes, we systematically examined a range of cross-correlation coefficient cutoff values, specifically 0.00 to 0.50 by 0.01 intervals. At each cutoff value, we recorded the set of genes exhibiting greater than threshold cross-correlation levels across all three comparisons. For each set of concordant probes, we then conducted the following procedures to assess their predictive performance in the BLA-40:
1) Selection of C1311-sensitive and -resistant NCI-60 training sets based on hierarchical clustering of cell lines across expression of C1311 sensitivity probes. Using a semisupervised approach, we discretized the continuous IC50 values in the NCI-60 data set into groupings of “sensitive” and “resistant” cells to provide categorical labels for the training data used in the development of a classifier. This was done by clustering the NCI-60 cell lines based on the expression of a given set of concordant C1311 sensitivity probes (described above) in an agglomerative hierarchical tree (e.g., see A in the following graph). Spearman correlation was used as the distance metric and the unweighted pair group method with arithmetic mean as the linkage function to construct the agglomerative hierarchical tree. Using the cluster function of Matlab, the hierarchical tree was used to divide the NCI-60 cells into two groups by drawing a horizontal cut through the tree such that only two clusters remain. This cluster grouping exhibited highly significant differences in NCI-60 IC50 values, as expected given our semisupervised approach (e.g., see B in the following graph). Examination of the central tendencies of the IC50 values from this grouping allowed us to appropriately label which cluster of the grouping represented sensitive (low IC50) versus resistant (high IC50) cell lines and use them for training data for the classifier.
2) Evaluation of significance of predictions on the BLA-40. With these two groups of NCI-60 cells in hand, we next predicted which class (sensitive or resistant) each of the BLA-40 cell lines was most like. To do this, the NCI-60 and BLA-40 gene expression data were first log transformed and then z-score standardized to enable intercohort comparisons in correlation space. A weighted k nearest-neighbor (weighted kNN) algorithm [7,8] was used as the classifier, with the NCI-60 groupings from 1) serving as the training data and predictions made for each cell line in the BLA-40 data set. A Spearman correlation distance metric was used to weight the influence that training samples had on the prediction of test samples, and the prediction of each test sample was based only on positively correlated training samples. The resulting predictions on the BLA-40 data set thus represent a binary classification of “sensitive” or “resistant,” and we tested for difference in distributions of observed IC50 values for C1311 between predicted sensitive and resistant classes by nonparametric t tests.
Following these two procedures systematically for each of the 0.00 to 0.50 cross-correlation cutoffs as outlined above, we identified a five-gene model that best allowed us to differentiate resistant and sensitive BLA-40 cell lines based on the expression patterns in the NCI-60 data set (cross-correlation cutoff, or COXEN coefficient = 0.25). Importantly, the weighted kNN prediction algorithm used (Matlab Code available on request) also provides a posterior probability estimate for the classification call, a technique that has been reported before [9]. The program then uses a threshold of greater than or less than 0.5 as the threshold for binary classification as sensitive or resistant. We have termed this score the “C1311 sensitivity score,” which ranges from 0 for sensitive to 1 for resistant, and it was the distributions of these scores for individual patient tumors that were tested among the different clinicopathologic characteristics in the data sets of Sanchez-Carbayo et al. and Als et al.
Significance of Best Classifier Performance by Random Permutation Testing
We next determined the exact statistical significance of these findings through permutation testing. To prove that the results we have generated thus far cannot be ascribed to “overtraining” or random chance, we carefully carried out the identical procedures described before but with 219 randomly selected probes. We then repeated this random resampling test 500 times and recorded the P values for the best-performing models, precisely as was done in 2) with the 219 genes significantly associated to C1311 IC50 values in the NCI-60. This allowed us to estimate the exact P value that results similar to or better than those observed could be attributed to chance alone (P = .012), as shown in the following graph:
Acknowledgments
The authors thank all members of the Theodorescu Laboratory for helpful thoughts and discussions, as well as Paul Williams, from the UVA Department of Public Health Sciences for his helpful suggestions.
Footnotes
This work was supported by the National Institutes of Health grant CA075115. The National Institutes of Health had no role in study design, data collection, and analysis, decision to publish, or preparation of the article.
This article refers to supplementary materials, which are designated by Figure W1 and Tables W1 to W4 and are available online at www.neoplasia.com.
References
- 1.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 2.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng A-C, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher DJ, Milowsky MI, Bajorin DF. Advanced bladder cancer: status of first-line chemotherapy and the search for active agents in the second-line setting. Cancer. 2008;113:1284–1293. doi: 10.1002/cncr.23692. [DOI] [PubMed] [Google Scholar]
- 4.Smith SC, Baras AS, Lee JK, Theodorescu D. The COXEN principle: translating signatures of in vitro chemosensitivity into tools for clinical outcome prediction and drug discovery in cancer. Cancer Res. 2010;70:1753–1758. doi: 10.1158/0008-5472.CAN-09-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JK, Havaleshko DM, Cho H, Weinstein JN, Kaldjian EP, Karpovich J, Grimshaw A, Theodorescu D. A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc Natl Acad Sci USA. 2007;104:13086–13091. doi: 10.1073/pnas.0610292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams PD, Cheon S, Havaleshko DM, Jeong H, Cheng F, Theodorescu D, Lee JK. Concordant gene expression signatures predict clinical outcomes of cancer patients undergoing systemic therapy. Cancer Res. 2009;69:8302–8309. doi: 10.1158/0008-5472.CAN-09-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 8.Berger B, Marquardt H, Westendorf J. Pharmacological and toxicological aspects of new imidazoacridinone antitumor agents. Cancer Res. 1996;56:2094–2104. [PubMed] [Google Scholar]
- 9.Burger AM, Jenkins TC, Double JA, Bibby MC. Cellular uptake, cytotoxicity and DNA-binding studies of the novel imidazoacridinone antineoplastic agent C1311. Br J Cancer. 1999;81:367–375. doi: 10.1038/sj.bjc.6690702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skladanowski A, Plisov SY, Konopa J, Larsen AK. Inhibition of DNA topoisomerase II by imidazoacridinones, new antineoplastic agents with strong activity against solid tumors. Mol Pharmacol. 1996;49:772–780. [PubMed] [Google Scholar]
- 11.Chau M, Otake Y, Christensen JL, Fernandes DJ, Ajami AM. The imidazoacridinone, C-1311 (Symadex™): the first of a potent new class of FLT3 inhibitors. AACR Meeting Abstracts. 2006;2006:B35. [Google Scholar]
- 12.Otake Y, Chau M, Ajami A, Fernandes D. Mechanism of topoisomerase II inhibition by the imidazoacridinone, C-1311. AACR Meeting Abstracts. 2007;2007:4041. [Google Scholar]
- 13.Thomas A, Anthoney A, Ahmed S, Drouin M, Major A, Capizzi RL, Grieshaber C, Loadman P, Twelves C. Evaluation of the safety of C-1311 administered in a phase 1 dose-escalation trial as a 1-hour infusion once every 3 weeks in patients with advanced solid tumors. ASCO Meeting Abstracts. 2006;24:12005. [Google Scholar]
- 14.Thomas AL, Anthoney A, Scott E, Ahmed S, Lundberg AS, Major A, Capizzi RL, Twelves CJ. C-1311, a novel inhibitor of FLT3 and topoisomerase II: a phase 1 trial of a once every three week schedule in patients with advanced solid tumors. J Clin Oncol. 2008;26:2576. [Google Scholar]
- 15.Havaleshko DM, Cho H, Conaway M, Owens CR, Hampton G, Lee JK, Theodorescu D. Prediction of drug combination chemosensitivity in human bladder cancer. Mol Cancer Ther. 2007;6:578–586. doi: 10.1158/1535-7163.MCT-06-0497. [DOI] [PubMed] [Google Scholar]
- 16.Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, Grever MR. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995;57:131–141. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- 17.Pan X, Yuan DS, Ooi S-L, Wang X, Sookhai-Mahadeo S, Meluh P, Boeke JD. dSLAM analysis of genome-wide genetic interactions in Saccharomyces cerevisiae. Methods. 2007;41:206–221. doi: 10.1016/j.ymeth.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce SE, Fung EL, Jaramillo DF, Chu AM, Davis RW, Nislow C, Giaever G. A unique and universal molecular barcode array. Nat Methods. 2006;3:601–603. doi: 10.1038/nmeth905. [DOI] [PubMed] [Google Scholar]
- 19.Boyle EI, Weng S, Gollub J, Jin H, Botstein D, Cherry JM, Sherlock G. GO::TermFinder—open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics. 2004;20:3710–3715. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blower PE, Yang C, Fligner MA, Verducci JS, Yu L, Richman S, Weinstein JN. Pharmacogenomic analysis: correlating molecular substructure classes with microarray gene expression data. Pharmacogenomics J. 2002;2:259–271. doi: 10.1038/sj.tpj.6500116. [DOI] [PubMed] [Google Scholar]
- 21.Shankavaram UT, Reinhold WC, Nishizuka S, Major S, Morita D, Chary KK, Reimers MA, Scherf U, Kahn A, Dolginow D, et al. Transcript and protein expression profiles of the NCI-60 cancer cell panel: an integromic micro-array study. Mol Cancer Ther. 2007;6:820–832. doi: 10.1158/1535-7163.MCT-06-0650. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein JN, Myers TG, O'Connor PM, Friend SH, Fornace AJ, Jr, Kohn KW, Fojo T, Bates SE, Rubinstein LV, Anderson NL, et al. An information-intensive approach to the molecular pharmacology of cancer. Science. 1997;275:343–349. doi: 10.1126/science.275.5298.343. [DOI] [PubMed] [Google Scholar]
- 23.Shah MA, Schwartz GK. Cell cycle-mediated drug resistance: an emerging concept in cancer therapy. Clin Cancer Res. 2001;7:2168–2181. [PubMed] [Google Scholar]
- 24.Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 25.Parsons AB, Brost RL, Ding H, Li Z, Zhang C, Sheikh B, Brown GW, Kane PM, Hughes TR, Boone C. Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nat Biotechnol. 2004;22:62–69. doi: 10.1038/nbt919. [DOI] [PubMed] [Google Scholar]
- 26.von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 27.Galsky MD. The role of taxanes in the management of bladder cancer. Oncologist. 2005;10:792–798. doi: 10.1634/theoncologist.10-10-792. [DOI] [PubMed] [Google Scholar]
- 28.Entwistle RA, Winefield RD, Foland TB, Lushington GH, Himes RH. The paclitaxel site in tubulin probed by site-directed mutagenesis of Saccharomyces cerevisiae β-tubulin. FEBS Lett. 2008;582:2467–2470. doi: 10.1016/j.febslet.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon R. The use of genomics in clinical trial design. Clin Cancer Res. 2008;14:5984–5993. doi: 10.1158/1078-0432.CCR-07-4531. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 31.Atiya AF. Estimating the posterior probabilities using the K-nearest neighbor rule. Neural Comput. 2005;17:731–740. doi: 10.1162/0899766053019971. [DOI] [PubMed] [Google Scholar]
- 32.Als AB, Dyrskjot L, von der Maase H, Koed K, Mansilla F, Toldbod HE, Jensen JL, Ulhoi BP, Sengelov L, Jensen KM, et al. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13:4407–4414. doi: 10.1158/1078-0432.CCR-07-0109. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isambert N, Campone M, Bourbouloux E, Drouin M, Major A, Loadman P, Capizzi R, Grieshaber C, Fumoleau P. Evaluation of the safety of C-1311 administered in a phase I dose-escalation trial as a weekly infusion for 3 consecutive weeks in patients with advanced solid tumors. ASCO Meeting Abstracts. 2006;24:2069. doi: 10.1016/j.ejca.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Konopa JK, Koba M, Dyrcz A. Interstrand crosslinking of DNA by C-1311 (Symadex) and other imidazoacridinones. AACR Meeting Abstracts. 2005;2005:1382-a. [Google Scholar]
- 36.Nguyen HB, Duncan K, Fang X, Chau M, Ajami A, Small D. FLT3 inhibition of leukemia cells by C-1311 and its analogs. AACR Meeting Abstracts. 2008;2008:4753. [Google Scholar]
- 37.Capizzi RL, Roman LA, Tjulandin S, Smirnova I, Manikhas A, Paterson JS, Major A, Lundberg AS, Fumoleau P. Phase II trial of C1311, a novel inhibitor of topoisomerase II in advanced breast cancer. J Clin Oncol. 2008;26:1055. [Google Scholar]
References
- 1.Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, Grever MR. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995;57:131–141. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- 2.Pierce SE, Fung EL, Jaramillo DF, Chu AM, Davis RW, Nislow C, Giaever G. A unique and universal molecular barcode array. Nat Methods. 2006;3:601–603. doi: 10.1038/nmeth905. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 4.Als AB, Dyrskjot L, von der Maase H, Koed K, Mansilla F, Toldbod HE, Jensen JL, Ulhoi BP, Sengelov L, Jensen KM, et al. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13:4407–4414. doi: 10.1158/1078-0432.CCR-07-0109. [DOI] [PubMed] [Google Scholar]
- 5.Farcomeni A. A review of modern multiple hypothesis testing, with particular attention to the false discovery proportion. Stat Methods Med Res. 2008;17:347–388. doi: 10.1177/0962280206079046. [DOI] [PubMed] [Google Scholar]
- 6.Pawitan Y, Michiels S, Koscielny S, Gusnanto A, Ploner A. False discovery rate, sensitivity and sample size for microarray studies. Bioinformatics. 2005;21:3017–3024. doi: 10.1093/bioinformatics/bti448. [DOI] [PubMed] [Google Scholar]
- 7.Berrar D, Bradbury I, Dubitzky W. Instance-based concept learning from multiclass DNA microarray data. BMC Bioinformatics. 2006;7:73. doi: 10.1186/1471-2105-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horlings HM, van Laar RK, Kerst J-M, Helgason HH, Wesseling J, van der Hoeven JJM, Warmoes MO, Floore A, Witteveen A, Lahti-Domenici J, et al. Gene expression profiling to identify the histogenetic origin of metastatic adenocarcinomas of unknown primary. J Clin Oncol. 2008;26:4435–4441. doi: 10.1200/JCO.2007.14.6969. [DOI] [PubMed] [Google Scholar]
- 9.Atiya AF. Estimating the posterior probabilities using the K-nearest neighbor rule. Neural Comput. 2005;17:731–740. doi: 10.1162/0899766053019971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.