SUMMARY
T cells can reject established tumors when adoptively transferred into patients, thereby demonstrating the power of the immune system for cancer therapy. However, it has proven difficult to maintain adoptively transferred T cells in the long term. Vaccines have the potential to induce tumor-specific effector and memory T cells. However, clinical efficacy of current vaccines is limited, possibly because tumors skew the immune system by means of myeloid-derived suppressor cells, inflammatory type 2 T cells and regulatory T cells (Tregs), all of which prevent the generation of effector cells. To improve the clinical efficacy of cancer vaccines in patients with metastatic disease, we need to design novel and improved strategies that can boost adaptive immunity to cancer, help overcome Tregs and allow the breakdown of the immunosuppressive tumor microenvironment. This can be achieved by exploiting the fast increasing knowledge about the dendritic cell (DC) system, including the existence of distinct DC subsets which respond differentially to distinct activation signals, (functional plasticity), both contributing to the generation of unique adaptive immune responses. We foresee that these novel cancer vaccines will be used as monotherapy in patients with resected disease, and in combination with drugs targeting regulatory/suppressor pathways in patients with metastatic disease.
Keywords: dendritic cells, cancer, vaccines, T cells
INTRODUCTION
The immune system is able to control cancer both in mice (1, 2) and humans (reviewed in (3)). Perhaps the most compelling evidence of tumor immunosurveillance is provided by the studies in breast cancer and paraneoplastic diseases. Onconeural antigens, which are normally expressed on neurons, immune privileged sites, are also expressed in some cases of breast cancer (4). In these patients, a strong antigen-specific CD8+ T cell response is generated, which provides effective tumor control but also an autoreactive neurologic disease, paraneoplastic cerebellar degeneration (5). In another example of tumor immunosurveillance, patients with pre-malignant monoclonal gammopathy of undetermined significance (MGUS) frequently display immune response against SOX2 (a gene critical for self-renewal in embryonal stem cells) (6). On the contrary, patients with malignancy such as multiple myeloma (MM) lack anti-SOX2 immunity (6).
Nevertheless, in the majority of cases, natural immunity to cancer is not protective, highlighting the need to develop strategies to boost patient resistance to cancer. This has been facilitated by the molecular identification of human cancer antigens, which in turn allowed the development of antigen specific immunotherapy (7–9). One strategy is adoptive T cell therapy (reviewed in (10, 11)). There, autologous antigen specific T cells are expanded ex vivo and reinfused to patients. Adoptive T cell therapy has been shown to be an effective treatment for EBV-associated lymphomas (12) and has induced tumor regression in patients with solid tumors (13, 14). Another strategy is to expand T cells in vivo by means of vaccination.
CANCER VACCINES: LESSONS FROM THE PAST AND KEY RECENT PROGRESS
Active immunization has been a successful strategy for the prevention of infectious diseases (15). One example showing great promise with regards to cancer is the prevention of HPV-positive cervical cancer by vaccinating with a recombinant viral capsid protein (16). Therapeutic vaccination is more difficult possibly because most cancer antigens are non-mutated self-proteins and thus the repertoire is depleted of high avidity clones through negative selection (17, 18). Numerous approaches for the therapeutic vaccination of humans with cancer have been developed including: autologous and allogeneic tumor cells (which are often modified to express various cytokines), peptides, proteins and DNA vaccines (reviewed in (19)). The observed results have been variable, yet in many cases, a tumor-specific immune response could be measured. The clinical efficacy of therapeutic vaccination in cancer has been questioned (20) because of the limited rate of objective tumor regressions observed in clinical trials. At least two issues need to be considered: 1) the quality of immune responses that these early cancer vaccines were capable of eliciting; this will be discussed later; and 2) definitions of clinical endpoints allowing assessment of the clinical efficacy of immunotherapy. The latter ones have been challenged by recent clinical trials testing anti-CTLA4 (ipilumimab) in patients with stage IV melanoma. There, in a randomized phase III clinical trial an improved overall survival in patients who received anti-CTLA4 was observed (21). In another indication an active immunotherapy product, sipuleucel-T (APC8015), based on the PBMCs activated with a fusion protein of prostate cancer antigen such as prostatic acid phosphatase PAP with GM-CSF, resulted in approximately 4 month-prolonged median survival in phase III trials in patients with prostate cancer (22). In both studies, the analysis of survival curves shows the separation only after 4–6 months suggesting a certain delay in the treatment effect. These clinical trials, therefore, bring forward basic principles of active immunotherapy which set this treatment modality apart from chemotherapy, radiotherapy, targeted therapies and even adoptive T cell transfer. Thus, during the time in which it takes to build tumor immunity tumors might progress before they actually regress; and tumors might appear clinically enlarged due to inflammation associated with active immune responses and lymphocyte infiltration. Thus, one of the lessons is that overall survival might be the only true parameter of clinical efficacy.
Nevertheless, cancer vaccines are entering a renaissance era prompted by a series of recent clinical trials showing promising clinical outcomes. Thus, sipuleucel-T discussed above has been approved by the FDA for treatment of metastatic prostate cancer thereby paving the clinical development and regulatory path for the next generation of active immunotherapy products. A randomized phase II trial of a poxviral-based vaccine targeting PSA (PROSTVAC) in men with metastatic castration-resistant prostate cancer showed improved overall survival in patients who received PROSTVAC compared to patients receiving control vectors (23). The list also includes positive reports from phase III trials in 1) follicular lymphoma testing idiotype vaccine therapy (BiovaxID); and 2) melanoma testing peptide vaccine in combination with IL-2 (24, 25). While these first generation positive randomized phase II/III clinical trials need further analysis and mechanistic studies, they underline the therapeutic potential of cancer vaccines.
Vaccines act through DCs which induce, regulate and maintain T cell immunity (15, 26). Therefore, understanding the DC system is essential for the design of novel cancer vaccines with improved clinical efficacy. Ex vivo-generated DCs have been used as therapeutic vaccines in patients with metastatic cancer for over a decade and early studies have been reviewed elsewhere (27, 28). Importantly, a number of studies have shown that DCs can expand in patients T cells specific for non-mutated self proteins that are over-expressed in cancer. As we will discuss below, the past five years have brought key findings relevant to DC biology and increased our understanding of how DCs regulate immune responses. This together with key progresses in tumor immunology and unraveling molecular pathways regulating T cell immunity (for example, CTLA-4 (29) and PD1 (30)), will allow us to refine and improve the immunogenicity and clinical efficacy of DC vaccination.
BUILDING ON DENDRITIC CELL SUBSETS FOR IMPROVED CANCER VACCINES
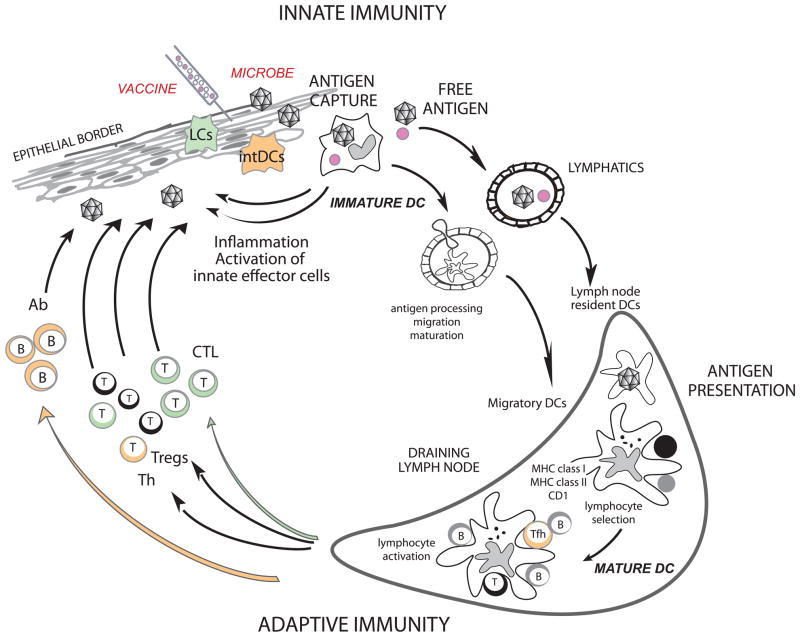
T cell priming is under the control of DCs (Figure 1). In the steady state, non-activated (immature) DCs present self-antigens to T cells, which leads to tolerance (31, 32). DCs induce immune tolerance in a number of ways including i) T cell deletion (33–35); ii) the induction of T cell unresponsiveness (36); and iii) the activation of regulatory T cells (Tregs) (37–40). Once activated (mature), antigen-loaded DCs are geared towards the launching of antigen-specific immunity (41, 42) leading to T cell proliferation and differentiation into helper and effector cells (Figure 1). DCs are also important in launching humoral immunity partly due to their capacity to directly interact with B cells (43, 44) and to present unprocessed antigens (45–48).
Figure 1. Dendritic cells.
DCs reside in the tissue where they are poised to capture antigens (118), be it microbes or vaccines. DCs recognize microbes/vaccines, and secrete cytokines (e.g. IFN-α), directly through pattern recognition receptors, or indirectly through stromal cells that sense microbes/vaccines. Cytokines secreted by DCs in turn activate effector cells of innate immunity such as eosinophils, macrophages and NK cells. Activation triggers DCs migration towards secondary lymphoid organs and simultaneous activation (maturation). These migratory DCs display antigens in the context of classical MHC class I and class II or non-classical CD1 molecules, which allow selection of rare antigen-specific T lymphocytes. Activated T cells drive DCs towards their terminal maturation, which induces further expansion and differentiation of lymphocytes. Activated T lymphocytes traverse inflamed epithelia and reach the injured tissue, where they eliminate microbes and/or microbe-infected cells. B cells, activated by DCs and T cells, differentiate into plasma cells that produce antibodies against the initial pathogen. Antigen can also drain into lymph nodes without involvement of peripheral tissue DCs and be captured and presented by lymph node resident DCs (119). Antigen capture by interstitial DCs (intDCs; orange) will preferentially lead to generation of humoral immunity whereas antigen capture by Langerhans cells (LCs; green) will preferentially lead to generation of cellular immunity (53).
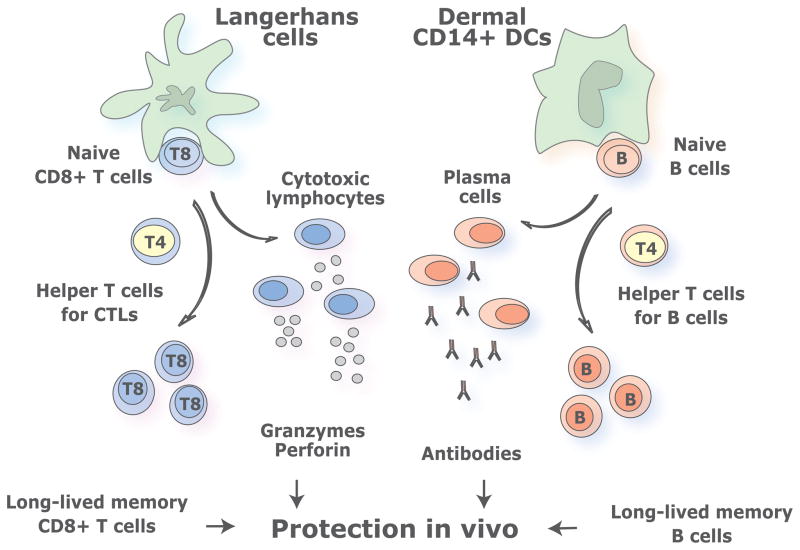
DCs are endowed with two critical features: subsets and functional plasticity (15). This diversity permits the adaptive immune system to mount functionally distinct types of responses (Figure 2). The two major DC subsets are the myeloid DCs (mDCs) and the plasmacytoid DCs (pDCs). pDCs are considered the front line in anti-viral immunity owing to their capacity to rapidly produce high amounts of type I interferon in response to viruses (49, 50). Human pDCs, in fact, are composed of two subsets with different functional properties, distinguished by the expression of CD2 (51). CD2high pDCs and CD2low pDCs display distinct transcription profiles, differential secretion of IL12 p40 and differential expression of co-stimulatory molecule CD80 on activation. The role of pDCs in active immunotherapy of cancer is largely undefined. mDCs are also composed of subsets displaying different phenotype and functions. For example, in human skin, epidermis hosts only Langerhans Cells (LCs) while the dermis contains two mDC subsets, CD1a+ DCs and CD14+ DCs, as well as macrophages (52–55). CD14+ dermal DCs specialize in the generation of humoral immunity with IL-12 being the major cytokine involved (53, 56–58), whereas LCs specialize in the priming of high avidity antigen-specific CD8+ T cells (53). Another mDC subset, BDCA-3+ DCs, present in blood and secondary lymphoid organs, was recently proposed to be the equivalent of mouse CD8+ DC subset (59–62). Accordingly, BDCA-3+ DCs can secrete IL-12, and cross-present exogenous antigens to CD8+ T cells. In line with this, the combination of cytokines used to differentiate monocytes into DCs, for the purpose of vaccination, play a critical role in determining the quality of the elicited T cell responses. For example, DCs generated with GM-CSF and IL-15 display the phenotype and characteristics of LCs. In particular, they are more efficient in priming melanoma-antigen specific CD8+ T cells in vitro than DCs derived with GM-CSF and IL-4 (63, 64). Thus, vaccination with IL15-DCs might elicit stronger CD8+ T cell responses that may lead to improved clinical responses. The selection of methods for activating DCs also represents a critical parameter in the design of DC vaccines. For example, IL-4 DCs activated with a cocktail of IFN-α, poly:IC, IL-1β, TNF, and IFN-γ induce up to 40 times more melanoma-specific CTLs in vitro than DCs matured with the “standard” cocktail of IL-1β/TNF/IL-6/prostaglandinE2 (PGE2)(65–67). Studies with the new generation of ex vivo DC vaccines will permit us to assess the type of immune responses elicited by human DCs generated in different cytokine environments in vivo.
Figure 2. Distinct DC subsets generate distinct types of T cell immunity.
DC system has two cardinal features: 1) subsets; and 2) plasticity. This yields distinct types of immunity thereby allowing DCs to cope with protection against a variety of microbes and maintenance of tolerance to self. Understanding these two features is fundamental to develop vaccines that elicit the desired type of immune responses. Novel vaccines rely on rational immunological approaches and aim at activating both the cellular and the humoral arm. We envision that targeting antigens and activation of distinct mDC subsets, with different specializations, will result in the generation of a broad and long lived immune protection. Thus, the most efficient vaccines might be those that will target both LCs and dermal CD14+ DCs thereby allowing the maximal stimulation of cellular and humoral immune responses and the generation of long-term memory protection.
Characterization of distinct DC subsets is in turn essential for building a novel in vivo approach to vaccination where antigens are directly delivered to DCs using chimeric proteins made of an anti-DC receptor antibody fused to a selected antigen (DC targeting). Pioneering studies in mice demonstrated that the specific targeting of model antigens to DCs in vivo results in considerable potentiation of antigen-specific CD4+ and CD8+ T cell immunity. The induction of immunity is observed only when the DC maturation signal is provided (31, 68, 69), and otherwise, tolerance ensues (31). These studies have already been extended to demonstrate the generation of therapeutic anti-tumor immunity (70, 71) in animal models through the targeting of tumor antigens to mDCs including LCs (72) (73). Furthermore, targeting both tumor and control antigens to human DCs ex vivo can lead to efficient antigen presentation and generation of CD4+ T cell (74, 75) and CD8+ T cell (76, 77) responses.
DENDRITIC CELLS IN TUMOR ENVIRONMENT
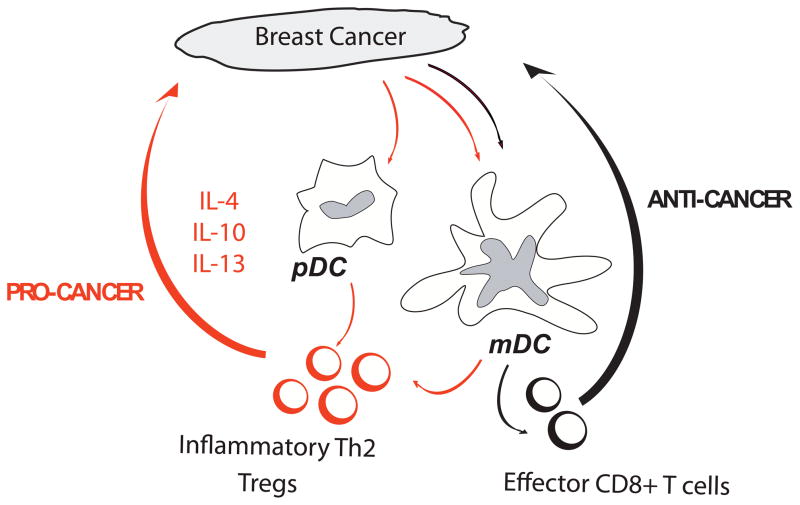
Essential to the success of next generation cancer vaccines based on fusion proteins targeting DCs in vivo is the understanding of the biology of DCs in the tumor environment. Numerous studies in humans have concluded that DCs can infiltrate tumors. We found that breast cancer tumor beds are infiltrated with immature DCs. In contrast, mature DCs are found in the peri-tumoral areas in ~60% of cases (78). A number of studies have suggested that DCs can contribute to tumor development. Our studies in breast cancer indicate that tumor cells polarize mDCs into a state that drives the differentiation of naïve CD4+ T cells into IL-13-secreting T cells (79). These Type 2 T cells in turn facilitate breast tumor development in xenograft model as it can be partly inhibited by administration of IL-13 antagonists (Figure 3). The role of Th2 cells was further established in a spontaneous mouse breast cancer model, where Th2 cells facilitate the development of lung metastasis through macrophage activation (80). In several other mouse tumor models, IL-13 produced by NKT cells induces myeloid cells to make TGF-β that inhibits CTL functions (81). Thus, type 2 cytokines are involved in tumorigenesis through various mechanisms. mDCs can also have direct interactions with tumor cells as shown in multiple myeloma where they directly promote the survival and clonogenicity of tumor cells (82, 83).
Figure 3. DCs in tumor environment.
Cancer cells attract immature DC possibly through chemokines such as MIP3 alpha and/or SDF-1. The DC can then be either blocked or skewed in their maturation, for example by VEGF, leading to induction of polarized CD4+T cells that promote the expansion of cancer cells (pro-cancer) at the expense of CD8+T cells that can cause tumor regression (anti-cancer). An interesting strategy would be to rewire their molecular pathways from “pro-cancer” DCs into “anti-cancer” DCs for example with antibodies or DC activators.
pDCs have been found in approximately 10% of breast carcinomas and are associated with poor prognosis (84). The infiltrating pDCs produce little type I IFN upon TLR ligation (85). This inhibition appears to depend on the ligation of ILT7 on pDCs binding by BST2 expressed on tumor cells (86). Likewise, in ovarian carcinoma, tumor-infiltrating pDCs do not induce effector CD8+ T cell responses, but rather promote the differentiation of IL10+ CCR7+ CD8+ Tregs (87). Finally, pDCs may promote tumor angiogenesis by the secretion of proangiogenic cytokines (88, 89).
DC can fight back tumors at least through two pathways: an indirect one with the induction of potent CTL responses, and a direct one through DC-dependent tumor cytotoxicity. For example, pDCs appear to directly contribute to the anti-tumor activity of in vivo-administered Imiquimod (TLR7 ligand), which is used for the treatment of basal cell carcinoma (90–92).
Clearly, understanding the functions of DCs in the tumor bed represents an important area of future investigations and exploitation for therapy. An interesting strategy would be to rewire their molecular pathways from “pro-tumor” DCs into “anti-tumor” DCs.
THE QUALITY OF IMMUNE RESPONSES
Four components of the immune response emerge as critical to whether the induced response will be therapeutic: 1) the quality of elicited CTLs; 2) the quality of induced CD4+ helper T cells; 3) the elimination and/or non-activation of Tregs; and 4) the breakdown of the immunosuppressive tumor microenvironment. Indeed, the immune responses elicited by the first generation DC vaccines might not be of the quality required to allow the rejection of bulky tumors. For example, the induced CD8+ T cells might not migrate into the tumor lesions (18, 93). Furthermore, low avidity CD8+ T cells might not be able to recognize peptide-MHC class I complexes on tumor cells and/or to kill them (18). Finally, the tumor micro-environment might inhibit effector CD8+ T cell functions, for example, by action of myeloid-derived suppressor cells and Tregs as summarized in recent reviews, respectively (94, 95). Besides the quality of CD8+ T cells, the quality of CD4+ T cells is one of the key parameters of immune efficacy. CD4+ T cells have long been known to be involved in anti-tumor immunity (96) and can act through different mechanisms including i) provision of help in the expansion of tumor antigen-specific CTLs (97), ii) activation of macrophages at tumor sites (98, 99), and iii) active killing of tumor cells (100, 101). Furthermore, it is now well established that antigen-specific CD4+ T cells are fundamental for the induction of long-term memory CD8+ T cells (102). However, CD4+ T cells can also be detrimental, be it in the form of regulatory/suppressor T cells that might dampen elicited CD8+ T cell responses (103) (104), or pro-tumor type 2 cytokine secreting CD4+ T cells that counteract anti-tumor immunity by promoting tumor development (79) and/or by polarizing tumor associated macrophages (80), as discussed above.
Furthermore, there is a need for the development and validation of tools to identify patients who can benefit from a particular form of immunotherapy including vaccination. Indeed, only a fraction of patients eligible for treatment responds to adoptive transfer of tumor-infiltrating lymphocyte (TIL) cells (105). Along the same lines, only a fraction of patients achieves durable regressions in response to vaccination (106).
COMBINING CANCER VACCINES WITH OTHER THERAPIES
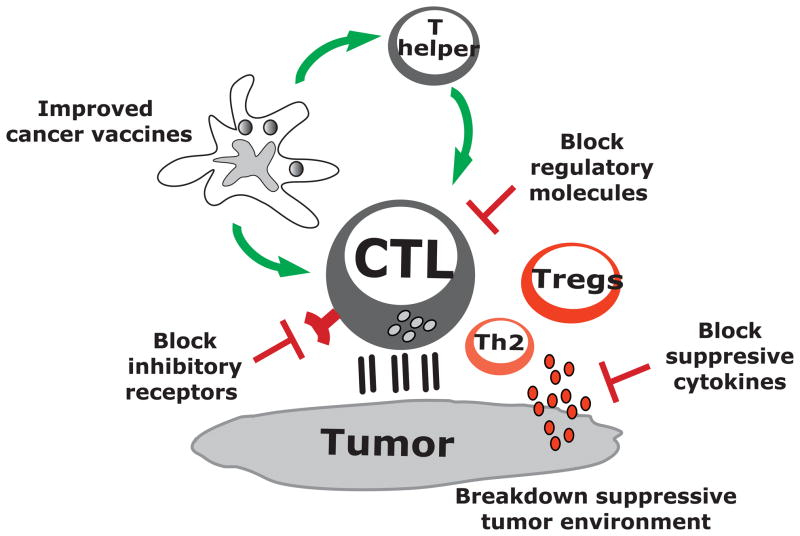
In view of the remarkable diversity of regulatory/suppressive pathways present in patients with metastatic cancer, any durable clinical response elicited by vaccination is already an achievement. However, to improve the outcomes, DC vaccines need to be combined, in particular for patients at advanced stages, with other therapies that offset the suppressive tumor environment (19). Such combination regimens will involve several intervention strategies that target different pathways (Figure 4).
Figure 4. DC vaccines in combination therapies.
Current active immunotherapy trials have shown durable tumor regressions in a fraction of patients. However, clinical efficacy of current approaches is limited, possibly because tumors invade the immune system by means of myeloid-derived suppressor cells, inflammatory type 2 T cells and regulatory T cells (Tregs). To improve the clinical efficacy of immunotherapies, we need to design novel and improved strategies that can boost adaptive immunity to cancer, help overcome Tregs and allow the breakdown of an immunosuppressive tumor microenvironment. This can be achieved by developing combination therapies targeting these three major components.
In particular, blocking antibodies or soluble receptors can be exploited for the blockade of suppressive cytokines in the tumor microenvironment such as IL-10 (107), IL-13 (108), TGF-β (109, 110) and VEGF (111, 112). Such strategies can be used to block immune-inhibitory signals in lymphocytes as illustrated by anti-CTLA-4 (29, 113) and/or anti-PD1 (30, 114, 115), or to block their ligands expressed on tumors or DCs (for example anti-PD-L1). In contrast, agonistic antibodies (111, 112) might further potentiate the function of effector T cells, for example, with anti-CD137 (116), a ligand for 4-1BB (117). Just as different tumors are currently treated with different combinations of cytostatic drugs and targeted therapies, we foresee the development of clinical protocols combining DC vaccines with individualized adjunct therapies.
CONCLUSIONS
There has never been a better and more exciting time to work on developing cancer vaccines. The considerable progress made in the understanding of DC biology as well as effector/regulatory T cell biology clearly has opened avenues for the development of vastly improved clinical protocols. These optimized DC vaccines eliciting strong and long-lived antigen-specific CD8+ and CD4+ T cell immunity will be offered to patients with early stage disease. For patients with late stage disease, strategies that combine novel highly immunogenic DC-based vaccines and immunomodulatory antibodies will have high impact on enhancing therapeutic immunity in cancer by simultaneously enhancing the potency of beneficial immune arms and offsetting immunoregulatory pathways.
Acknowledgments
Dedicated to patients and volunteers who participated in our studies. We thank former and current members of the Institute for their contributions, in particular: Joseph Fay, Lee Roberts, Gerard Zurawski, and Virginia Pascual. Supported by the NIH (P01 CA084514, U19 AIO57234, R01 CA089440 and CA078846), the Dana Foundation, the Susan Komen Foundation, the Baylor Health Care System; the Baylor Health Care System Foundation, the ANRS and the INSERM. KP holds the Michael A. Ramsay Chair for Cancer Immunology Research. JB holds the Caruth Chair for Transplant Immunology Research.
Footnotes
Conflict of interest statement
Jacques Banchereauis employed by Roche.
References
- 1.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 2.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 3.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 4.Darnell RB. Onconeural antigens and the paraneoplastic neurologic disorders: at the intersection of cancer, immunity, and the brain. Proc Natl Acad Sci U S A. 1996;93:4529–4536. doi: 10.1073/pnas.93.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- 6.Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, Jagannath S, Zebroski HA, Simpson AJ, Ritter G, Durie B, Crowley J, Shaughnessy JD, Jr, Scanlan MJ, Gure AO, Barlogie B, Dhodapkar MV. Frequent and specific immunity to the embryonal stem cell associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–840. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 8.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997;18:175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 10.Heslop HE, Rooney CM. Adoptive cellular immunotherapy for EBV lymphoproliferative diseases. Immuno Rev. 1997;157:217–222. doi: 10.1111/j.1600-065x.1997.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 11.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–1212. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N Engl J Med. 1994;331:679–680. doi: 10.1056/NEJM199409083311017. [DOI] [PubMed] [Google Scholar]
- 13.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002 doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 16.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 17.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 18.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 19.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010 doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 23.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall Survival Analysis of a Phase II Randomized Controlled Trial of a Poxviral-Based PSA-Targeted Immunotherapy in Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartzentruber DJ, Lawson D, Richards J, Conry RM, Miller D, Triesman J, Gailani F, Riley LB, Vena D, Hwu P. A phase III multi-institutional randomized study of immunization with the gp100:209–217 (210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma. J Clin Oncol. 2009;27:18s. [Google Scholar]
- 25.Schuster SJ, Neelapu SS, Gause BL, Muggia FM, Gockerman JP, Sotomayor EM, Winter JN, Flowers CR, Stergiou AM, Kwak LW. Idiotype vaccine therapy (BiovaxID) in follicular lymphoma in first complete remission: Phase III clinical trial results. ASCO Meeting Abstract 2009 [Google Scholar]
- 26.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 28.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 29.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006 doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 31.Hawiger D, Inaba K, Dorsett Y, Guo K, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 33.Volkmann A, Zal T, Stockinger B. Antigen-presenting cells in the thymus that can negatively select MHC class II-restricted T cells recognizing a circulating self antigen. J Immunol. 1997;158:693–706. [PubMed] [Google Scholar]
- 34.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fairchild PJ, Austyn JM. Thymic dendritic cells: phenotype and function. Int Rev Immunol. 1990;6:187–196. doi: 10.3109/08830189009056629. [DOI] [PubMed] [Google Scholar]
- 36.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 41.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–1414. [PubMed] [Google Scholar]
- 42.Brimnes MK, Bonifaz L, Steinman RM, Moran TM. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J Exp Med. 2003;198:133–144. doi: 10.1084/jem.20030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun. 2005;8:124–139. doi: 10.1159/000082101. [DOI] [PubMed] [Google Scholar]
- 44.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 45.Zhong G, Reis e Sousa C, Germain RN. Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility class II complexes after soluble protein exposure in vivo or in vitro. J Exp Med. 1997;186:673–682. doi: 10.1084/jem.186.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- 47.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 49.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 50.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 51.Matsui T, Connolly JE, Michnevitz M, Chaussabel D, Yu CI, Glaser C, Tindle S, Pypaert M, Freitas H, Piqueras B, Banchereau J, Palucka AK. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol. 2009;182:6815–6823. doi: 10.4049/jimmunol.0802008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 55.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 57.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dubois B, Massacrier C, Vanbervliet B, Fayette J, Briere F, Banchereau J, Caux C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol. 1998;161:2223–2231. [PubMed] [Google Scholar]
- 59.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, Henn V, Kloetzel PM, Gurka S, Kroczek RA. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, Vu Manh TP, Baranek T, Storset AK, Marvel J, Boudinot P, Hosmalin A, Schwartz-Cornil I, Dalod M. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, Vulink AJ, Hart DN, Radford KJ. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, Le Moine A, Faure F, Donckier V, Sancho D, Cerundolo V, Bonnet D, Reis e Sousa C. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, Bridges G, Palucka AK, Banchereau J. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8(+) T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 65.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 66.Fujita M, Zhu X, Ueda R, Sasaki K, Kohanbash G, Kastenhuber ER, McDonald HA, Gibson GA, Watkins SC, Muthuswamy R, Kalinski P, Okada H. Effective immunotherapy against murine gliomas using type 1 polarizing dendritic cells--significant roles of CXCL10. Cancer Res. 2009;69:1587–1595. doi: 10.1158/0008-5472.CAN-08-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, Gooding W, Kalinski P. Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol Immunother. 2009;58:1329–1336. doi: 10.1007/s00262-008-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In Vivo Targeting of Antigens to Maturing Dendritic Cells via the DEC-205 Receptor Improves T Cell Vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei H, Wang S, Zhang D, Hou S, Qian W, Li B, Guo H, Kou G, He J, Wang H, Guo Y. Targeted delivery of tumor antigens to activated dendritic cells via CD11c molecules induces potent antitumor immunity in mice. Clin Cancer Res. 2009;15:4612–4621. doi: 10.1158/1078-0432.CCR-08-3321. [DOI] [PubMed] [Google Scholar]
- 72.Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol. 2009;39:931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- 73.Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P. Targeting of epidermal Langerhans cells with antigenic proteins: attempts to harness their properties for immunotherapy. Cancer Immunol Immunother. 2009;58:1137–1147. doi: 10.1007/s00262-008-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tacken PJ, I, de Vries J, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, Figdor CG. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106:1278–1285. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- 75.Birkholz K, Schwenkert M, Kellner C, Gross S, Fey G, Schuler-Thurner B, Schuler G, Schaft N, Dorrie J. Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation. Blood. 2010 doi: 10.1182/blood-2010-02-268425. [DOI] [PubMed] [Google Scholar]
- 76.Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, Nussenzweig MC, Piperno AG, Steinman RM. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci U S A. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O’Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S, Reiter Y, Palucka K, Zurawski G, Banchereau J. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010 doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berzofsky JA, Terabe M. A novel immunoregulatory axis of NKT cell subsets regulating tumor immunity. Cancer Immunol Immunother. 2008;57:1679–1683. doi: 10.1007/s00262-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kukreja A, Hutchinson A, Dhodapkar K, Mazumder A, Vesole D, Angitapalli R, Jagannath S, Dhodapkar MV. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bahlis NJ, King AM, Kolonias D, Carlson LM, Liu HY, Hussein MA, Terebelo HR, Byrne GE, Jr, Levine BL, Boise LH, Lee KP. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood. 2007;109:5002–5010. doi: 10.1182/blood-2006-03-012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, Bremond A, Goddard S, Pin JJ, Barthelemy-Dubois C, Lebecque S. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 85.Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, Giese T, Gires O, Endres S, Hartmann G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–6487. [PubMed] [Google Scholar]
- 86.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 88.Coukos G, Benencia F, Buckanovich RJ, Conejo-Garcia JR. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer. 2005;92:1182–1187. doi: 10.1038/sj.bjc.6602476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, Wei S, Zou L, Kryczek I, Hoyle G, Lackner A, Carmeliet P, Zou W. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535–5538. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]
- 90.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Urosevic M, Dummer R, Conrad C, Beyeler M, Laine E, Burg G, Gilliet M. Disease-independent skin recruitment and activation of plasmacytoid predendritic cells following imiquimod treatment. J Natl Cancer Inst. 2005;97:1143–1153. doi: 10.1093/jnci/dji207. [DOI] [PubMed] [Google Scholar]
- 92.Panelli MC, Stashower ME, Slade HB, Smith K, Norwood C, Abati A, Fetsch P, Filie A, Walters SA, Astry C, Arico E, Zhao Y, Selleri S, Wang E, Marincola FM. Sequential gene profiling of basal cell carcinomas treated with imiquimod in a placebo-controlled study defines the requirements for tissue rejection. Genome Biol. 2007;8:R8. doi: 10.1186/gb-2007-8-1-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Menetrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69:7895–7898. doi: 10.1158/0008-5472.CAN-09-1642. [DOI] [PubMed] [Google Scholar]
- 96.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 97.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen Is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 99.Mumberg D, Monach PA, Wanderling S, Philip M, Toledano AY, Schreiber RD, Schreiber H. CD4+ T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-γ. Proc Natl Acad Sci USA. 1999;96:8633–8638. doi: 10.1073/pnas.96.15.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010 doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010 doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 104.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 108.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 109.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming Growth Factor-beta Regulation of Immune Responses. Annu Rev Immunol. 2005 doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 110.Terabe M, Ambrosino E, Takaku S, O’Konek JJ, Venzon D, Lonning S, McPherson JM, Berzofsky JA. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res. 2009;15:6560–6569. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 112.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of Programmed Death Ligand 1 Enhances the Therapeutic Efficacy of Combination Immunotherapy against Melanoma. J Immunol. 2010 doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 117.Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, Riley JL, June CH. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 118.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]