Abstract
Investigation of the endemic Madagascar plant Leptadenia madagascariensis Decne. (Apocynaceae) for antiproliferative activity against the A2780 ovarian cancer cell line led to the isolation of the four new cardenolides 1–4. The structure elucidations of these compounds were based on analyses of their 1D and 2D NMR spectra and mass spectrometric data. The cardenolides were strongly antiproliferative to the A2780 ovarian cancer cell line, with IC50 values of 0.18, 0.21, 0.17 and 0.29 μM line, and to the H460 human lung cancer cell line, with IC50 values of 0.16, 0.68, 0.37 and 0.48 μM respectively.
Keywords: Antiproliferative activity, cardenolide, biodiversity, NMR
1. Introduction
In our continuing search for biologically active natural products from tropical rainforests as part of an International Cooperative Biodiversity Group (ICBG) program,2,3 we obtained an EtOH extract from the roots of the plant Leptadenia madagascariensis Decne. (Apocynaceae) from Madagascar. The extract exhibited good antiproliferative activity against the A2780 human ovarian cancer cell line, with an IC50 value of 10 μg/mL. On the basis of this activity and the absence of any previous phytochemical studies on this species, the extract was selected for fractionation to isolate its active components by bioassay-guided fractionation.
There are about twenty species in the genus Leptadenia, some of which are used in traditional medicine in Africa and India. 4 – 6 Previous phytochemical investigations reported the presence of flavonoids5,7,8 terpenoids1–10 polyoxypregnane esters,4,11 pregnane glycosides,12,13 cardiac glycosides14 and alkaloids15 in these species. The medicinal use of plants containing cardiac glycosides was recorded as early as 1500 years ago. As an important class of natural products, cardiac glycosides are widely used for treating cardiac failure,16 and their cardiac activities and cytotoxicities are well known.6,17,18 What is less well known is the fact that they are also beginning to find use in cancer chemotherapy, and the first generation of anticancer cardiac glycosides is in clinical trials.19,20
2. Results and Discussion
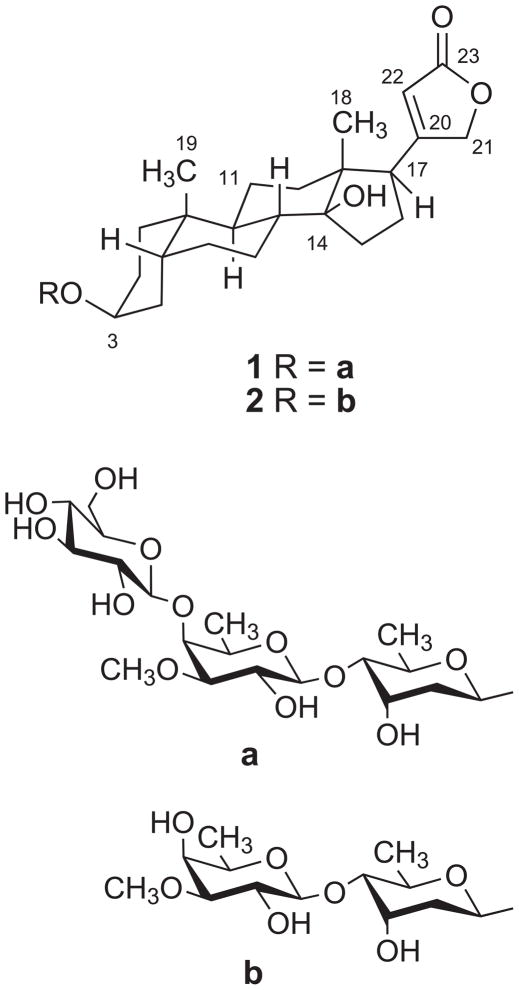
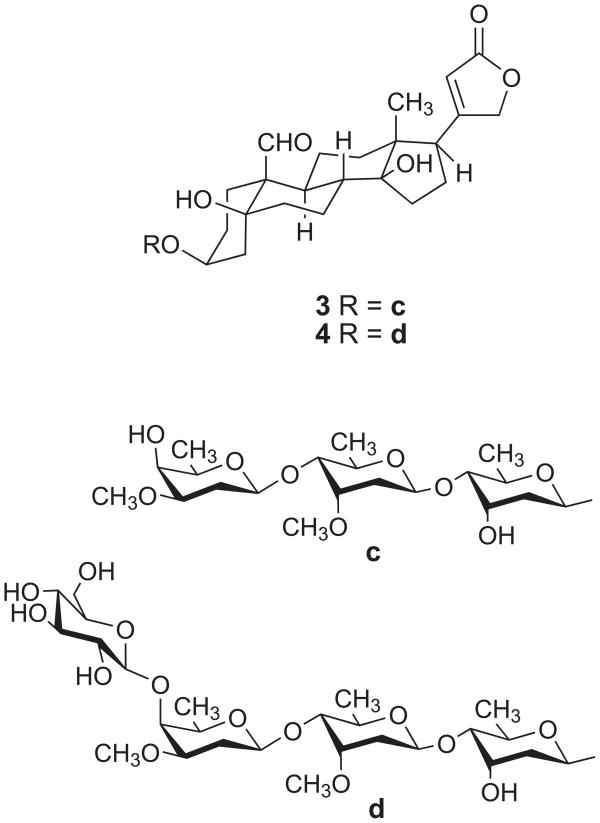
The EtOH extract of the stems and leaves of L. madagascariensis was subjected to liquid-liquid partitioning to give active dichloromethane and MeOH fractions with IC50 values in the A2780 assay of 0.28 and 2.4 μg/mL, respectively. Fractionation by C18 open column and High Performance Liquid Chromatography (HPLC) on the MeOH fraction yielded two new cardenolides named madagascarensilide A (1) and madagascarensilide B (2) (Figure 1). Similar purification of the CH2Cl2 fraction yielded the two additional new compounds madagascarensilides C and D (3 and 4, Figure 3). Herein we report the structural elucidation and the antiproliferative properties of the four isolates.
Figure 1.
Chemical structures of madagascarensilides A (1) and B (2).
Figure 3.
Chemical structures of madagascarensilides C (3) and D (4)
Madagascarensilide A (1) was obtained as a white amorphous solid. Its positive ion HRESIMS revealed a pseudomolecular ion peak at m/z 849.4255 [M+Na]+, corresponding to a molecular formula of C42H66O16 for 1. Its 1H NMR spectrum in CD3OD showed signals at δH 5.04 dd (J = 18.6, 1.8 Hz), 4.92 dd (J = 18.6,1.8 Hz), and 5.90 s, characteristic of an α,β–unsaturated γ–lactone (Table 1). In addition, three anomeric proton signals were observed at δH 4.91 dd (J = 9.5, 1.8 Hz), 4.57 d (J = 7.7) and 4.37 d (J = 7.7). The 13C NMR spectrum contained 42 signals, which included signals for one methoxyl, four methyls, 12 methylenes (including one oxymethylene), 20 methines (including 15 oxymethines and one olefinic carbon), and five quaternary carbons (including one oxyquaternary carbon, one olefinic carbon and one carbonyl carbon), as indicated by an HMQC spectrum (Table 1). The above data suggested that 1 is a cardiac glycoside with three sugar moieties.
Table 1.
1H and 13C NMR chemical shifts of madagascarensilides A (1), B (2), C (3) and D (4)a
| position | 1b | 1c | 2b | 3c | 3b | 4b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1H (J, Hz) | 13C | 1H (J, Hz) | 13C | 1H (J, Hz) | 13C | 1H (J, Hz) | 13C | 1H (J, Hz) | 13C | 1H (J, Hz) | 13C | |
| Aglycone | ||||||||||||
| 1 | 1.46 m | 31.0 | 30.9 | 31.0 | 2.59m, 1.91m | 18.9 | 1.72 m, 1.60m | 18.9 | 18.9 | |||
| 2 | 1.63 m | 27.5 | 27.5 | 28.1 | 2.19m, 1.68m | 26.0 | 1.94m, 1.61m | 25.9 | 25.9 | |||
| 3 | 4.02 m | 74.5 | 4.27 m | 73.6 | 4.26 m | 74.5 | 4.33 m | 75.3 | 4.15 m | 76.3 | 4.15 m | 76.3 |
| 4 | 1.83 m, 1.46 m | 31.4 | 31.2 | 31.4 | 2.19m, 1.73m | 36.2 | 2.17m, 1.62m | 36.8 | 36.8 | |||
| 5 | 1.66 m | 38.0 | 37.4 | 38.0 | OH 4.89s | 74.1 | 75.2 | 75.2 | ||||
| 6 | 1.88 m, 1.26 m | 27.9 | 27.5 | 27.5 | 2.34m, 1.82m | 37.3 | 2.17m, 1.62m | 37.2 | 37.2 | |||
| 7 | 1.78 m, 1.25 m | 22.4 | 22.0 | 22.4 | 2.33 m, 1.46m | 25.2 | 2.12m, 1.32m | 25.2 | 25.2 | |||
| 8 | 1.63 m | 42.7 | 42.3 | 42.7 | 2.31 m | 42.3 | 1.94 m | 42.6 | 42.6 | |||
| 9 | 1.73 m | 36.9 | 36.3 | 36.9 | 1.78m | 39.9 | 1.66m | 40.4 | 40.4 | |||
| 10 | 36.3 | 35.9 | 36.3 | 56.1 | 56.1 | 56.1 | ||||||
| 11 | 1.43 m, 1.24 m | 22.6 | 22.4 | 22.6 | 1.59m, 1.40m | 23.0 | 1.56m, 1.51m | 23.3 | 23.3 | |||
| 12 | 1.51 m | 41.0 | 40.3 | 41.0 | 1.45 m, 1.35m | 39.9 | 1.49 m, 143m | 40.5 | 40.5 | |||
| 13 | 51.1 | 50.5 | 51.1 | 50.2 | 50.7 | 50.7 | ||||||
| 14 | 86.5 | 85.0 | 86.5 | −OH, 5.66 | 84.8 | 85.9 | 85.9 | |||||
| 15 | 2.18, 1.73 | 33.4 | 33.6 | 33.4 | 2.08m, 1.86m | 32.5 | 2.17 m, 1.72 m | 32.4 | 32.4 | |||
| 16 | 2.18, 1.88 | 28.1 | 27.7 | 27.9 | 2.10m, 2.02m | 27.6 | 2.19 m, 2.14 m | 27.9 | 27.9 | |||
| 17 | 2.83 m | 52.1 | 2.79 m | 51.9 | 2.83 m | 52.1 | 2.79 m | 51.5 | 2.82 m | 51.7 | 2.82 m | 51.8 |
| 18 | 0.88 s | 16.4 | 0.89 s | 16.6 | 0.88 s | 16.4 | 1.01 | 16.4 | 0.85 s | 16.2 | 0.85 s | 16.2 |
| 19 | 0.94 s | 24.3 | 1.02 s | 24.3 | 0.95 s | 24.3 | 10.42 | 208.9 | 10.05 s | 209.9 | 10.05 s | 209.9 |
| 20 | 178.5 | 176.4 | 178.5 | 176.1 | 178.2 | 178.2 | ||||||
| 21 | 5.04 dd (18.6, 1.8) | 75.4 | 5.34 dd (18.2, 1.4) | 74.1 | 5.03 dd (18.4, 1.5) | 75.4 | 5.31 dd (18.2, 1.7) | 74.4 | 5.03 dd (18.5, 1.7) | 75.3 | 5.03 dd (18.5, 1.7) | 75.3 |
| 4.92 dd (18.6,1.8) | 5.06 dd (18.2,1.4) | 4.92 dd (18.4,1.5) | 5.05 dd (18.2, 1.7) | 4.91dd (18.5, 1.7) | 4.91dd (18.5, 1.7) | |||||||
| 22 | 5.90 s | 117.8 | 6.15 s | 118.1 | 5.90 s | 117.8 | 6.14 | 118.2 | 5.90 | 117.9 | 5.90 | 117.9 |
| 23 | 177.3 | 174.9 | 177.3 | 174.9 | 177.2 | 177.2 | ||||||
| Sugar I | ||||||||||||
| 1′ | 4.91 dd (9.5, 1.8) | 96.8 | 5.44 dd (9.5, 1.8) | 96.9 | 4.91 dd (9.4, 1.5) | 96.8 | 5.39 dd (9.6, 1.9) | 98.0 | 4.91 dd (8.9, 1.9) | 98.3 | 4.91 dd (8.9, 1.9) | 98.3 |
| 2′ | 1.95 m, 1.73 m | 39.0 | 2.43 m, 2.11 m | 39.9 | 1.95 m, 1.73 m | 38.9 | 2.34 m, 1.93 m | 39.1 | 1.98 m, 1.71 m | 38.7 | 1.98 m, 1.71 m | 38.7 |
| 3′ | 4.24 m | 68.7 | 4.71 m | 68.1 | 4.26 m | 68.7 | 4.61 m -OH, 5.52 |
67.8 | 4.24 m | 68.3 | 4.24 m | 68.3 |
| 4′ | 3.23 m | 84.3 | 3.67 dd (9.7, 2.7) | 84.7 | 3.23 dd (9.5, 2.9) | 84.1 | 3.50 dd (9.7, 3.0) | 83.2 | 3.23 dd (9.5, 2.9) | 83.5 | 3.23 m | 83.5 |
| 5′ | 3.85 m | 69.7 | 4.37 m | 69.3 | 3.85 m | 69.7 | 4.26 m | 69.2 | 3.80 m | 69.6 | 3.80 m | 69.6 |
| 6′ | 1.31 d (6.5) | 18.6 | 1.67 d (6.2). | 19.2 | 1.29 d (6.2) | 18.5 | 1.41d (6.3) | 18.9 | 1.21 d (6.2) | 18.4 | 1.21 d (6.1) | 18.4 |
| Sugar II | ||||||||||||
| 1″ | 4.37 d (7.7) | 106.2 | 4.77 d (7.7) | 106.7 | 4.34 (7.7) | 106.2 | 5.17 dd (9.7, 1.8) | 100.1 | 4.84 m | 100.7 | 4.84 m | 100.6 |
| 2″ | 3.66 m | 71.4 | 4.42 m | 71.7 | 3.55 dd (9.7, 7.7) | 71.4 | 2.28 m, 1.76m | 36.9 | 2.15 m, 1.62 m | 36.2 | 2.15 m, 1.62 m | 35.8 |
| 3″ | 3.25 m | 85.3 | 3.57 dd (9.7, 2.9) | 85.6 | 3.12 dd (9.7, 3.1) | 84.4 | 4.07 m | 78.1 | 3.87 m | 78.5 | 3.87 m | 78.2 |
| 4″ | 4.16 m | 76.3 | 4.28 m | 77.8 | 3.85 m | 68.6 | 3.48 dd (9.7, 2.7) | 83.3 | 3.30 m | 83.5 | 3.30 m | 83.7 |
| 5″ | 3.66 m | 71.8 | 3.75 m | 71.0 | 3.63 bq (6.4) | 71.6 | 4.20 m | 69.5 | 3.86 m | 70.1 | 3.86 m | 70.0 |
| 6″ | 1.29 d (6.5) | 17.4 | 1.55 d (6.4) | 17.9 | 1.27 d (6.4) | 17.0 | 1.34 d (6.3) | 18.9 | 1.22 d (6.2) | 18.6 | 1.22 d (6.2) | 18.6 |
| Sugar III | ||||||||||||
| 1‴ | 4.57 d (7.7) | 103.9 | 5.10 d (7.8) | 106.4 | 4.69 dd (9.7, 2.1) | 103.1 | 4.55 dd (9.7, 2.0) | 103.4 | 4.57 dd (9.7, 1.9) | 103.4 | ||
| 2‴ | 3.22 m | 76.0 | 4.02 t (7.8) | 76.6 | 2.17 m, 2.29 m | 33.1 | 1.93 m, 1.65 m | 32.9 | 2.00 m, 1.81m | 33.2 | ||
| 3‴ | 3.37 m | 78.2 | 4.26 m | 79.1 | 3.41 ddd (12.1, 4.7, 2.9) | 79.3 | 3.35 m | 79.2 | 3.47 m | 80.6 | ||
| 4‴ | 3.26 m | 71.8 | 4.25 m | 72.2 | 3.91 m -OH 6.00 |
67.1 | 3.67 m | 67.7 | 3.99 m | 74.6 | ||
| 5‴ | 3.26 m | 78.0 | 3.98 m | 78.9 | 3.56 qd (6.4, 1.3) | 71.9 | 3.49 qd (6.6, 1.3) | 72.0 | 3.53 m | 71.8 | ||
| 6‴ | 3.88 m, 3.65 m | 63.0 | 4.59 m, 4.38 m | 63.4 | 1.55 d (6.4) | 17.9 | 1.28 d (6.6) | 17.2 | 1.30 d (6.4) | 17.7 | ||
| Sugar IV | ||||||||||||
| 1″″ | 4.56 d (7.7) | 104.6 | ||||||||||
| 2″″ | 3.22 m | 76.0 | ||||||||||
| 3″″ | 3.36 m | 78.2 | ||||||||||
| 4″″ | 3.27 m | 71.8 | ||||||||||
| 5″″ | 3.25 m | 78.0 | ||||||||||
| 6″″ | 3.86m, 3.64m | 63.0 | ||||||||||
| 3″-OCH3 | 3.52 s | 58.8 | 3.69 s | 59.5 | 3.46 | 57.3 | 3.51 s | 59.1 | 3.44 s | 58.4 | 3.42 s | 58.0 |
| 3‴-OCH3 | 3.40 s | 55.7 | 3.38 s | 55.8 | 3.41 s | 56.6 | ||||||
δ (ppm) 500 MHz for 1H and 125 MHz for 13C; multiplicities; J values (Hz) in parentheses.
In CD3OD
In deuterated pyridine
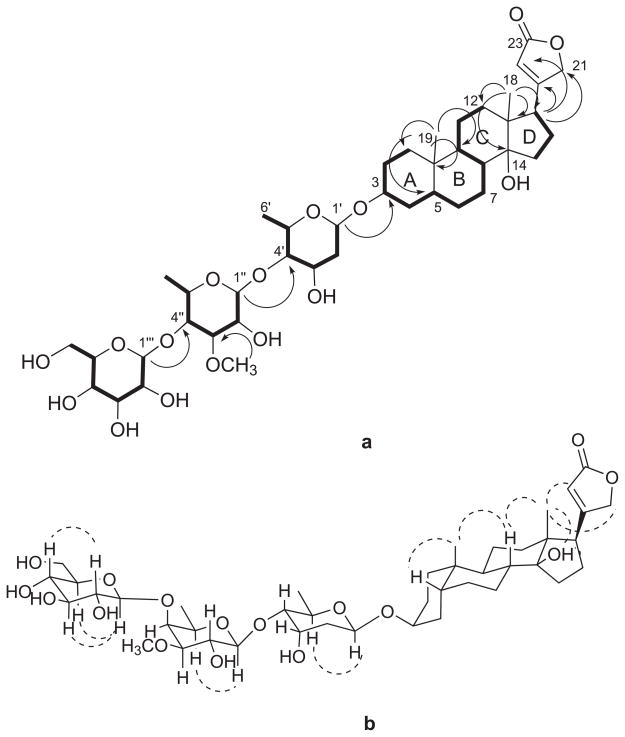
In the aglycone of 1, two spin systems CH2-CH2-CH-CH2-CH-CH2-CH2-CH-CH-CH2-CH2 (H2-1 through H2-2, H-3, H2-4, H-5, H2-6, H2-7, H-8, H-9 and H2-11 to H2-12) in rings A, B and C, and CH2-CH2-CH (H2-15 through H2-16 to H-17) in ring D (Figure 2) were identified in the COSY and TOCSY spectra. The connectivities of rings A, B, C and D were assigned based on the interpretation of the HMBC spectrum. Long-range correlations from H3-19 to C-1, C-5, C-9 and C-10, and from H2-1 to C-9 indicated the connectivity of rings A and B. The relationship between rings C and D was established by the observation of correlations from H3-18 to C-12, C-13, the oxygenated quaternary carbon at C-14 and C-17, as well as those observed from H2-12 to C-17, and H2-15 to C-8. Moreover, the α,β–unsaturated γ–lactone was deduced to be connected to C-17 by the HMBC correlation from H-17 to C-20, C21 and C22. The Rotating frame Overhauser Effect SpectroscopY (ROESY) correlation between H3-19 and H-5 indicated that rings A and B are cis fused, while the trans orientation of H-8 and H-9 was deduced from the presence of correlations between H3-18 and H3-19 to H-8 (Figure 2). The correlations of H3-18 to C-14-OH, H-21, and H-22 in the ROESY spectrum in deuterated pyridine indicated cis fused C and D rings and the β-orientation of the γ-lactone at C-17. These data, together with a comparison of the 13C NMR data of 1 with those of the aglycone of coroloside and similar digitoxigenin glycosides, established the aglycone of 1 as digitoxigenin.21
Figure 2.
a) Key COSY (bold) and HMBC (arrows) correlations for 1.
b) Key ROESY correlations for 1.
The presence of three sugar units in 1 was indicated by the presence of three anomeric proton signals at δH 4.91, 4.57 and 4.37. Their spin systems were determined by COSY and TOCSY correlations: H-1′-H2-2′-H-3′-H-4′-H-5′-H3-6′, H-1″-H-2″-H-3″-H-4″-H-5″-H3-6″, and H-1‴-H-2‴-H-3‴-H-4‴-H-5‴-H2-6‴. In addition, HMBC correlations from H-1′ to C-3, H-1″ to C-4′, and H-1‴ to C-4″ built up the connectivity of the sugar units from C-1′ to C-3, C-1″ to C-4′ and C-1‴ to C-4″. The relative conformations of the sugar moieties were determined by the coupling constants of the sugar protons and by analysis of the ROESY data. In the 1H NMR spectrum of 1 in deuterated pyridine, the coupling constants observed for H-1′ (d, J = 9.5, 1.8) and for H-4′ (dd, J = 9.7, 2.7) as well as the clear ROESY correlation between H-1′ and H-5′ indicated that H-1′, H-4′ and H-5′ are all axial, and so H-3′ must be equatorial based on its 2.7 Hz coupling constant with H-4′. The HMBC correlation between the methoxy protons at 3.69 ppm and C-3″ (δC = 85.6) placed the methoxy group at C-3″. In the same manner, the coupling constants observed for H-1″ (d, J = 7.7), H-3″ (dd, J = 9.7, 2.9) and the ROESY correlation of H-5″ with H-1″ indicated that H-1″, H-2″, H-3″, H-5″ are axial, while H-4″ is equatorial. The coupling constants observed for the third sugar unit for H-1‴ (d, J = 7.8) and H-2‴ (t, J = 7.8), with ROESY correlations from H-1‴ to H-3‴ and H-5‴, as well as from H-2‴ to H-4‴, led to the conclusion that all the protons in this sugar unit must be axial, indicating it to be glucopyranose. Therefore, the structure of 1 was determined to be digitoxigenin 3-O-β-glucopyranosyl-(1→4)-O-β-digitalopyranosyl-(1→4)-O-β-digitoxopyranoside. The absolute stereochemistry of the glucose unit was assigned as D since L-glucose has never been observed in cardenolides. The digitoxose and digitalose units were also assigned as D-sugars based on their occurrence in other cardenolides in the D-form.21,22
Madagascarensilide B (2) was obtained as a white amorphous solid. Its positive ion HRESIMS revealed a pseudomolecular ion peak at m/z 687.3728, corresponding to a molecular formula of C36H56O11 for 2. The 1H NMR data of 2 in CD3OD were very similar to those of 1, and thus its structure was indicated to be a cardiac glycoside with two sugar units. The 1H and 13C NMR data arising from the aglycone and the sugar moiety attached at C-3 of 2 were essentially superposable with those of 1. In addition, the spin system H-1″-H-2″-H-3″-H-4″-H-5″-H3-6″ was identified by analysis of the COSY data (Table 1). The HMBC correlation observed between the methoxy protons and C-3″ indicated that the second sugar moiety shares the same planar structure as the second sugar of 1. The coupling constants observed at H-1″ (d, J = 7.7), H-2″ (dd, J = 9.7, 7.7), H-3″ (dd, J = 9.7, 3.1) and H-5″ (brq, J = 6.4), together with the ROESY correlation between H-1″ and H-5″ indicated that H-1″, H-2″, H-3″ and H-5″ are axial while H-4″ is equatorial. Thus the structure of 2 was assigned as digitoxigenin 3-O-β-digitalopyranosyl-(1→4)-O-β-digitoxopyranoside.
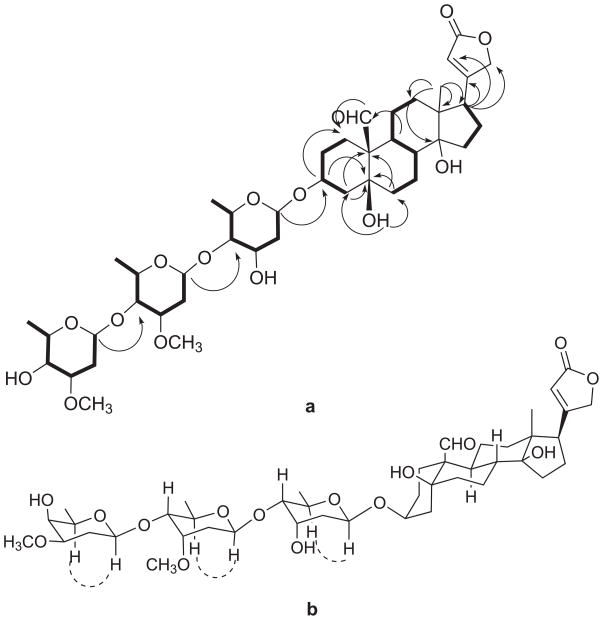
Madagascarensilide C (3) was obtained as a white amorphous solid. Its positive ion HRESIMS revealed a pseudomolecular ion peak at m/z 845.4315 [M+Na]+ corresponding to a molecular formula of C43H66O15 for 3. The 1H and 13C NMR spectra in deuterated pyridine indicated compound 3 to be a cardenolide with three sugar units, with signals for three anomeric protons at δH 5.39, 5.17, and 4.69 ppm and one aldehyde at δH 10.42 (Table 1). In the aglycone of 3, three spin systems: CH2-CH2-CH-CH2 (H2-1 through H2-2, H-3 to H2-4) for ring A; CH2-CH2-CH-CH-CH2-CH2 (H2-6 through H2-7, H-8, H-9, H2-11 to H2-12) in rings B and C; and CH2-CH2-CH (H2-15 through H2-16 to H-17) in ring D were identified by COSY and TOCSY spectra. The connectivities of rings A, B, C and D were assigned based on an analysis of HMBC data. The long-range correlations arising from H-19 at δH 10.42 to C-1 and H-9 at δH 1.78 to C-19 at δC 208.9, as well as the correlations from Ha-4 and Ha-6 to C-10, and from the hydroxyl group signal at C-5 to C-4 and C-6, indicate the connectivity of rings A and B. Meanwhile, the relationship between rings C and D was established by the observation of correlations from H3-18 to C-12, C-13, C-14 and C-17, and from the hydroxy group at C-14 to C-8, C-13, C-14 and C-15. The α,β-unsaturated γ-lactone was determined to be at C-17 by the HMBC correlation from H-17 to C-20, C21 and C22. Moreover the assigned 13C NMR chemical shifts of the aglycone of 3 in CD3OD (Table 1) are very similar to those of strophanthidin.23 From the above data, the planar structure of the aglycone of 3 was deduced to be strophanthidin.
The spin systems H-1′-H2-2′-H-3′-H-4′-H-5′-H3-6′, H-1″-H2-2″-H-3″-H-4″-H-5″-H3-6″, and H-1‴-H2-2‴-H-3‴-H-4‴-H-5‴-H3-6‴ of the sugar units were assigned by COSY and TOCSY correlations. Long-range correlations from H-1′ to C-3, H-1″ to C-4′, and H-1‴ to C-4″ established the connectivity of the sugar units as depicted in Figure 3. The methoxy groups at C-3″ and C-3‴ were substantiated by observation of HMBC correlations in deuterated pyridine between the methoxy signals (δH 3.51 and 3.40, each singlet) and the two carbon signals at δC 78.1 and 79.3 (C-3″ and C-3‴, respectively). The relative conformations of the sugar moieties were determined by analysis of the ROESY data of 3 and the coupling constants of the sugar protons. The values of the coupling constants of H-1′ (J = 9.6, 1.9 Hz) and H-4′ (J = 9.7, 3.0 Hz) and the clear ROESY correlation between H-1′ and H-5′ indicated that H-1′, H-4′ and H-5′ are axial, while H-3′ is equatorial. The coupling constants of H-1″ (dd, J = 9.7, 1.8) and H-4″ (dd, J = 9.7, 2.7) and the ROESY correlation between H-5″ and H-1″ suggested that H-1″, H-4″, and H-5″ are axial, while H-3″ is equatorial. Similarly, the coupling constants of H-1‴ (dd, J = 9.7, 2.1), H-3‴ (ddd, J = 12.1, 4.7, 2.9) and H-5‴ (qd, J = 6.4, 1.3), and the ROESY correlation between H-1‴ and H-5‴ indicate that H-1‴, H-3‴ and H-5‴ are axial and H-4‴ is equatorial. Therefore, the structure of 3 was determined as strophanthidin 3-O-β-diginopyranosyl-(1→4)-O-β-cymaropyranosyl-(1→4)-O-β-digitoxopyranoside.
Madagascarensilide D (4) was obtained as a white amorphous solid. Its positive ion HRESIMS revealed a pseudomolecular ion peak at m/z 1007.4844 [M+Na]+ corresponding to a molecular formula of C49H76O20 for 4. The 1H and 13C NMR spectroscopic data of 4 were very similar to those of 3, suggesting that 4 is a cardenolide derivative with four sugar units (Table 2). Inspection of the carbon chemical shifts of 4 revealed a close similarity to those of 3 except for the downfield shifts of the signals arising from C-3‴ (+1.4) and C-4‴ (+6.9) and the upfield shift of the signal of C-5‴ (−0.2). These data indicated that the additional sugar unit in 4 is linked to the third sugar, and the position of attachment of this fourth sugar unit was confirmed to be at C-4‴ due to observation of a clear HMBC correlation between H-1″″ and C-4‴. The 13C NMR data of the fourth sugar unit (δC 104.6, 76.0, 78.2, 71.8, 78.0, 63.0) were very similar to the those of the terminal β-glucopyranosyl unit of the tetrasaccharide adoligose B (β-Glc-β-Dgn-β-Cym-α-Cym),24 indicating the sugar moieties of 4 to be β-Glc-β-Dgn-β-Cym-β-Dgx. Compound 4 is thus strophanthidin 3-O-β-glucopyranosyl-(l→4)-O-β-diginopyranosyl-(1→4)-O-β-cymaropyranosyl-(1→4)-O-β-digitoxopyranoside.
3. Bioassay Data
Madagascarensilide A (1), B (2), C (3) and D (4) were tested for antiproliferative activity against the A2780 human ovarian cancer cell line. Compounds 1 and 3 were the most potent, having an IC50 value of 0.18 and 0.17 μM, while compounds 2 and 4 were slightly less potent, with IC50 values of 0.21 and 0.29 μM, respectively. It appears that the aldehyde group at position 10 between rings A and B on the aglycone does not significantly affect the activity of cardenolides against A2780 cells.
Cardenolides 1–4 were also evaluated in the H460 human lung cancer cell line. Madagascarensilide A (1) showed strong activity with a IC50 value of 0.16 μM. Madagascarensilide B (2), C (3) and D (4) were also active with IC50 values of 0.68, 0.37, and 0.48 μM, respectively.
4. Experimental
4.1 General experimental procedures
Optical rotations were recorded on a JASCO P-2000 polarimeter. UV and IR spectra were measured on a Shimadzu UV-1201 spectrophotometer and a MIDAC M-series FTIR spectrophotometer, respectively. NMR spectra were obtained in CD3OD or deuterated pyridine on either JEOL Eclipse 500 or Bruker Avance 600 spectrometers. The chemical shifts are given in δ (ppm) and coupling constants (J) are reported in Hz. Mass spectra were obtained on an Agilent 6220 TOF Mass Spectrometer. HPLC was performed on a Shimadzu LC-10AT instrument with a semi-preparative C18 Varian Dynamax column (5 μm, 250 × 10 mm).
4.2 Antiproliferative Bioassays
4.2.1 A2780 ovarian cancer cell line
A2780 human ovarian cancer cell25 were grown to 95% confluency and harvested and resuspended in growth medium (RPMI-1640 supplemented with 10% fetal bovine serum and 2 mM L-glutamine). Cells were counted using a hemacytometer and a solution containing 2.5×105 cells per ml was prepared in growth media. Eleven columns of a 96 well microtiter plate were seeded with 199 μL of cell suspension per well, and the remaining column contained media only (one hundred percent inhibition control). The plate was incubated for 3 hours at 37°C/5% CO2 to allow the cells to adhere to the wells. Following this incubation, potential cytotoxic agents, prepared in DMSO, were added to the wells in an appropriate series of concentrations, 1 μL per well. One column of wells was left with no inhibitor (zero percent inhibition control), and 4 dilutions of a known compound (taxol or actinomycin) was included as a positive control. The plate was incubated for 2 days at 37ºC/5% CO2, then the media gently shaken from the wells and replaced with reaction media (supplemented growth medium containing 1% alamarBlue), and incubated for another 3 hours. The level of alamarBlue converted to a fluorescent compound by living cells was then analyzed using a Cytofluor Series 4000 plate reader (Perseptive Biosystems) with an excitation wavelength of 530 nm, an emission wavelength of 590 nm, and gain of 45. The percent inhibition of cell growth was calculated using the zero percent and one hundred percent controls present on the plate, and an IC50 value (concentration of cytotoxic agent which produces 50% inhibition) was calculated using a linear extrapolation of the data which lie either side of the 50% inhibition level. Samples were analyzed in triplicate on at least two separate occasions to produce a reliable IC50 value.
4.2.2 H460 NSCLC cell line
The cell growth inhibition assay was performed using the H460 (NSCLC) cell line at Eisai Inc. Andover, MA. The cells were cultured in 96-well plates in the absence or continuous presence of test samples at various concentrations for 96 hours. Cell growth was assessed using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega). Luminescence was read on the EnVision 2102 Multilabel Reader (Perkin-Elmer). IC50 values were determined as the concentration of test sample at which cell growth was inhibited by 50% compared to untreated cell population; vinblastine was used as a reference compound.
4.3 Plant material
A sample of the roots of Leptadenia madagascariensis Decne. (Apocynaceae) was collected in 2007 2 km west of the village of Ambolobozobe, Madagascar, in degraded dry forest. Collection coordinates were 12° 31′ 26″ S, 49° 31′ 29″ E, and elevation 20 m. Voucher specimens have been deposited at the Parc Botanique and Zoologique de Tsimbazaza (TAN) and at the Centre National d’Application des Recherches Pharmaceutiques (CNARP) in Antananarivo, Madagascar; the Missouri Botanical Garden in St. Louis, Missouri (MO); and the Muséum National d’Histoire Naturelle in Paris, France (P), voucher number SR1092.
4.4 Extraction and Isolation
Dried roots of Leptadenia madagascariensis (275 g) were ground in a hammer mill, then extracted with EtOH by percolation for 24 hours at room temperature to give the crude extract MG 4294 (12.7 g), of which 3.34 g was shipped to Virginia Polytechnic Institute and State University (VPISU) for bioassay guided isolation. The extract MG 4294 (IC50 3.6 μg/mL, 2.0 g) was suspended in aqueous MeOH (MeOH-H2O, 9:1, 100 mL) and extracted with hexane (3 × 100 mL portions). The aqueous layer was then diluted to 60% MeOH (v/v) with H2O and extracted with CH2Cl2 (3 × 150 mL portions). The hexane extract was evaporated in vacuo to leave 352 mg with an IC50 value of 15 μg/mL. The residue from the CH2Cl2 extract (223 mg) had an IC50 of 0.28 μg/mL and the aqueous MeOH extract (1.22 g) had an IC50 of 2.4 μg/mL. Fractionation of the aqueous MeOH extract by C18 open column gave the four fractions I - IV (987.6, 70.4, 11.9 and 5.8 mg), with IC50 values of 10, 0.14, 16 and 14 μg/mL, respectively. The most active fraction (fr-II) was separated further by C-18 HPLC (solvent system: MeOH-H2O 70:30), and compounds 1 (2.8 mg, tR = 15.5 min) and 2 (0.7 mg, tR = 21.4 min) were isolated. Fractionation of the CH2Cl2 extract on a C18 open column gave the five fractions A - E (11.5, 67.3, 47.8, 44.6, and 8.8 mg) with IC50 values: >20, 0.11, 0.12, 0.97, and 6.9 μg/mL, respectively. Fraction B was selected for further purification by C18-HPLC (solvent system: gradient from MeOH: H2O 60:40 to 70:30 for 40 min) to afford compounds 3 (1.5 mg, tR 38.7 min) and 4 (5.0 mg, tR = 28.0 min).
4.5 Digitoxigenin 3-O-β-glucopyranosyl-(1→4)-O-β-digitalopyranosyl(1→4)-O-β-digitoxopyranoside (1, madagascarensilide A)
Compound 1 was a white amorphous solid; [α]D23 +11 (c 0.28, MeOH); UV (MeOH) λmax nm (ε) 215 (4.1); IR νmax cm-1: 3396, 2934, 1739, 1449, 1372, 1073 cm-1. 1H NMR (500 MHz, CD3OD, d-pyridine) and 13C NMR (125 MHz, CD3OD, d-pyridine), see Table 1; HRESI-MS m/z 849.4255 [M+Na]+ (calcd for C42H66NaO16 849.4249).
4.6 Digitoxigenin 3-O-β-digitalopyranosyl-(1→4)-O-β-digitoxopyranoside (2, madagascarensilide B)
Compound 2 was a white amorphous solid; [α]D23 +16 (c 0.07, MeOH); UV (MeOH) λmax nm (ε) 215 (3.6); IR νmax cm-1: 3224, 2940, 1739, 1595, 1355, 1078 cm-1. 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD), see Table 1; HRESI-MS m/z 687.3728 [M+Na]+ (calcd for C36H56NaO11 687.3720).
4.7 Strophanthidin 3-O-β-diginopyranosyl-(1→4)-O-β-cymaropyranosyl-(1→4)-O-β-digitoxopyranoside (3, madagascarensilide C)
Compound 3 was a white amorphous solid; [α]D23 +21 (c 0.15, MeOH); UV (MeOH) λmax nm (ε) 215 (3.9); IR νmax cm-1: 3459, 2939, 1740, 1374, 1072 cm-1. 1H NMR (500 MHz, d-pyridine) and 13C NMR (125 MHz, d-pyridine), see Table 1; HRESI-MS m/z 845.4315 [M+Na]+ (calcd for C43H66NaO15 845.4299).
4.8 Strophanthidin 3-O-β-glucopyranosyl-(l→4)-O-β-diginopyranosyl-(1→4)-O-β-cymaropyranosyl-(1→4)-O-β-digitoxopyranoside (4, madagascarensilide D)
Compound 4 was a white amorphous solid; [α]D23 +16 (c 0.51, MeOH); UV (MeOH) λmax nm (ε) 216 (4.1); IR νmax cm-1: 3445, 2937, 1736, 1372, 1074 cm-1. 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD), see Table 1; HRESI-MS m/z 1007.4844 [M+Na]+ (calcd for C49H76NaO20 1007.4828).
Supplementary Material
Figure 4.
a) Key COSY (bold) and HMBC (arrows) correlations for 3.
b) Key ROESY correlations for 3.
Acknowledgments
This project was supported by the Fogarty International Center, the National Cancer Institute, the National Science Foundation, the National Heart, Lung and Blood Institute, the National Institute of Mental Health, the Office of Dietary Supplements, and the Office of the Director of NIH, under Cooperative Agreement U01 TW000313 with the International Cooperative Biodiversity Groups. This project was also supported by the National Research Initiative of the Cooperative State Research, Education and Extension Service, USDA, Grant #2008-35621-04732. These supports are gratefully acknowledged. This work was also supported by the National Science Foundation under Grant No CHE-0619382 for purchase of the Bruker Avance 600 NMR spectrometer and Grant No. CHE-0722638 for the purchase of the Agilent 6220 mass spectrometer. We thank Mr. B. Bebout for obtaining the mass spectra and Dr. Hugo Azurmendi for assistance with the NMR spectra. Field work essential for this project was conducted under a collaborative agreement between the Missouri Botanical Garden and the Parc Botanique et Zoologique de Tsimbazaza and a multilateral agreement between the ICBG partners, including the Centre National d’Applications des Recherches Pharmaceutiques. We gratefully acknowledge courtesies extended by the Government of Madagascar (Ministère des Eaux et Forêts).
Footnotes
Biodiversity Conservation and Drug Discovery in Madagascar, Part 46. For Part 45, see Ref. 1.
Supporting Information Available
1H, 13C, COSY, TOCSY, HMBC, HMQC, and ROESY spectra of madagascarensilides A-D (1–4) are provided as Supplementary Data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and note
- 1.Pan E, Harinantanaina L, Brodie PJ, Miller JS, Callmander MW, Rakotonandrasana S, Rakotobe E, Rasamison VE, Kingston DGI. J Nat Prod. doi: 10.1021/np100411d. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harinantenaina L, Brodie PJ, Slebodnick C, Callmander MW, Rakotobe E, Randrianasolo S, Randrianaivo R, Rasamison VE, TebDyke K, Shen Y, Suh EM, Kingston DGI. J Nat Prod. 2010 doi: 10.1021/np100430r. np-2010-00430r, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao S, Kingston DGI. Pharm Biol. 2009;47:809–823. doi: 10.1080/13880200902988629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aquino R, Peluso G, DeTommasi N, DeSimone F, Pizza C. J Nat Prod. 1996;59:555. doi: 10.1021/np960251e. [DOI] [PubMed] [Google Scholar]
- 5.El-Moghazy AM, Ali AA, El-Sayyad SM, Sayed HM. Fitoterapia. 1980;51:321. [Google Scholar]
- 6.Lhinhatrakool T, Sutthivaiyakit S. J Nat Prod. 2006;69:1249. doi: 10.1021/np060249f. [DOI] [PubMed] [Google Scholar]
- 7.Sankara Subramanian S, Nair AGR. Phytochemistry. 1968;7:1703. [Google Scholar]
- 8.Krishna PVG, Rao EV, Rao DV. Planta Med. 1975;27:395. doi: 10.1055/s-0028-1097821. [DOI] [PubMed] [Google Scholar]
- 9.Nikiema JB, VanhaelenFastre R, Vanhaelen M. Planta Med. 1997;63:486–486. doi: 10.1055/s-2006-957747. [DOI] [PubMed] [Google Scholar]
- 10.Nikiema JB, Vanhaelen-Fastre R, Vanhaelen M, Fontaine J, De Graef C, Heenen M. Phytother Res. 2001;15:131–134. doi: 10.1002/ptr.700. [DOI] [PubMed] [Google Scholar]
- 11.Aquino R, Pizza C, Detommasi N, Desimone F. J Nat Prod. 1995;58:672–679. doi: 10.1021/np50119a004. [DOI] [PubMed] [Google Scholar]
- 12.Srivastav S, Deepak D, Khare A. Tetrahedron. 1994;50:789–798. [Google Scholar]
- 13.Cioffi G, Sanogo R, Vassallo A, Dal Piaz F, Autore G, Marzocco S, De Tommasi N. J Nat Prod. 2006;69:625–635. doi: 10.1021/np050493r. [DOI] [PubMed] [Google Scholar]
- 14.Moustafa AMY, Khodair AI, Saleh MA. Pharm Biol. 2009;47:826–834. [Google Scholar]
- 15.Moustafa AMY, Khodair AI, Saleh MA. Pharm Biol. 2009;47:994–1003. [Google Scholar]
- 16.Mehanna AS. Cardiac Agents: Cardiac Glycosides, Antianginal, and Antiarrhythmic Drugs. In: Lemke TL, Williams DA, editors. Foye’s Principles of Medicinal Chemistry. 6. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. pp. 698–721. [Google Scholar]
- 17.Rao RV, Vaidyanathan CS. J Indian Inst Sci. 1991;71:329–64. [Google Scholar]
- 18.Deepak D, Srivastava S, Khare NK, Khare A. Prog Chem Org Nat Prod. 1996;69:71–155. doi: 10.1007/978-3-7091-6578-2_2. [DOI] [PubMed] [Google Scholar]
- 19.Newman RA, Yang PY, Pawlus AD, Block KI. Mol Interv. 2008;8:36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- 20.Prassas I, Diamandis EP. Nat Rev Drug Discovery. 2008;7:926–935. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Goda Y, Sakai S, Kondo K, Akiyama H, Toyoda M. Phytochemistry. 1998;49:2097–2101. doi: 10.1016/s0031-9422(98)00421-x. [DOI] [PubMed] [Google Scholar]
- 22.Abe F, Yamauchi T. Chem Pharm Bull. 1979;27(7):1604–1610. doi: 10.1248/cpb.27.1604. [DOI] [PubMed] [Google Scholar]
- 23.Kopp B, Krenn L, Kubelka E, Kubelka W. Phytochemistry. 1992;31:3195–3198. doi: 10.1016/0031-9422(92)83473-c. [DOI] [PubMed] [Google Scholar]
- 24.Pauli GF. J Nat Prod. 1995;58:483–494. doi: 10.1021/np50118a002. [DOI] [PubMed] [Google Scholar]
- 25.Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Cancer Res. 1985;45:2110–2115. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.