Abstract
The aim of this study was to determine if the neutral cannabinoid CB1 receptor antagonist, AM4113, regulates body weight in the rat via changes in food intake. We confirmed that the AM4113-induced reduction in food intake is mediated by CB1 receptors using CB1 receptor knockout mice. In rats, intraperitoneally administered AM4113 (2, 10 mg kg−1) had a transient inhibitory effect on food intake, while body weight gain was suppressed for the duration of the study. AM4113-induced hypophagia was no longer observed once the inhibitory effect of AM4113 on body weight stabilized, at which time rats gained weight at a similar rate to vehicle-treated animals, yet at a lower magnitude. Pair-feeding produced similar effects to treatment with AM4113. Food intake and body weight gain were also inhibited in rats by oral administration of AM4113 (50 mg kg−1). Dual energy x-ray absorptiometry (DEXA) was used to measure lean and fat mass. The AM4113 treated group had 29.3 ± 11.4 % lower fat mass than vehicle treated rats; this trend did not reach statistical significance. There were no differences in circulating levels of the endogenous cannabinoid 2-arachidonoyl glycerol (2-AG), glucose, triglycerides, or cholesterol observed between treatment groups. Similarly, 2-AG hypothalamic levels were not modified by AM4113 treatment. These data suggest that blockade of an endocannabinoid tone acting at CB1 receptors induces an initial, transient reduction in food intake which results in long-term reduction of body weight gain.
Keywords: 2-AG, cannabinoid, food intake, metabolism, neutral cannabinoid antagonist, pair-feeding, weight loss
1. Introduction
The endogenous cannabinoid (CB) system consists of CB1 and CB2 receptors, endogenous ligands for these receptors, and the enzymes responsible for the synthesis and degradation of these ligands (Sugiura et al. 2002; Matias and Di Marzo 2006). Anandamide and 2-arachidonoyl glycerol (2-AG) were the first endogenous cannabinoid ligands to be discovered, and, like the exogenous ligands for these receptors, they display a range of actions including those on food intake and appetite (Kirkham and Williams 2001). Cannabinoids affect appetite and body weight by acting at CB1 receptors, where agonist stimulation increases food intake (Cota 2007). Treatment of rodents with a CB1 antagonist/inverse agonist, such as rimonabant, causes reductions in daily food intake, but this effect is transient, and food intake soon returns to the level of vehicle-treated controls (Colombo et al. 1998; Hildebrandt et al. 2003; Vickers et al. 2003; Ravinet Trillou et al. 2003; Bensaid et al. 2003; Liu et al. 2005). This observation led to the hypothesis that the effect of CB1 receptor antagonist/inverse agonists on body weight is maintained through actions on peripheral metabolic pathways (Ravinet Trillou et al. 2003; Cota et al. 2003; Jbilo et al. 2005; Horvath 2006; Nogueiras et al. 2008; Cota et al. 2009; Koolman et al. 2010).
Endogenous cannabinoid signaling could affect metabolism by acting on lipogenic pathways, or on pathways that affect lipolysis and energy expenditure. CB1 receptors are expressed in liver (Osei-Hyiaman et al. 2005), adipose tissue (Bensaid et al. 2003; Cota et al. 2003; Jbilo et al. 2005; Starowicz et al. 2008), gastrointestinal tract (Gómez et al. 2002; Duncan et al. 2005), pancreas (Starowicz et al. 2008) and skeletal muscle (Liu et al. 2005). In rodents, CB1 receptor antagonist/inverse agonists increase in vivo resting energy expenditure (Liu et al. 2005; Herling et al. 2008; Kunz et al. 2008) and glucose uptake in isolated soleus muscle (Liu et al. 2005). CB1 receptor agonists increase the expression of lipogenic transcription factors and de novo lipogenesis in liver (Osei-Hyiaman et al. 2005)and cultured adipocytes (Cota et al. 2003);effects that are blocked by a CB1 receptor antagonist/inverse agonist.
Pair-feeding studies, specifically designed to test the hypothesis that changes in metabolism maintain weight loss induced by a CB1 receptor antagonist/inverse agonist, have produced conflicting results (Vickers et al. 2003; Ravinet Trillou et al. 2003; Thornton-Jones et al. 2006; Janiak et al. 2007; Irwin et al. 2008; Herling et al. 2008; Cota et al. 2009). In some studies, differences in body weight between pair-fed rodents, and rodents treated with a CB1 receptor antagonist/inverse agonist implied the presence of an effect on metabolism (Ravinet Trillou et al. 2003; Herling et al. 2008; Cota et al. 2009). In others, pair-fed rodents weighed the same as treated animals indicating that changes in body weight induced by a CB1 receptor antagonist/inverse agonist result solely from the inhibition of food intake (Vickers et al. 2003; Thornton-Jones et al. 2006; Janiak et al. 2007; Irwin et al. 2008). The effects of a CB1 receptor antagonist/inverse agonist on energy expenditure have also produced inconsistent findings. In one study, mice treated with SR141716 had higher basal oxygen consumption rates than vehicle treated mice (Liu et al. 2005), but the body weight in these animals was the same as pair-fed mice. In another study, SR141716A significantly increased oxygen consumption in rat, but only for a brief time and only after the first treatment (Kunz et al. 2008).
We investigated whether the effect of a neutral CB1 receptor antagonist, AM4113, on body weight in rat was due solely to effects on food intake or whether effects on metabolism may contribute to the effect. The potential advantage of a neutral CB1 receptor antagonist is that the effects are specific to the pharmacological blockade of endogenous cannabinoid signaling (Chambers et al. 2007; Sink et al. 2008), without affects on constitutive receptor activity. Recently, it was shown that AM4113 inhibited food intake to a similar degree as the CB1 receptor antagonist/inverse agonist AM251 in rat (Chambers et al. 2007; Sink et al. 2008), but unlike AM251, AM4113 did not potentiate vomiting in the ferret (Chambers et al. 2007)or promote nausea in rat (Sink et al. 2008). We examined the role that food intake plays in the actions of AM4113 on body weight by measuring food intake and body weight in rats that were pair-fed to an AM4113 treatment group. The effects of AM4113 on body composition and on fasting glucose and lipid levels were assessed using dual energy x-ray absorptiometry (DEXA) and blood analysis, respectively to investigate which tissues and metabolic pathways were potentially altered by AM4113 administration. We also examined hypothalamic and plasma levels of the endogenous cannabinoid 2-AG following each treatment to determine whether AM4113 was modifying endocannabinoid levels as well as antagonizing CB1 receptors to exert its effects. Furthermore, we investigated the effects of orally administered AM4113 to gain an insight into the therapeutic potential of a neutral cannabinoid antagonist.
2. Materials and Methods
2.1. Compounds
The pyrazole-based neutral antagonist AM4113 (Chambers et al. 2007; Sink et al. 2008) was synthesized at Northeastern University. For intraperitoneal (i.p.) administration AM4113 was dissolved in DMSO using gentle heating and sonication before being diluted with Tween 80 and saline (4% DMSO; 1% Tween 80; 95% saline) to a final concentration of 2 and 10 mg ml−1 (Chambers et al. 2007). Injections were administered to mice at 100 μl 10 g−1 body weight and to rats at 100 μl 100 g−1 body weight. For oral (p.o.) administration AM4113 was dissolved in 4% DMSO before being diluted with extra light olive oil (Safeway, Calgary, Canada) to a final concentration of 25 mg ml−1 and was delivered in a volume of 200 μl 100 g−1 body weight.
2.2. Animal Experiments
Animals were individually housed in transparent plastic cages, with sawdust bedding, in a temperature-controlled room maintained on a 12 -h:12-h light/dark cycle and were allowed access to water ad libitum. Animal use for these studies was approved by the University of Calgary Animal Care Committee and all protocols were carried out in accordance with the guidelines of the Canadian Council on Animal Care.
2.3. Acute feeding experiments in CB1 receptor knockout (−/−) and wild type mice
Two breeding pairs of heterozygous CB1+/−C57BL/6N mice were obtained from B. Lutz (University Medical Center, Mainz, Germany) and bred at the University of Calgary to obtain CB1−/−C57BL/6N mice (Marsicano et al. 2002). Animals used in these studies were backcrossed to C57BL/6N for 6 generations and were used at the same age (10–16 weeks) and maintained under the same conditions as the wild type mice. All CB1−/− mice were genotyped using established protocols and were confirmed as homozygous gene deficient animals (CB1−/−C57BL/6N) prior to inclusion in the study.
Female CB1 receptor knockout mice weighing between 20–30 g at the start of the study were placed on a medium fat, palatable diet (51.4% carbohydrate, 31.8% fat, 16.8% protein; 4.41 kcal/g; Diet # D12266B, Research Diets, New Brunswick, NJ, USA) at least 4 days prior to the experiment. Food was available for 18 h each day starting at 16:30 h (lights off 18:00 h). One day prior to the experiment mice were assigned to either vehicle (wild type mice, mean body weight ± S.E.M.; 26.3± 1.2 g, n =5; CB1−/− mice: 22.1 ± 0.4 g, n =6) or 10 mg kg−1 AM4113 (wild type mice: 26.5 ± 1.1 g, n =5. CB1−/− mice: 22.3 ± 0.5 g, n =6) treatment groups. Mice were injected i.p. with 10 mg kg−1 AM4113 or vehicle immediately prior to the addition of food and their food intake was measured 1, 2, 3 and 18 hours after injection.
2.4. 14 d chronic feeding study in rats
Male Sprague-Dawley rats (Charles River, Montreal, Quebec; 180–280 g at the start of the study) were used to examine the effect of i.p. administered AM4113 on food intake and body weight. Animals were fed chocolate flavored Ensure plus liquid diet (53.3% carbohydrate, 29% fat, 16.7% protein; 1.41 kcal/g) (Abbot Laboratories, Abbott Park, IL, USA) to promote food intake and control for spillage. Rats were habituated to testing and handling procedures for 7 d prior to the start of the study. Food and water were presented in drip free inverted glass bottles that attached to the outside of the cage. Food was available for 18 h each day starting at 16:00 h (lights off 16:00 h). Prior to the first day of treatment, rats were assigned to either vehicle (1 ml kg−1, mean body weight ± SEM.; 229 ± 14 g, n =6), 2 mg kg−1 (231 ± 12 g; n = 6), or 10 mg kg−1 AM4113 (231± 11 g, n =5) treatment groups. Treatments were given i.p. once each day, for 14 d, immediately prior to the addition of food. An additional group of rats were treated with vehicle and pair-fed (232± 15 g, n =5) to rats in the 10 mg kg−1 treatment group. Food intake was time delayed in these rats by 1 d. Food intake and body weight were measured daily.
On day 15, after the 6 h period of food deprivation, under light anesthesia with isoflurane, body composition of each animal was analyzed using dual energy x-ray absorptiometry (DEXA) in conjunction with Hologic QDR software for small animals (Hologic QDR 4500, Holgic, Inc., Bedford, MA, U.S.A.). After DEXA analysis was complete rats were decapitated and trunk blood was collected in heparinized and non-heparinized tubes for the collection of plasma and serum, respectively. Heparinized tubes were immediately centrifuged for 10 min at 13,000 RCF after which 500 μl of plasma was aliquoted and flash-frozen in liquid nitrogen. At the same time the hypothalamus from each animal was isolated and flash-frozen. The level of 2-AG in plasma and hypothalamic samples was determined using the liquid chromatography-mass spectrometry (triple quadrupole)method described below. Non-heparinized tubes were placed on ice for at least 30 min before being centrifugated and aliquoted into two 300 μl serum samples. These samples were analyzed by Calgary Laboratory Services (Calgary, Alberta) for glucose, triglycerides, total cholesterol, and HDL cholesterol levels. Serum was analyzed for glucose (Gluco-quant/HK reagent, Roche Diagnostics) using an enzymatic UV assay. Circulating triglycerides (Triglycerides GPO-PAP reagent, Roche Diagnostics) and cholesterol (Cholesterol CHOD-PAP Reagent and HDL-C Plus 3rd generation Reagent, Roche Diagnostics) were analyzed by enzymatic colorimetric assays using a Roche/Hitachi Modular P800. All samples were collected using coded tubes and were analyzed blindly.
2.5. 2-AG levels
Mixtures of the analytes and the deuterated analogs that had been stored at −80°C were further diluted in a 20 mg ml−1 solution of fatty acid free bovine serum albumin to simulate analyte-free plasma and in ethanol to make the calibration standards, quality control samples and reference samples, as previously described (Williams et al. 2007). Calibration standards, reference extraction and tissue samples were extracted using a modified version of the Folch extraction, as previously described (Folch et al. 1957; Williams et al. 2007; Wood et al. 2010). Frozen tissue samples were weighed prior to extraction. Calibration curves were constructed from the ratios of the peak areas of the analytes versus the internal standard. Chromatographic separation was achieved using an Agilent Zorbax SB-CN column (2.1×50 mm, 5 mm) on a Finnigan TSQ Quantum Ultra triple quad mass spectrometer (Thermo Electron, San Jose CA) with an Agilent 1100 HPLC on the front end (Agilent Technologies, Wilmington, DE) (Williams et al. 2007; Wood et al. 2010). The mobile phase consisted of 10 mM ammonium acetate, pH 7.3 (A) and methanol (B) in the following gradient: initial conditions are held at 10% B for 1 min, increased to 60% B in 0.5 min, then from 60% to 75% from 1.5 to 10 min, finally to 95% in 0.5 min (flow rate = 0.5 ml min−1); the autosampler was kept at 4°C to prevent analyte degradation. Eluted peaks were ionized via atmospheric pressure chemical ionization in multiple reaction monitoring mode.
2.7. 7 d orally administered AM4113 feeding study
Rats, weighing 400–530 g at the start of the study, were habituated to testing and handling procedures as described above. The day before the experiment rats were assigned to either vehicle (2 ml kg−1, 459 ±23 g, n = 5), 50 mg kg−1(457 ± 24 g, n = 4), or pair-fed (459± 21 g, n = 4) treatment groups. Treatments were given orally once each day, for 7 d, immediately prior to the addition of food. Pair -fed rats were treated with vehicle. Food intake and body weight was measured daily during the treatment period and for a further 7 d following the cessation of treatment.
2.8. Statistics
Food intake and body weight data were analyzed using a 2-way mixed design ANOVA with time as the repeated measure. Significant differences were analyzed using Bonferroni’s post hoc test at each time point. Food intake data are expressed as mean food intake (g) ± S.E.M. Body weight data are expressed as mean weight change (g) ± S.E.M. or as a mean percentage body weight of vehicle treated rats. DEXA data was analyzed using a 1-way independent measures ANOVA. Data are expressed as the mean fat (g) or lean (g) body mass ± S.E.M. 2-AG levels were analyzed in triplicate using a 1-way independent measures ANOVA and are presented as mean μg ml −1 and mean ng g−1 ± S.E.M. in plasma and hypothalamus respectively. Glucose and lipid profiles were analyzed using a 1-way ANOVA and are expressed as mean mmol L−1± S.E.M.
3. Results
3.1. Feeding experiments in CB1 receptor knockout and wild type mice
AM4113 (10 mg kg−1) significantly reduced food intake in wild type mice, F=13.6, p=0.006 (Figure 1A), at 2, 3 and 18 h, p<0.05. In comparison, there were no significant differences in food intake between vehicle and AM4113 treated CB1 receptor knockout mice, F=0.6, p>0.05 (Figure 1B).
Figure 1.
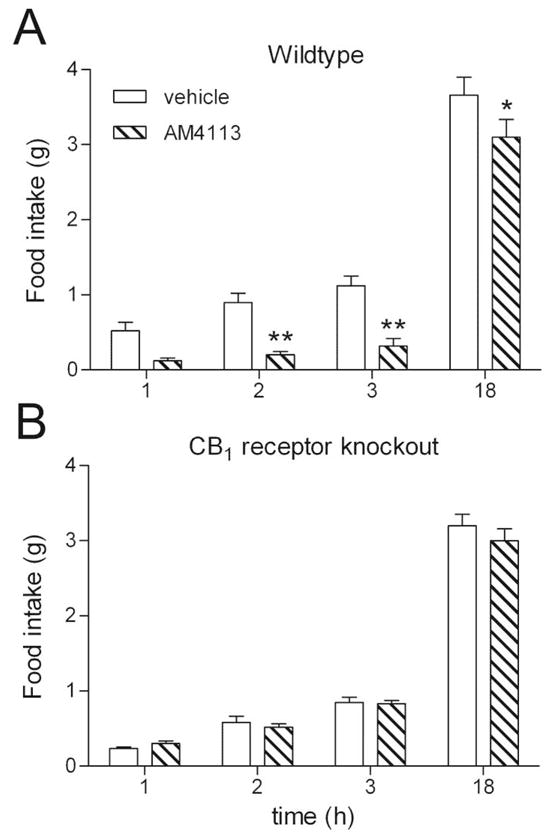
The effect of AM4113 (10 mg kg−1; i.p.) on food intake compared to vehicle (4% DMSO; 1% Tween 80; 95% saline; i.p.) treated animals in wildtype (A) and CB1 receptor knockout mice (B). Bars represent the mean ± S.E.M, n = 5–6 per group. * p < 0.05 and ** p<0.01 represent a significant difference to vehicle treatment analyzed by 2-way ANOVA followed by Bonferroni’s post hoc test.
3.2. 14 d chronic feeding study
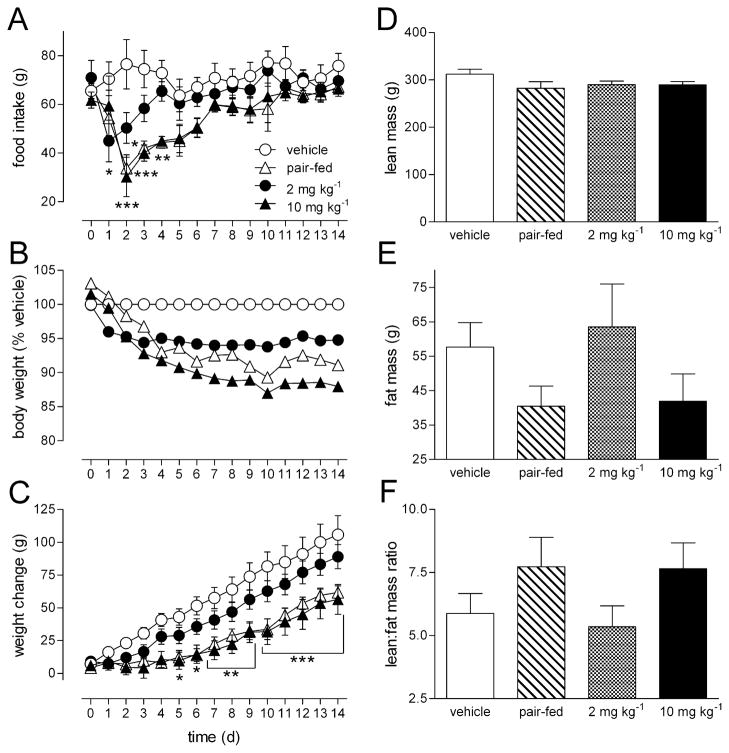
AM4113 produced a transient reduction in food intake (Figure 2A) seen as an effect of treatment F=3.93, p=0.026, accompanied by a sustained reduction in weight gain (Figure 2C), F=5.74, p=0.006. Post-hoc analysis revealed that food intake was significantly reduced on days 1 and 2 in 2 mg kg−1 treated rats ( p<0.05), and on days 2 to 4 in 10 mg kg−1 treated rats ( p< 0.01), compared to vehicle controls (Figure 2A). AM4113 (10 mg kg−1) significantly (p<0.05) reduced body weight gain on treatment days 5 to 14 (Figure 2C). Body weight of rats treated with 2 and 10 mg kg−1 AM4113 stabilized at 5% and 10% less than vehicle treated rats, respectively (Figure 2B). The anorectic effect of AM4113 on food intake was lost at the same time as the effect of AM4113 on body weight stabilized (Figure 2B; 2 mg kg−1, day 3 and 10 mg kg−1, day 5). Changes in body weight were similar between the pair-fed rats and the treated rats demonstrating that the effect of AM4113 on body weight was caused by reductions in food intake. When the anorectic effect of AM4113 was no longer observed (Figure 2A), rats gained weight at a similar rate to vehicle treated rats (Figure 2C). DEXA analysis showed that lean body mass was comparable between treatment groups (Figure 2D). There was a strong trend for fat mass (Figure 2E) to be reduced in AM4113 treated rats and their pair-fed controls, although these differences were not statistically significant alone or when data were expressed as lean: fat mass ratio (Figure 2F). Rats in the 10 mg kg−1 treatment group and pair-fed rats had 29.3 ± 11.4 % less fat mass, and 9.0 ± 2.2 % less lean body mass, than vehicle treated rats.
Figure 2.
The effect of daily AM4113 (2 or 10 mg kg−1; i.p.) administration, over 14 d, on food intake (A), body weight (B) and weight change (C) in rats compared to vehicle (4% DMSO; 1% Tween 80; 95% saline; i.p.)and rats pair -fed to the 10 mg kg−1 treated group. The effect of AM4113 (2 or 10 mg kg−1), vehicle or being pair-fed on lean mass (D), fat mass (E) and the lean: fat mass ratio (F). Data points or bars represent the mean ± S.E.M, n = 5–6 per group. * p <0.05, ** p<0.01 and *** p < 0.001 represent a significant difference to vehicle treatment analyzed by 2-way ANOVA (A, B, C) or 1-way ANOVA (D, E, F) followed by Bonferroni’s post hoc test.
3.3. 2-AGlevels and lipid profiles
Endogenous 2-AG and lipid profiles were examined in each group following the 14 d chronic feeding study. Following AM4113 (2 or 10 mg kg−1) treatment, plasma and hypothalamic levels of 2-AG were comparable to those measured in vehicle treated rats. Serum levels of glucose, triglycerides, total cholesterol, and HDL cholesterol in treated rats were also comparable to those in vehicle treated rats. The results are summarized in Table 1.
Table 1.
Plasma and brain 2-AG levels and serum lipid profiles following the 14 d i.p. chronic feeding study. 2-AG levels are presented as mean μg ml−1 and mean ng g−1 ± S.E.M. in plasma and brain respectively. Serum glucose and lipid profiles are expressed as mean mmol/L ± S.E.M.
| Vehicle | 2 mg kg−1 | 10 mg kg−1 | Pair-fed | |
|---|---|---|---|---|
| Plasma 2-AG (μg ml−1) | 18.1 ± 3.5 | 28.4 ± 7.9 | 12.7 ± 1.0 | 22.3 ± 4.6 |
| Hypothalamic 2-AG (ng g−1) | 5.1 ± 0.4 | 7.1 ± 0.7 | 6.4 ± 0.4 | 6.0 ± 0.4 |
| Glucose (mmol L−1) | 12.0 ± 1.2 | 12.0 ± 0.2 | 13.4 ± 0.8 | 11.5 ± 0.5 |
| Triglycerides (mmol L−1) | 1.4 ± 0.4 | 1.2 ± 0.3 | 1.4 ± 0.3 | 1.1 ± 0.3 |
| Total Cholesterol (mmol L−1) | 1.7 ± 0.09 | 1.65 ± 0.1 | 1.65 ± 0.15 | 1.75 ± 0.12 |
| HDL Cholesterol (mmol L−1) | 1.24 ± 0.07 | 1.19 ± 0.01 | 1.13 ± 0.11 | 1.33 ± 0.13 |
3.4. The effect of orally administered AM4113 on food intake
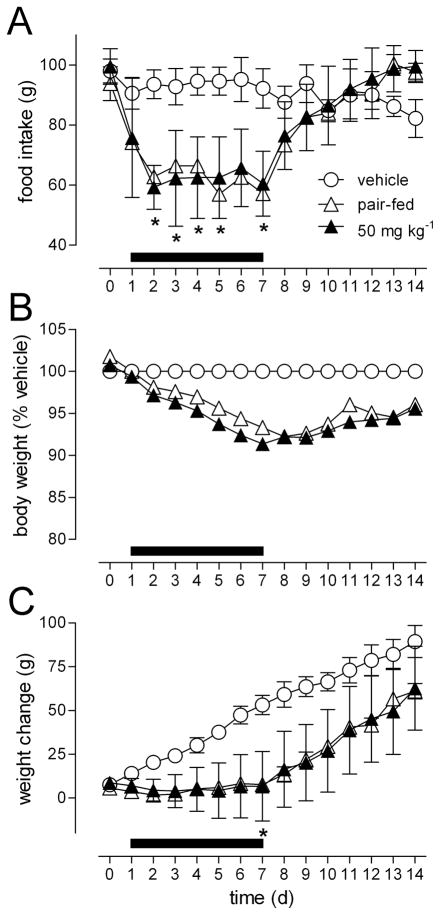
Preliminary studies showed that 2 and 10 mg kg−1 AM4113 administered orally produced transient reductions in food intake, that were not significant 24 h after treatment, compared with vehicle treatment (data not shown). Seven day treatment with 50 mg kg−1 AM4113 significantly reduced food intake and body weight change compared with vehicle treated rats, with 2-way ANOVA revealing a treatment by time interaction (food intake, F=9.0, p<.0001; body weight, F=2.5, p=.0003) (Figure 3A and 3C). Rats were orally treated with AM4113 on days 1 through 7 and post hoc analysis revealed that food intake was significantly reduced (p<0.05) on days 1 to 5 and day 7. On days 8 to 14, following cessation of treatment, food intake increased to become comparable to that recorded in vehicle treated control rats (Figure 3A). Body weight change was significantly (p<0.05) reduced in AM4113 treated rats on day 7 (Figure 3C). Comparison to the pair-fed rats showed that the effect of AM4113 on body weight (Figure 3Band C) was associated with a reduction in food intake. The anorectic effect of AM4113 given orally was sustained throughout the 7 d treatment period, while body weight continued to drop, and did not stabilize, in AM4113 treated rats relative to vehicle treated controls (Figure 3B).
Figure 3.
The effect of daily AM4113 (50 mg kg−1; p.o.) administration, over 7 d (treatments days denoted by the black horizontal bar) on food intake (A), body weight (B) and weight change(C) in rats compared to vehicle (4% DMSO; 96% olive oil; p.o.) and rats pair-fed to the 50 mg kg−1 treated group. Data points represent the mean ±S.E.M, n = 4–5 per group. * p <0.05 represents a significant difference to vehicle treatment analyzed by 2-way ANOVA followed by Bonferroni’s post hoc test.
4. Discussion
We tested the hypothesis that endogenous cannabinoid signaling at CB1 receptors regulates body weight by affecting food intake. Using CB 1 receptor knockout mice we confirmed that the neutral CB1 receptor antagonist AM4113 reduced food intake through an action at CB1 receptors. In rats, when administered intraperitoneally, AM4113 transiently reduced food intake and induced a sustained reduction in body weight gain in a dose-dependent manner. Oral administration of this compound also reduced food intake and body weight in rat, and a higher dose was required than with the i.p. route of administration. Contrary to some of the literature, changes in metabolism did not appear to contribute to the actions of the CB1 receptor antagonist on body weight, since pair-feeding produced almost identical effects to rats treated with AM4113. Chronic treatment with AM4113 had no effect on hypothalamic or circulating levels of the endogenous cannabinoid 2-AG in fasted rats, and there were also no significant differences in circulating levels of glucose, triglycerides, or cholesterol.
Following 14 days of daily, i.p. administration of AM4113, body weight was approximately 5% (2 mg kg−1) and 10% (10 mg kg−1) lower relative to vehicle-treated rats, which is comparable to the effect of CB1 receptor antagonist/inverse agonists in animals (Colombo et al. 1998; Vickers et al. 2003; Chambers et al. 2006; Herling et al. 2007; Janiak et al. 2007) and humans (Despres et al. 2005; Van Gaal et al. 2005; Pi-Sunyer et al. 2006). Moreover, data from pair-fed rats showed that the effect of AM4113 on body weight had been, and continued to be, maintained by changes in food intake rather than through a metabolic action of the endocannabinoid system. Our interpretation of these data differs from the idea that differences in body weight are maintained by an effect on metabolism (Ravinet Trillou et al. 2003; Jbilo et al. 2005; Liu et al. 2005; Horvath 2006; Osei-Hyiaman et al. 2006; Cota 2007; Nogueiras et al. 2008; Herling et al. 2008; Cota et al. 2009).
Studies have shown significant differences in body weight between rodents treated with a CB1 receptor antagonist/inverse agonist and their pair-fed controls (Ravinet Trillou et al. 2003; Herling et al. 2008) suggesting that the reduction in body weight is due to factors other than a reduction in food intake. In one of those studies the effect of rimonabant (SR14176A) was examined in diet-induced obese mice that were treated over 3 d (Ravinet Trillou et al. 2003). In another study, rats treated with rimonabant for 5 weeks demonstrated a lowered body weight and it was concluded that this was due to an increase in energy expenditure (Herling et al. 2008). However, it was also postulated that differences in body weight between pair-fed rats and rats treated with rimonabant could result from a reduced metabolic rate in pair-fed rats (Herling et al. 2008). Differences in energy expenditure between vehicle-treated rats and rats treated with rimonabant were significant on the first day of the study, but not at later times (Herling et al. 2008). A similar effect of rimonabant on energy expenditure had been reported previously in rats (Kunz et al. 2008) yet in another study this CB1 receptor antagonist/inverse agonist had no effect on energy expenditure in mice (Koolman et al. 2010). These studies highlight the controversy (Vickers et al. 2003; Thornton-Jones et al. 2006; Janiak et al. 2007; Kunz et al. 2008)surrounding the proposed role of energy expenditure in weight changed induced by changes in CB1 receptor activity (Ravinet Trillou et al. 2003; Bensaid et al. 2003; Jbilo et al. 2005; Liu et al. 2005; Osei-Hyiaman et al. 2005; Horvath 2006; Cota 2007; Herling et al. 2008; Nogueiras et al. 2009; Cota et al. 2009).
In the present study, the inhibitory effect of AM4113 on body weight was initiated by a dramatic reduction in food intake. When the hypophagic effect of AM4113 was no longer observed, and food intake returned to control levels, body weight no longer continued to decrease. This resulted in a rate of growth in rats treated with AM4113 that paralleled that of vehicle-treated animals, although at a lower level (see Figure 2B). These data support the notion that AM4113 had lowered defended body weight.
DEXA analysis showed that the effect of AM4113 on body weight was likely due to reduced fat mass as treated rats had a lower fat mass than vehicle treated rats. Rimonabant (Ravinet Trillou et al. 2003; Doyon et al. 2006; Herling et al. 2008; Cota et al. 2009)and AM251 (Hildebrandt et al. 2003) also preferentially reduce fat mass compared with lean body mass. This suggest that the actions on fat mass may be due to blockade of endocannabinoid tone on CB1 receptors as AM4113 is a neutral antagonist and, unlike rimonabant and AM251, has no effect on constitutive activity of the receptors. The fact that weight loss induced by cannabinoid antagonists is fairly specific to reduced fat stores seems significant given the expression of CB1 receptors on adipocytes (Bensaid et al. 2003), increased fat oxidation by rimonabant (Liu et al. 2005; Herling et al. 2008; Kunz et al. 2008), the proposed role of CB1 receptors in lipogenesis (Cota et al. 2003; Osei-Hyiaman et al. 2005), and lipolysis (Jbilo et al. 2005). The observation that tolerance to the anorectic effect of CB1 receptor antagonist or antagonist/inverse agonist is linked to existing energy stores, i.e. is delayed in heavier animals (Vickers et al. 2003; Doyon et al. 2006) seems to suggest that endogenous cannabinoid signaling plays a specific role in the maintenance and regulation of adipose tissue mass.
In humans, circulating levels of the endogenous cannabinoid 2-AG are positively correlated with intra-abdominal obesity (Bluher et al. 2006; Cote et al. 2007). In rat, 2-AG levels are increased in the hypothalamus of fasted animals and normalized by feeding (Kirkham et al. 2002). We examined circulating and hypothalamic levels of 2-AG to see if they were affected by AM4113 or weight loss in pair-fed rats. No differences were seen between treatments. More recently Martin-Garcia et al. showed that CB 1 receptor density and activity was decreased by chronic treatment with a CB1 receptor antagonist in rats (Martin-Garcia et al. 2010). Thus, it may be that CB1 receptor down-regulation mediates tolerance to the anorectic effect of these compounds. CB1 receptor antagonist/inverse agonist treatment has been reported to improve circulating glucose and cholesterol levels in humans(Despres et al. 2005; Van Gaal et al. 2005; Pi-Sunyer et al. 2006) and rodents (Doyon et al. 2006; Herling et al. 2007; Janiak et al. 2007; Irwin et al. 2008). These effects may be induced by the inverse agonist properties of these agents because in the current study these levels were not altered by neutral antagonism of CB1 receptors by AM4113. CB1 receptor expression in peripheral tissues(Cota 2007), on vagal afferent fibers (Burdyga et al. 2004; Burdyga et al. 2006), and in areas of the brain that regulate food intake (Cota 2007) may explain how endogenous cannabinoid signaling is able to regulate short term and long term caloric intake. Furthermore, recent studies have reported that neutral antagonism of peripheral CB1 receptors inhibits food reinforced behaviour (Randall et al. 2010)and food intake, and that receptors located on vagal afferents may not involved in the mediation of these effects (Cluny et al. 2010).
In summary, we show that pharmacological blockade of endogenous cannabinoid signaling resulted in a new lower body weight that was defended by changes in food intake. Induced by a transient reduction in food intake, body weight was reduced and resumed a revised rate of growth. DEXA analysis showed that lower body weights in AM4113 treated rats resulted primarily from reductions in fat mass. In our study, there were no differences in circulating glucose and lipid levels, or levels of the endogenous cannabinoid 2-AG, between treatments. Future studies will be needed that examine the effect of a CB1 receptor antagonist at later time points to determine if circulating endogenous cannabinoid levels are related to fat mass in rodents, and if so, how this is affected by treatment with pharmacological tools which act at cannabinoid CB1 receptors.
Acknowledgments
The authors thank Beat Lutz for supplying the breeding stock of cannabinoid CB1 receptor gene-deficient mice and Winnie Ho for the genotyping of the cannabinoid CB1 receptor gene-deficient mice. These studies were supported by grants from Canadian Institutes of Health Research (KAS) and from National Institutes of Health, U.S.A (DA09158, DA7215, DA3801 to AM). APC is an Alberta Heritage Foundation for Medical Research (AHFMR) Graduate Student. KAS is an AHFMR Medical Scientist and the Crohn’s and Colitis Foundation of Canada Chair in Inflammatory Bowel Disease Research. LKE is an NSERC and Canadian Diabetes Association Graduate Student.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Archer ZA, Brown YA, Rayner DV, Stubbs RJ, Mercer JG. Effect of flavour of liquid Ensure diet supplement on energy intake in male SD rats. Physiol Behav. 2006;89:414–419. doi: 10.1016/j.physbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrie P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- Bluher M, Engeli S, Kloting N, Berndt J, Fasshauer M, Batkai S, Pacher P, Schon MR, Jordan J, Stumvoll M. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1289–G1297. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Koopmans HS, Pittman QJ, Sharkey KA. AM 251 produces sustained reductions in food intake and body weight that are resistant to tolerance and conditioned taste aversion. Br J Pharmacol. 2006;147:109–116. doi: 10.1038/sj.bjp.0706439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, Makriyannis A, Sharkey KA. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–R2193. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, Lutz B, Zimmer A, Parker LA, Makriyannis A, Sharkey KA. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol. 2010 doi: 10.1111/j.1476-5381.2010.00908.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:L113–L117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Cota D. CB1 receptors: emerging evidence for central and peripheral mechanisms that regulate energy balance, metabolism, and cardiovascular health. Diabetes Metab Res Rev. 2007;23:507–517. doi: 10.1002/dmrr.764. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Sandoval DA, Olivieri M, Prodi E, D’Alessio DA, Woods SC, Seeley RJ, Obici S. Food intake-independent effects of CB1 antagonism on glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1641–1645. doi: 10.1038/oby.2009.84. [DOI] [PubMed] [Google Scholar]
- Cote M, Matias I, Lemieux I, Petrosino S, Almeras N, Despres JP, Di MV. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007;31:692–699. doi: 10.1038/sj.ijo.0803539. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Doyon C, Denis RG, Baraboi ED, Samson P, Lalonde J, Deshaies Y, Richard D. Effects of rimonabant (SR141716) on fasting-induced hypothalamic-pituitary-adrenal axis and neuronal activation in lean and obese Zucker rats. Diabetes. 2006;55:3403–3410. doi: 10.2337/db06-0504. [DOI] [PubMed] [Google Scholar]
- Duncan M, Davison JS, Sharkey KA. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005;22:667–683. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gessa GL, Orru A, Lai P, Maccioni P, Lecca R, Lobina C, Carai MA, Colombo G. Lack of tolerance to the suppressing effect of rimonabant on chocolate intake in rats. Psychopharmacology (Berl) 2006;185:248–254. doi: 10.1007/s00213-006-0327-1. [DOI] [PubMed] [Google Scholar]
- Gómez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodriguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herling AW, Gossel M, Haschke G, Stengelin S, Kuhlmann J, Muller G, Schmoll D, Kramer W. CB1 receptor antagonist AVE1625 affects primarily metabolic parameters independently of reduced food intake in Wistar rats. Am J Physiol Endocrinol Metab. 2007;293:E826–E832. doi: 10.1152/ajpendo.00264.2007. [DOI] [PubMed] [Google Scholar]
- Herling AW, Kilp S, Elvert R, Haschke G, Kramer W. Increased energy expenditure contributes more to the body weight-reducing effect of rimonabant than reduced food intake in candy-fed wistar rats. Endocrinology. 2008;149:2557–2566. doi: 10.1210/en.2007-1515. [DOI] [PubMed] [Google Scholar]
- Hildebrandt AL, Kelly-Sullivan DM, Black SC. Antiobesity effects of chronic cannabinoid CB1 receptor antagonist treatment in diet-induced obese mice. Eur J Pharmacol. 2003;462:125–132. doi: 10.1016/s0014-2999(03)01343-8. [DOI] [PubMed] [Google Scholar]
- Horvath TL. The unfolding cannabinoid story on energy homeostasis: central or peripheral site of action? Int J Obes (Lond) 2006;30 (Suppl 1):S30–S32. doi: 10.1038/sj.ijo.0803275. [DOI] [PubMed] [Google Scholar]
- Irwin N, Hunter K, Frizzell N, Flatt PR. Antidiabetic effects of sub-chronic administration of the cannabinoid receptor (CB1) antagonist, AM251, in obese diabetic (ob/ob) mice. Eur J Pharmacol. 2008;581:226–233. doi: 10.1016/j.ejphar.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Janiak P, Poirier B, Bidouard JP, Cadrouvele C, Pierre F, Gouraud L, Barbosa I, Dedio J, Maffrand JP, Le FG, O’Connor S, Herbert JM. Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int. 2007;72:1345–1357. doi: 10.1038/sj.ki.5002540. [DOI] [PubMed] [Google Scholar]
- Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le FG, Galiegue S, Casellas P. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 2005;19:1567–1569. doi: 10.1096/fj.04-3177fje. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM. Endogenous cannabinoids and appetite. Nutr Res Rev. 2001;14:65–86. doi: 10.1079/NRR200118. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di MV. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolman AH, Bloks VW, Oosterveer MH, Jonas I, Kuipers F, Sauer PJ, van DG. Metabolic responses to long-term pharmacological inhibition of CB1-receptor activity in mice in relation to dietary fat composition. Int J Obes (Lond) 2010;34:374–384. doi: 10.1038/ijo.2009.219. [DOI] [PubMed] [Google Scholar]
- Kunz I, Meier MK, Bourson A, Fisseha M, Schilling W. Effects of rimonabant, a cannabinoid CB1 receptor ligand, on energy expenditure in lean rats. Int J Obes (Lond) 2008;32:863–870. doi: 10.1038/ijo.2008.3. [DOI] [PubMed] [Google Scholar]
- Liu YL, Connoley IP, Wilson CA, Stock MJ. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond) 2005;29:183–187. doi: 10.1038/sj.ijo.0802847. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin-Garcia E, Burokas A, Martin M, Berrendero F, Rubi B, Kiesselbach C, Heyne A, Gispert JD, Millan O, Maldonado R. Central and peripheral consequences of the chronic blockade of CB1 cannabinoid receptor with rimonabant or taranabant. J Neurochem. 2010;112:1338–13351. doi: 10.1111/j.1471-4159.2009.06549.x. [DOI] [PubMed] [Google Scholar]
- Matias I, Di Marzo V. Endocannabinoid synthesis and degradation, and their regulation in the framework of energy balance. J Endocrinol Invest. 2006;29:15–26. [PubMed] [Google Scholar]
- Nogueiras R, Diaz-Arteaga A, Lockie SH, Velasquez DA, Tschop J, Lopez M, Cadwell CC, Dieguez C, Tschop MH. The endocannabinoid system: role in glucose and energy metabolism. Pharmacol Res. 2009;60:93–98. doi: 10.1016/j.phrs.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschop J, Caldwell C, Woods SC, Wittmann G, Watanabe M, Liposits Z, Fekete C, Reizes O, Rohner-Jeanrenaud F, Tschop MH. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57:2977–2991. doi: 10.2337/db08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Depetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Harvey-White J, Batkai S, Kunos G. The role of the endocannabinoid system in the control of energy homeostasis. Int J Obes (Lond) 2006;30 (Suppl 1):S33–S38. doi: 10.1038/sj.ijo.0803276. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Randall PA, Vemuri VK, Segovia KN, Torres EF, Hosmer S, Nunes EJ, Santerre JL, Makriyannis A, Salamone JD. The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacol Biochem Behav. 2010 doi: 10.1016/j.pbb.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Peng Y, Olszewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:946–955. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz KM, Cristino L, Matias I, Capasso R, Racioppi A, Izzo AA, Di MV. Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring) 2008;16:553–565. doi: 10.1038/oby.2007.106. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Kennett GA, Benwell KR, Revell DF, Misra A, Sellwood DM, Vickers SP, Clifton PG. The cannabinoid CB1 receptor inverse agonist, rimonabant, modifies body weight and adiponectin function in diet-induced obese rats as a consequence of reduced food intake. Pharmacol Biochem Behav. 2006;84:353–359. doi: 10.1016/j.pbb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Williams J, Wood J, Pandarinathan L, Karanian DA, Bahr BA, Vouros P, Makriyannis A. Quantitative method for the profiling of the endocannabinoid metabolome by LC-atmospheric pressure chemical ionization-MS. Anal Chem. 2007;79:5582–5593. doi: 10.1021/ac0624086. [DOI] [PubMed] [Google Scholar]
- Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi-Keefe CJ, Makriyannis A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J Lipid Res. 2010;51:1416–1423. doi: 10.1194/jlr.M002436. [DOI] [PMC free article] [PubMed] [Google Scholar]