Abstract
Growing evidence suggests that altered function of the GABAergic system can contribute to the pathophysiology of depression. Many GABAergic effects are mediated via ionotropic GABAA receptors, which are functionally defined by their α subunit (α1–α6). Although it remains unknown which specific GABAA receptor population mediates depressive-like effects, we posit that α2-containing GABAA receptors, which are highly expressed in limbic regions, may underlie these behaviors. We hypothesized that genetic inactivation of α2-containing GABAA receptors would generate a depressive-like phenotype in mice. Male and female wild type, α2 heterozygous, and α2 homozygous knockout mice generated on the 129×1/SvJ background were examined in the novelty-suppressed feeding (NSF) test, the forced swim test (FST) and the tail suspension test (TST). Male α2 knockout mice took longer to eat in the NSF test and became immobile faster and remained immobile longer when challenged in the FST and the TST compared to wild types. In females significant genotypic differences were only observed in the FST. We conclude that GABAergic inhibition acting via α2-containing GABAA receptors has an antidepressant-like effect in vivo and that these receptors represent a specific molecular substrate that can regulate depressive-like states. α2-containing GABAA receptors may therefore represent a novel target for the development of more effective antidepressants.
Keywords: GABRA2, depression, novelty-suppressed feeding, tail suspension test, forced swim test, 129×1/SvJ mice
1. Introduction
Evidence from clinical and preclinical studies suggests a relationship between γ-aminobutyric acid (GABA) and depression (Brambilla et al., 2003). GABA levels in the plasma and the corticospinal fluid are reduced in patients with major depressive disorder (Sanacora and Saricicek, 2007) and corticospinal fluid GABA levels increased after administration of selective serotonin reuptake inhibitors (Bhagwagar, 2004). Administration of the benzodiazepines alprazolam and adinazolam elicits antidepressant responses similar to widely prescribed antidepressants in patients with major depressive disorder (Amsterdam et al., 1986; Petty et al., 1995). Even though clinically used benzodiazepines are non-subunit-selective allosteric modulators of GABAA receptors and are most commonly prescribed as anxiolytics, these findings suggest that GABAA receptors may also have a role in the treatment of depression.
Many GABAergic effects are mediated via ionotropic GABAA receptors, whose subunit composition determines the receptor's physiological and pharmacological characteristics. Preclinical work has shown that GABAA receptor γ2 heterozygous knockout mice exhibit a depressive-like phenotype compared to wild type mice (Earnheart et al., 2007; Shen et al., 2010) providing evidence for the involvement of GABAA receptors in depression. However, since the γ2 subunit is contained in about 90% of all GABAA receptors, it still remains unknown which GABAA receptor subtype, as defined by its α subunit, mediates these effects.
Here, we focus on α2-containing GABAA receptors since these receptors are highly expressed in limbic regions (Fritschy and Möhler, 1995) that are involved in emotional stimulus processing and are also implicated in the pathophysiology of depression (Kaplan et al., 1994). GABRA2, the gene encoding the GABAA receptor α2 subunit, has also been linked to other mental health disorders including post-traumatic stress disorder (Nelson et al., 2009), conduct disorder (Dick et al., 2006), alcohol and illicit drug dependence (Agrawal et al., 2006; Edenberg et al., 2004). α2-containing GABAA receptors have also been linked to the improvement of cognitive deficits in schizophrenia (Lewis et al., 2008). Additionally, this GABAA receptor subtype mediates the anxiolytic-like action of the benzodiazepine diazepam in mice (Löw et al., 2000). Given the high comorbidity between anxiety disorders and depression (Rapaport, 2001), it is likely that these two diseases share common neural structures and circuits. While strong evidence suggests that α2-containing GABAA receptors mediate anxiety-like behaviors (Löw et al., 2000), we posit that they may also regulate mood. Mice globally lacking the α2 subunit of the GABAA receptor were examined in preclinical tests modeling aspects of depressive-like symptomatology. We hypothesized that the genetic inactivation of this receptor would generate a depressive-like phenotype.
2. Materials and methods
2.1. Animals
Male and female wild type, α2 heterozygous (α2+/−), and α2 homozygous (α2−/−) knockout mice (Gabra2tm2.2Uru) were generated on the 129×1/SvJ background (origin: RCC Fuellinsdorf, Switzerland; the global α2 knockout was generated by excision of exon 5; the mutant allele was backcrossed for 12 generations before heterozygous breedings were established) (n=12–16/genotype). Subjects were group housed (3–4 mice/cage) in Super Mouse 750™ cages containing a LifeSpan™ Rodent Enrichment insert (Lab Products Inc., Seaford, DE, USA); these cages are covered by micro-isolator non-wire bar lids and can be maintained either on or off individual ventilation. Subjects were maintained on a reverse 12:12 h light-dark cycle (lights off 0900 h) with food (Purina Lab Diet 5P76, PMI Nutrition International, Brentwood, MO, USA) and water available ad libitum unless stated otherwise. Mice were ear tagged and genotyped by PCR analysis of tail biopsies at 4 weeks of age. Female estrous cycle was not assessed with vaginal smears. Experiments were approved by the McLean Hospital Institutional Animal Care and Use Committee and in accordance with the NIH Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
2.2. Behavioral tests
Subjects were tested sequentially in the novelty-suppressed feeding (NSF) test, the forced swim test (FST), and the tail suspension test (TST) during the dark phase of the daily cycle with one week separating each test. Locomotor activity in a familiar open field (OF) was assessed with separate groups of male wild type and α2−/− mice only (n=6/genotype).
2.2.1. Novelty-suppressed feeding test
Percent weight loss was determined by weighing subjects before and after a 24-hour food (but not water) deprivation period. Subjects were placed in a clean cage 1 h before being tested in a clear Plexiglas box (42 × 42 × 31 cm; 20 lux) lined with clean bedding and a food pellet placed in the center on an inverted petri dish. Latencies to bite and eat the food were recorded. Subjects were removed from the apparatus after they began eating, or a maximum latency of 6 min, and returned to their home cage where consumption of a pre-weighed food pellet was determined after 5 min.
2.2.2. Forced swim test
A clear Plexiglas cylinder (diameter, 20 cm) was filled with water (24–25°C) and illuminated by overhead room lighting (~100 lux). After a 6 min test session, mice were placed in a clean cage containing paper towels under a heat lamp until dry. Subject behavior was videotaped (Sony Handycam DCR-DVD108, Sony Electronics Inc., San Diego, CA, USA) and the latency to first immobility and time spent immobile in the first 2 and last 4 minutes of the test were scored manually. Immobility was defined as floating motionless or using movements only necessary to keep the head above water (Porsolt et al., 1978).
2.2.3. Tail suspension test
Mice were suspended for a 6 min test session by taping the tail to the edge of a table (height, 70 cm). Subjects were videotaped and the latency to first immobility and total time spent immobile were manually scored. Immobility was defined as the complete cessation of movement while suspended.
2.2.4. Familiar open field
Subjects were habituated to the testing arena (clear Plexiglas box, 42 × 42 × 31 cm) for 30 min and then, 24 hours later, were placed in the same arena (now familiar) for another 30 min. Total distance traveled (cm) was measured using the EthoVision XT (Noldus Information Technology, Netherlands) tracking system.
2.3. Statistical analysis
NSF, TST, and FST data were analyzed by one-way analysis of variance (ANOVA) followed by a Dunnett's post hoc t-test comparing α2+/− and α2−/− mice to wild types. Overall mean locomotor activity in the OF was analyzed by an independent t-test; the time course of activity in 5 min intervals across the 30 min test session was analyzed by a two-way ANOVA (genotype × time interval) with time as a within-subjects factor. Data are expressed as mean ± SEM. The significance level for all tests was set at p<0.05. Males and females were analyzed separately using the SPSS statistical software version 18 (SPSS Inc., Chicago, IL, USA).
3. Results
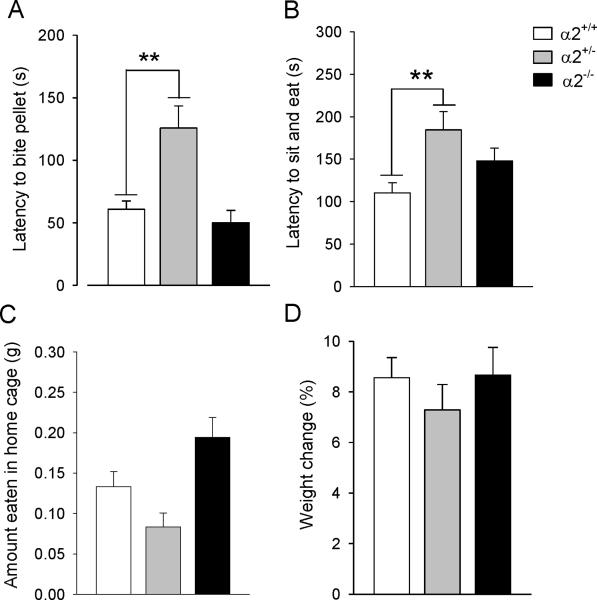
The most salient behavioral differences in the NSF test were observed in male α2+/− mice which took significantly longer to bite (F(2,38)=10.96, p=0.0001; Dunnett's post hoc, p<0.01) (Figure 1A) and to eat (F(2,38)=5.65, p=0.007; post hoc, p<0.01) (Figure 1B) the food pellet compared to wild type mice. While α2−/− mice ate the most food in the home cage (F(2,38)=6.60, p=0.03), total food consumption by α2+/− and α2−/− mice was not significantly different from wild types (p=0.108 and p=0.134, respectively) (Figure 1C). There were no significant genotypic differences in percent body weight loss after the 24-hour food deprivation (F(2,38)=0.66, p=0.53) (Figure 1D).
Figure 1.
Novelty-suppressed feeding test. (A) Latency to bite and (B) to sit and eat the food pellet. (C) Amount of food eaten in the home cage in 5 min and (D) percent weight loss after 24-hour food deprivation. α2+/− = heterozygous α2 knockout mice; α2−/− = homozygous α2 knockout mice.
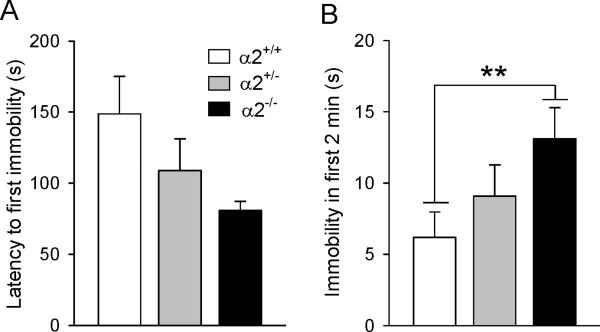
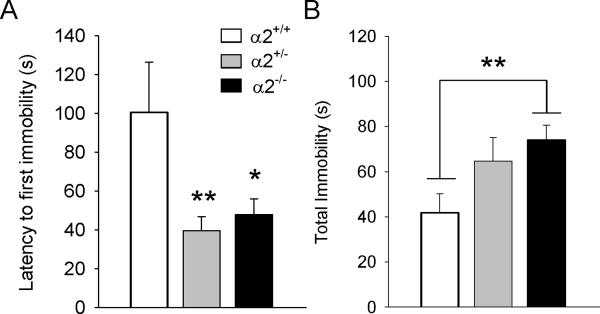
Male α2−/− mice most rapidly attained immobility in the FST (Figure 2A) and remained immobile twice as long as wild types in the first 2 min of the test session (F(2,35)=4.78, p=0.015; post hoc, p<0.01) (Figure 2B). No genotypic differences were detected for time spent immobile in the last 4 min of the test (data not shown). In the TST, both α2+/− and α2−/− males became immobile significantly faster than wild types (F(2,36)=6.83, p=0.003; post hoc, p<0.01 and p<0.05, respectively) (Figure 3A). While these same groups remained immobile longest during test (F(2,36)=3.52, p=0.04), only α2−/− mice were significantly different from wild types (post hoc p<0.01) (Figure 3B).
Figure 2.
Forced swim test. (A) Latency to become immobile and (B) immobility in the first two minutes of the test session. α2+/− = heterozygous α2 knockout mice; α2−/− = homozygous α2 knockout mice.
Figure 3.
Tail suspension test. (A) Latency to first immobility and (B) total immobility. * p ≤ 0.05; ** p ≤ 0.01; α2+/+ = wild type; α2+/− = heterozygous α2 knockout mice; α2−/− = homozygous α2 knockout mice.
Female mice were also examined in the same tests. While no genotypic differences were observed in females in the NSF test and the TST (Table 1) significant differences were observed in the FST (Table 2). Here a main effect of genotype was found for the latency to become immobile (F(2,38)=5.52, p=0.008) (Table 2); both the α2+/− (p<0.05) and the α2−/− (p<0.01) mice became immobile sooner than wild types. α2−/− also remained immobile longer in the first 2 minutes of the test (F(2,38)=7.332, p=0.02; post hoc p<0.001) (Table 2).
Table 1.
Female α2KO Mice in the Novelty-Suppressed Feeding Test
| Genotype | n | Latency to bite pellet (s) | Latency to sit and eat pellet (s) | Amount food eaten in home cage (g) | Weight loss (%) |
|---|---|---|---|---|---|
| α2+/+ | 16 | 150 ± 33 | 199 ± 26 | 0.16 ± 0.28 | 10 ± 0.7 |
| α2+/− | 12 | 136 ± 26 | 204 ± 28 | 0.14 ± 0.19 | 11 ± 0.4 |
| α2−/− | 12 | 132 ± 38 | 169 ± 35 | 0.13 ± 0.17 | 9.7 ± 0.4 |
No genotypic differences were observed for any of the measures recorded. α2+/+ = wild type; α2+/− = heterozygous α2 knockout mice; α2−/− = homozygous α2 knockout mice.
Table 2.
Female α2KO Mice in the Tail Suspension and Forced Swim Tests
| Tail Suspension Test | Forced Swim Test | ||||
|---|---|---|---|---|---|
| Genotype | n | Latency to first immobility (s) | Total immobility (s) | Latency to first immobility (s) | Immobility in first 2 min (s) |
| α2+/+ | 16 | 41 ± 5 | 116 ± 13 | 109 ± 12 | 6 ± 2 |
| α2+/− | 12 | 70 ± 27 | 78 ± 18 | 80 ± 4* | 10 ± 2 |
| α2−/− | 12 | 40 ± 6 | 107 ± 21 | 69 ± 6** | 16 ± 2** |
No genotypic differences were found in the tail suspension test for both measures. In the forced swim test, a main effect of genotype was found for the latency to become immobile.
p ≤ 0.05
p ≤ 0.01.
α2+/+ = wild type; α2+/− = heterozygous α2 knockout mice; α2−/− = homozygous α2 knockout mice.
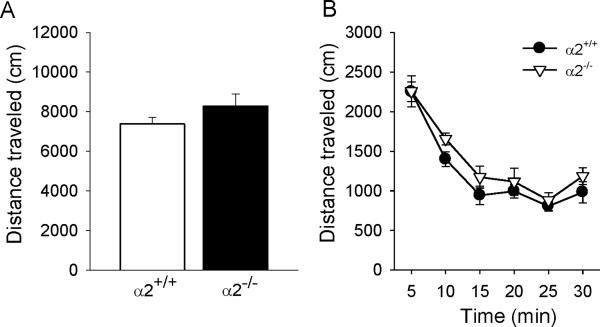
Wild type and α2−/− mice explored a familiar open field equally; there were no genotypic differences in the overall mean total distance traveled (t(10)=1.28, p=0.22) (Figure 4A) or in the pattern of activity across the 30 min test session (no significant main effect of genotype: F(1,10)=1.64, p=0.23) (Figure 4B). Activity was highest in the first 5 min of the test and decreased to a stable level by 15 min (significant main effect of time interval: F(5,50)=55.65, p<0.0001) (Figure 4B).
Figure 4.
Locomotor activity in a familiar open field. (A) Overall mean total distance traveled and (B) the time course of activity in 5 min intervals across the 30 min test session. α2+/+ = wild type; α2−/− = homozygous α2 knockout mice.
4. Discussion
GABAergic dysregulation has been linked to depression in preclinical and clinical studies (Brambilla et al., 2003; Earnheart et al., 2007; Shen et al., 2010; Petty, 1995; Sanacora and Saricicek, 2007; Sanacora et al., 2000). Using genetically-modified mice as a preclinical tool to examine components of depressive-like behavior, we show that α2-containing GABAA receptors represent a specific molecular substrate that can regulate depressive-like states. Our findings suggest that GABAergic inhibition acting via α2-containing GABAA receptors has an antidepressant-like effect in vivo and are important for emotional regulation.
α2−/− mice took longer to eat in a novel environment and exhibited greater immobility when challenged in inescapable situations (FST and TST) compared to wild types. These results suggest a profile of increased sensitivity to novelty and elevated behavioral despair in α2KO mice that was not confounded by baseline differences in locomotor activity. α2−/− mice also exhibited a profound pro-depressant profile in the TST and FST whereas α2+/− mice surprisingly had a more pronounced phenotype in the NSF test. Compensatory adaptations in the α2−/− mice combined with the specific characteristics of the NSF test may explain why heterozygous mice exhibited behavioral differences compared to wild types.
While a depressive-like phenotype was observed in genetically modified males in all three tests, this was not the case in females where a clear phenotype was only observed in the FST; it is possible that the chosen experimental conditions more effectively revealed genotypic differences in males than females. However, it is known that general food consumption and TST-related behaviors in female rodents are sensitive to hormonal fluctuations induced by the estrous cycle (Meziane et al., 2007; Parker et al., 2001). Ovarian-cycle linked changes in GABAA receptors are known to influence anxiety-related behaviors (Maguire and Mody, 2009; Maguire et al., 2005) and it has also been shown that steroids modulate the expression of α2 subunit mRNA in vitro (Pierson et al., 2005). Here, we posit that the female estrous state may potentially represent a confounding variable that may have masked genotype-dependent effects in the TST and NSF test.
The results of this study are consistent with previously published preclinical work demonstrating a role for GABAA receptors in depressive-like behavior (Earnheart et al., 2007; Shen et al., 2010) but extend this finding by identifying a specific GABAA receptor subtype that mediates antidepressant-like effects generated from stressful situations (e.g., food deprivation) in vivo. The Gabra2 gene is expressed in distinct brain areas including the amygdala, hippocampus and nucleus accumbens (Fritschy and Möhler, 1995), anatomical structures considered to be key components in the pathophysiology of depression (Kaplan et al., 1994). Inhibitory GABAergic circuits acting via α2-containing GABAA receptors may critically modulate the output of these brain regions and a dysregulation of these circuits could lead to disturbances in emotional processing and mood regulation.
While α2-containing GABAA receptors are known to be involved in regulating anxiety-like behaviors (Löw et al., 2000), our results further suggest that they may be a common neural substrate linking comorbid anxiety disorders and depression in humans. Given that that the behavioral tests used here to assess depressive-like states are derived from subjecting the animal to a stressful state, and that the responses observed are also fundamentally related to anxiety, α2-containing GABAA receptors may play a particularly salient role for both anxiety and depression. α2-containing GABAA receptors may therefore represent a novel target for the development of more effective, non-monoamine-based antidepressants that are also effective anxiolytics.
Acknowledgements
The project described was supported by Award Number R03MH085149 from the National Institute of Mental Health to UR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. We thank Dr. Elif Engin for her critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures The authors declare that they have no conflict of interest to disclose and no competing financial interests.
References
- Agrawal A, Edenberg HJ, Foroud T, Bierut LJ, Dunne G, Hinrichs AL, Nurnberger JI, Crowe R, Kuperman S, Schuckit MA, Begleiter H, Porjesz B, Dick DM. Association of GABRA2 with drug dependence in the collaborative study of the genetics of alcoholism sample. Behav. Genet. 2006;36:640–650. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Kaplan M, Potter L, Bloom L, Rickels K. Adinazolam, a new triazolobenzodiazepine, and imipramine in the treatment of major depressive disorder. Psychopharmacology. 1986;88:484–488. doi: 10.1007/BF00178511. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z. Increased Brain GABA Concentrations Following Acute Administration of a Selective Serotonin Reuptake Inhibitor. Am. J. Psychiatry. 2004;161:368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares J. GABAergic dysfunction in mood disorders. Mol. Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Jr., Xuei X, Edenberg HJ, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav. Genet. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Möhler H, Luscher B. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J. Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr., O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and brain oscillations. Am. J. Hum. Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Möhler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Kaplan HI, Grebb JA, Sadock BJ. Kaplan and Sadock's synopsis of psychiatry: behavorial sciences/clinical psychiatry. seventh ed. Williams & Wilkins; Baltimore: 1994. [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am. J. Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Mody I. Steroid hormone fluctuations and GABAAR plasticity. Psychoneuroendocrinology. 2009;34S:S84–S90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Meziane H, Ouagazzal A, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy; Washington, D.C.: 1996. [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Lynskey MT, Todorov AA, Wang JC, Todd RD, Martin NG, Heath AC, Goate AM, Montgomery GW, Madden PA. Association of childhood trauma exposure and GABRA2 polymorphisms with risk of posttraumatic stress disorder in adults. Mol. Psychiatry. 2009;14:234–235. doi: 10.1038/mp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GC, McKee ME, Bishop C, Coscina DV. Whole-body metabolism varies across the estrous cycle in Sprague-Dawley rats. Physiol. Behav. 2001;74:399–403. doi: 10.1016/s0031-9384(01)00599-6. [DOI] [PubMed] [Google Scholar]
- Petty F. GABA and mood disorders: a brief review and hypothesis. J. Affect. Disord. 1995;34:275–281. doi: 10.1016/0165-0327(95)00025-i. [DOI] [PubMed] [Google Scholar]
- Petty F, Trivedi M, Fulton M, Rush A. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol. Psychiatry. 1995;38:578–591. doi: 10.1016/0006-3223(95)00049-7. [DOI] [PubMed] [Google Scholar]
- Pierson RC, Lyons AM, Greenfield LJ., Jr. Gonadal steroids regulate GABAA receptor subunit mRNA expression in NT2-N neurons. Mol Brain Res. 2005;138:105–115. doi: 10.1016/j.molbrainres.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Porsolt R, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978;47:379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Rapaport M. Prevalence, recognition, and treatment of comorbid depression and anxiety. J. Clin. Psychiatry. 2001;62(Suppl 24):6–10. [PubMed] [Google Scholar]
- Sanacora G, Mason G, Krystal J. Impairment of GABAergic transmission in depression: new insights from neuroimaging studies. Crit. Rev. Neurobiol. 2000;14:23–45. doi: 10.1615/critrevneurobiol.v14.i1.20. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Saricicek A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol. Disord. Drug Targets. 2007;6:127–40. doi: 10.2174/187152707780363294. [DOI] [PubMed] [Google Scholar]
- Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. gamma-Aminobutyric acid-Type A receptor deficits cause hypothalamic-pituitary-adrenal Axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol. Psychiatry. 2010;68:500–502. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]