Abstract
The copper chaperone for superoxide dismutase (CCS) binds to both the β-site AβPP cleaving enzyme (BACE1) and to the neuronal adaptor protein X11α. BACE1 initiates AβPP processing to produce the amyloid-β (Aβ) peptide deposited in the brains of Alzheimer’s disease patients. X11α also interacts directly with AβPP to inhibit Aβ production. However, whether CCS affects AβPP processing and Aβ production is not known. Here we show that loss of CCS increases Aβ production in both CCS knockout neurons and CCS siRNA-treated SHSY5Y cells and that this involves increased AβPP processing at the BACE1 site.
Keywords: BACE1, copper, copper chaperone for superoxide dismutase, Cu/Zn superoxide dismutase, munc18 interacting protein-1, X11α
INTRODUCTION
Altered copper homeostasis is strongly implicated in Alzheimer’s disease. Copper is enriched in amyloid plaques and binds to both the amyloid-β protein precursor (AβPP) and to amyloid-β (Aβ), and copper binding to Aβ can promote its aggregation into amyloid fibrils. Moreover, changes in copper metabolism including copper binding to Aβ are linked to the generation of reactive oxygen species and to increased oxidative stress in the brains of Alzheimer’s disease patients (see reviews [1,2]). While copper is an essential nutrient, the ability of copper ions to exchange electrons makes copper highly toxic and so its intracellular levels are tightly regulated via a variety of transporters, chelators, and chaperones [3]. CCS is a copper-binding protein that delivers copper to several proteins including the antioxidant enzyme Cu/Zn superoxide dismutase (SOD1), X-linked inhibitor of apoptosis protein (XIAP), and possibly BACE1 [4-6].
A number of lines of evidence suggest that CCS may impact on AβPP processing and Aβ production in Alzheimer’s disease. Firstly, CCS binds to the intracellular domain of BACE1 and may deliver copper to BACE1 [4]. BACE1 is a key enzyme required for the processing of AβPP to produce Aβ [7]. Secondly, CCS binds to the neuronal adaptor protein X11α (also known as munc-18 interacting protein-1) [8]. X11α also interacts directly with AβPP and overexpression of X11α inhibits Aβ production in AβPP transgenic mice [9-11]. Finally, modulating SOD1 levels alters Aβ production [12]. However, whether CCS affects Aβ production is not known.
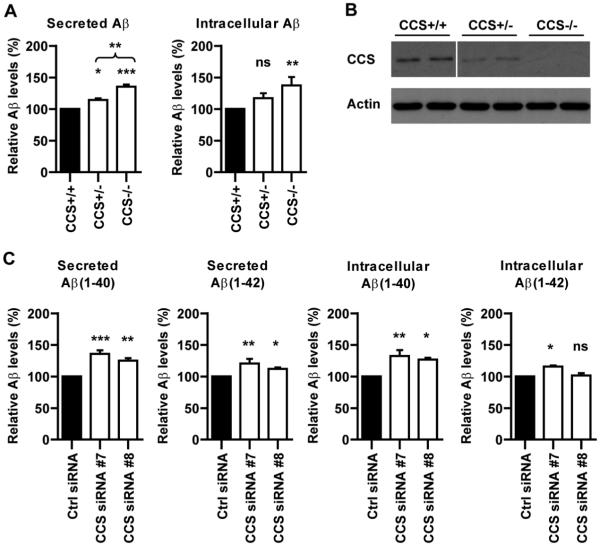
To address this question, we monitored how loss of CCS influences neuronal Aβ production via analyses of neurons derived from CCS homozygous and heterozygous knockout, and wild-type littermate mice (CCS−/−; CCS+/−; CCS+/+) [5]. The levels of both secreted and intracellular Aβ were significantly higher in CCS−/− compared to CCS+/+ neurons (Fig. 1A). Moreover, elevated levels of secreted Aβ were also detected in CCS+/− neurons and this was significantly less than in CCS−/− neurons (Fig. 1A). CCS+/− neurons displayed the predicted 50% reduction in CCS protein levels (Fig. 1B).
Fig. 1.
Loss of CCS increases Aβ production in both CCS knockout neurons (A) and SHSY5Y-AβPP siRNA treated cells (C). (A) Male and female CCS+/− mice were mated and cortical neurons prepared from E14.5 embryos and cultured as described [22]. Samples were harvested for analyses at DIV5. Cultures were genotyped by PCR analyses of the remaining carcasses as described [5]. Secreted and intracellular Aβ1–40 levels were determined using mouse/rat high specific Aβ ELISA (IBL International) according to the manufacturer’s instructions. Secreted Aβ levels were significantly increased in both CCS−/− and CCS+/− compared to CCS+/+ neurons; secreted Aβ levels in CCS−/− neurons were also significantly increased compared to CCS+/− neurons. Intracellular Aβ levels were significantly increased in CCS−/− neurons. Secreted and intracellular Aβ levels in CCS+/+ neurons were 230 pg/ml and 32 pg/ml respectively. (B) Immunoblot showing the levels of CCS in CCS+/+, CCS+/− and CCS−/− neurons; two different samples are shown for each genotype (line in CCS immunoblot indicates removal of portion of the blot for clarity but the samples shown are all from the same blot and are presented at the same exposure). Analyses of the signal intensities for CCS in this and other samples (Fig. 2A) were performed as described [23] and revealed an approximate 50% reduction in CCS levels in CCS+/− compared to CCS+/+ neurons. Actin levels are shown as a loading control. CCS was detected using antibody FL-274 (Santa Cruz); actin using antibody AC-40 (Sigma). (C) Stably expressing SHSY5Y-AβPP cells were prepared by transfection of AβPP in plasmid pCIneo (Promega) and selection with G418. For siRNA knockdown experiments, G418 was removed and cells transfected with non-targeting control or CCS siRNAs (On-TARGETplus; Dharmacon) using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. 48 hours post-transfection, the media was replaced with Opti-MEM (Invitrogen) and the cells harvested for analyses after a further 48 hours in culture. siRNA sequences were: CCS siRNA#7 5′-GGAAUCACUUUAACCCUGA-3′, CCS siRNA#8 5′-GGCCAUCCCUUAUCCAAGA-3′. Secreted and intracellular Aβ1–40 and Aβ1–42 levels were determined using TKHS-ELISA kits (Millipore) according to the manufacturer’s instructions. Both CCS siRNAs significantly increased secreted Aβ1–40 and Aβ1–42, and intracellular Aβ1–40 levels; CCS siRNA#7 (which induced a greater reduction in CCS levels) also significantly increased intracellular Aβ1–42 levels. Secreted and intracellular Aβ levels in control cells were: secreted Aβ1–40 1327 pg/ml; secreted Aβ1–42 316 pg/ml; intracellular Aβ1–40 31 pg/ml; intracellular Aβ1–42 8 pg/ml. Data were analysed using one-way ANOVA tests with LSD post-hoc test; * indicate significant differences between CCS−/− and CCS+/+ in (A) and between control and CCS siRNAs in (B). *p < 0.05; **p < 0.01; ***p < 0.001; not significant (ns). n = 6–8 for individual experiments which were repeated a further two times; data are normalized to controls. Error bars are SEM.
We also assayed how loss of CCS influenced Aβ production in SHSY5Y neuroblastoma cells stably expressing human AβPP-695 isoform (SHSY5Y-AβPP); CCS was depleted by use of siRNAs. The higher levels of Aβ in SHSY5Y-AβPP cells enabled robust determination of both Aβ1–40 and Aβ1–42 isoforms. Two different CCS siRNAs both significantly increased secreted A1–40 and Aβ1–42 levels, and increased the level of intracellular Aβ1–40; one siRNA (the most potent at reducing CCS levels; siRNA#7) also increased intracellular Aβ1–42 levels (Fig. 1C). The two CCS siRNAs reduced CCS levels to approximately 10% (CCS siRNA#7) and 25% (CCS siRNA#8) of control levels (Fig. 2A). Thus, loss of CCS increases Aβ production in two different experimental systems. Cell counts revealed no loss of viability in either CCS−/− neurons or CCS siRNA treated SHSY5Y-AβPP cells compared to controls. Likewise no noticeable morphological changes were observed in CCS depleted cells.
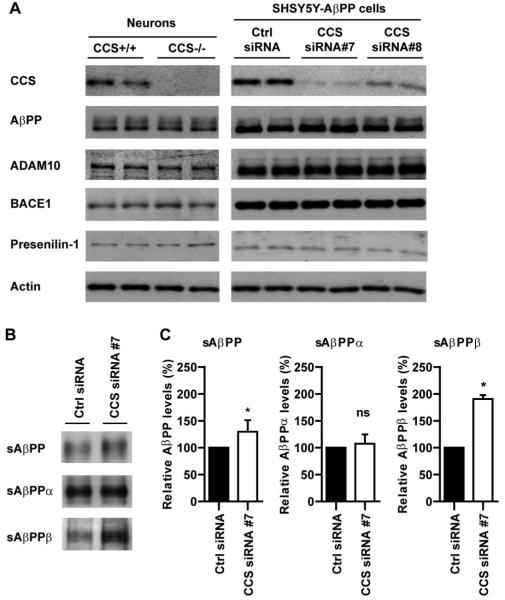
Fig. 2.
Loss of CCS does not induce detectable changes in the steady state levels of AβPP or its secretases but selectively increases processing of AβPP at the BACE1 site. (A) Immunoblots of total cell lysates from SHSY5Y-AβPP siRNA treated cells and CCS knockout neurons probed for AβPP, ADAM10, BACE1, and presenilin-1. Two different samples are shown for each treatment/genotype but two further samples were analyzed with similar results. Also shown are immunblots for CCS and as a loading control, actin. AβPP was detected using a C-terminal antibody [24], presenilin-1 using an N-terminal antibody [25], the ADAM10 antibody was from Calbiochem and the BACE1 antibody (EE-17) was from Sigma. (B) Immunoblots for total sAβPP, sAβPPα, and sAβPPβ in conditioned media from SHSY5Y-AβPP expressing cells treated with control or CCS siRNA#7. Total sAβPP was detected using antibody 22C11 (Millipore), sAβPPα was detected using antibody 6E10 (Covance) and sAβPPβ detected using antibody 1A9 that detects the sAβPP neo-epitope generated after cleavage of AβPP by BACE1 [26]. Quantification of signal intensities on the immunoblots (performed as described [23]) are shown in (C). CCS knockdown increased total sAβPP and sAβPPβ levels but had no effect on sAβPPα levels. The larger relative increase in sAβPPβ compared to total sAβPP levels in CCS depleted cells is consistent with known AβPP processing events; most AβPP is cleaved by α-secretase and not BACE1 such that sAβPPα is the major and sAβPPβ the minor species secreted by cells. Data analysed by t-test; *p < 0.05; not significant (ns). n = 3; error bars are SEM.
Processing of AβPP by BACE1 and γ-secretase releases Aβ, whereas α- and γ-secretase processing precludes Aβ production [13]. To determine whether loss of CCS induced changes in expression of AβPP or its major secretases, we probed immunoblots of CCS+/+ and CCS−/− mouse neurons, and siRNA treated SHSY5Y-AβPP cells for AβPP, ADAM10 (α-secretase), BACE1, and presenilin-1 (γ-secretase component). However, loss of CCS did not induce detectable changes in the levels of any of these proteins (Fig. 2A).
We next investigated whether loss of CCS influenced processing of AβPP at the BACE1 or α-secretase cleavage sites. To do so we monitored the levels of secreted ectodomain fragments of AβPP (sAβPP) present in the culture medium from SHSY5Y-AβPP cells by immunoblotting. Processing of AβPP by BACE1 and α-secretase induces release of sAβPPβ and sAβPPα fragments respectively into the media. siRNA knockdown of CCS induced significant increases in the levels of both total sAβPP and sAβPPβ; no change in sAβPPα levels were detected (Fig. 2B, C).
Our results described here demonstrate that loss of CCS increases Aβ production and that this is accompanied by increased processing of AβPP at the BACE1 site. The precise mechanisms by which this occurs are unclear. Oxidative stress increases BACE1 expression [14-16] but we did not detect any changes in BACE1 levels in either CCS siRNA-treated or CCS knockout cells. Indeed, although CCS loss can influence SOD1 activity, there are other routes whereby SOD1 can obtain copper for its antioxidant activity [17]. Alternatively, CCS may increase Aβ production via an effect on BACE1 activity, trafficking, or its association with AβPP. The intracellular domain of BACE1 that binds CCS is known to mediate its trafficking [7,18]. A further possibility is that CCS delivers copper to BACE1 since its intracellular domain binds a single copper atom and that bound copper modulates BACE1 activity or trafficking [4]. Copper is known to influence the activity and trafficking of other proteins [3]. Finally, CCS may influence AβPP and/or BACE1 trafficking via its binding to X11α. Overexpression of X11α inhibits Aβ production and X11α is known to be involved in protein trafficking including the trafficking of AβPP [9,19-21]. Whatever the precise mechanism, our results demonstrate a role for CCS in BACE1 mediated processing of AβPP and production of Aβ.
ACKNOWLEDGMENTS
Supported by grants from the Wellcome Trust, MRC, Alzheimer’s Association, and Alzheimer’s Research Trust. We thank Philip Wong, Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore USA for supplying CCS knockout mice and GSK for antibody 1A9.
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=453).
REFERENCES
- [1].Zhu X, Su B, Wang X, Smith MA, Perry G. Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci. 2007;64:2202–2210. doi: 10.1007/s00018-007-7218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hung YH, Bush AI, Cherny RA. Copper in the brain and Alzheimer’s disease. J Biol Inorg Chem. 2010;15:61–76. doi: 10.1007/s00775-009-0600-y. [DOI] [PubMed] [Google Scholar]
- [3].Banci L, Bertini I, Cantini F, Ciofi-Baffoni S. Cellular copper distribution: a mechanistic systems biology approach. Cell Mol Life Sci. 2010;67:2563–2589. doi: 10.1007/s00018-010-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Angeletti B, Waldron KJ, Freeman KB, Bawagan H, Hussain I, Miller CC, Lau KF, Tennant ME, Dennison C, Robinson NJ, Dingwall C. BACE1 cytoplasmic domain interacts with the copper chaperone for superoxide dismutase-1 and binds copper. J Biol Chem. 2005;280:17930–17937. doi: 10.1074/jbc.M412034200. [DOI] [PubMed] [Google Scholar]
- [5].Wong PC, Waggoner D, Subramaniam JR, Tessarollo L, Bartnikas TB, Culotta VC, Price DL, Rothstein J, Gitlin JD. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc Natl Acad Sci U S A. 2000;97:2886–2891. doi: 10.1073/pnas.040461197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brady GF, Galban S, Liu X, Basrur V, Gitlin JD, Elenitoba-Johnson KS, Wilson TE, Duckett CS. Regulation of the copper chaperone CCS by XIAP-mediated ubiquitination. Mol Cell Biol. 2010;30:1923–1936. doi: 10.1128/MCB.00900-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer’s disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009;29:12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McLoughlin DM, Standen CL, Lau K-F, Ackerley S, Bartnikas TP, Gitlin JD, Miller CCJ. The neuronal adaptor protein X11alpha interacts with the copper chaperone for SOD1 and regulates SOD1 activity. J Biol Chem. 2001;276:9303–9307. doi: 10.1074/jbc.M010023200. [DOI] [PubMed] [Google Scholar]
- [9].Miller CC, McLoughlin DM, Lau KF, Tennant ME, Rogelj B. The X11 proteins, Abeta production and Alzheimer’s disease. Trends Neurosci. 2006;29:280–285. doi: 10.1016/j.tins.2006.03.001. [DOI] [PubMed] [Google Scholar]
- [10].Lee JH, Lau KF, Perkinton MS, Standen CL, Shemilt SJ, Mercken L, Cooper JD, McLoughlin DM, Miller CC. The neuronal adaptor protein X11alpha reduces Abeta levels in the brains of Alzheimer’s APPswe Tg2576 transgenic mice. J Biol Chem. 2003;278:47025–47029. doi: 10.1074/jbc.M300503200. [DOI] [PubMed] [Google Scholar]
- [11].Mitchell JC, Perkinton MS, Yates DM, Lau KF, Rogelj B, Miller CC, McLoughlin DM. Expression of the neuronal adaptor protein X11alpha protects against memory dysfunction in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;20:31–36. doi: 10.3233/JAD-2009-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice over-expressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- [13].Selkoe DJ. Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- [14].Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- [15].Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF, Xu H, Zhang YW. Hypoxia-inducible factor 1a (HIF-1a)-mediated hypoxia increases BACE1 expression and b-amyloid generation. J Biol Chem. 2007;282:10873–10880. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- [17].Carroll MC, Girouard JB, Ulloa JL, Subramaniam JR, Wong PC, Valentine JS, Culotta VC. Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone. Proc Natl Acad Sci U S A. 2004;101:5964–5969. doi: 10.1073/pnas.0308298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walter J, Fluhrer R, Hartung B, Willem M, Kaether C, Capell A, Lammich S, Multhaup G, Haass C. Phosphorylation regulates intracellular trafficking of beta-secretase. J Biol Chem. 2001;276:14634–14641. doi: 10.1074/jbc.M011116200. [DOI] [PubMed] [Google Scholar]
- [19].He X, Cooley K, Chung CH, Dashti N, Tang J. Apolipoprotein receptor 2 and X11alpha/beta mediate apolipoprotein E-induced endocytosis of amyloid-beta precursor protein and beta-secretase, leading to amyloid-beta production. J Neurosci. 2007;27:4052–4060. doi: 10.1523/JNEUROSCI.3993-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Setou M, Nakagawa T, Seog D-H, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- [21].Saito Y, Sano Y, Vassar R, Gandy S, Nakaya T, Yamamoto T, Suzuki T. X11 proteins regulate the translocation of APP into detergent resistant membrane and suppress the amyloidogenic cleavage of APP by BACE in brain. J Biol Chem. 2008;283:35763–35771. doi: 10.1074/jbc.M801353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ackerley S, Grierson AJ, Brownlees J, Thornhill P, Anderton BH, Leigh PN, Shaw CE, Miller CCJ. Glutamate slows axonal transport of neurofilaments in transfected neurons. J Cell Biol. 2000;150:165–175. doi: 10.1083/jcb.150.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lau KF, Howlett DR, Kesavapany S, Standen CL, Dingwall C, McLoughlin DM, Miller CCJ. Cyclin-dependent kinase-5/p35 phosphorylates Presenilin 1 to regulate carboxy-terminal fragment stability. Mol Cell Neurosci. 2002;20:13–20. doi: 10.1006/mcne.2002.1108. [DOI] [PubMed] [Google Scholar]
- [24].Hoey SE, Williams RJ, Perkinton MS. Synaptic NMDA receptor activation stimulates alpha-secretase amyloid precursor protein processing and inhibits amyloid-beta production. J Neurosci. 2009;29:4442–4460. doi: 10.1523/JNEUROSCI.6017-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lau K-F, McLoughlin DM, Standen C, Miller CCJ. X11alpha and X11beta interact with presenilin-1 via their PDZ domains. Mol Cell Neurosci. 2000;16:555–563. doi: 10.1006/mcne.2000.0898. [DOI] [PubMed] [Google Scholar]
- [26].Parkin ET, Watt NT, Hussain I, Eckman EA, Eckman CB, Manson JC, Baybutt HN, Turner AJ, Hooper NM. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer’s amyloid precursor protein. Proc Natl Acad Sci U S A. 2007;104:11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]