Synopsis
For in-transit melanoma confined to the extremities, regional chemotherapy in the form of hyperthermic isolated limb perfusion and isolated limb infusion are effective treatment modalities carrying superior response rates to current standard systemic therapy. Despite high response rates, most patients will eventually recur, supporting the role for novel research aimed at improving durable responses and minimizing toxicity. Although the standard cytotoxic agent for regional chemotherapy is melphalan, alternative agents such as temozolomide are currently being tested with promising preliminary results. Current strategies for improving chemosensitivity to regional chemotherapy are aimed at overcoming classic resistance mechanisms such as drug metabolism and DNA repair, increasing drug delivery, inhibiting tumor-specific angiogenesis, and decreasing the apoptotic threshold of melanoma cells. Concurrent with development and testing of these agents, genomic profiling and biomolecular analysis of acquired tumor tissue may define patterns of tumor resistance and sensitivity from which personalized treatment may be tailored to optimize efficacy. Here, rational strategies for treatment of in-transit melanoma are outlined with special emphasis on current translational and clinical research efforts.
Keywords: Melanoma, In-transit, Regional Chemotherapy, Isolated Limb Infusion, Isolated Limb Perfusion
Introduction
Epidemiology
While the incidence of several other cancers decline, the incidence of melanoma continues to rise and is now the most common fatal malignancy of young adults, and overall the sixth most common cancer amongst Americans.1 In fact, an estimated 1 in 50 people will be diagnosed with melanoma over the course of their lifetime.2 In 2009, there were an estimated 68,720 people newly diagnosed with invasive melanoma, and over 8,650 people died of melanoma in the United States.2 Unfortunately, mortality rates from melanoma have remained stable because overall poor response of patients with metastatic disease to systemic therapy.3
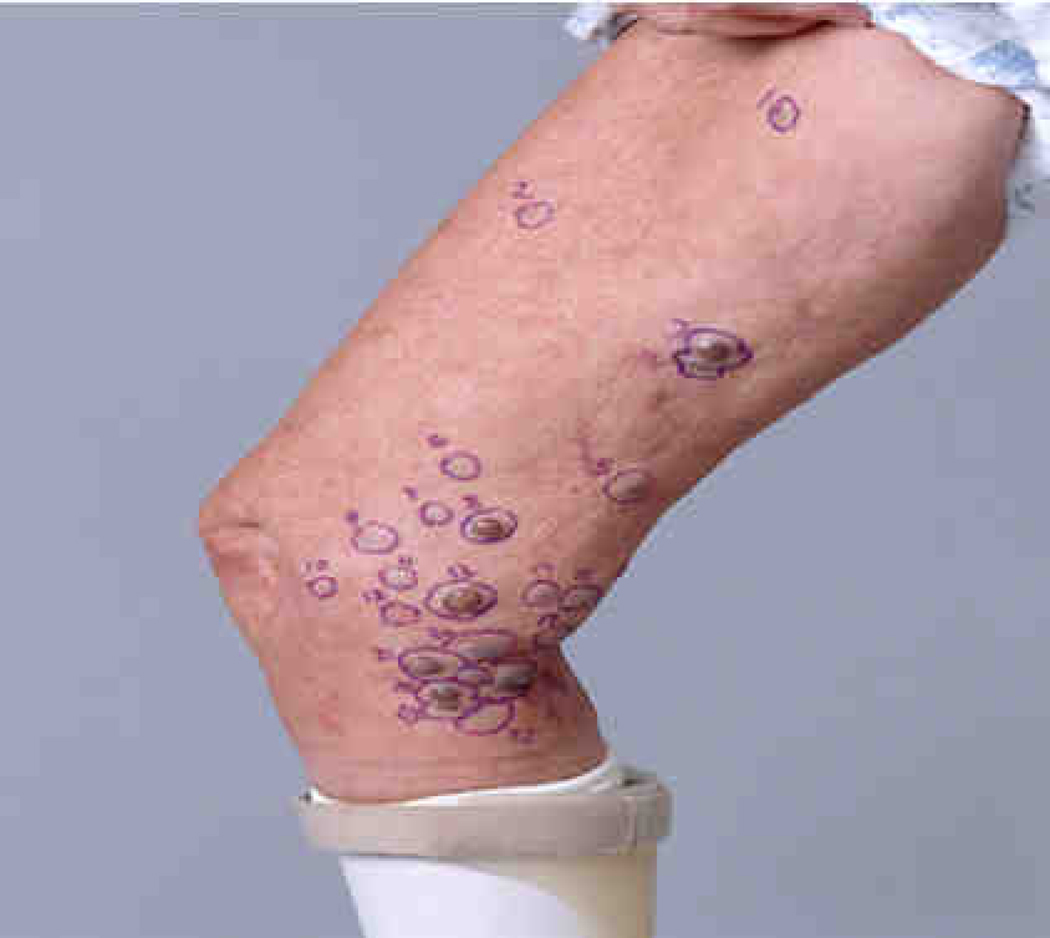
In-transit metastases represent multifocal metastases that spread through the lymphatic system and occur between the site of the primary lesion and the regional draining lymph node basin (Figure 1).4 Recently, the 2009 AJCC Cancer Staging Manual was published which recommended the retention of the previous (2002) edition’s staging definition for the in-transit metastases.5 Patients with in-transit metastases are classified as stage IIIB or IIIC, depending on their regional lymph node status (B=negative, C=positive), regardless of the number of lesions.5–6 The number of patients that develop in-transit metastases is not insignificant with a 30-year German study showing 21% of recurrences are in the form of in-transit or satellite metastases.7 Another study reports that 2–10% of initially treated melanomas of the extremity recur in an in-transit fashion.4 Historically, this pattern of recurrence is associated with an unfavorable prognosis, with 5-year survival rates ranging from 25% to 30%.8–10 However, patients with IIIB in-transit disease seem to have better survival when compared to the rest of their cohort.5–6 In the minority of patients, surgical excision of in-transit metastases can be utilized when the in-transit disease is limited to a few tumor deposits.11 Unfortunately, the majority of patients have multifocal disease for which standard of care systemic chemotherapy or immunotherapy has had limited benefit.12 However, if patients have in-transit disease confined to the extremities, regional chemotherapy delivered by isolated limb perfusion or isolated limb infusion is an effective treatment option associated with complete response rates ranging from 23–82% (Table 1).13–27 Regional chemotherapy for in-transit disease is a rapidly progressing field and a platform for ongoing research aimed at delineating underlying melanoma tumor biology. This review will describe the current status of regional chemotherapy in treating patients with in-transit disease of the extremity and the novel approaches being developed using targeted agents and immune modulators in an effort to improve efficacy while minimizing toxicity.
Figure 1. In-transit melanoma metastases.
Right leg with advanced, in-transit metastases of melanoma occurring between site of primary on lower leg and draining lymph node basin. Reproduced with permission from Beasley et al. Surg Oncol Clin N Am. 2008 Oct;17(4):731–58.
Table 1.
Response rates following melphalan-based HILP and ILI in patients with melanoma.
| Study | N | CR (%) | PR (%) | SD (%) | PD (%) | Condition | |
|---|---|---|---|---|---|---|---|
| HILP | Minor 198513 | 18 | 82 | 18 | 0 | 0 | Hyperthermia |
| Storm 198514 | 26 | 50 | 31 | 19* | 0 | Hyperthermia | |
| Kroon 198715 | 18 | 38 | 44 | 17* | 0 | Normothermia | |
| Kroon 199316 | 43 | 77 | 14 | 9* | 0 | Normothermia | |
| Klaase 199417 | 120 | 54 | 25 | 21* | 0 | Normothermia | |
| Grünhagen 200418 | 100 | 69 | 26 | 5* | 0 | Hyperthermia | |
| Aloia 200519 | 59 | 57 | 31 | 12* | 0 | Hyperthermia | |
| Cornett 200620 | 58 | 25 | 39 | 28 | 11 | Hyperthermia | |
| Sanki 200721 | 120 | 69 | 16 | 0 | 15 | Hyperthermia | |
| ILI | Mian 200122 | 9 | 44 | 56 | 0 | 0 | |
| Lindner 200223 | 128 | 41 | 44 | 12 | 4 | ||
| Bonenkamp 200424 | 13 | 31 | 61 | 0 | 8 | ||
| Brady 200625** | 22 | 23 | 27 | 0 | 50 | ||
| Beasley 200830 | 50 | 30 | 14 | 10 | 46 | ||
| Kroon 200826*** | 185 | 38 | 46 | 10 | 6 | ||
| Beasley 200927 | 128 | 31 | 33 | 7 | 29 | ||
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
No response.
Includes one patient with advanced sarcoma.
Includes 128 patients reviewed in Lindner 2002.
Isolated Limb Perfusion
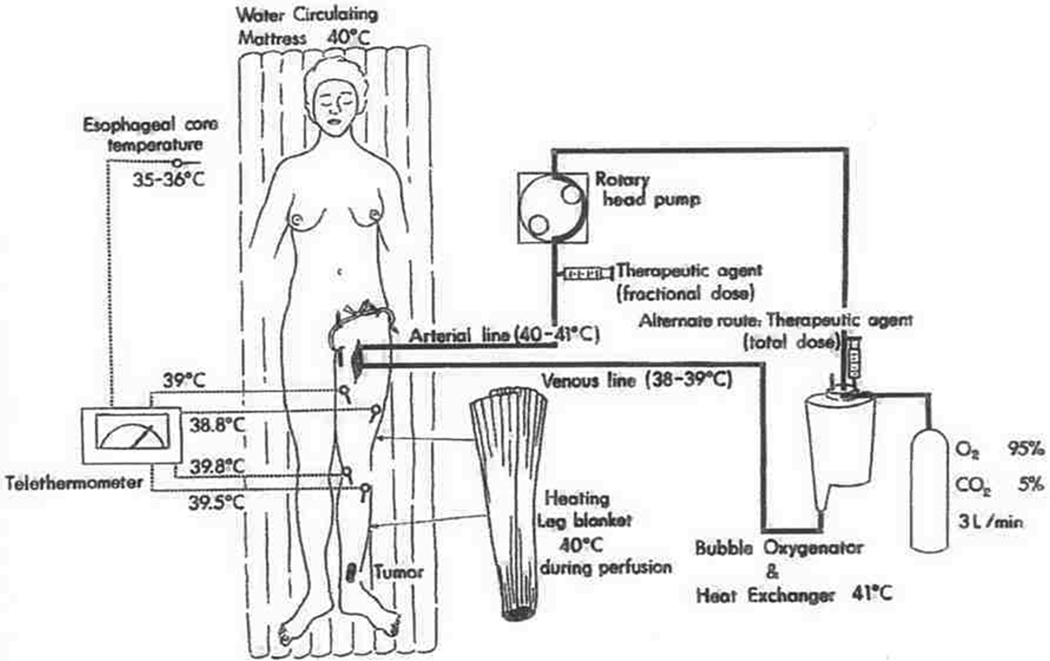
Regional treatment, in the form of hyperthermic isolated limb perfusion (HILP) was first performed for in-transit melanoma over 50 years ago by Creech.28–29 This technique is still used today and overall remains unchanged from its original components. In brief, the femoral or subclavian vessels are surgically exposed and cannulated. When indicated, lymphadenectomy can also be performed during the vascular exposure. The artery and vein are cannulated at the root of the limb and an esmarch tourniquet is placed proximal to the cannulated vessels. Perfusion proceeds with the use of a high-flow, melphalan-based perfusate using a membrane oxygenator to maintain the acid-base status and oxygenation of the isolated limb in the physiologic range (Figure 2). Creech et al. used melphalan as their chemotherapeutic agent based on in vivo mouse data, and this has remained the standard agent for I LP.29 Hyperthermia of the limb is achieved due to the heated, high-flow perfusate, as well as warming blankets wrapped around the extremity for the duration of the procedure.
Figure 2. Schematic of hyperthermic isolated limb perfusion.
The affected limb’s main artery and vein are surgically exposed and openly cannulated. Warming blankets maintain hyperthermia and temperature is monitored with temperature probes. The tourniquet is applied proximally and the melphalan chemotherapy perfusate is circulated with heated, high-flow membrane oxygenator to maintain acid-base status of the limb. Reproduced with permission from Muchmore et al., Surg Oncol Clin N Am. 2008 Oct;17(4):709–30, vii.
Retrospective studies have shown up to 82% of patients experience a complete response after ILP depending on the patient population and particular adjuncts, but larger studies seem to demonstrate complete response rates in the 50–70% range (Table 1).13–16, 18–21 For instance, the Sydney Melanoma Unit has reported an overall response rate of 75%, with 69% of patients experiencing a complete response when treated with ILP with regional melphalan ± actinomycin D or regional cisplatin.21 In our Duke University experience of melphalan based ILP, 88% of patients responded and 57% were complete responders.30 One of the larger series by Grunhagen et al. reported an overall response rate of 95%, with 69% complete responders who received HILP with melphalan and adjunctive tumor necrosis factor-α (TNF- α). The overall 5-year survival rate for this cohort was 32%; the median survival was 25 months.18
Isolated Limb Infusion
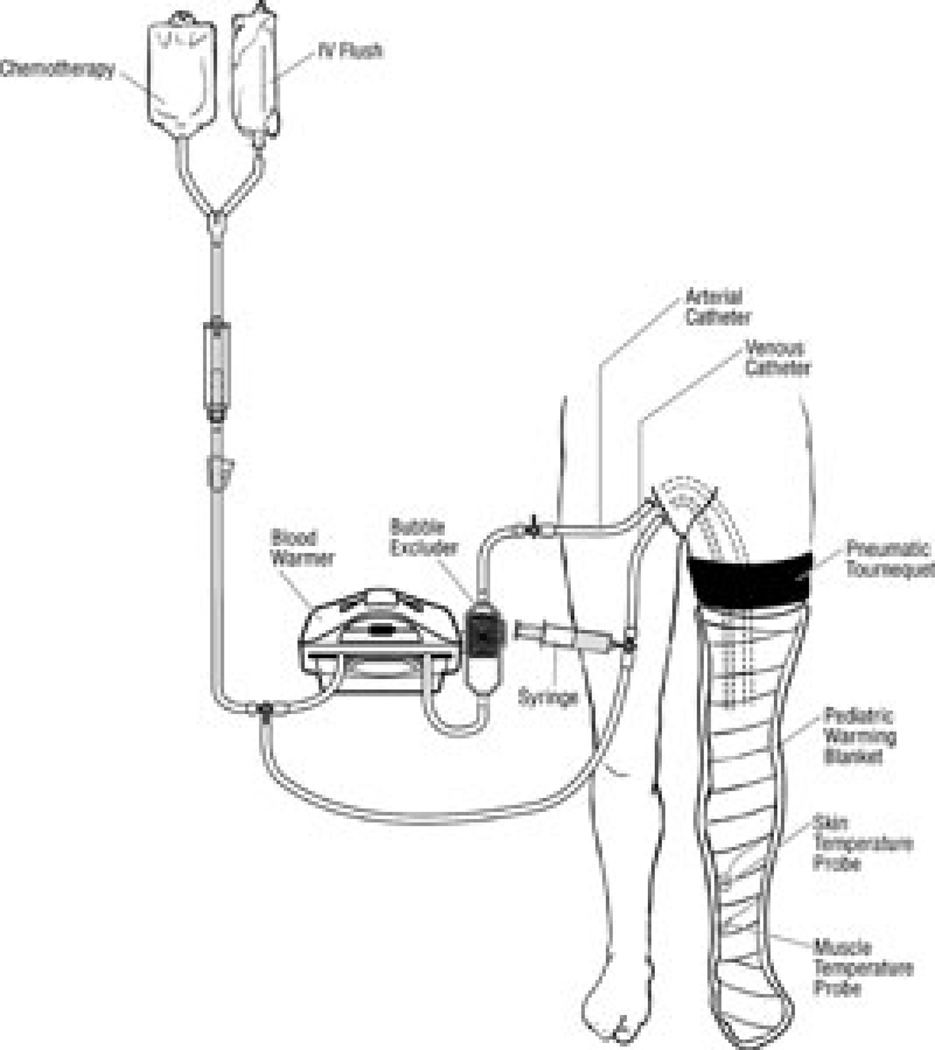
More recently, Thompson and colleagues at the Sydney Melanoma Unit (SMU) developed an alternative to HILP, called isolated limb infusion (ILI).31–32 ILI is less invasive compared to HILP as it is performed via percutaneous catheterization of the involved limb. Using the Seldinger technique under fluoroscopic guidance, arterial and venous catheters are placed into the involved limb (Figure 3). A pneumatic or esmarch tourniquet is then positioned at the most proximal portion of the limb and inflated, thereby isolating the limb from systemic circulation. The extremity is wrapped with warming blankets using circulated heated water for the duration of the procedure. Next, melphalan is rapidly infused into the arterial catheter and manually circulated through a blood warmer syringe and a 3-way stopcock. After circulating for 30 minutes, a washout procedure using crystalloid fluids removes the chemotherapy from the limb via venous outflow extraction.
Figure 3. Schematic of isolated limb infusion.
Catheters are percutaneously inserted into the affected limb. Warming blankets are applied to the limb, but the same degree of hyperthermia in HILP cannot be achieved with ILI. The tourniquet is applied proximally and chemotherapy is circulated manually through a blood warmer using a syringe and 3-way stopcock. Reproduced with permission from Brady et al. Ann Surg Oncol. 2006 Aug; 13(8): 1123–9.
In contrast to ILP, ILI is a low-flow circuit with no oxygenator, resulting in the limb becoming normothermic, hypoxic, and acidotic. It is postulated that the limb acidosis and hypoxia may increase melphalan activity.26 The simplicity of ILI has several advantages over traditional HILP. First of all, it does not require a membrane oxygenator or pump priming with blood. It is also a shorter procedure, is repeatable, and is associated with less regional toxicity when correcting for ideal body weight.30, 33 ILI is the preferred treatment for more frail patients with multiple comorbidities who may not tolerate the more involved HILP procedure. To be fair, there are also potential disadvantages of ILI. Namely, the same degree of hyperthermia as HILP cannot be routinely achieved by ILI. Furthermore, ILI uses a lower dose of melphalan, and its duration is half as long as compared to HILP.34 These differences probably contribute to the lower overall response rates of ILI compared to HILP in a recent multicenter combined analysis (79% in 294 patients versus 64% in 313 patients).27 While low to moderate grade toxicities are similar for both ILI and HILP, HILP appears to be associated with more treatment-related limb loss.26–27,30
Regional Chemotherapy
The major difference between systemic and regional chemotherapy treatments is the ability to deliver very high doses of chemotherapy through an isolated extremity circuit while minimizing systemic leakage and resultant systemic toxicity. Since the typical leak rate is less than 1% when performing HILP/ILI with conventional cytotoxic drugs, there are few systemic side effects and organ toxicity is rarely dose-limiting.35 As a result, plasma melphalan can safely reach levels 10- to 100-fold times higher following regional compared to systemically delivered chemotherapy.13,36 Drug delivery is also enhanced through its local delivery and avoidance of hepatic metabolism and renal clearance.37 Adjuvant treatments, such as hyperthermia, may impact the pharmacokinetics of the chemotherapeutic agent and can be applied more safely and effectively to the extremity for a regional treatment as compared to systemic chemotherapy.38–40 HILP/ILI are also unique compared to systemic therapy in that after chemotherapy has circulated in the extremity, a washout procedure eliminates the remaining drug, reducing the risk of systemic toxicity before the tourniquet is released. Melphalan remains the most common agent used in regional chemotherapy for melanoma and is considered standard of care for the procedures.34 However, alternative agents including cisplatin and temozolomide may offer significant advantages over melphalan in certain circumstances.
Melphalan
The standard chemotherapy agent for regional chemotherapy of melanoma is melphalan (L-phenylalanine mustard (LPAM)). LPAM is a bifunctional alkylating agent and remains the most common drug used for HILP/ILI procedures.41 The rationale in using melphalan stems from the essential role phenylalanine plays in melanin synthesis. In theory, the phenylalanine-derivative melphalan should provide selective toxicity to melanocytes and melanoma cells in which melanin is synthesized.42 Despite its theoretical selective toxicity for metastatic melanoma, systemic melphalan is relatively ineffective since the maximally tolerated dose is much lower than the effective dose.34 This limitation is overcome with regional delivery techniques since much higher doses can be achieved in the extremity than would be tolerated when administered systemically.
Regional melphalan dosing is based in part on the report by Roberts and colleagues who determined that treatment responses tend to plateau at achieved melphalan levels of 25 µg/ml.43 Melphalan concentrations above this value are routinely achieved by HILP/ILI, thus increasing the dose of melphalan to achieve levels higher than this is not likely to impact the response rate and may only increase toxicity.35 Properties of melphalan that make it particularly effective as a regionally delivered chemotherapy include its short half-life, limited cell cycle specificity, low vascular endothelium and soft tissue toxicity, and linear dose-response relationship with respect to cytotoxicity.13
Melphalan has historically been dosed and administered based on body weight over a wide dose range (0.25 to 2.8 g/kg) without accounting for body mass distribution.44–45 Dosing is now based on limb volume, with a target melphalan dose of 7.5 mg/L and 10 mg/L for a lower extremity ILI and HILP, respectively, and 10 mg/L and 13 mg/L for an upper extremity ILI and HILP respectively. 23, 46 Based on our experience, we have recommended a total dose not exceeding 100 mg for the lower extremity or 50 mg for the upper extremity, although there have been reports of maximum doses as high as 120 mg for the lower extremity and 80 mg for the upper extremity. 30, 47–48
Limb volume can be estimated using volume displacement or limb circumference. Volume displacement may offer a more accurate estimation; however, it can be more cumbersome and may necessitate the position of the tourniquet be pre-determined.49 Our preferred method is using the limb circumference measurement by which the volume of the limb is calculated based of serial circumference measurements (e.g., every 1.5 cm). Despite the fact that this is a calculated estimate rather than direct measurement, it allows the volume to be determined in the clinic or even during the procedure once the final position of the tourniquet has been decided. We find this method to be more flexible by facilitating a more real-time calculation of limb volume at the time of the actual procedure. If the hand or foot of the involved extremity is free of disease, a second optional esmarch or pneumatic tourniquet may be used to exclude these areas from the circuit. In this situation, the hand or foot volume should be subtracted from the total limb volume.50 Some groups, including our own, now correct melphalan dosing for ideal body weight based on evidence that this dose modification is associated with lower toxicity without altering the complete response rate.50
Cisplatin
Cisplatin is an attractive agent as it is commonly used systemically for the treatment of melanoma. By crosslinking DNA, cisplatin interferes with mitosis, leading to eventual apoptosis. Essential to its utility as a regional chemotherapy agent, cisplatin does not require metabolic transformation to become an active anti-neoplastic agent and it is cell-cycle independent.51 Indeed, initial animal studies using cisplatin as a regional chemotherapy agent were promising and were quickly translated into clinical trials.52 Unfortunately, these subsequent clinical studies have produced mixed results.
Early, small clinical studies reported favorable response rates after regional cisplatin perfusion without significant toxicities.53–56 In a larger series of 58 melanoma patients reported by Hajarizadeh et al., 41 patients received prophylactic HILPs with cisplatin for Stage I disease and 17 others were treated stage II, III, or IV disease.57 In this study, HILP was followed by wide local excision of the primary tumor or re-excision of any remaining melanoma in all patients. After a median follow-up of 29 months, local recurrence rates of 12%, 33%, and 30% were observed for Stage I, II, and III disease respectively. Unfortunately, significant complications occurred in eight patients, leaving two with permanent deficits. This study concluded that HILP with cisplatin was more effective than surgery alone to achieve local control and in the short-term appeared to be at least as effective as HILP with melphalan.
However, subsequent studies have shown disappointing results for HILP with cisplatin both in terms of efficacy and toxicity. Santinami et al. discontinued a study over concerns about toxicity after only nine patients with melanoma had been treated.58 In a phase I trial of 15 patients of HILP with cisplatin, Coit and colleagues reported only three patients having a complete remission lasting more than 2 years as well as three patients developing severe limb-threatening toxicity.59 These authors eventually concluded that HILP with cisplatin was not justified as a standard therapy for metastatic in-transit melanoma. Similarly, Thompson et al. suggested cisplatin was not the drug of choice for HILP treatment of melanoma confined to the limb when five of six patients receiving therapeutic HILPs with cisplatin did not achieve complete responses and two of four patients receiving prophylactic treatments developed early recurrence.51 Confounding these results, toxicity was very significant, resulting in an amputation in one patient and leaving another with a permanent foot drop. In the patient who had the leg amputation, melanoma remained despite extensive necrosis of normal tissue. Presently, cisplatin is not utilized due to the incidence of complications and lack of a significant improvement in response over melphalan.
Temozolomide
Temozolomide (TMZ) is considered to have some activity for metastatic melanoma as an oral agent. A new intravenous (IV) formulation of temozolomide has been developed and appears to hold tremendous potential for use in regional chemotherapeutic treatments. Temozolomide is an alkylating agent that is metabolized to the active metabolite, methyldiazonium ion (MTIC), which methylates guanine residues in DNA at the O6 and N7 positions.60 Cellular apoptotic pathways become activated when DNA mismatch enzymes attempt to repair the O6-methylguanine generated by temozolomide, resulting in single and double-stranded DNA breaks.61 Temozolomide is suitable to the regional model because unlike dacarbazine (DTIC), temozolomide has 100% bioavailability under physiologic conditions, and does not require hepatic conversion.62 Preclinical in vivo studies in nude rats implanted with melanoma xenografts demonstrated that regional infusion with temozolomide resulted in prolonged tumor growth delay compared to rats that received systemic temozolomide or regional melphalan, especially in tumors with low O6-alkylguanine-DNA alkyltransferase (AGT) activity, the predominant mechanism of resistance of temozolomide.62 Analysis across a panel of xenografts suggested that approximately 20% of tumors preferentially respond to temozolomide, 20% preferentially respond to regional melphalan, and 60% respond equally to temozolomide or melphalan.63 The FDA recently approved the IV formulation of temozolomide; a phase I clinical trial is underway at Duke, The University of Texas MD Anderson (MDACC), and Moffitt Cancer Center to define the toxicity profile and maximally tolerated dose to utilize in regional therapy.
Algorithm
Despite the frequency with which HILP and ILI are performed for in-transit melanoma, no consensus exists as to which treatment is preferable or what strategies surgeons should follow should a patient progress after regional treatment. In general, we perform surgical excision for small volume disease, usually defined as 1–2 lesions which we can resected with negative surgical margins. Often, in patients who undergo surgical excision, we will also perform a simultaneous sentinel lymph node biopsy, which reports suggest will be positive about 50% of the time.64 Regional therapy is utilized in patients who have failed surgical excision, have non-resectable extremity disease, or have multifocal disease of 3 or more lesions. We do not recommend excision of lesions in the field of treatment if patients are undergoing regional therapy. The rationale for this two-fold: (1) potential wound healing issues related to the chemotherapy that can cause limb swelling and tissue damage, and (2) leaving the lesions in place provides an opportunity for the surgeon to monitor the effectiveness of the treatment.
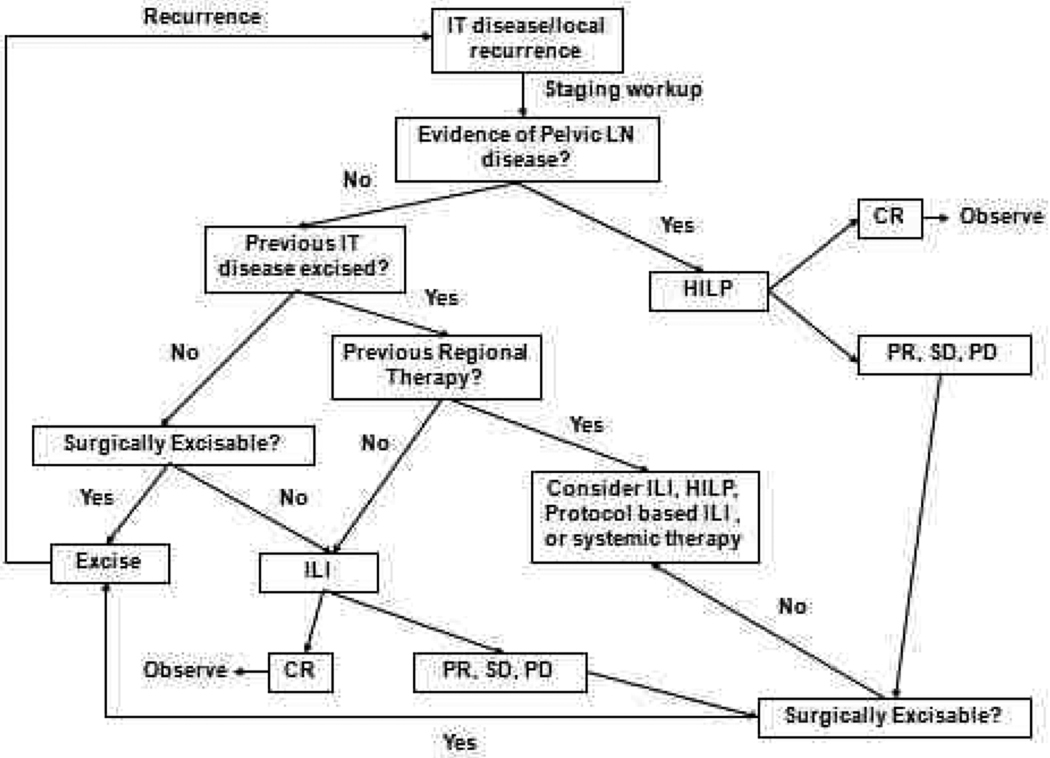
The algorithm for which type of regional treatment (HILP or ILI) we utilize is as follows. Patients with local recurrence or with evidence of pelvic, femoral, or axillary nodal involvement may be candidates for HILP, as excision of the tumor-containing lymph nodes exposes the vessels utilized for this procedure. For patients with femoral or axillary nodal involvement,65 a less invasive alternative is to treat these patients with an ILI, and then perform the lymphadenectomy at the end of the ILI once the heparinization is reversed and after the infusion catheters are removed. Some centers may preferentially offer patients with high disease burden isolated limb perfusion as the initial treatment, as these patients may be more likely to have regional nodal disease. However, there is not strong data suggesting that patients with high disease burden are more likely to response to HILP as compared to ILI. Our group has favored using ILI first, even in high disease burden patients, leaving HILP as a potential salvage therapy for those who do not respond to ILI. Additionally, we have several protocol-based ILI trials (such as temozolomide, or melphalan in combination with other targeted agents), or systemic therapy (Figure 4) for patients who either fail regional treatment or progress.
Figure 4. Regional treatment algorithm for in-transit extremity melanoma.
Patient entry is denoted at the box labeled “IT disease/local recurrence.” IT, in-transit disease; LN, lymph node; HILP, hyperthermic isolated limb perfusion; ILI, isolated limb infusion; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease. Reproduced with permission from Beasley et al. J Am Coll Surg. 2009 May;208(5):706–15;.
Strategies to optimize response
As highlighted above, overall and complete response rates, as well as durability of response, are quite variable depending on the regional chemotherapy platform as well adjunctive therapies. In the context of regional chemotherapy, standard adjuncts to optimize therapy include the addition of hyperthermia and TNF-α to isolated limb perfusion. Newer strategies under active investigation include novel agents which overcome traditional drug resistance mechanisms, target tumor vasculature, and lower the apoptotic threshold of malignant melanoma.
Hyperthermia
Hyperthermia may augment or sensitize melanoma to cytotoxic therapy by increasing blood flow, membrane permeability, local metabolism, and drug uptake.66–69 Indeed, hyperthermia continues to be an essential component of ILP since its introduction by Dr. Cavaleire in 1967.38 Based on preclinical experiments suggesting heat to be tumoricidal in a rat melanoma model, Cavaleire et al. first explored the effects of heat alone in 22 patients with recurrent extremity tumors. The morbidity of the procedure was quite high, with six immediate post-operative deaths. Of the remaining 16, 12 patients were alive without evidence of disease at 3 to 28 months. Two years later, Stehlin and colleagues combined regional chemotherapy with heated perfusion and established hyperthermic isolated limb perfusion (HILP) in a reported experience of 37 patients.70 Of the 37 melanoma patients, only 12 were evaluated for measurable disease. Of these, 10 of the 12 patients (83%) had a response described as “pronounced regression” for greater than 3 months. Interestingly, this report used very high perfusate temperatures reaching up to 46°C. As a modern comparison, we initiate HILP at 38.5°C and with a goal temperature of 40°C.
In 1989, the first prospective, randomized trial was conducted to evaluate the role of hyperthermia and regional extremity perfusion of in-transit melanoma.71 In this study, 107 patients were randomized to receive either surgical excision or surgical excision followed by HILP with melphalan. With a primary end-point of disease-free survival, the study was stopped prematurely (median follow-up 550 days) due to a highly significant advantage in disease-free survival in the HILP arm (89% vs. 52%). In addition, there was an improvement in overall survival (98% vs. 86%) with minimal local and systemic complications.
There is also strong animal model data in the context of regional therapy with the alkylating agents melphalan and temozolomide that hyperthermia augments their anti-tumor efficacy against both melanoma and sarcomas.72–73 To date, no randomized controlled trial has compared hyperthermic ILP versus normothermic ILP. Despite lacking level-I evidence, hyperthermia remains a standard component of ILP due to strong indirect clinical evidence and theoretical merit.
TNF-α
The first study of tumor necrosis factor-α (TNF-α) in a human trial of HILP was performed by Posner and colleagues in 1995. In this study of six patients receiving escalating doses of TNF-α alone during HILP, only 1 patient had a complete response.74 A subsequent study combined TNF-α with interferon-γ (IFN-γ) and melphalan delivered via HILP.75 Interferon was added for potential anti-tumor synergism. Overall, this treatment strategy was quite toxic: all patients required dopamine infusions for a severe inflammatory response and two patients even required an amputation due to limb toxicity. Despite the toxicity, 89% of patients had a complete response and 11% had a partial response to the combination treatment at 11 months follow-up.76
Based on this study, interest in TNF-α in addition to HILP began to rise, eventually resulting in a randomized trial of 103 patients conducted by the American College of Surgeons Oncology Group (ACOSOG).20 This trial was stopped early after an interim analysis showed no evidence of improved patient responses with significant increases in severe toxicities in the TNF-α plus melphalan arm. At six months, although there remained no significant improvement with the addition of TNF-α in the 89 patients still in the study, a difference in response rates was noted (42% CR with combination versus 20% in single therapy; P = 0.101). Critics of this trial have cited an early time point for assessing response (3 months) as the reason the lower response rates observed with TNF-α compared with prior trials.77–78 However, a recent large single institutional series from the United States has also failed to demonstrate an improvement in regional in-field progression free survival with the use of TNF-α.65 Due to the inability to clearly demonstrate any benefit from the addition of TNF to regional therapy, it has not been FDA approved as an adjunct for HILP in the United States.
Targeting traditional drug resistance mechanisms
Drug Metabolism
One mechanism of melanoma chemoresistance is hypermetabolism of alkylating chemotherapy agents by glutathione-S-transferases (GSTs).79 GSTs are a family of phase-II detoxification enzymes that catalyze glutathione (GSH) to electrophiles including carcinogens, mutagens, and anti-cancer reagents. Melanoma cells as well as tumor types have been shown to express elevated GST and GSH levels,80 which in turn have been associated with resistance to chemotherapy. Further supporting its critical role in tumor chemoresistance, melphalan exposures appears to increase tumor GSH levels by more than two-thirds.81 Conversely, reducing GSH levels confers or restores sensitivity in vitro.81–83 The GST enzymes are polymorphic and exist in both membrane-bound and cytosolic forms are divided into six classes; Alpha, Mu, Pi, Theta, Omega and Zeta.84–85 Through conjugation of GSH to intracellular melphalan, GSTs effectively neutralize melphalan by inhibiting the alkylating agent access to DNA.86–87 Given their great potential to augment the chemosensitivity of melanoma as well as other tumor types, there exists significant interest in developing novel inhibitors of GST mediated metabolism.
One strategy in overcoming GST-mediated drug metabolism is to deplete the tumor cells of available GSH, thereby preventing conjugation of the GSH by GSTs to alkylating chemotherapy agents.88 L-S,R-buthionine sulfoximine (BSO) is a potent and specific inhibitor of the enzyme γ-glutamylcysteine synthetase (GCS), which is the rate-limiting step in the production of GSH.89 BSO has been shown to decrease intracellular GSH levels, increase DNA cross-linking, and sensitize the cells to cytotoxicity.90 Early, small clinical studies of continuous BSO infusion with intravenous melphalan were limited by occasional severe myelosuppression. While the addition of BSO to systemic melphalan may be associated with myelosuppression, one might predict that the combination of BSO and melphalan in regional therapy would not be limited by systemic toxicity. In a nude rat xenograft model of ILI, systemic BSO treatment reduced resistance to ILI melphalan.81 Intraperitoneal administration of BSO was well tolerated and was associated with an approximately 72% reduction in tumor GSH levels. BSO treatment was associated with an increase in tumor growth delay relative to saline and melphalan-alone controls. Based on these studies, a phase I trial at Duke using a 3-day infusion of BSO around the time of melphalan ILI was opened for enrollment but was closed prematurely due to restricted availability of the intravenous formulation of BSO.
One of the more promising targets for inhibiting the GST pathway drug metabolism is enzyme Glutathione-s-transferase Pi (GSTP1). In addition to its metabolizing function, GSTP1 also serves as a major regulator of cell signaling in response to stress, hypoxia, growth factors, and other stimuli. GSTP1 inhibits downstream mitogen activated protein (MAP) kinase signaling mediated by binding c-Jun N-terminal kinase (JNK), thereby preventing the phosphorylation of c-Jun.91 The novel GSTP1 inhibitor 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX), has recently been shown to inhibit JNK pathway activation leading to melanoma cell apoptosis in vitro as well as to potently inhibit tumor growth in vivo. 92 The exact role of GSTP1 in melanoma tumorigenesis and its potential for augmenting currently available alkylating chemotherapy agents remains an active area of investigation.
DNA Repair
Should temozolomide be shown to be an effective regional chemotherapy agent upon completion of phase I and II trials, strategies to impair melanoma DNA repair induced by this compound will become important. TMZ confers its cytotoxicity by alkylating or methylating DNA at O6-guanine, N7-guanine, and N3-adenine residues.93 This methylation results in DNA damage and eventual cell death. Temozolomide cytotoxicity can be overcome, however, in tumor cells through several different DNA repair mechanisms including the O6-methylguanine-DNA methyltransferase (MGMT), O6-alkylguanine-DNA alkyltransferase (AGT), the DNA-mismatch repair system (MMR), and the base excision repair pathway (BER) pathways.94–97 Two drugs, the Poly (ADP-ribose) Polymerase (PARP) inhibitor INO-1001 and the AGT inhibitor O6-benzylguanine (O6-BG) have been tested in a pre-clinical setting in an effort to delineate the importance of DNA repair inhibition in augmenting regionally delivered temozolomide.
Poly (ADP-ribose) Polymerase (PARP) recognizes DNA damage and activates the base excision repair pathway by recruiting the DNA repair proteins XRCC1, DNA ligase III, and DNA polymerase beta.93 Cells without PARP activity have been to shown to be more sensitive to alkylating chemotherapy demonstrating increased apoptosis and chromosomal instability.98–100 Based on this data, the novel PARP inhibitor INO-1001 was tested in a melanoma model to determine whether it could augment the response to temozolomide. Toshimitsu et al. recently tested this hypothesis using a rat xenograft model of regional chemotherapy. In his study, systemic administration of the PARP inhibitor INO-1001, followed by regional infusion of temozolomide, did not significantly decrease tumor growth in melanoma xenografts with intact mismatch repair mechanisms and high MGMT activity. However, in the xenograft that exhibited deficient mismatch repair without MGMT activity, termed PRMel, systemic INO-1001 followed by regional infusion of temozolomide significantly decreased tumor growth compared to regional temozolomide alone (22.6% versus 322.8% increase in tumor volume at day 40).93
Inhibition of the DNA repair enzyme AGT using the drug O6-benzylguanine (O6-BG) has also be tested and shown to reduce tumor AGT activity by 93.5%. Regionally administered temozolomide in conjunction with systemic O6-BG led to significant tumor growth delay compared to either regional temozolomide alone, or systemic temozolomide plus systemic O6-BG. Two of six (2/6) rats had tumor regression in the group treated with 750 mg/kg temozolomide alone versus 6/6 with regression in the group treated with O6-BG for five days plus 750 mg/kg temozolomide.62
Vascular targeting agents
ADH-1
ADH-1 (Exherin) is a cyclic pentapeptide that disrupts N-cadherin binding interactions.101 The cadherins are a large supergene family of proteins involved in cell to cell adhesion.102 Malignant transformation in melanoma is characterized by a 'switch' from E-cadherin to N-cadherin altering intracellular signaling pathways leading to increased proliferation, survival and angiogenesis as well as decreased apoptosis.102–103 In preclinical studies, the use of systemic ADH-1 in conjunction with regional melphalan showed marked increases in tumor responses even in the most melphalan-resistant tumors.101 Subsequent mechanistic studies suggest that the efficacy of ADH-1 in augmenting tumor responses may result from disrupting tumor cell interactions with its microenvironment. In the rat xenograft ILI model, ADH-1 significantly increased melphalan-DNA adduct formation in tumor cells, suggesting ADH-1 may augment tumor response to melphalan by increasing delivery of the relatively small melphalan molecule into tumor cells. This has been further supported by in vitro and in vivo permeability assays showing increasing permeability of both normal and tumor microvasculature after treatment with ADH-1. The exact role of N-cadherin in melanoma tumor signaling remains unclear and is an active area of investigation in our laboratory.
As a direct result of the marked responses seen in pre-clinical experiments, a phase I trial was conducted. Sixteen patients were treated without dose-limiting toxicities using a single dose of ADH-1 prior to ILI and a second dose one week after ILI. The ADH-1 was dose-escalated while the ILI was kept at its standard dosing. In-field responses included eight complete responders and two partial responders.47 Given lack of additional toxicity from ADH-1 and a surprising 50% complete response rate, a multi-center phase II trial of systemic ADH-1 in combination with melphalan via ILI for patients who have AJCC Stage IIIB or IIIC extremity melanoma was performed and recently completed. This trial marks the first prospective multi-center (Duke, MDACC, Moffit Cancer Center, and University of Florida) trial of ILI as well as the first to investigate whether a targeting agent could augment regional chemotherapy responses. In total, 45 patients were enrolled over 15 months at the four institutions. ADH-1 was well-tolerated without significant additional toxicity. Pretreatment using ADH-1 prior to ILI with melphalan resulted in an increase in overall and complete response rates compared to historical controls as measured at three months. However, many of these patients recurred five to six weeks after the three-month time point such that there was no significant difference in regional progression-free survival curves between the study participants and patients treated with ILI alone off this study at the same institutions.104
Bevacizumab
Another promising vascular targeting agent for augmenting regional chemotherapy is bevacizumab, a monoclonal antibody to vascular endothelial growth factor (VEGF). Bevacizumab has been used in combination with standard chemotherapies to treat patients with metastatic colorectal, breast, brain kidney, and lung cancers.105–108 The classic mechanism of bevacizumab is sequestration of VEGF and inhibition of aberrant tumor vasculature formation. Moreover, increasing evidence suggests inhibition of VEGF via bevacizumab can augment chemotherapy agents by ‘normalizing’ tumor vasculature leading to greater delivery of chemotherapy to tumor cells. Bevacizumab is currently being investigated in combination with other chemotherapy agents for metastatic melanoma in multiple clinical trials across the United States.109 A recent phase II study examining bevacizumab in combination with the mammalian target of rapamycin (mTOR) inhibitor everolimus demonstrated moderate activity in 57 patients with metastatic melanoma.110 When combined with dacarbazine and IFN-α-2a in 26 patients, bevacizumab had moderate activity, with an overall response rate of 23% and two complete responders. The median overall survival for all patients in this study was 11.5 months.111 In another phase II trial of 53 patients, bevacizumab in combination with paclitaxel and carboplatin was found to be moderately well tolerated and resulted in 9 (17%) patients achieving partial regression and 30 (57%) achieving stable disease at 8 weeks.112 Given multiple phase II trials suggesting improved overall response rates in combination with systemic chemotherapy as well as its inherent potential to increase drug delivery through tumor vasculature ‘normalization’, we hypothesized that systemically administered bevacizumab prior to regionally delivered melphalan would significantly enhance the efficacy of regionally delivered melphalan. Indeed, preclinical evidence in a rat melanoma xenograft model suggests that a single injection bevacizumab given three days prior to isolated limb infusion with melphalan markedly improved response rates. (Manuscript in preparation). Tumors pre-treated with bevacizumab demonstrated decreased tumor vasculature density and permeability yet had increased melphalan-DNA adducts formation. Taken together, these studies support the concept that bevacizumab results in tumor vascular normalization, thereby enhancing drug delivery and improving overall response to regional chemotherapy (manuscript in preparation). A phase I trial combining bevacizumab with melphalan via ILI is currently being planned at Duke for 2011.
Altering apoptotic threshold
Sorafenib
Altered cellular signaling cascades in melanoma have been shown to increase proliferation and decrease apoptosis leading to greater chemoresistance.113 One of the more common oncogenic pathways altered in melanoma is the BRaf serine/threonine kinase pathway. In fact, 50–70% of melanomas express the constitutively active mutant BRaf serine/threonine kinase.114 Although more than 30 mutations of the BRAF gene have been identified and associated with malignancy, the most frequent mutation (~90%) is a single substitution of glutamine for valine at residue 599 which is referred as V600E.115 The end effect of this mutation is transformation of the BRaf kinase from the inactive to constitutionally active state, resulting in continuous stimulation of mitogen-activated protein (MAP) kinase and extracellular regulated (ERK) kinase pathways with resultant augmentation of proliferation, differentiation, angiogenesis, and apoptosis.116 Given the high incidence of BRAF mutation in melanoma and its oncogenic effects, the BRaf kinase is a promising target for enhancing systemic as well as regional chemotherapy.
Sorafenib (Nexavar®) is a commercially available drug approved for the treatment of renal cell and hepatocellular carcinomas.117–118 This small molecule inhibits multiple tyrosine kinase pathways including the VEGF receptor-2 and −3 kinases, c-Kit, and the MAP kinases pathways including BRaf.119 Through its inhibition of the Raf-MEK-ERK pathway, sorafenib has been shown to inhibit cell proliferation and angiogenesis116 as well as induce apoptosis by down-regulating the anti-apoptotic protein Mcl-1 and preventing the nuclear translocation of apoptosis-inducing factor (AIF).120–122 Although effective in pre-clinical in vitro and in vivo models,123–124 clinical trials with sorafenib as a single agent in melanoma have produced disappointing results.125 However, subsequent phase I-II trials showed greater overall tumor response rates when sorafenib was combined with cytotoxic therapies including carboplatin and paclitaxel, temozolomide, dacarbazine, or interferon α-2a.126–129 Unfortunately, in a phase III randomized, controlled trial, the combination of sorafenib with carboplatin plus paclitaxel for patients with advanced melanoma failed to demonstrate improvements in any clinical endpoints.130 Although sorafenib failed to synergistically enhance systemically administered cytotoxic therapy, whether it could augment regionally delivered chemotherapy where dose of chemotherapy can be an order of magnitude higher, remained unclear.
In preclinical studies, sorafenib significantly augmented melanoma chemosensitivity to melphalan and temozolomide across a panel of 24 cell lines independent of BRaf or NRas mutational status. Using a rat xenograft model of ILI, the combination of sorafenib and either melphalan or temozolomide significantly reduced tumor growth after ILI compared to melphalan or temozolomide alone. On immunohistochemical analysis of tumor tissue acquired 24 hours after ILI, rats pre-treated with sorafenib exhibited increased apoptosis with associated decreases in ERK activation and Mcl-1 protein levels.131 Overall, the results of these pre-clinical studies suggested that sorafenib reduces tumor apoptotic threshold and thereby sensitizes melanoma tumor cells to the cytotoxic agents melphalan and temozolomide. These promising pre-clinical results led to an open-label, multi-center, phase I dose escalation study to evaluate safety, tolerability and anti-tumor activity of oral sorafenib in combination with normothermic ILI with melphalan at Duke University and Memorial Sloan Kettering Cancer. This study has been recently completed and involved 20 patients. From this trial, a maximally tolerated oral dose of sorafenib was defined as 200 mg twice daily. Overall response and complete response rates in this small study did not exceed historic measures of standard melphalan ILI alone, and patients pre-treated with higher doses of sorafenib did seem to have an increased risk of local toxicity (i.e. ulceration and myositis) without an obvious improvement in response rates. Interestingly, preliminary analyses have not associated response to sorafenib and melphalan with BRaf or N-ras mutational status. Final analyses of this trial are ongoing and soon to be published.
Dasatinib
Another potential target in melanoma is the src family of kinases. Src kinase overexpression leads to increased cellular proliferation and decreased adhesion.132 Src signals through its downstream intermediaries called signal tranducers and activators of transcription (STAT) factors. One of these factors, STAT3, has been found to be activated in the majority (85%) of melanoma cell lines.133 STAT3 has been shown to not only promote tumor growth and surviva,l but also appears to up regulate VEGF and promote tumor angiogenesis.134 Inhibition of src kinase blocked the growth of human melanoma cell lines with elevated STAT3 activity and induced apoptosis by inhibiting the expression of anti-apoptotic genes Bcl-xL and Mcl-1.134
By mitigating the unchecked STAT3 activity leading to expression of pro-survival genes, Src inhibitors such as dasatinib (Sprycel®) may also potentiate the effectiveness of traditional chemotherapy by lowering the apoptotic threshold. Dasatinib is manufactured by Bristol-Myers Squibb and is a BCR-Abl and Src family tyrosine kinase inhibitor currently approved for the treatment of chronic myelogenous leukemia (CML) refractory to imatinib treatment as well as acute lymphoblastic leukemia positive for the Philadelphia chromosome.135 Recent in vitro studies of have shown dasatinib to synergistically enhance the effects of cisplatin, but not temozolomide or paclitaxel in melanoma.136 In regards to the role of dasatinib in augmenting regional chemotherapy for in-transit melanoma, preclinical studies are currently ongoing and promising. Using nude rat xenografts model of ILI, rats pre-treated with orally administered dasatinib followed by regionally delivered melphalan have shown superior tumor responses as compared to rats receiving regional melphalan therapy alone (unpublished data). Based on this preliminary data, we are planning for a phase I clinical trial for melanoma patients with in-transit disease.
Immune Modulation and Regional Therapy
Immune-based therapies have demonstrated some efficacy in melanoma patients.12, 137–138 To what degree the immune system may contribute to the magnitude or durability of a tumor response to chemotherapy is currently unclear. The immune system may play a significant role in regionally treated patients especially in light of data that response to a regional treatment, more so than lymph node status, is the strongest predictor of long term survival.19, 26, 139 Regionally treated patients do not have the immune suppression associated with systemic chemotherapy treatments and may respond differently to tumor cytotoxicity induced by the administered chemotherapeutic agent. Current studies at Duke University are trying to quantify the nature of the immune response in patient with in-transit disease and how the immune response might contribute to regional tumor response and durability of response. The group at Memorial Sloan Kettering Cancer Center (MSKCC) has recently proposed an ILI trial where patients will receive four doses of the CTLA-4 antibody ipilimumab after ILI. This trial is currently undergoing IRB approval and will help determine if immune regulation can either augment a regional chemotherapy response or help generate a more robust anti-tumor response to it.
Personalization
With an ever growing number of available drugs targeting specific molecular pathways, rational methods will be required to tailor individual tumor biology with appropriate molecular interventions. Although many targeting drugs have modest effect when given alone, many can sensitize in-transit melanoma to regional chemotherapy when targeted therapies are matched correctly with the tumor’s oncogenic aberration. Gene signature profiling through microarray technology is one possible method through which optimal therapy combinations for regional chemotherapy can be tailored for each patient. Gene expression profiling provides a powerful method of classifying tumors based on their underlying biology and has already been used to predict oncogenic signaling,140 prognosis,141, and progression.142 In-transit melanoma is, in many ways, an ideal platform for tailored therapy since most patients will, by definition, have multiple lesions available for tissue sampling. In theory, tissue could be acquired relatively easily in the outpatient setting and then analyzed to guide optimal therapeutic regimen selection, verify tumor response to systemic targeted therapies prior to HILP/ILI, and even confirm response after regional chemotherapy. The practicality of this approach assumes homogeneity of gene expression across individual tumor nodules in a single patient. Recently, our group tested whether gene expression differed across individual tumor nodules in a single patient using from a gene expression analysis across 55 lesions in 29 patients. Patterns of gene expression were highly similar (P < 0.006; average r = 0.979) across pretreatment lesions from a single patient compared with the significantly different patterns observed across patients (P < 0.05).143
Whether gene expression profiling can predict melphalan and temozolomide sensitivity for in-transit melanoma remains an area of active investigation. For temozolomide, the O6- methylguanine-DNA methyltransferase (MGMT) is considered by many to be the primary resistance mechanism of melanoma cells. We have found quantified MGMT activity and expression across 26 melanoma cells lines correlates highly with temozolomide sensitivity.95 Yet other studies attempting to correlate MGMT activity144–145, expression,146 and promoter methylation147 to temozolomide resistance have produced mixed results. Since multiple cellular events have the potential to alter chemosensitivity of tumor cells, large-scale gene expression profiling, as opposed to single -pathway analyses, could potentially lend more insight on differential temozolomide chemosensitivity. In this regard, we recently tested whether a gene signature derived from larger-scale gene expression analysis could be used to create a more robust predictor of temozolomide resistance as compared to the MGMT activity and expression. Using 60 cell lines from the NCI-60 panel of cancer cell lines, forty-five genes were identified as predictors of temozolomide “resistance” or “sensitivity” and used to create a gene signature of temozolomide resistance. When validated against a separate set of 26 melanoma cells lines, the temozolomide resistance gene signature did significantly correlate with measured temozolomide resistance. This correlation, however, was inferior to that derived from a single analysis of MGMT expression in the same cell lines.95 Whether a temozolomide gene signature or MGMT expression alone best predicts response of in-transit melanoma patients to temozolomide infusion will require validation in a clinical trial.
Creating a melphalan-based ILI signature has been more challenging. Initial attempts of creating a gene signature predictor from RNA extracted from multiple melanoma cell lines have been arduous and have yet to produce a signature which can be validated. More recent attempts using RNA extracted from patient samples have been more fruitful. Using tissue samples, we have correlated 100 genes to response to ILI with melphalan and are currently in the process of validating this gene signature as a predictor of response to melphalan-based regional therapy (unpublished data).
Conclusions
Regional chemotherapy for in-transit melanoma is an effective therapy for melanoma isolated to the extremity with response rates far exceeding those seen with current systemic therapy. ILI is a well-tolerated technique of regional chemotherapy and is considered less technically demanding to perform compared to HILP. Complete response rates are similar for ILI and HILP, but HILP trends to produce a higher overall response rate as well as more durable response. Novel therapeutic agents given systemically with regional ILI may augment the complete rates and improve its therapeutic index. On a grander scale, ILI is an excellent platform for conducting research aimed at improving regional as well as systemic therapy for metastatic melanoma by gaining further insight on the underlying biology of melanoma as well as understanding the mechanisms of action of novel targeted therapies. With a proven and reproducible animal model for ILI,101 novel targeted agents can be readily tested and quickly translated into clinical trials.47
In the future, the regional chemotherapy armamentarium will include agents capable of overcoming classic chemotherapy resistance pathways, normalizing abnormal tumor vasculature, modulating the immunological response, as well as correcting altered cell-signaling pathways which lead to unchecked proliferation and decreased apoptosis. As the efficacy of these agents in augmenting regional chemotherapy to melanoma become more apparent, it will become essential to generate predictors to personalize appropriate therapy for each particular patient. As more trials with targeted agents are performed, patterns of gene and/or protein expression of responders and non-responders can potentially be elucidated to give us a better understanding of how targeted treatments work. The future of regional chemotherapy is not just in augmenting regional tumor responses, but in also serving as a platform to help us develop better therapeutic strategies to utilize in patients with distant metastatic disease.
Acknowledgments
Grant Support: Duke Melanoma Research Fund and VA Merit Review Grant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr Tyler receives grant support from Adherex Technologies. Dr. Tyler is also a coinventor on a patent entitled “Cancer treatment methods using cadherin antagonists in combination with anticancer agents”. The patent application number is 60/848,624 and this patent was filed on 9/27/06. Dr Tyler’s rights to this patent have been signed over to the United States Government. Dr. Tyler has material transfer agreements with Bayer, Schering, and Genta pharmaceuticals. Adherex Technologies funded the phase I and II clinical trials of systemic ADH-1 and regional melphalan. Bayer provided drug only (sorafenib) for the phase I trial of systemic sorafenib and regional melphalan
Contributor Information
Ryan S. Turley, Duke University, Department of Surgery, DUMC 3443, Durham, NC 27710, ryan.turley@duke.edu
Amanda K. Raymond, Duke University, School of Medicine, DUMC 3443, Durham, NC 27710, amanda.raymond@duke.edu
Douglas S. Tyler, Professor of Surgery, Duke University, Department of Surgery, DUMC 3118, Durham, NC 27710, tyler002@duke.edu
References
- 1.Weinstock MA. Do sunscreens increase or decrease melanoma risk: an epidemiologic evaluation. J Investig Dermatol Symp Proc. 1999 Sep;4(1):97–100. doi: 10.1038/sj.jidsp.. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009 Jul-Aug;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.de Vries E, Bray FI, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1953-1997: rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int J Cancer. 2003 Oct 20;107(1):119–126. doi: 10.1002/ijc.11360. [DOI] [PubMed] [Google Scholar]
- 4.Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol. 2005 Aug;12(8):587–596. doi: 10.1245/ASO.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009 Dec 20;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001 Aug 15;19(16):3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 7.Meier F, Will S, Ellwanger U, et al. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol. 2002 Jul;147(1):62–70. doi: 10.1046/j.1365-2133.2002.04867.x. [DOI] [PubMed] [Google Scholar]
- 8.Cascinelli N, Bufalino R, Marolda R, et al. Regional non-nodal metastases of cutaneous melanoma. Eur J Surg Oncol. 1986 Jun;12(2):175–180. [PubMed] [Google Scholar]
- 9.Calabro A, Singletary SE, Balch CM. Patterns of relapse in 1001 consecutive patients with melanoma nodal metastases. Arch Surg. 1989 Sep;124(9):1051–1055. doi: 10.1001/archsurg.1989.01410090061014. [DOI] [PubMed] [Google Scholar]
- 10.Zogakis TG, Bartlett DL, Libutti SK, et al. Factors affecting survival after complete response to isolated limb perfusion in patients with in-transit melanoma. Ann Surg Oncol. 2001 Dec;8(10):771–778. doi: 10.1007/s10434-001-0771-4. [DOI] [PubMed] [Google Scholar]
- 11.Dong XD, Tyler D, Johnson JL, DeMatos P, Seigler HF. Analysis of prognosis and disease progression after local recurrence of melanoma. Cancer. 2000 Mar 1;88(5):1063–1071. doi: 10.1002/(sici)1097-0142(20000301)88:5<1063::aid-cncr17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009 May;23(6):488–496. [PMC free article] [PubMed] [Google Scholar]
- 13.Minor DR, Allen RE, Alberts D, Peng YM, Tardelli G, Hutchinson J. A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer. 1985 Jun 1;55(11):2638–2644. doi: 10.1002/1097-0142(19850601)55:11<2638::aid-cncr2820551118>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Storm FK. Morton DL Value of therapeutic hyperthermic limb perfusion in advanced recurrent melanoma of the lower extremity. Am J Surg. 1985 Jul;150(1):32–35. doi: 10.1016/0002-9610(85)90006-6. [DOI] [PubMed] [Google Scholar]
- 15.Kroon BB, Van Geel AN, Benckhuijsen C, Wieberdink J. Normothermic isolation perfusion with melphalan for advanced melanoma of the limbs. Anticancer Res. 1987 May-Jun;7(3 Pt B):441–442. [PubMed] [Google Scholar]
- 16.Kroon BB, Klaase JM, van Geel BN, Eggermont AM, Franklin HR, van Dongen JA. Results of a double perfusion schedule with melphalan in patients with melanoma of the lower limb. Eur J Cancer. 1993;29A(3):325–328. doi: 10.1016/0959-8049(93)90377-r. [DOI] [PubMed] [Google Scholar]
- 17.Klaase JM, Kroon BB, van Geel AN, Eggermont AM, Franklin HR, Hart AA. Prognostic factors for tumor response and limb recurrence-free interval in patients with advanced melanoma of the limbs treated with regional isolated perfusion with melphalan. Surgery. 1994 Jan;115(1):39–45. [PubMed] [Google Scholar]
- 18.Grunhagen DJ, Brunstein F, Graveland WJ, van Geel AN, de Wilt JH, Eggermont AM. One hundred consecutive isolated limb perfusions with TNF-alpha and melphalan in melanoma patients with multiple in-transit metastases. Ann Surg. 2004 Dec;240(6):939–947. doi: 10.1097/01.sla.0000146147.89667.ed. discussion 947–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aloia TA, Grubbs E, Onaitis M, et al. Predictors of outcome after hyperthermic isolated limb perfusion: role of tumor response. Arch Surg. 2005 Nov;140(11):1115–1120. doi: 10.1001/archsurg.140.11.1115. [DOI] [PubMed] [Google Scholar]
- 20.Cornett WR, McCall LM, Petersen RP, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol. 2006 Sep 1;24(25):4196–4201. doi: 10.1200/JCO.2005.05.5152. [DOI] [PubMed] [Google Scholar]
- 21.Sanki A, Kam PC, Thompson JF. Long-term results of hyperthermic, isolated limb perfusion for melanoma: a reflection of tumor biology. Ann Surg. 2007 Apr;245(4):591–596. doi: 10.1097/01.sla.0000251746.02764.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mian R, Henderson MA, Speakman D, Finkelde D, Ainslie J, McKenzie A. Isolated limb infusion for melanoma: a simple alternative to isolated limb perfusion. Can J Surg. 2001 Jun;44(3):189–192. [PMC free article] [PubMed] [Google Scholar]
- 23.Lindner P, Doubrovsky A, Kam PC, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002 Mar;9(2):127–136. doi: 10.1007/BF02557363. [DOI] [PubMed] [Google Scholar]
- 24.Bonenkamp JJ, Thompson JF, de Wilt JH, Doubrovsky A, de Faria Lima R, Kam PC. Isolated limb infusion with fotemustine after dacarbazine chemosensitisation for inoperable loco-regional melanoma recurrence. Eur J Surg Oncol. 2004 Dec;30(10):1107–1112. doi: 10.1016/j.ejso.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Brady MS, Brown K, Patel A, Fisher C, Marx W. A phase II trial of isolated limb infusion with melphalan and dactinomycin for regional melanoma and soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006 Aug;13(8):1123–1129. doi: 10.1245/ASO.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008 Nov;15(11):3003–3013. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 27.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009 May;208(5):706–715. doi: 10.1016/j.jamcollsurg.2008.12.019. discussion 715–707. [DOI] [PubMed] [Google Scholar]
- 28.Creech O, Jr, Ryan RF, Krementz ET. Regional chemotherapy by isolated perfusion in the treatment of melanoma of the extremities. Plast Reconstr Surg Transplant Bull. 1961 Oct;28:333–346. doi: 10.1097/00006534-196110000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Creech O, Jr, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958 Oct;148(4):616–632. doi: 10.1097/00000658-195810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008 Aug;15(8):2195–2205. doi: 10.1245/s10434-008-9988-9. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998 Apr-May;14(3):238–247. doi: 10.1002/(sici)1098-2388(199804/05)14:3<238::aid-ssu8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JF, Waugh RC, Saw RPM, et al. Isolated limb infusion with melphalan for recurrent limb melanoma: a simple alternative to isolated limb perfusion. Regional Cancer Treat. 1994;7:188–192. [Google Scholar]
- 33.Santillan AA, Delman KA, Beasley GM, et al. Predictive factors of regional toxicity and serum creatine phosphokinase levels after isolated limb infusion for melanoma: a multi-institutional analysis. Ann Surg Oncol. 2009 Sep;16(9):2570–2578. doi: 10.1245/s10434-009-0563-9. [DOI] [PubMed] [Google Scholar]
- 34.Padussis JC, Steerman SN, Tyler DS, Mosca PJ. Pharmacokinetics & drug resistance of melphalan in regional chemotherapy: ILP versus ILI. Int J Hyperthermia. 2008 May;24(3):239–249. doi: 10.1080/02656730701816410. [DOI] [PubMed] [Google Scholar]
- 35.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Factors predictive of acute regional toxicity after isolated limb infusion with melphalan and actinomycin D in melanoma patients. Ann Surg Oncol. 2009 May;16(5):1184–1192. doi: 10.1245/s10434-009-0323-x. [DOI] [PubMed] [Google Scholar]
- 36.Benckhuijsen C, Kroon BB, van Geel AN, Wieberdink J. Regional perfusion treatment with melphalan for melanoma in a limb: an evaluation of drug kinetics. Eur J Surg Oncol. 1988 Apr;14(2):157–163. [PubMed] [Google Scholar]
- 37.Padussis JC, Steerman SN, Tyler DS, Mosca PJ. Pharmacokinetics and drug resistance of melphalan in regional chemotherapy: ILP versus ILI. International Journal of Hyperthermia. 2008;24(3):239–249. doi: 10.1080/02656730701816410. [DOI] [PubMed] [Google Scholar]
- 38.Cavaliere R, Ciocatto EC, Giovanella BC, et al. Selective heat sensitivity of cancer cells Biochemical and clinical studies. Cancer. 1967 Sep;20(9):1351–1381. doi: 10.1002/1097-0142(196709)20:9<1351::aid-cncr2820200902>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Thompson JF, Lai DT, Ingvar C, et al. Maximizing efficacy and minimizing toxicity in isolated limb perfusion for melanoma. Melanoma Res. 1994;4S:45–50. [PubMed] [Google Scholar]
- 40.Wu ZY, Smithers BM, Roberts MS. Tissue and perfusate pharmacokinetics of melphalan in isolated perfused rat hindlimb. J Pharmacol Exp Ther. 1997 Sep;282(3):1131–1138. [PubMed] [Google Scholar]
- 41.Eggermont AM, de Wilt JH, ten Hagen TL. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol. 2003 Jul;4(7):429–437. doi: 10.1016/s1470-2045(03)01141-0. [DOI] [PubMed] [Google Scholar]
- 42.Luce JK. Chemotherapy of malignant melanoma. Cancer. 1972 Dec;30(6):1604–1615. doi: 10.1002/1097-0142(197212)30:6<1604::aid-cncr2820300629>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 43.Roberts MS, Wu ZY, Siebert GA, Thompson JF, Smithers BM. Saturable dose-response relationships for melphalan in melanoma treatment by isolated limb infusion in the nude rat. Melanoma Res. 2001 Dec;11(6):611–618. doi: 10.1097/00008390-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Taber SW, Polk HC., Jr Mortality, major amputation rates, and leukopenia after isolated limb perfusion with phenylalanine mustard for the treatment of melanoma. Ann Surg Oncol. 1997 Jul-Aug;4(5):440–445. doi: 10.1007/BF02305559. [DOI] [PubMed] [Google Scholar]
- 45.Cheng TY, Grubbs E, Abdul-Wahab O, et al. Marked variability of melphalan plasma drug levels during regional hyperthermic isolated limb perfusion. Am J Surg. 2003 Nov;186(5):460–467. doi: 10.1016/j.amjsurg.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 46.Vrouenraets BC, Hart GA, Eggermont AM, et al. Relation between limb toxicity and treatment outcomes after isolated limb perfusion for recurrent melanoma. J Am Coll Surg. 1999 May;188(5):522–530. doi: 10.1016/s1072-7515(99)00018-6. [DOI] [PubMed] [Google Scholar]
- 47.Beasley GM, McMahon N, Sanders G, et al. A phase 1 study of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with locally advanced in-transit malignant melanoma. Cancer. 2009 Jul 27; doi: 10.1002/cncr.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Filippo F, Calabro A, Giannarelli D, et al. Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer. 1989 Jun 15;63(12):2551–2561. doi: 10.1002/1097-0142(19890615)63:12<2551::aid-cncr2820631233>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 49.Engler HS, Sweat RD. Volumetric arm measurements: technique and results. Am Surg. 1962 Jul;28:465–468. [PubMed] [Google Scholar]
- 50.McMahon N, Cheng TY, Beasley GM, et al. Optimizing melphalan pharmacokinetics in regional melanoma therapy: does correcting for ideal body weight alter regional response or toxicity? Ann Surg Oncol. 2009 Apr;16(4):953–961. doi: 10.1245/s10434-008-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson JF, Gianoutsos MP. Isolated limb perfusion for melanoma: effectiveness and toxicity of cisplatin compared with that of melphalan and other drugs. World J Surg. 1992 Mar-Apr;16(2):227–233. doi: 10.1007/BF02071525. [DOI] [PubMed] [Google Scholar]
- 52.Wile AG, Guilmette E, Friedberg H, Mason GR. A model of experimental isolation perfusion using cis-platinum. J Surg Oncol. 1982 Sep;21(1):37–41. doi: 10.1002/jso.2930210110. [DOI] [PubMed] [Google Scholar]
- 53.Aigner K, Hild P, Henneking K, Paul E, Hundeiker M. Regional perfusion with cis-platinum and dacarbazine. Recent Results Cancer Res. 1983;86:239–245. doi: 10.1007/978-3-642-82025-0_40. [DOI] [PubMed] [Google Scholar]
- 54.Roseman JM. Effective management of extremity cancers using cisplatin and etoposide in isolated limb perfusions. J Surg Oncol. 1987 Jul;35(3):170–172. doi: 10.1002/jso.2930350306. [DOI] [PubMed] [Google Scholar]
- 55.Klein ES, Ben-Ari GY. Isolation perfusion with cisplatin for malignant melanoma of the limbs. Cancer. 1987 Mar 15;59(6):1068–1071. doi: 10.1002/1097-0142(19870315)59:6<1068::aid-cncr2820590604>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 56.Pommier RF, Moseley HS, Cohen J, Huang CS, Townsend R, Fletcher WS. Pharmacokinetics, toxicity, and short-term results of cisplatin hyperthermic isolated limb perfusion for soft-tissue sarcoma and melanoma of the extremities. Am J Surg. 1988 May;155(5):667–671. doi: 10.1016/s0002-9610(88)80140-5. [DOI] [PubMed] [Google Scholar]
- 57.Hajarizadeh H, Mueller CR, Woltering EA, Small K, Fletcher WS. Phase I-II trial of hyperthermic isolated limb perfusion with cisplatin in the treatment of high risk malignant melanoma of the extremities. Melanoma Res. 1991 Apr-May;1(1):55–61. doi: 10.1097/00008390-199104000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Santinami M, Belli F, Cascinelli N, Rovini D, Vaglini M. Seven years experience with hyperthermic perfusions in extracorporeal circulation for melanoma of the extremities. J Surg Oncol. 1989 Nov;42(3):201–208. doi: 10.1002/jso.2930420315. [DOI] [PubMed] [Google Scholar]
- 59.Coit DG, Bajorin DF, Menendez-Botet C. A phase I trial of hyperthermic isolation limb perfusion (HILP) using cisplatin (CDDP) for metastatic melanoma. Proceedings of ASCO. 1991;10:294. [Google Scholar]
- 60.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000 Jan;18(1):158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 61.Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist. 2000;5(2):144–151. doi: 10.1634/theoncologist.5-2-144. [DOI] [PubMed] [Google Scholar]
- 62.Ueno T, Ko SH, Grubbs E, et al. Modulation of chemotherapy resistance in regional therapy: a novel therapeutic approach to advanced extremity melanoma using intra-arterial temozolomide in combination with systemic O6-benzylguanine. Mol Cancer Ther. 2006 Mar;5(3):732–738. doi: 10.1158/1535-7163.MCT-05-0098. [DOI] [PubMed] [Google Scholar]
- 63.Yoshimoto Y, Augustine CK, Yoo JS, et al. Defining regional infusion treatment strategies for extremity melanoma: comparative analysis of melphalan and temozolomide as regional chemotherapeutic agents. Mol Cancer Ther. 2007 May;6(5):1492–1500. doi: 10.1158/1535-7163.MCT-06-0718. [DOI] [PubMed] [Google Scholar]
- 64.Yao KA, Hsueh EC, Essner R, Foshag LJ, Wanek LA, Morton DL. Is sentinel lymph node mapping indicated for isolated local and in-transit recurrent melanoma? Ann Surg. 2003 Nov;238(5):743–747. doi: 10.1097/01.sla.0000094440.50547.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alexander HR, Jr, Fraker DL, Bartlett DL, et al. Analysis of factors influencing outcome in patients with in-transit malignant melanoma undergoing isolated limb perfusion using modern treatment parameters. J Clin Oncol. 2010 Jan 1;28(1):114–118. doi: 10.1200/JCO.2009.23.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dahl O, Mella O. Hyperthermia and chemotherapeutic agents. New York: Taylor and Francis; 1990. [Google Scholar]
- 67.Hahn GM. Potential for therapy of drugs and hyperthermia. Cancer Res. 1979 Jun;39(6 Pt 2):2264–2268. [PubMed] [Google Scholar]
- 68.Wallah DFH. Basic mechanisms of tumor thermotherapy. J. Mol. Med. 1977;17:381–403. [Google Scholar]
- 69.Huang SK, Stauffer PR, Hong K, et al. Liposomes and hyperthermia in mice: increased tumor uptake and therapeutic efficacy of doxorubicin in sterically stabilized liposomes. Cancer Res. 1994 Apr 15;54(8):2186–2191. [PubMed] [Google Scholar]
- 70.Stehlin JS., Jr Hyperthermic perfusion with chemotherapy for cancers of the extremities. Surg Gynecol Obstet. 1969 Aug;129(2):305–308. [PubMed] [Google Scholar]
- 71.Ghussen F, Kruger I, Smalley RV, Groth W. Hyperthermic perfusion with chemotherapy for melanoma of the extremities. World J Surg. 1989 Sep-Oct;13(5):598–602. doi: 10.1007/BF01658878. [DOI] [PubMed] [Google Scholar]
- 72.Ko SH, Ueno T, Yoshimoto Y, et al. Optimizing a novel regional chemotherapeutic agent against melanoma: hyperthermia-induced enhancement of temozolomide cytotoxicity. Clin Cancer Res. 2006 Jan 1;12(1):289–297. doi: 10.1158/1078-0432.CCR-05-0210. [DOI] [PubMed] [Google Scholar]
- 73.Abdel-Wahab OI, Grubbs E, Viglianti BL, et al. The role of hyperthermia in regional alkylating agent chemotherapy. Clin Cancer Res. 2004 Sep 1;10(17):5919–5929. doi: 10.1158/1078-0432.CCR-04-0096. [DOI] [PubMed] [Google Scholar]
- 74.Posner MC, Lienard D, Lejeune FJ, Rosenfelder D, Kirkwood J. Hyperthermic isolated limb perfusion with tumor necrosis factor alone for melanoma. Cancer J Sci Am. 1995 Nov-Dec;1(4):274–280. [PubMed] [Google Scholar]
- 75.Brouckaert PG, Leroux-Roels GG, Guisez Y, Tavernier J, Fiers W. In vivo anti-tumour activity of recombinant human and murine TNF, alone and in combination with murine IFN-gamma, on a syngeneic murine melanoma. Int J Cancer. 1986 Nov 15;38(5):763–769. doi: 10.1002/ijc.2910380521. [DOI] [PubMed] [Google Scholar]
- 76.Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992 Jan;10(1):52–60. doi: 10.1200/JCO.1992.10.1.52. [DOI] [PubMed] [Google Scholar]
- 77.Lejeune FJ, Eggermont AM. Hyperthermic isolated limb perfusion with tumor necrosis factor is a useful therapy for advanced melanoma of the limbs. J Clin Oncol. 2007 Apr 10;25(11):1449–1450. doi: 10.1200/JCO.2006.09.8459. author reply 1450-1441. [DOI] [PubMed] [Google Scholar]
- 78.Garrido-Laguna I, Ponz M, Espinos J. Is there any reason to delay introduction of tumor necrosis factor in the management of in-transit metastasis of unresectable melanoma? J Clin Oncol. 2007 Mar 20;25(9):1149–1151. doi: 10.1200/JCO.2006.09.4862. 1149; author reply. [DOI] [PubMed] [Google Scholar]
- 79.Depeille P, Cuq P, Passagne I, Evrard A. Vian L Combined effects of GSTP1 and MRP1 in melanoma drug resistance. Br J Cancer. 2005 Jul 25;93(2):216–223. doi: 10.1038/sj.bjc.6602681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harkey MA, Czerwinski M, Slattery J, Kiem HP. Overexpression of glutathione-S-transferase, MGSTII, confers resistance to busulfan and melphalan. Cancer Invest. 2005;23(1):19–25. [PubMed] [Google Scholar]
- 81.Grubbs EG, Abdel-Wahab O, Cheng TY, et al. In-transit melanoma: the role of alkylating-agent resistance in regional therapy. J Am Coll Surg. 2004 Sep;199(3):419–427. doi: 10.1016/j.jamcollsurg.2004.05.271. [DOI] [PubMed] [Google Scholar]
- 82.Buller AL, Clapper ML, Tew KD. Glutathione S-transferases in nitrogen mustard-resistant and -sensitive cell lines. Mol Pharmacol. 1987 Jun;31(6):575–578. [PubMed] [Google Scholar]
- 83.Suzukake K, Petro BJ, Vistica DT. Reduction in glutathione content of L-PAM resistant L1210 Cells confers drug sensitivity. Biochem Pharmacol. 1982 Jan 1;31(1):121–124. doi: 10.1016/0006-2952(82)90249-0. [DOI] [PubMed] [Google Scholar]
- 84.Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003 Oct 20;22(47):7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okamura T, Singh S, Buolamwini J, et al. Tyrosine phosphorylation of the human glutathione S-transferase P1 by epidermal growth factor receptor. J Biol Chem. 2009 Jun 19;284(25):16979–16989. doi: 10.1074/jbc.M808153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McClean S, Hill BT. An overview of membrane, cytosolic and nuclear proteins associated with the expression of resistance to multiple drugs in vitro. Biochim Biophys Acta. 1992 Dec 16;1114(2–3):107–127. doi: 10.1016/0304-419x(92)90010-v. [DOI] [PubMed] [Google Scholar]
- 87.Suzukake K, Vistica BP, Vistica DT. Dechlorination of L-phenylalanine mustard by sensitive and resistant tumor cells and its relationship to intracellular glutathione content. Biochem Pharmacol. 1983 Jan 1;32(1):165–167. doi: 10.1016/0006-2952(83)90671-8. [DOI] [PubMed] [Google Scholar]
- 88.Strumeyer DH, Bloch K. Some properties of gamma-glutamylcysteine synthetase. J Biol Chem. 1960 Jun;235:PC27. [PubMed] [Google Scholar]
- 89.Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- 90.Biroccio A, Benassi B, Fiorentino F, Zupi G. Glutathione depletion induced by c-Myc downregulation triggers apoptosis on treatment with alkylating agents. Neoplasia. 2004 May-Jun;6(3):195–206. doi: 10.1593/neo.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McIlwain CC, Townsend DM, Tew KD. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006 Mar 13;25(11):1639–1648. doi: 10.1038/sj.onc.1209373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pellizzari Tregno F, Sau A, Pezzola S, et al. In vitro and in vivo efficacy of 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX) on human melanoma. Eur J Cancer. 2009 Sep;45(14):2606–2617. doi: 10.1016/j.ejca.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 93.Toshimitsu H, Yoshimoto Y, Augustine CK, et al. Inhibition of Poly(ADP-Ribose) Polymerase Enhances the Effect of Chemotherapy in an Animal Model of Regional Therapy for the Treatment of Advanced Extremity Malignant Melanoma. Ann Surg Oncol. 2010 Feb 24; doi: 10.1245/s10434-010-0971-x. [DOI] [PubMed] [Google Scholar]
- 94.Bignami M, O'Driscoll M, Aquilina G, Karran P. Unmasking a killer: DNA O(6)-methylguanine and the cytotoxicity of methylating agents. Mutat Res. 2000 Apr;462(2–3):71–82. doi: 10.1016/s1383-5742(00)00016-8. [DOI] [PubMed] [Google Scholar]
- 95.Augustine CK, Yoo JS, Potti A, et al. Genomic and molecular profiling predicts response to temozolomide in melanoma. Clin Cancer Res. 2009 Jan 15;15(2):502–510. doi: 10.1158/1078-0432.CCR-08-1916. [DOI] [PubMed] [Google Scholar]
- 96.Pegg AE. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990 Oct 1;50(19):6119–6129. [PubMed] [Google Scholar]
- 97.Cheng CL, Johnson SP, Keir ST, et al. Poly(ADP-ribose) polymerase-1 inhibition reverses temozolomide resistance in a DNA mismatch repair-deficient malignant glioma xenograft. Mol Cancer Ther. 2005 Sep;4(9):1364–1368. doi: 10.1158/1535-7163.MCT-05-0128. [DOI] [PubMed] [Google Scholar]
- 98.Boulton S, Pemberton LC, Porteous JK, et al. Potentiation of temozolomide-induced cytotoxicity: a comparative study of the biological effects of poly(ADP-ribose) polymerase inhibitors. Br J Cancer. 1995 Oct;72(4):849–856. doi: 10.1038/bjc.1995.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haince JF, Rouleau M, Hendzel MJ, Masson JY, Poirier GG. Targeting poly(ADP-ribosyl)ation: a promising approach in cancer therapy. Trends Mol Med. 2005 Oct;11(10):456–463. doi: 10.1016/j.molmed.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 100.Tentori L, Leonetti C, Scarsella M, et al. Systemic administration of GPI 15427, a novel poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clin Cancer Res. 2003 Nov 1;9(14):5370–5379. [PubMed] [Google Scholar]
- 101.Augustine CK, Yoshimoto Y, Gupta M, et al. Targeting N-cadherin enhances antitumor activity of cytotoxic therapies in melanoma treatment. Cancer Res. 2008 May 15;68(10):3777–3784. doi: 10.1158/0008-5472.CAN-07-5949. [DOI] [PubMed] [Google Scholar]
- 102.Hsu MY, Wheelock MJ, Johnson KR, Herlyn M. Shifts in cadherin profiles between human normal melanocytes and melanomas. J Investig Dermatol Symp Proc. 1996 Apr;1(2):188–194. [PubMed] [Google Scholar]
- 103.Hsu MY, Meier FE, Nesbit M, et al. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol. 2000 May;156(5):1515–1525. doi: 10.1016/S0002-9440(10)65023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beasley G, Riboh JC, Augustine CK, Zager JS, Hochwald SN, Grobmyer SR, Peterson B, Royal R, Ross MI, Tyler DS. A Prospective Multi-Center Phase II Trial of Systemic ADH-1 in Combination with Melphalan via Isolated Limb Infusion (M-ILI) in Patients with Advanced Extremity Melanoma. In Submission. 2010 doi: 10.1200/JCO.2010.32.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab Alone and in Combination With Irinotecan in Recurrent Glioblastoma. J Clin Oncol. 2009 Aug 31; doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 106.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004 Jun 3;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 107.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007 Dec 27;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 108.Pallis AG, Serfass L, Dziadziusko R, et al. Targeted therapies in the treatment of advanced/metastatic NSCLC. Eur J Cancer. 2009 Sep;45(14):2473–2487. doi: 10.1016/j.ejca.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 109.Woolam GL. Cancer statistics, 2000: a benchmark for the new century. CA Cancer J Clin. 2000 Jan-Feb;50(1):6. doi: 10.3322/canjclin.50.1.6. [DOI] [PubMed] [Google Scholar]
- 110.Hainsworth JD, Infante JR, Spigel DR, et al. Bevacizumab and everolimus in the treatment of patients with metastatic melanoma: a phase 2 trial of the Sarah Cannon Oncology Research Consortium. Cancer. 2010 May 28; doi: 10.1002/cncr.25320. [DOI] [PubMed] [Google Scholar]
- 111.Vihinen PP, Hernberg M, Vuoristo MS, et al. A phase II trial of bevacizumab with dacarbazine and daily low-dose interferon-alpha2a as first line treatment in metastatic melanoma. Melanoma Res. 2010 Apr 1; doi: 10.1097/CMR.0b013e3283390365. [DOI] [PubMed] [Google Scholar]
- 112.Perez DG, Suman VJ, Fitch TR, et al. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group study, N047A. Cancer. 2009 Jan 1;115(1):119–127. doi: 10.1002/cncr.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smalley KS, Herlyn M. Targeting intracellular signaling pathways as a novel strategy in melanoma therapeutics. Ann N Y Acad Sci. 2005 Nov;1059:16–25. doi: 10.1196/annals.1339.005. [DOI] [PubMed] [Google Scholar]
- 114.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007 Feb 22;445(7130):851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 115.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002 Jun 27;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 116.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445(7130):851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 117.Lang L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology. 2008 Feb;134(2):379. doi: 10.1053/j.gastro.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 118.Stein MN, Flaherty KT. CCR drug updates: sorafenib and sunitinib in renal cell carcinoma. Clin Cancer Res. 2007 Jul 1;13(13):3765–3770. doi: 10.1158/1078-0432.CCR-06-2844. [DOI] [PubMed] [Google Scholar]
- 119.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008 Oct;7(10):3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 120.Panka DJ, Wang W, Atkins MB, Mier JW. The Raf inhibitor BAY 43–9006 (sorafenib) induces caspase-independent apoptosis in melanoma cells. Cancer Res. 2006 February 1;66(3):1611–1619. doi: 10.1158/0008-5472.CAN-05-0808. 2006. [DOI] [PubMed] [Google Scholar]
- 121.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43–9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J. Biol.Chem. 2005 October 21;280(42):35217–35227. doi: 10.1074/jbc.M506551200. 2005. [DOI] [PubMed] [Google Scholar]
- 122.Yu C, Bruzek LM, Meng XW, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43–9006. Oncogene. 2005 Oct 20;24(46):6861–6869. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 123.Gray-Schopfer VC, Karasarides M, Hayward R, Marais R. Tumor necrosis factor-alpha blocks apoptosis in melanoma cells when BRAF signaling is inhibited. Cancer Res. 2007 Jan 1;67(1):122–129. doi: 10.1158/0008-5472.CAN-06-1880. [DOI] [PubMed] [Google Scholar]
- 124.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005 March 15;65(6):2412–2421. doi: 10.1158/0008-5472.CAN-04-2423. 2005. [DOI] [PubMed] [Google Scholar]
- 125.Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–586. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Escudier B, Lassau N, Angevin E, et al. Phase I trial of sorafenib in combination with IFN {alpha}-2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma. Clin.Cancer. Res. 2007 March 15;13(6):1801–1809. doi: 10.1158/1078-0432.CCR-06-1432. 2007. [DOI] [PubMed] [Google Scholar]
- 127.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003 Feb 1;101(3):1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 128.McDermott DF, Sosman JA, Gonzalez R, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients With advanced melanoma: A report from the 11715 Study Group. J. Clin. Oncol. 2008 May 1;26(13):2178–2185. doi: 10.1200/JCO.2007.14.8288. 2008. [DOI] [PubMed] [Google Scholar]
- 129.Amaravadi RK, Schuchter LM, McDermott DF, et al. Phase II Trial of Temozolomide and Sorafenib in Advanced Melanoma Patients with or without Brain Metastases. Clin Cancer Res. 2009 Dec;8 doi: 10.1158/1078-0432.CCR-09-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009 Jun 10;27(17):2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 131.Augustine CK, Toshimitsu H, Jung SH, et al. Sorafenib, a Multikinase Inhibitor, Enhances the Response of Melanoma to Regional Chemotherapy. Mol Cancer Ther. 2010 Jun 22; doi: 10.1158/1535-7163.MCT-10-0073. [DOI] [PubMed] [Google Scholar]
- 132.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004 Jun;4(6):470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 133.Niu G, Bowman T, Huang M, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002 Oct 10;21(46):7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 134.Niu G, Wright KL, Huang M, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002 Mar 27;21(13):2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 135.Brave M, Goodman V, Kaminskas E, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res. 2008 Jan 15;14(2):352–359. doi: 10.1158/1078-0432.CCR-07-4175. [DOI] [PubMed] [Google Scholar]
- 136.Homsi J, Cubitt CL, Zhang S, et al. Src activation in melanoma and Src inhibitors as therapeutic agents in melanoma. Melanoma Res. 2009 Jun;19(3):167–175. doi: 10.1097/CMR.0b013e328304974c. [DOI] [PubMed] [Google Scholar]
- 137.Yuan J, Gnjatic S, Li H, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008 Dec 23;105(51):20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alexandrescu DT, Ichim TE, Riordan NH, et al. Immunotherapy for melanoma: current status and perspectives. J Immunother. 2010 Jul-Aug;33(6):570–590. doi: 10.1097/CJI.0b013e3181e032e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barbour AP, Thomas J, Suffolk J, Beller E, Smithers BM. Isolated limb infusion for malignant melanoma: predictors of response and outcome. Ann Surg Oncol. 2009 Dec;16(12):3463–3472. doi: 10.1245/s10434-009-0717-9. [DOI] [PubMed] [Google Scholar]
- 140.Bild A, Febbo PG. Application of a priori established gene sets to discover biologically important differential expression in microarray data. Proc Natl Acad Sci U S A. 2005 Oct 25;102(43):15278–15279. doi: 10.1073/pnas.0507477102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.John T, Black MA, Toro TT, et al. Predicting clinical outcome through molecular profiling in stage III melanoma. Clin Cancer Res. 2008 Aug 15;14(16):5173–5180. doi: 10.1158/1078-0432.CCR-07-4170. [DOI] [PubMed] [Google Scholar]
- 142.Winnepenninckx V, Lazar V, Michiels S, et al. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006 Apr 5;98(7):472–482. doi: 10.1093/jnci/djj103. [DOI] [PubMed] [Google Scholar]
- 143.Augustine CK, Jung SH, Sohn I, et al. Gene expression signatures as a guide to treatment strategies for in-transit metastatic melanoma. Mol Cancer Ther. 2010 Apr;9(4):779–790. doi: 10.1158/1535-7163.MCT-09-0764. [DOI] [PubMed] [Google Scholar]
- 144.Middleton MR, Lunn JM, Morris C, et al. O6-methylguanine-DNA methyltransferase in pretreatment tumour biopsies as a predictor of response to temozolomide in melanoma. Br J Cancer. 1998 Nov;78(9):1199–1202. doi: 10.1038/bjc.1998.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Middleton MR, Lee SM, Arance A, Wood M, Thatcher N, Margison GP. O6-methylguanine formation, repair protein depletion and clinical outcome with a 4 hr schedule of temozolomide in the treatment of advanced melanoma: results of a phase II study. Int J Cancer. 2000 Nov 1;88(3):469–473. [PubMed] [Google Scholar]
- 146.Ma S, Egyhazi S, Martenhed G, Ringborg U, Hansson J. Analysis of O(6)-methylguanine-DNA methyltransferase in melanoma tumours in patients treated with dacarbazine-based chemotherapy. Melanoma Res. 2002 Aug;12(4):335–342. doi: 10.1097/00008390-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 147.Rietschel P, Wolchok JD, Krown S, et al. Phase II study of extended-dose temozolomide in patients with melanoma. J Clin Oncol. 2008 May 10;26(14):2299–2304. doi: 10.1200/JCO.2007.14.5292. [DOI] [PubMed] [Google Scholar]