Abstract
Previous research has found that hyperbaric oxygen (HBO2) produces an acute antinociceptive effect that is dependent on nitric oxide (NO). The present study was undertaken to determine whether HBO2-induced acute antinociception might involve a NO–cyclic GMP–protein kinase G–ATP-sensitive potassium (KATP) channel pathway. Male NIH Swiss mice were subjected to a 5-min HBO2 treatment (100% oxygen at 3.5 absolute atmospheres) and antinociception was assessed over the next 6 min still under HBO2 using the acetic acid abdominal constriction test. Pretreatment with 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide (carboxy-PTIO, an NO scavenger), 1H-[1,2,4]-oxadiazolo-[4,3-a]quinoxalin-1-one) (a soluble guanylyl cyclase-inhibitor, Rp-8-(4-chlorophenylthio)-guanosine-3',5'-cyclic monophosphorothioate (a protein kinase G-inhibitor) or glibenclamide (an ATP-sensitive potassium channel-inhibitor) all led to antagonism of the HBO2-induced acute antinociception in a dose-dependent manner. These findings suggest that HBO2-induced acute antinociception might be due to activation of a NO–cyclic GMP–protein kinase G–KATP channel pathway.
Keywords: Hyperbaric oxygen, antinociception, nitric oxide, cyclic GMP, protein kinase G, potassium channels, mice
1. Introduction
Previously we implicated the biological regulator nitric oxide (NO) in the antinociceptive response of mice to different conditions of hyperbaric oxygen (HBO2) treatment (Ohgami et al., 2009; Zelinski et al., 2009; Chung et al., 2010). The acute antinociceptive response to HBO2 was significantly reduced by pharmacological inhibition of nitric oxide synthase (NOS) activity or suppression of neuronal NOS (nNOS) expression by an anti-sense oligodeoxynucleotide directed against nNOS (Ohgami et al., 2009). Developmental suppression of nNOS in mice homozygous for a defective nNOS gene (nNOS–/–) also caused a significantly diminished antinociceptive response to HBO2, when compared to wild-type (nNOS+/+) mice (Ohgami et al., 2009).
Other investigations have shown that the main signal transduction pathway activated by NO likely involves the activation of soluble guanylyl cyclase (sGC), which increases intracellular levels of 3',5'-cyclic guanosine monophosphatecyclic (cyclic GMP) (Southam and Garthwaite, 1993). The NO signal can then be passed downstream to a variety of signaling targets, including cyclic GMP-dependent protein kinases, cyclic nucleotide-gated ion channel protein kinases, and cyclic AMP-specific phosphodiesterases (MacFarland, 1995).
This study tested the hypothesis that the acute antinociceptive effect of HBO2 is mediated by a signaling pathway in which there is sequential activation of NOS, sGC, protein kinase G and ATP-sensitive potassium (KATP) channels. A pharmacological approach was adopted to assess the involvement of each of these components of the signaling pathway.
2. Results
2.1. HBO2-induced antinociception
An intraperitoneal injection of 1.0% glacial acetic acid typically induced abdominal constrictions that were counted for a 6-min period commencing 5 min after the injection. On average, mice exhibited 16.4±1.1 abdominal constrictions (N=21). Exposure of mice to 3.5 ATA HBO2 during that 11-min period evoked a robust antinociceptive effect, reducing the number of abdominal constriction typically by 60-70%.
2.2. Effect of carboxy-PTIO on the antinociceptive activity of HBO2
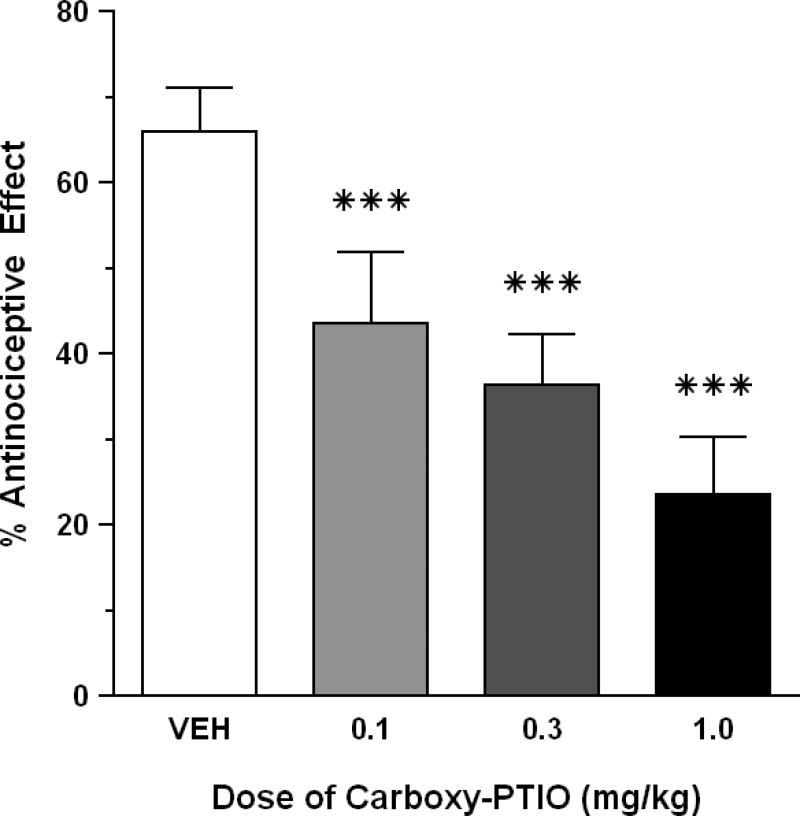
Control mice pretreated with saline vehicle reacted to HBO2 (3.5 absolute atmospheres, ATA) with a 66.0% antinociceptive response. I.p. pretreatment with three doses of carboxy-PTIO (0.1-1.0 mg/kg) caused a significant (P < 0.05) dose-related antagonism of HBO2-induced antinociception when compared to saline-pretreated mice (P < 0.001) (Fig. 1). A control dose of 0.3 mg/kg carboxy-PTIO alone (i.e., in room air) produced minimal suppression of abdominal constrictions (data not shown).
Fig. 1.
Effect of carboxy-PTIO on the antinociceptive effect of HBO2 at 3.5 ATA. Each bar represents the mean antinociceptive effect and each vertical line represents the SEM of at least 8 mice per group. Significance of difference: ***, P < 0.001, compared to the vehicle pretreatment group (post-hoc Bonferroni's multiple comparison test).
2.3. Effect of ODQ on the antinociceptive activity of HBO2
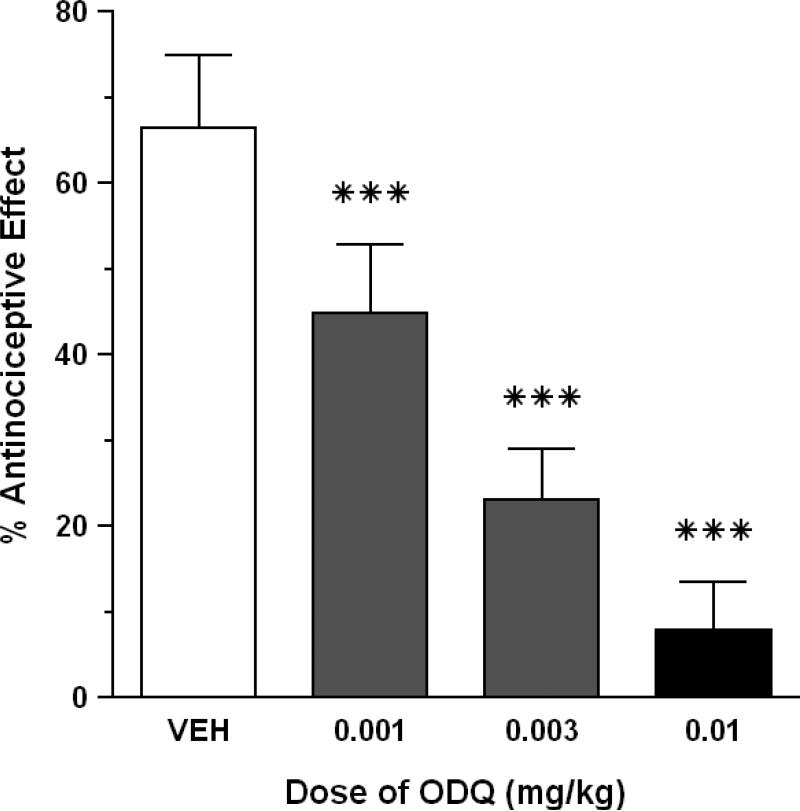
HBO2 elicited a 66.5% antinociceptive effect in control mice that were pretreated with 1% DMSO vehicle. In an earlier experiment, i.p. pretreatment with three doses of ODQ (0.1-1.0 mg/kg) virtually abolished the antinociceptive effect of HBO2 at each dose: 5.7% antinociceptive effect at 1.0 mg/kg ODQ; 7.9% antinociceptive effect at 0.3 mg/kg ODQ; and 3.0% antinociceptive effect of 0.1 mg/kg ODQ. Therefore, we resorted to a lower dose range and found that ODQ (0.001-0.01 mg/kg) produced a dose-dependent reduction in HBO2-induced antinociception (P < 0.05) (Fig. 2). All three doses of ODQ significantly reduced the magnitude of the HBO2-induced antinociceptive effect (P < 0.001) compared to a control group that was pretreated with the 1% DMSO vehicle. A control dose of 1.0 mg/kg ODQ had little effect on the number of abdominal constrictions (data not shown).
Fig. 2.
Effect of ODQ on the antinociceptive effect of HBO2 at 3.5 ATA. Each bar represents the mean antinociceptive effect and each vertical line represents the SEM of at least 8 mice per group. Significance of difference: ***, P < 0.001, compared to the vehicle pretreatment group (post-hoc Bonferroni's multiple comparison test).
2.4. Effect of Rp-8-pCPT-cGMPS on the antinociceptive activity of HBO2
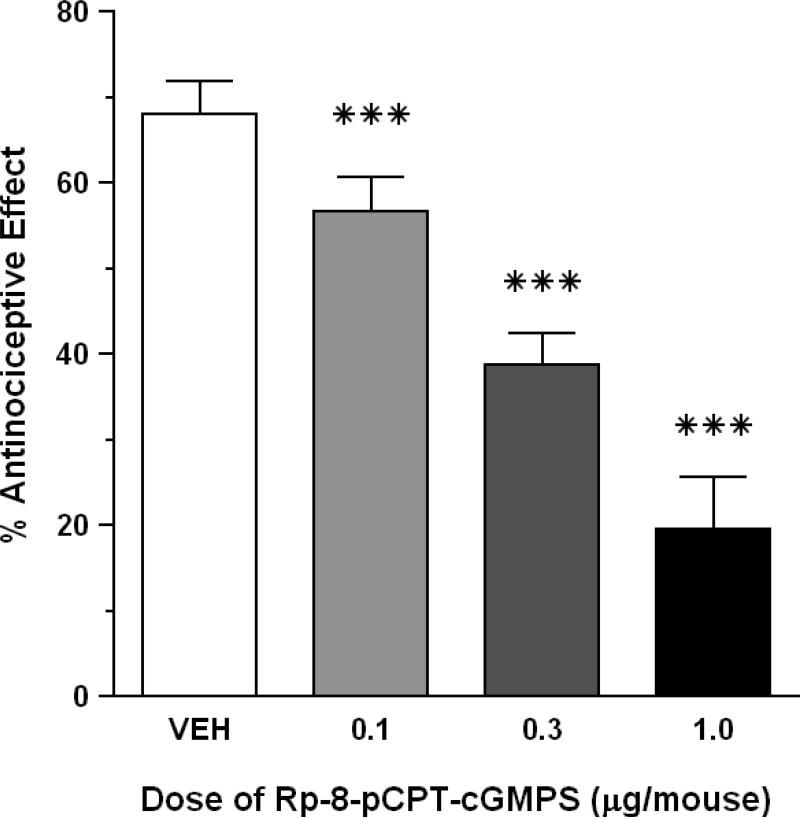
Control mice pretreated with sterile saline i.c.v. responded to HBO2 with 68.0% antinociception. I.c.v. pretreatment with three doses of Rp-8-pCPT-cGMPS (0.1-1.0 μg/mouse) also resulted in a dose-related antagonism of HBO2-induced antinociception (P < 0.05) (Fig. 3). All three doses of Rp-8-pCPT-cGMPS significantly reduced the magnitude of the HBO2-induced antinociception (P < 0.05) compared to a control group pre-treated i.c.v. with the vehicle. A control dose of 0.3 μg Rp-8-pCPT-cGMPS per mouse produced a slight but statistically insignificant reduction in the number of abdominal constrictions (data not shown).
Fig. 3.
Effect of Rp-8-pCPT-cGMPS on the antinociceptive effect of HBO2 at 3.5 ATA. Each bar represents the mean antinociceptive effect and each vertical line represents the SEM of at least 8 mice per group. Significance of difference: ***, P < 0.001, compared to the vehicle pretreatment group (post-hoc Bonferroni's multiple comparison test).
2.5. Effect of glibenclamide on the antinociceptive activity of HBO2
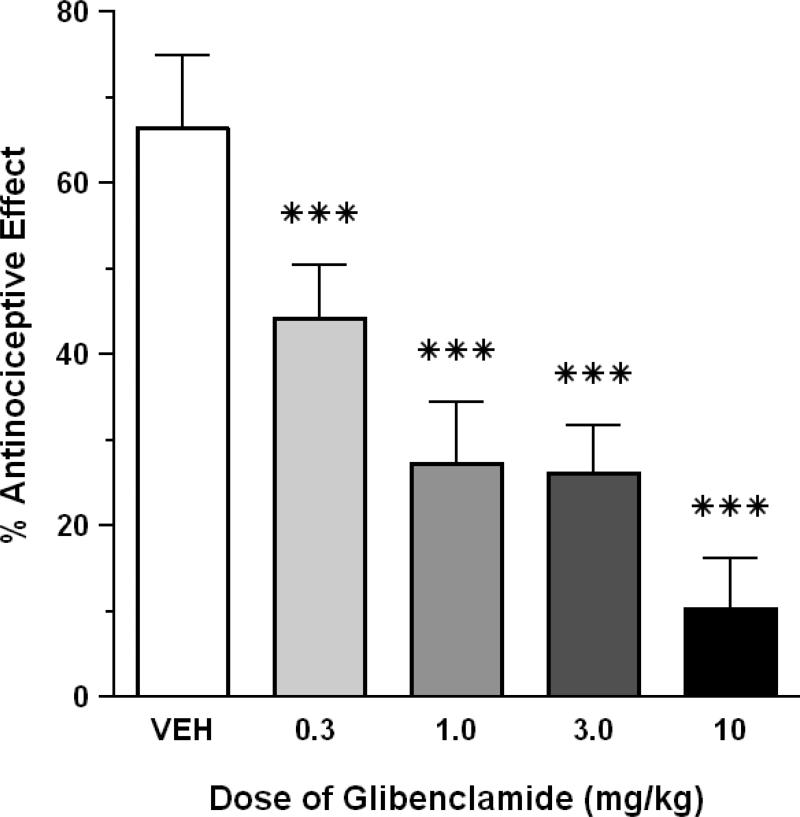
HBO2 elicited a 66.5% antinociceptive effect in control mice that were pretreated with 1% DMSO vehicle. I.p. pretreatment with four doses of glibenclamide (0.3-10 mg/kg) caused a dose-dependent attenuation of HBO2-induced antinociception (P < 0.05) (Fig. 4). All doses of glibenclamide significantly reduced the magnitude of the HBO2-induced antinociception compared to a control group pretreated i.p. with 1% DMSO vehicle (P < 0.05). However, the level of antagonism at 1.0 and 3.0 mg/kg glibenclamide was virtually identical. The highest dose of glibenclamide (10 mg/kg) alone had no effect on the number of abdominal constrictions (data not shown).
Fig. 4.
Effect of glibenclamide on the antinociceptive effect of HBO2 at 3.5 ATA. Each bar represents the mean antinociceptive effect and each vertical line represents the SEM of at least 8 mice per group. Significance of difference: ***, P < 0.001, compared to the vehicle pretreatment group (post-hoc Bonferroni's multiple comparison test).
3. Discussion
Findings from our laboratory and others have demonstrated that HBO2 treatment can increase the production of NO in brain (Elayan et al., 2000; Thom and Buerk, 2003; Ohgami et al., 2008) and other tissues (Thom et al., 2003). We also found that inhibiting either the activity or the expression of nNOS can interfere with the antinociceptive effect of HBO2 following 11- and 60-min treatments. This suggests that NO initiates a process that culminates in the relief of pain (Ohgami et al., 2009; Zelinski et al., 2009) in the experimental pain model used.
There are several molecular targets of NO, but the most studied is sGC, which catalyzes the conversion of GTP to cyclic GMP (Southam and Garthwaite, 1993). Cyclic GMP, in turn, has several possible targets, including cyclic GMP-dependent protein kinases (PKG) (Ota et al., 2008), cyclic GMP-gated cation channels (Barnstable et al., 2004) and cyclic GMP-regulated phophodiesterases (PDE) (Lin et al., 2010).
Recent studies have also indicated that NO and cyclic GMP can activate ATP-sensitive potassium (KATP) channels, which have been implicated in drug-induced antinociception in the periphery (Lázaro-Ibáñez et al., 2001; Ortiz et al., 2003; Sachs et al., 2004; Brito et al., 2006; Hernández-Pacheco et al., 2008). There is also evidence that KATP channels may also be implicated in the central antinociceptive effect of morphine (Ocaña et al., 1990; Narita et al., 1992) and other μ or δ but not κ opioid agonists (Ocaña et al., 1993; Marker et al., 2005).
Against this backdrop, the present study was conducted to determine the possible involvement of a NO–cyclic GMP–PKG–KATP pathway in the acute antinociceptive response produced by HBO2. Accordingly, we examined the influence of pretreatment drugs that would disrupt the NO–cyclic GMP–PKG–KATP pathway at different chokepoints and determined their influence on HBO2-induced antinociception.
Carboxy-PTIO is an NO scavenger that exhibits an inhibitory action against the NO free radical (Akaike et al., 1993). Its ability to antagonize the acute antinociceptive effect of HBO2 replicates the antagonism of HBO2 by inhibition of NOS enzyme activity or expression of neuronal NOS in mice (Ohgami et al., 2009). This reaffirms the important role played by NO in HBO2-induced antinociception.
ODQ is a highly selective, irreversible inhibitor of sGC (Garthwaite et al., 1995). Its ability to antagonize HBO2-induced acute antinociception supports the contention that HBO2-stimulated production of NO leads to activation of soluble guanylyl cyclase to stimulate the production of cyclic GMP. But it was surprising how exquisitely sensitive was HBO2-induced acute antinociception to antagonism by ODQ. In the present study, i.p. doses of ODQ between 0.001 and 0.01 mg/kg caused a dose-related antagonism of the antinociceptive effect. By comparison, ODQ has been reported to antagonize the antinociceptive effect of sildenafil in a dose range of 0.1-1.0 mg/kg (Vale et al., 2007). The explanation for this different in antagonistic potency of ODQ is not presently known.
Rp-8-pCPT-cGMPS is an inhibitor of PKG or cyclic GMP-dependent protein kinase (Butt et al., 1994). Its ability to antagonize HBO2-induced acute antinociception suggests that the HBO2-stimulated production of cyclic GMP results in activation of the cyclic GMP-dependent protein kinase enzyme to produce antinociception.
Zaprinast is an inhibitor of cyclic GMP-sensitive PDE (Gibson, 2001). Taken together with the ODQ antagonism of HBO2-induced antinociception, the observed enhancement of HBO2 by zaprinast verifies that cyclic GMP is an important determinant of the magnitude of the antinociceptive response to HBO2.
Glibenclamide (also known as glyburide) is a sulfonylurea compound that inhibits KATP channels (Silva-Santos et al., 2002). The dose-related antagonism of HBO2-induced antinociception by glibenclamide indicates that the activation of PKG in HBO2-exposed animals, in turn, activates KATP channels to elicit the acute antinociceptive response.
The findings from this study clearly implicate the involvement of NO–cyclic GMP–protein kinase G–KATP channel pathway in the acute antinociceptive effect of HBO2-induced antinociception. It is acknowledged that there is increasing evidence of both pronociceptive and antinociceptive roles for NO, which is a ubiquitous and unique biological messenger molecule (see reviews by Miclescu and Gordh, 2009 and Schmidtko et al., 2009). The literature reports an exasperating dichotomy of NO modulation of neurological, pathophysiological and psychological functions. For example, NO is a physiological inhibitor of neurogenesis, yet NO has also been found to activate neurogenesis (Cárdenas et al., 2005). There is evidence that NO participates in both neurotoxicity and neuroprotection (Calabrese et al., 2007). NO can exert both proconvulsant and anticonvulsant influences (Ferraro and Sardo, 2004). Research findings show that NO mediates drug-induced hypothermia (Quock et al., 2007). But other studies show that NO is required for the production of fever (Scammell et al., 1996). NO also appears to have a dual role in the modulation of depression as well as anxiety (Li et al., 2003; Spolidório et al., 2007; Spiacci et al., 2008). Therefore, it may not be surprising that NO is likely to be involved in modulation of pronociceptive as well as antinociceptive pathways. This is a plausible explanation for contradictory experimental findings that NO promotes pain in some studies and inhibits pain in others.
In summary, the findings of this study provide support for the involvement of an NO-cyclic GMP-PKG-KATP channel pathway in mediation of HBO2-induced antinociception.
4. Experimental procedures
4.1. Animals
Male NIH Swiss mice, weighing 18-22 g, were purchased from Harlan Laboratories (Indianapolis, IN) and used in these experiments, which were approved by an institutional animal care and use committee and carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996). All measures to minimize pain or discomfort were taken by the investigators.
Mice were housed five per cage in the Animal Resource Unit at Washington State University with access to food and water ad libitum. The facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), was maintained on a 12-h light:dark cycle (lights on 07:00-19:00 h) under standard conditions (22 ± 1°C room temperature, 33% humidity). Mice were kept in the holding room for at least four days after arrival in the facility for acclimation prior to experimentation.
4.2. Exposure to Hyperbaric Oxygen (HBO2)
Mice were placed in a B-11 research hyperbaric chamber (Reimers Systems, Inc., Lorton, VA) as previously described (Zelinski et al., 2009). The chamber was ventilated with 100% O2, U.S.P. (A-L Compressed Gases Inc., Spokane, WA) at a flow rate of 20 L/min to minimize CO2 accumulation. The pressure within the cylindrical clear acrylic chamber (27.9 cm diameter × 55.9 cm L) was increased from 1.0-3.5 absolute atmosphere (ATA) over 2 min. The pressure was held for another 3 min prior to the start of the 6-min observation period. The mice breathed spontaneously during HBO2 treatment. After completion of the HBO2 exposure, mice were then decompressed over 2-3 min. Hence, the actual exposure time to 3.5 ATA HBO2 was approximately 9 min. Control groups of mice were exposed to room air, and experimental groups of mice were exposed to 100% O2 circulated through the chamber at either 1.0 or 3.5 ATA according to the same time schedule as above.
4.3. Antinociceptive Testing
Antinociceptive responsiveness was assessed using the abdominal constriction test (Siegmund et al., 1957). This nociceptive model was selected over tests employing a thermal noxious stimulus because this paradigm is significantly more sensitive to detection of κ opioid antinociceptive activity than are thermal tests (Tyers, 1980) and because our previous studies have implicated κ opioid mechanisms in mediation of HBO2-induced antinociception (Zelinski et al., 2009).
Mice were treated i.p. with 0.1 ml per 10 g body weight of 0.6% glacial acetic acid and placed into the hyperbaric chamber. Exactly 5 min later, the number of abdominal constrictions—lengthwise stretches of the torso with concave arching of the back—in each animal was counted for 6 min while under HBO2. Multiple raters were used for some but not all experiments; at least one of the raters was blinded to the drug treatment. All experiments were generally conducted between 0900 and 1200 h. The control reference group was exposed to room air. The degree of antinociception (inhibition of abdominal constrictions) produced in various treatment groups of mice was calculated as:
4.4. Drugs
The following drugs were used in this research: 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide, potassium salt (carboxy-PTIO) and glibenclamide (Tocris Bioscience, Ellisville, MO); 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ) (Axxora LLC, San Diego, CA); and Rp-8-(4-chlorophenylthio)-guanosine-3',5'-cyclic monophosphorothioate, sodium salt (Rp-8-pCPT-cGMPS) (Enzo Life Sciences, Plymouth Meeting, PA).
Carboxy-PTIO was dissolved in bacteriostatic saline. ODQ and glibenclamide were dissolved in dimethylsulfoxide (DMSO), and distilled water was added to attain a final DMSO concentration of 1%. All three pretreatment drugs were administered i.p. 30 min prior to testing. The volume of i.p. injection was 0.1 ml/10 g body weight with an equivalent volume of the respective vehicles administered to control animals. The dose ranges were 0.1-1.0 mg/kg of carboxy-PTIO, 0.001-1.0 mg/kg of ODQ and 0.3-10 mg/kg of glibencalmide. It should be noted that the original dose range of ODQ was 1.0-10 mg/kg but was adjusted when these doses proved to be supramaximal in antagonizing HBO2.
Rp-8-pCPT-cGMPS was dissolved in distilled water and administered within a dose range of 0.1-1.0 μg/mouse via the intracerebroventricular (i.c.v.) route according to the method of Haley and McCormick (1958). Briefly, mice were anesthetized with IsoFlo® (isoflurane, U.S.P., Abbott Laboratories, N. Chicago, IL). A short incision was made along the midline of the scalp using a scalpel, and the skin was pulled back to expose the calvarium. The i.c.v. microinjection was made using a 10-μl microsyringe (Hamilton, Reno, NV) with a 26-gauge cemented needle. The microsyringe was held vertically by hand at a point on the calvarium 2.0 mm lateral and 1.0 mm caudal from bregma to a depth of −2.0 mm from the skull surface. Penetration was controlled by a large-bore needle through which the microsyringe needle was inserted; the larger hypodermic needle served as a collar to limit penetration of the microsyringe needle to 2.0 mm. A volume of 4.0 μl of drug solution or vehicle was delivered directly into the lateral cerebral ventricle over 30 sec.
4.5. Statistical Analysis of Data
One-way ANOVA with a post-hoc Bonferroni multiple comparison test was used to compare the antinociceptive responsiveness of different drug pretreatment groups to HBO2-induced antinociception.
5. Conclusion
Competitive antagonism of the antinociceptive response of mice to HBO2 by scavenging of NO, inhibition of cyclic GMP production, inhibition of PKG enzyme and blockade of KATP channel indicates that HBO2-induced antinociception is mediated a NO–cyclic GMP–PKG–KATP channel pathway.
Acknowledgements
This research was supported by NIH Grant GM-77153 (R.M.Q.), the Allen I. White Distinguished Professorship and an Institutional Summer Undergraduate Research Fellowship (SURF) Award in Pharmacology/Toxicology from the American Society for Pharmacology and Experimental Therapeutics (ASPET).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Hyperbaric oxygen (HBO2) induces antinocicepton in the mouse abdominal constriction test.
- HBO2 antinociception depends on NO, cyclic GMP, PKG and the KATP channel.
References
- 1.Akaike T, Yoshida M, Miyamoto Y, Sato K, Kohno M, Sasamoto K, Miyazaki K, Ueda S, Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/•NO through a radical reaction. Biochemistry. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- 2.Barnstable CJ, Wei JY, Han MH. Modulation of synaptic function by cGMP and cGMP-gated cation channels. Neurochem. Int. 2004;45:875–884. doi: 10.1016/j.neuint.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Brito GAC, Sachs D, Cunha FQ, Vale ML, Lotufo CMC, Ferreira SH, Ribeiro RA. Peripheral antinociceptive effect of pertussis toxin: activation of the arginine/NO/cGMP/PKG/ATP-sensitive K+ channel pathway. Eur. J. Neurosci. 2006;24:1175–1181. doi: 10.1111/j.1460-9568.2006.04991.x. [DOI] [PubMed] [Google Scholar]
- 4.Butt E, Eigenthaler M, Genieser HG. (Rp)-8-pCPT-cGMPS, a novel cGMP-dependent protein kinase inhibitor. Eur. J. Pharmacol. 1994;269:265–268. doi: 10.1016/0922-4106(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 6.Cárdenas A, Moro MA, Hurtado O, Leza JC, Lizasoain I. Dual role of nitric oxide in adult neurogenesis. Brain Res. Rev. 2005;50:1–6. doi: 10.1016/j.brainresrev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Chung E, Zielinski LM, Ohgami Y, Shirachi DY, Quock RM. Hyperbaric oxygen treatment induces a two-phase antinociceptive response of unusually long duration in mice. J. Pain. 2010;11:847–853. doi: 10.1016/j.jpain.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elayan IM, Axley MJ, Prasad PV, Ahlers ST, Auker CR. Effect of hyperbaric oxygen treatment on nitric oxide and oxygen free radicals in rat brain. J. Neurophysiol. 2000;83:2022–2029. doi: 10.1152/jn.2000.83.4.2022. [DOI] [PubMed] [Google Scholar]
- 9.Ferraro G, Sardo P. Nitric oxide and brain hyperexcitability. In Vivo. 2005;18:357–366. [PubMed] [Google Scholar]
- 10.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 11.Gibson A. Phosphodiesterase 5 inhibitors and nitrergic transmission-from zaprinast to sildenafil. Eur. J. Pharmacol. 2001;411:1–10. doi: 10.1016/s0014-2999(00)00824-4. [DOI] [PubMed] [Google Scholar]
- 12.Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injections of drugs in the conscious mouse. Br. J. Pharmacol. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández-Pacheco A, Araiza-Saldana CI, Granados-Soto V, Mixcoatl-Zecuatl T. Possible participation of the nitric oxide–cyclic GMP–protein kinase G–K+ channels pathway in the peripheral antinociception of melatonin. Eur. J. Pharmacol. 2008;596:70–76. doi: 10.1016/j.ejphar.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 14.Lázaro-Ibáñez GG, Torres-López JE, Granados-Soto V. Participation of the nitric oxide-cyclic GMP-ATP-sensitive K+ channel pathway in the antinociceptive action of ketorolac. Eur. J. Pharmacol. 2001;426:39–44. doi: 10.1016/s0014-2999(01)01206-7. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Ohgami Y, Dai Y, Quock RM. Antagonism of nitrous oxide-induced behavior in mice by pharmacologic disruption of endogenous nitric oxide function. Psychopharmacology. 2003;166:366–372. doi: 10.1007/s00213-002-1363-0. [DOI] [PubMed] [Google Scholar]
- 16.Lin DT, Fretier P, Jiang C, Vincent SR. Nitric oxide signaling via cGMP-stimulated phosphodiesterase in striatal neurons. Synapse. 2010;64:460–466. doi: 10.1002/syn.20750. [DOI] [PubMed] [Google Scholar]
- 17.MacFarland RT. Molecular aspects of cyclic GMP signaling. Zool. Sci. 1995;12:151–163. doi: 10.2108/zsj.12.151. [DOI] [PubMed] [Google Scholar]
- 18.Marker CL, Luján R, Loh HH, Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of μ- and δ- but not κ-opioids. J. Neurosci. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miclescu A, Gordh T. Nitric oxide and pain: “Something old, something new.”. Acta Anaesthesiol. Scand. 2009;53:1107–1120. doi: 10.1111/j.1399-6576.2009.02054.x. [DOI] [PubMed] [Google Scholar]
- 20.Narita M, Suzuki T, Misawa M, Nagase H, Nabeshima A, Ashizawa T, Ozawa H, Saito T, Takahata N. Role of central ATP-sensitive potassium channels in the analgesic effect and spinal noradrenaline turnover-enhancing effect of intracerebroventricularly injected morphine in mice. Brain Res. 1992;596:209–214. doi: 10.1016/0006-8993(92)91549-t. [DOI] [PubMed] [Google Scholar]
- 21.Ocaña M, Del Pozo E, Baeyens JM. ATP-dependent K+ channel blockers antagonize morphine- but not U-504,88H-induced antinociception. Eur. J. Pharmacol. 1993;230:203–207. doi: 10.1016/0014-2999(93)90803-p. [DOI] [PubMed] [Google Scholar]
- 22.Ocaña M, Del Pozo E, Barrios M, Robles LI, Baeyens JM. An ATP-dependent potassium channel blocker antagonizes morphine analgesia. Eur. J. Pharmacol. 1990;186:377–378. doi: 10.1016/0014-2999(90)90466-j. [DOI] [PubMed] [Google Scholar]
- 23.Ohgami Y, Chung E, Shirachi DY, Quock RM. Influence of hyperbaric oxygen on regional brain levels and spinal cord levels of nitric oxide metabolites in rat. Brain Res. Bull. 2008;75:668–673. doi: 10.1016/j.brainresbull.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Ohgami Y, Zylstra CC, Quock LP, Chung E, Shirachi DY, Quock RM. Nitric oxide in hyperbaric oxygen-induced acute antinociception in mice. NeuroReport. 2009;20:1325–1329. doi: 10.1097/WNR.0b013e3283305a49. [DOI] [PubMed] [Google Scholar]
- 25.Ortiz MI, Granados-Soto V, Castañeda-Hernández G. The NO-cGMP-K+ channel pathway participates in the antinociceptive effect of diclofenac, but not of indomethacin. Pharmacol. Biochem. Behav. 2003;76:187-195. doi: 10.1016/s0091-3057(03)00214-4. [DOI] [PubMed] [Google Scholar]
- 26.Ota KT, Pierre VJ, Ploski JE, Queen K, Schafe GE. The NO-cGMPPKG signaling pathway regulates synaptic plasticity and fear memory consolidation in the lateral amygdala via activation of ERK/MAP kinase. Learn. Mem. 2008;15:792–805. doi: 10.1101/lm.1114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quock RM, Butt S, Prall CW, Ramsay DS, Kaiyala KJ. Role of nitric oxide in nitrous oxide-induced thermolysis in the rat. Proc. West. Pharmacol. Soc. 2007;50:206. [Google Scholar]
- 28.Sachs D, Cunha FQ, Ferreira SH. Peripheral analgesic blockade of hyper-nociception: activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc. Natl. Acad. Sci. USA. 2004;101:3680–3685. doi: 10.1073/pnas.0308382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scammell TE, Elmquist JK, Saper CB. Inhibition of nitric oxide synthase produces hypothermia and depresses lipopolysaccharide fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996;271:R333–R338. doi: 10.1152/ajpregu.1996.271.2.R333. [DOI] [PubMed] [Google Scholar]
- 30.Schmidtko A, Tegeder I, Geisslinger G. No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci. 2009;32:339–346. doi: 10.1016/j.tins.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Siegmund E, Cadmus R, Lu G. A method for evaluating both non-narcotic and narcotic analgesics. Proc. Soc. Exp. Biol. Med. 1957;95:729–731. doi: 10.3181/00379727-95-23345. [DOI] [PubMed] [Google Scholar]
- 32.Silva-Santos JE, Santos-Silva MA, Cunha FQ, Assreuy J. The role of ATP-sensitive potassium channels in neutrophil migration and plasma exudation. J. Pharmacol. Exp. Ther. 2002;300:946–951. doi: 10.1124/jpet.300.3.946. [DOI] [PubMed] [Google Scholar]
- 33.Southam E, Garthwaite J. The nitric oxide-cyclic GMP signalling pathway in rat brain. Neuropharmacol. 1993;32:1267–1277. doi: 10.1016/0028-3908(93)90021-t. [DOI] [PubMed] [Google Scholar]
- 34.Spiacci A, Jr., Kanamaru F, Guimarães FS, Oliveira RM. Nitric oxide-mediated anxiolytic-like and antidepressant-like effects in animal models of anxiety and depression. Pharmacol. Biochem. Behav. 2008;88:247–255. doi: 10.1016/j.pbb.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Spolidório PC, Echeverry MB, Iyomasa M, Guimarães FS, Del Bel EA. Anxiolytic effects induced by inhibition of the nitric oxide-cGMP pathway in the rat dorsal hippocampus. Psychopharmacol. 2007;195:183–192. doi: 10.1007/s00213-007-0890-0. [DOI] [PubMed] [Google Scholar]
- 36.Thom SR, Buerk DG. Nitric oxide synthesis in brain is stimulated by oxygen. Adv. Exp. Med. Biol. 2003;510:133–137. doi: 10.1007/978-1-4615-0205-0_22. [DOI] [PubMed] [Google Scholar]
- 37.Thom SR, Fisher D, Zhang J, Bhopale VM, Ohnishi ST, Kotake Y, Ohnishi T, Buerk DG. Stimulation of perivascular nitric oxide synthesis by oxygen. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1230–1239. doi: 10.1152/ajpheart.01043.2002. [DOI] [PubMed] [Google Scholar]
- 38.Tyers MB. A classification of opiate receptors that mediate antinociception in animals. Br. J. Pharmacol. 1980;69:503–512. doi: 10.1111/j.1476-5381.1980.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vale ML, Rolim DE, Cavalcante IF, Ribeiro RA, Souza MHLP. Role of NO/cGMP/KATP pathway in antinociceptive effect of sildenafil in zymosan writhing response in mice. Inflamm. Res. 2007;56:1023–3830. doi: 10.1007/s00011-006-6109-8. [DOI] [PubMed] [Google Scholar]
- 40.Zelinski LM, Ohgami Y, Chung E, Shirachi DY, Quock RM. A prolonged NO-dependent, opioid-mediated antinociceptive effect of hyperbaric oxygen in mice. J. Pain. 2009;10:167–172. doi: 10.1016/j.jpain.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]