Abstract
Heart diseases are major causes of morbidity and mortality linked to extensive loss of cardiac cells. Embryonic stem cells (ESCs) give rise to cardiomyocyte-like cells, which may be used in heart cell replacement therapies. Most cardiogenic differentiation protocols involve the culture of ESCs as embryoid bodies (EBs). Stirred-suspension bioreactor cultures of ESC aggregates may be employed for scaling up the production of cardiomyocyte progeny but the wide range of EB sizes and the unknown effects of the hydrodynamic environment on differentiating EBs are some of the major challenges in tightly controlling the differentiation outcome. Here, we explored the cardiogenic potential of mouse ESCs (mESCs) and human ESCs (hESCs) encapsulated in poly-L-lysine (pLL)-coated alginate capsules. Liquefaction of the capsule core led to the formation of single ESC aggregates within each bead and their average size depended on the concentration of seeded ESCs. Encapsulated mESCs were directed along cardiomyogenic lineages in dishes under serum-free conditions with the addition of bone morphogenetic protein 4 (BMP4). Human ESCs in pLL-layered liquid core (LC) alginate beads were also differentiated towards heart cells in serum-containing media. Besides the robust cell proliferation, higher fractions of cells expressing cardiac markers were detected in ESCs encapsulated in LC than in solid beads. Furthermore, we demonstrated for the first time that ESCs encapsulated in pLL-layered LC alginate beads can be coaxed towards heart cells in stirred-suspension bioreactors. Encapsulated ESCs yielded higher fractions of Nkx2.5- and GATA4-positive cells in the biore-actor compared to dish cultures. Differentiated cells formed beating foci that responded to chronotropic agents in an organotypic manner. Our findings warrant further development and implementation of microencapsulation technologies in conjunction with bioreactor cultivation to enable the production of stem cell-derived cardiac cells appropriate for clinical therapies and applications.
Keywords: Embryonic stem cells (ESCs), Heart cells, Directed differentiation, Encapsulation, Bioreactor
INTRODUCTION
Heart disease is associated with extensive cardiomyocyte attrition and impaired heart function, leading to high morbidity and mortality (38). Given the scarcity of donor organs for transplantation, cardiac cell replacement therapy has emerged as an appealing alternative, fueling efforts into renewable sources of functional cardiomyocytes. Embryonic stem cells (ESCs) give rise to cardiomyocyte-like cells, which reverse heart injury in rodents (50) after implantation. To date, most protocols for in vitro cardiogenic differentiation of ESCs entail the formation of multicellular aggregates or embryoid bodies (EBs) (13,21). Typically, EBs are generated by the hanging drop method, methylcellulose culture, or spontaneous aggregation of ESCs on low-adhesion surfaces (21,49). These systems, however, yield EBs with large variations in size, contributing to the heterogeneity of the differentiated cells.
Several studies have linked the EB size to stem cell fate decisions, including the adoption of a cardiac cell phenotype (6,24). Various methods have been proposed for generating EBs of specific size, including centrifugation in 96-well plates (34), seeding in 3D microwells with defined geometric size (20,31), and micropatterned surfaces (41). These systems allow extensive and precise control of the stem cell niche and are suitable for studying the effect of EB size on the differentiation outcome. Yet, most of them are challenging to scale up for producing EBs with uniform size and in sufficient amounts for seeding commercial-scale bioreactors.
Reports of ESC differentiation as EBs to heart cells in stirred-suspension vessels serve as a proof of principle that such bioreactors can be used for the production of medically relevant quantities of heart cells. The scalability of EB formation in petri dishes (51) prior to their inoculation in spinner flasks is limited and the resulting EBs are uneven in size. When single, dispersed mouse ESCs (mESCs) are loaded in a stirred-suspension bioreactor (42) EBs are formed, but their size and concentration depend largely on the agitation rate. Low stirring speeds result in a few oversized aggregates exhibiting poor cell growth, whereas more intense agitation induces high shear, affecting cell viability, morphology, gene expression, and the differentiation potential of ESCs, as shown also in rotary orbital cultures (40).
To that end, Niebruegge et al. (35) reported that hESC colonies from micropatterned slides inoculated in spinner flasks with serum-containing medium lead to the emergence of cells expressing cardiac cell genes after 2 weeks of culture. Among the different pattern sizes examined, EBs from 400-μm microprints exhibit the highest cell proliferation and yield of heart cells. Besides the intimate link between EB size and cardiogenic differentiation, these findings show that coupling methods for generating particular size EBs to bioreactor cultures enhances significantly the differentiation of ESCs to heart cells. Given the practical considerations stemming from loading large-scale bioreactors with cells from micropatterned slides, alternative methods may be more attractive.
One such approach is the encapsulation of stem cells prior to their differentiation in stirred-suspension vessels to heart cells. Hydrogels such as agarose (5), dextran (15), and gelatin (1) have been used for culturing ESCs. Alginate (19,29,30) remains the encapsulation material of choice because of its intrinsic properties (36), including biocompatibility, biosafety, adjustable permeability, and the benign conditions employed during its preparation (e.g., physiological temperature and the lack of cytotoxic solvents). Alginate-based scaffolds have also been employed in cardiac tissue engineering (2). Interestingly enough, various high-throughput designs are currently available [e.g., electrostatic generator (8), air-driven multinozzle droplet generator (12)] for the encapsulation of a wide range of mammalian cells (7,11,32), even under good manufacturing practice (GMP) conditions (43).
Encapsulation of ESCs in solid alginate beads for differentiation to various lineages (28,30,47) results in a dispersed cell phase. As such, encapsulated ESCs have the propensity to form multiple EBs within each bead, limiting control over their size. Lowering the alginate concentration may increase the internal fluidity of the bead, facilitating the formation of a single EB per bead and the exchange of nutrients through the bead. However, these capsules have reduced mechanical stability, making them unsuitable for culture in bioreactors under constant stirring. Furthermore, the ability of ESCs encapsulated in alginate beads to give rise to h'eart cell progeny has not been analyzed in detail.
Here, we explored the cardiogenic potential of mESCs and human ESCs (hESCs) encapsulated in alginate beads coated with poly-L-lysine (pLL) (23). We hypothesized that aggregates with specific size can be formed by encapsulating ESCs in pLL-coated capsules and dissolving their core. Indeed, liquefaction of the core allowed the formation of a single ESC aggregate per bead and its size was adjusted via the ESC seeding density. The cardiogenic differentiation of encapsulated ESCs was directed with a physiologically relevant factor under serum-free conditions as well as in serum-supplemented media. Most importantly, encapsulated mESCs and hESCs cultured in spinner flasks also gave rise to heart cell phenotypes with higher efficiency compared to their static culture counterparts. Our results support further development of this culture modality for the scalable differentiation of stem cells to therapeutically useful cells such as cardiomyocytes.
MATERIALS AND METHODS
Human Embryonic Stem Cell Culture
Human H9 ESCs (WiCell Research Institute, Madison, WI) were maintained as described previously (27). Briefly, hESCs were cultured on mitomycin C-treated (Sigma-Aldrich, St. Louis, MO) mouse embryonic fibroblasts (mEFs) in Dulbecco's modified Eagle's medium/Ham's F-12 medium (DMEM/F12) with 20% KnockOut serum replacement (KSR), 2 mM L-glutamine, 1 mM nonessential amino acids, 100 U/ml penicillin and 100 mg/ml streptomycin, and 4 ng/ml basic fibroblast growth factor (bFGF; all from Invitrogen, Carlsbad, CA).
For differentiation experiments, the cells were maintained free of mEFs on dishes coated with growth factor-reduced Matrigel (BD Biosciences, San Jose, CA) for 1–2 passages and in mEF-conditioned medium (CM) (27). The CM was filter sterilized and supplemented with 4 ng/ml bFGF before its addition to hESCs.
Cultures were manually passaged every 6–7 days with 2 mg/ml collagenase IV (GIBCO, Grand Island, NY) in DMEM/F12 and split 1:4–1:6. The cultures were maintained in 5% CO2/95% air at 37°C and media were replaced daily.
Mouse Embryonic Stem Cell Culture
mESCs (E14Tg2a; Mutant Mouse Regional Resource Centers, University of California-Davis, CA) were maintained in tissue culture dishes coated with 0.1% gelatin-phosphate-buffered saline (PBS; Sigma) solution in 5% CO2/95% air at 37°C (22). The culture medium consisted of DMEM (Mediatech, Manassas, VA) with 10% ESC-screened FBS (Hyclone, Logan, UT), 2 mM L-glutamine, 0.1 mM nonessential amino acids, 0.055 mM β-mercaptoethanol, penicillin (100 U/ml), streptomycin (100 μg/ml), and 1,000 U/ml leukemia inhibitor factor (LIF; Chemicon, Temecula, CA). This medium is termed serum-containing mESC medium (SCMM). Medium was replaced every day and the mESCs were passaged every 2–3 days by incubation with TrypLE (GIBCO) and gentle pipetting to dissociate clumps into single cells. For the experiments performed in this study, mESCs were adapted in defined serum-free medium (DSFM) over 6–10 passages (22). The composition of DSFM is similar to that of SCMM above except that 10% KSR is added instead of FBS.
Cell Encapsulation and Release
For encapsulation experiments, undifferentiated hESC colonies were incubated with 10 μM Y27632 [a Rho-associated protein kinase (ROCK) inhibitor (EMD, Gibbstown, NJ)] for 1 h prior to their dissociation into single hESCs upon treatment with Accutase (Millipore, Billerica, MA). Mouse ESCs were harvested as single cells as described above. The cells were counted in a hemocytometer and resuspended in sterile sodium alginate (FMC Biopolymer, Philadelphia, PA) solution in DMEM at room temperature, resulting in densities of 105–107 cells/ml and 1.5% final alginate concentration. Beads were generated by passing the cell/alginate solution through a 27-gauge syringe needle coaxially with an air jet (25) into a sterile 100 mM CaCl2 solution. The airflow was adjusted so as to produce beads with a 500–600-μm diameter. After a 5-min incubation in CaCl2, the beads were washed twice with basal medium and immersed into 0.05% (w/v) pLL solution (Sigma) for 2 min. The core was liquefied by incubating the beads in 0.055 M sodium citrate solution (a Ca2+ chelator) for 5 min followed by washing twice with culture medium. To assess the viability of cells immediately after their encapsulation, the beads were incubated with 20 μg/ml fluorescein diacetate (FDA; live cells) and 10 μg/ml propidium iodide (PI; dead cells; both from Sigma) in PBS for 5–15 min (27). The beads were washed three times with PBS and observed on a fluorescence inverted microscope.
Encapsulated cells were released from beads as follows: (i) pLL-coated beads with a liquefied core were incubated with TrypLE (which is formulated in Dulbecco's PBS with 1 mM EDTA) for 5 min at room temperature. Breakage of the beads was facilitated by gentle pipetting using 1000-μl pipette tips as reported (16). (ii) Solid beads were incubated with 0.055 M sodium citrate solution for 5 min and the cells were washed with culture medium and centrifuged twice. (iii) Cells in pLL-coated beads with a solid core were released by combining steps (i) and (ii) and we noted >95% recovery of live cells. Cell release from the beads was confirmed by observation under a microscope. Aggregate dissociation into single cells was described previously (22).
Differentiation of ESCs
Single dispersed hESCs pretreated with 10 μM Y27632 were seeded in petri dishes and formed EBs in differentiation medium consisting of KnockOut-DMEM (KO-DMEM; Invitrogen), 1 mM L-glutamine, 0.1 mM β-mercaptoethanol, 1% nonessential amino acids, and 20% FBS (Hyclone, Logan, UT). Cells were incubated with differentiation medium for up to 2 weeks.
mESCs were cultured as monolayers or EBs in SCMM with conventional FBS instead of ESC-screened FBS and without LIF. All-trans retinoic acid (RA, 1 nM; Sigma) was added as stated. For serum-free differentiation, the EBs were cultured in DSFM without LIF (DSFM-L) supplemented with 10 ng/ml recombinant bone morphogenetic protein 4 (BMP4) (R&D Systems, Minneapolis, MN) for the first 6 days of differentiation. Then, cells were maintained in DSFM-L alone. Medium was changed every 2 days.
Encapsulated mESCs and hESCs were coaxed towards the cardiomyogenic lineage using the same differentiation media and time of incubation as for their nonencapsulated counterparts. At different times, the aggregates were released from the beads for further analysis.
The aggregates were transferred to gelatin-coated plates at ~1–3 EBs/cm2 for hESCs and ~4–6 EBs/cm2 for mESCs. Spontaneously beating foci emerged as early as 2 days after plating.
Bioreactor Culture and Sampling
Encapsulated ESCs were seeded in 125 ml ProCulture spinner flasks (Corning, Corning, NY) at 2 × 104 cells/ml. The agitation rate was kept constant at 45 rpm throughout each run. Encapsulated mESCs in spinner flasks were incubated with DSFM-L containing 10 ng/ml BMP4 for 6 days followed by DSFM-L alone. Capsules with hESCs in stirred suspension were incubated in the same serum-supplemented differentiation medium as described for static hESC cultures. Media were replaced every 2 days. Samples (~0.5 ml) were withdrawn from spinner flasks under continuous stirring to ensure that similar numbers of beads per unit volume of medium were collected each time. Samples were obtained every 1–2 days and an equal amount of fresh medium was added to keep the total culture volume constant. After acquiring images of cell-laden beads, encapsulated cells were released, stained with the trypan blue dye (GIBCO), and counted in a hemocytometer.
Image Analysis
Samples were transferred to dishes and examined under an inverted microscope. Images of the beads were acquired via a Nikon digital camera connected to the microscope and analyzed with the NIH ImageJ (http://rsb.info.nih.gov/ij/). At least 20 images were analyzed per condition. The diameter of each aggregate or bead was calculated by taking the average of two perpendicular diameters.
The permeability of pLL-coated alginate beads was determined by incubation with a 106 kDa fluorescein isothiocyanate conjugated-immunoglobulin (IgG-FITC; Jackson ImmunoResearch Laboratories, West Grove, PA) and observation under a Zeiss Axio Observer Z1 fluorescence microscope (Carl Zeiss, Thornwood, NY) at different times. Transmission and green fluorescence images were acquired at the equatorial plane of the beads with the AxioVision software and the corresponding fluorescence intensity profiles were generated in MATLAB (The MathWorks, Natick, MA) using the surf and contour functions.
XTT and Lactate Dehydrogenate (LDH) Activity Assays
The XTT assay (Roche, Indianapolis, IN) was carried out according to the manufacturer's instructions. Briefly, XTT labeling solution (200 μl) was added to each well of a 24-well plate loaded with equal number of cell-laden beads/well. After a 24-h incubation, absorbance was measured at 490 nm with a microplate reader (Synergy HT, BioTek, Winooski, VT).
Analysis of LDH activity in supernatant samples was carried out with a LDH-based cytotoxicity detection kit (Roche) as we described previously (22). Calibration curves to convert absorbance to activity units (U/ml) were generated using bovine LDH (Sigma) and human LDH (Cell Sciences, Canton, MA) for mESCs and hESCs, respectively. The specific rate of LDH release was calculated by normalizing the LDH activity (U/ml) detected at each interval with the corresponding logarithmic mean of the viable cell concentration.
RNA Extraction, RT-PCR, and Quantitative PCR (qPCR)
Total RNA was isolated from cells using Trizol (Invitrogen) according to the manufacturer's instructions. Reverse transcription was performed using the ImProm-II reverse transcriptase (Promega, Madison, WI) with 1 μg of total RNA and 250 ng random primers or oligo(dT)12–18 primers (Fermentas, Glen Burnie, MD). Reverse transcription was carried out at 42°C for 60 min. PCR runs were performed with the resulting complementary DNA for 35–40 cycles at an annealing temperature of 58–60°C depending on the primer set. Primer sequences are shown in Table 1.
Table 1.
Human and Mouse Gene Primer Sequences (5′ to 3′ Orientation)
| Gene | Forward Primer | Reverse Primer | Amplicon Size (bp) |
|---|---|---|---|
| Human primers | |||
| OCT3/4A | AAGCTGGAGAAGGAGAAGCTG | AATAGAACCCCCAGGGTGAG | 158 |
| NANOG | AGATGCCTCACACGGAGACT | ACACAGCTGGGTGGAAGAGA | 194 |
| TBX5 | GTCCCAATACCAGTGTGAGAATG | CTGTGGATAGCTAGAGCGGTAGA | 217 |
| NKX2.5 | AGGACCCTAGAGCCGAAAAG | AGATCTTGACCTGCGTGGAC | 243 |
| GATA4 | TCATCTCACTACGGGCACAG | GGGAAGAGGGAAGATTACGC | 233 |
| ANF | TCTGCCCTCCTAAAAAGCAA | ATCACAACTCCATGGCAACA | 249 |
| α-MHC | GTGGACAAGCTGCAACTGAA | TGTCACTCCTCATCGTGCAT | 215 |
| β-MHC | AGCTAAAGGTCAAGGCCTACAAG | AGATCAAGATGTGGCAAAGCTACT | 213 |
| TBX20 | CAATGCATAAGTACCAGCCAAG | TCAGTGAGCCTGGAGGAATC | 214 |
| β-actin | CTTCCTGGGCATGGAGTCCT | AGGAGCAATGATCTTGATCTTC | 202 |
| Mouse primers | |||
| rex1 | GGCCAGTCCAGAATACCAGA | GAACTCGCTTCCAGAACCTG | 232 |
| nanog | GCAGGTTAAGACCTGGTTTCA | TTCACCTGGTGGAGTCACAG | 499 |
| brachyury | TGCTGCAGTCCCATGATAAC | TGTGCGTCAGTGGTGTGTAA | 228 |
| nkx2.5 | CAAGTGCTCTCCTGCTTTCC | GACAGGTACCGCTGTTGCTT | 252 |
| anf | CCTGTGTACAGTGCGGTGTC | GCCCTCAGTTTGCTTTTCAA | 272 |
| α-mhc | CCTGATGACAAGGAGGAGTATGTTA | TTGTACACAGGCAGCCACTTATAG | 287 |
| gata4 | CTGTGCCAACTGCCAGACTA | GCATCTCTTCACTGCTGCTG | 281 |
| β-actin | GCTCTTTTCCAGCCTTCC | GCTCAGGAGGAGCAATGA | 226 |
Real-time polymerase chain reactions were performed on an iCycler quantitative PCR (qPCR) machine (Bio-Rad, Hercules, CA) with the DyNAmo SYBR Green qPCR mix (Finnzymes, Woburn, MA) under the following conditions: denaturation and polymerase activation at 95°C for 2 min; amplification for 40 cycles at 95°C for 15 s, 58°C or 60°C for 30 s, and 72°C for 1 min. All reactions were run in triplicate on samples from at least three experiments. Amplification specificity was verified by a melting curve method. Relative gene expression was calculated using the ΔΔCT method (26). β-Actin was used as the housekeeping gene and its CT did not vary under different experimental conditions when equal amounts of RNA were used. PCR efficiencies for different primer pairs were ~1 as determined by a standard curve method on serially diluted templates.
Immunocytochemistry
Cells were washed in PBS and fixed with 4% paraformaldehyde (Sigma) in PBS for 20 min at room temperature (22). After washing with PBS, cells were permeabilized and blocked with 1% bovine serum albumin (BSA, BioFX, Owings Mills, MD)/0.1% Triton X-100 (Mallinckrodt Baker, Phillipsburg, NJ) in PBS for 30 min. Incubation was carried out overnight at 4°C with a primary antibody such as goat anti-cardiac troponin I (cTnI), rabbit anti-Nkx2.5, anti-GATA4, anti-α-actinin (all from Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-cardiac myosin heavy chain (MHC) (Abcam, Cambridge, MA), and anti-cardiac troponin T (cTnT; Sigma). After three 5-min washes with PBS, the samples were incubated with secondary antibodies for 1 h at room temperature. Donkey anti-rabbit, anti-goat, and anti-mouse secondary antibodies conjugated with FITC or Cy3 (Jackson ImmunoResearch Laboratories) were used. Nuclear DNA was stained with DAPI (Sigma). Samples were viewed with a Zeiss Axio Observer Z1 fluorescence microscope.
Flow Cytometry
Cells were prepared for and analyzed by flow cytometry as we reported previously (27). Cells were fixed for 10 min with 3.7% formaldehyde solution (Sigma-Aldrich), washed with PBS, and blocked with 3% normal donkey serum (NDS) for 30 min. The samples were incubated with the primary antibodies rabbit anti-GATA4 and anti-Nkx2.5 for 1 h at room temperature in 1% NDS. Cells were washed three times with 1% NDS and incubated with appropriate Cy3- or FITC-conjugated donkey secondary antibodies for 30 min at room temperature. The cells were washed again three times and analyzed with a FACS Calibur flow cytometer with the Cell Quest software (Becton Dickinson, Franklin Lakes, NJ). Undifferentiated mESCs and hESCs were used as negative controls and the mouse cardiac cell line HL-1 (10) served as a positive control to confirm gatings. Cells were registered as positive for a particular antigen if their emitted fluorescence level was higher than 99% of that of cells stained only with the corresponding secondary antibodies.
Statistical Analysis
Data are expressed as mean ± SD unless stated otherwise. ANOVA and the post-hoc Tukey test were performed using Minitab (Minitab Inc, State College, PA). Values of p < 0.05 were considered as significant.
RESULTS
Stem Cell Encapsulation in pLL-Coated Alginate Beads With a Liquefied Core
Our goal was to examine if ESCs encapsulated in alginate beads can be coaxed towards cardiac cell progeny. Hence, we first explored whether encapsulation in alginate beads affects the viability and proliferation of ESCs while allowing the formation of aggregates within a certain size range.
Initially, 105–107 dispersed hESCs and mESCs were encapsulated in beads per milliliter of 1–2% (w/v) alginate solution. Generation of beads by the coaxial air jet method did not affect significantly the viability of ESCs as evidenced by live (FDA)/dead (PI) cell staining (Fig. 1). However, the cells remained dispersed and their proliferation was considerably retarded within the solid beads (Fig. 1B–D). Lowering the concentration of alginate resulted repeatedly in compromised capsule structure and cells leaked out of the beads (Fig. 1E). Increasing the concentration of alginate helped to avert cell leakage but this affects adversely the viability and proliferation of encapsulated cells (47).
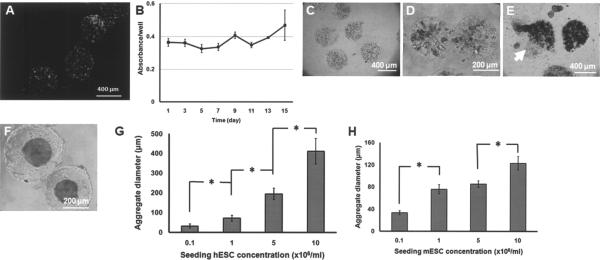
Figure 1.
(A) Mouse ESCs stained with FDA (green; live cells) and PI (red; dead cells) after their encapsulation in 1.5% alginate beads. (B) XTT assay for hESCs cultured in solid alginate (1.5%) beads. (C) Mouse ESCs entrapped in nonliquefied beads remain dispersed even after (D) 8 days of culture. In many instances, (E) the bead structure was compromised and cells leaked out (arrow). (F) Mouse ESCs in pLL-coated alginate beads treated with a Ca2+ chelating agent (LC beads) form single aggregates. A representative image is shown of beads 7 days after encapsulation/liquefaction of mESCs. (G, H) Average aggregate size versus concentration of seeded hESCs and mESCs (cells/ml of 1.5% alginate solution), respectively. Aggregate size was measured 24 h after encapsulation. Values are shown as mean ± SD. *p < 0.001.
These observations prompted us to coat the alginate capsules with pLL. This not only improved the structural integrity of the beads but also allowed the reduction of alginate concentration (≤1.5%). Furthermore, incubation with a Ca2+ chelating agent resulted in beads with a liquefied core (LC). This facilitated the formation of single ESC aggregates (Fig. 1F). Given the importance of the EB size on the differentiation of ESCs, we explored whether the average diameter of aggregates formed in the LC beads could be controlled via an easily adjustable variable such as the seeding cell density. The average aggregate diameter was determined 24 h after encapsulation and core liquefaction to allow the completion of cluster formation. For hESCs seeded at 105–107 cells/ml of alginate solution, the average diameter of the resulting clusters increased from 31 ± 10 to 411.4 ± 65.4 μm (Fig. 1G). Similarly, aggregates with a larger average diameter were observed as the seeding mESC density increased (Fig. 1H).
An aggregate diameter of ~210 μm resulting from H9 hESC colonies on 400-μm patterns was reported as optimal for cardiac induction (6). Therefore, for the experiments henceforth we chose a seeding density of 5 × 106 H9 hESCs/ml, resulting in an average size of 194 ± 28.8 μm for 1-day-old EBs. The same seeding density was selected for mESCs.
Our results show that ESCs can be encapsulated in pLL-coated LC alginate beads while maintaining their viability. Cells also proliferated more extensively in LC than in solid beads (see Differentiation of Encapsulated mESCs in Serum-free Medium and Cardiogenic Differentiation of hESCs below). Moreover, the fluid environment inside the pLL-coated LC capsules allows ESCs to form aggregates and their size can be controlled in a straightforward manner.
Serum-Free Cardiogenic Differentiation of mESCs
Our aim was to explore if ESCs in pLL-coated capsules can be coaxed to heart cells via either directed differentiation or serum-based protocols. For this purpose, we developed a method for guiding the cardiomyogenic commitment of mESCs in serum-free medium with physiologically relevant factors. mESCs (22) can be adapted and maintained in culture with DSFM, allowing the use of soluble agents (e.g., growth factors) for differentiation without interference from serum. BMP4 drives mESCs toward heart cells (17) but the necessity for serum activation prior to this differentiation is unsettled (44).
We hypothesized that serum activation is not needed given that mESCs in DSFM retain their ability for mesoderm differentiation (22). To test our hypothesis, mESCs were maintained for 8–10 passages in DSFM and cultured for 6 days either as monolayers or EBs in DSFM without LIF (DSFM-L) but supplemented with 10 ng/ml BMP4. Subsequently, BMP4 was withdrawn and the cells were cultured in DSFM-L alone. The expression of Brachyury (T or Bry) was apparent by day 4, signifying mesendodermal commitment of mESCs (Fig. 2A) and was downregulated by days 7 and 16, denoting the departure from an early mesoderm fate and further maturation (33). At the same time differentiating mESCs displayed a decline in the expression of the pluripotent markers Rex-1 and Nanog (Fig. 2B) and a marked upregulation of cardiomyocyte genes such as α-myosin heavy chain (α-MHC), atrial natriuretic factor (anf), and nkx2.5 (Fig. 2C). The adoption of a cardiac muscle cell fate by the differentiating mESCs was also evident by α-actinin immunoreactivity (Fig. 2D).
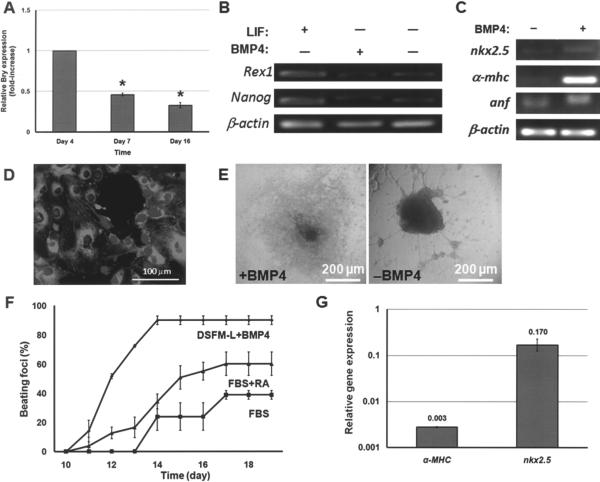
Figure 2.
(A) Mouse ESCs were cultured with DSFM-L and 10 ng/ml BMP4 for 6 days and in DSFM-L alone thereafter. The expression profile of Bry in these cells was determined by qPCR. *p < 0.001 compared to day 4 Bry expression. (B) Expression of pluripotency genes by mESCs cultured in DSFM (+LIF), DSFM-L with BMP4 (day 16), and DSFM-L without BMP4 (day 16). (C) Expression of cardiomyocyte genes after 16 days of mESC differentiation in DMSF-L with or without BMP4. (D) Cells differentiated with BMP4 exhibit α-actinin (costaining with DAPI). Day 14 cells are shown. (E) Beating clusters emerged after 10–11 days of differentiation with BMP4. However, mESCs cultured in DMSF-L alone (−BMP4) were morphologically different and no contractile foci were observed. (F) Beating curves for mESCs differentiated under different conditions. (G) Exposure to 150 ng/ml noggin (+DSFM-L + BMP4) resulted in reduced cardiac cell gene expression. Expression from cells cultured in DSFM-L + BMP4 without noggin was set to 1. Values are reported as mean ± SD.
Beating was readily seen when EBs were plated in tissue culture dishes. In contrast, cells differentiating in monolayer cultures exhibited contractile activity only after being replated at higher densities, promoting the formation of clusters. Contractile foci were not observed when EBs were transferred to plates before day 6. Similarly, beating foci did not appear in mESC clusters cultured in DSFM-L without BMP4. Interestingly enough, outgrowths were noticed in these cultures with morphological resemblances to neuronal-type cells (Fig. 2E). Therefore, allowing cells to form aggregates in conjunction with BMP4 treatment for the first 6 days was necessary for the cardiogenic differentiation of mESCs to proceed and beating activity to emerge.
More than 90% of the foci exposed to BMP4 exhibited contractile activity 14 days after the differentiation started (Fig. 2F). In contrast, mESCs coaxed in serum-containing medium gave rise to less than 40% contractile clusters. This increased to ~60% with the addition of RA, which is known to enhance the commitment of mESCs to cardiomyocyte-like cells (48). Of note is the fact that, unlike the serum-based differentiation, the beating outcome in cultures of cells exposed to DSFM-L and BMP4 was reproducible in terms of the fraction of beating EBs, time of appearance of the first beating clusters (7–10 days after the start of the differentiation), and time point at which beating reached a maximum percentage (14–15 days).
The effect of BMP4 was abolished when cultured cells were treated the BMP antagonist noggin. Beating areas were not observed when the cells were exposed to 150 ng/ml noggin in DSFM-L with 10 ng/ml BMP4. Instead, neuronal-like outgrowths were observed similar to those in cultures with DSFM-L alone. Compared to BMP4-treated cells, there was a markedly lower expression of heart muscle genes α-MHC and nkx2.5 in mESCs exposed to noggin (Fig. 2G). This illustrates the direct role of BMP4 in inducing the cardiomyogenic differentiation of mESCs. Our results show that BMP4 is sufficient to direct mESCs under serum-free conditions towards cells expressing heart cell markers. Cell aggregation is also important for these cells to exhibit contractile activity.
Differentiation of Encapsulated mESCs in Serum-Free Medium
Next, we applied the BMP4-based protocol for directing encapsulated mESCs to cardiac cells. First, however, we examined the permeability of pLL-coated alginate beads to determine whether BMP4 from the medium can reach the encapsulated cells. For this purpose, LC and nonliquefied core (NLC) pLL-coated beads were incubated with 10 ng/ml of IgG-FITC (MW ~106 kDa) similar to the concentration of BMP4. Diffusion of the IgG-FITC molecules through the beads was tracked by fluorescence microscopy. Within 1 h after adding the IgG-FITC, the level of fluorescence was uniform across the interior of both LC and NLC pLL-coated capsules and did not differ significantly from that outside the beads (Fig. 3A). Hence, we concluded that diffusion of the IgG-FITC through the pLL-coated alginate beads is not hindered. Given the lower molecular weight of BMP4 (~20–22 kDa as a homodimer) compared to IgG-FITC, we concluded that BMP4 from the medium permeates the beads reaching the cells inside.
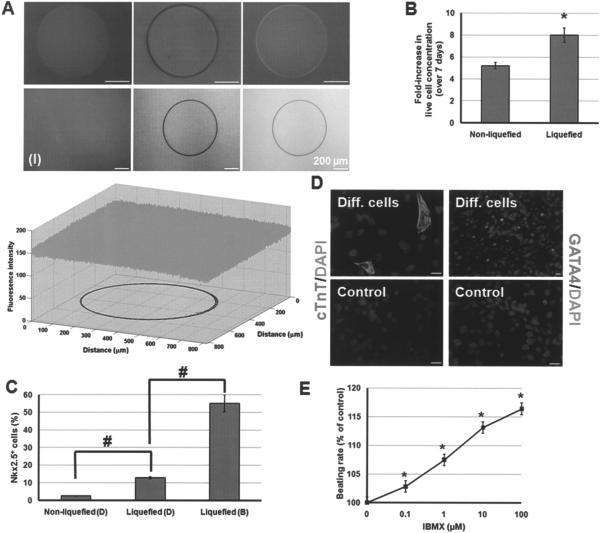
Figure 3.
(A) Poly-L-lysine-coated NLC (upper row) and LC (lower row) beads were incubated with IgG-FITC for 1 h. The green and transmission channel images as well as the merged images of representative beads are shown. The plot depicts the fluorescence intensity (8-bit dynamic range) of image (I). The contour delineating the bead boundary is also shown. (B) Increase in the concentration of live cells differentiated in dishes while encapsulated in solid and LC capsules. *p < 0.05. (C) Fraction of Nkx2.5+ progeny from cells in solid and LC capsules cultured in dishes (marked D) or spinner flasks (marked B). #p < 0.001. (D) Cells differentiated in LC beads with DSFM-L + BMP4 (Diff. cells) exhibit cTnT and GATA4. Cells treated in LC beads with DSFM-L alone are also shown (Control). Scale bars: 20 μm. (E) Beating foci of differentiated mESCs increase their beating rate when incubated with increased concentrations of IBMX. *p < 0.001 compared to baseline response.
Then, mESCs in pLL-coated alginate beads were incubated in dishes with DSFM-L and 10 ng/ml BMP4. Over 7 days of differentiation, cells in LC beads grew 8 ± 0.65-fold compared to 5.2 ± 0.29-fold (p < 0.05) in NLC beads (Fig. 3B). Under both conditions, the viability of encapsulated cells remained above 95% (data not shown). Aggregates, released from the LC capsules and plated, yielded contractile bodies within 2 days. This is the same time frame in which beating bodies appeared after plating nonencapsulated, differentiating mESCs. In contrast, no beating bodies were formed by cells released from NLC capsules. In line with this finding, examination by flow cytometry revealed that ~13% of differentiating cells in LC beads were Nkx2.5+ compared to only ~2.5% from NLC beads (Fig. 3C). The limited mESC aggregation in solid beads may have contributed to the absence of beating bodies.
Cells cultured in LC capsules with DSFM-L and BMP4 were immunopositive for cTnT and GATA4 (Fig. 3D). Plated cells formed spontaneously contracting clusters and their beating rate was modulated by IBMX in a dose-dependent manner (Fig. 3E). When mESCs were cultured in pLL-coated LC beads with DSFM-L but no BMP4, they did not display cardiac cell proteins.
These findings illustrate that mESC encapsulation in pLL-coated LC beads does not hinder the cardiogenic differentiation using physiologically relevant factors under serum-free conditions. In fact, higher fractions of cells positive for cardiac cell markers emerged in LC capsules, which permit cell aggregation.
Cardiogenic Differentiation of hESCs
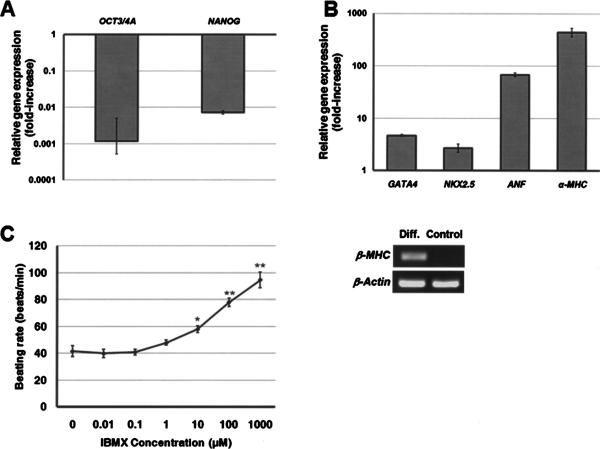
Differentiation of hESCs to cardiomyocytes was carried out in EB cultures with medium containing FBS (49). The expression of OCT3/4A and NANOG was reduced during the 2 weeks of hESC differentiation (Fig. 4A), whereas cardiac genes such as NKX2.5, GATA4, ANF, α- and β-MHC (Fig. 4B) were notably upregulated. Actually, β-MHC was not detectable in hESCmonolayers differentiated in the same medium. Yet, the expression of β-MHC was pronounced after 14 days of hESC differentiation as EBs. The hESC-derived progeny was also immunoreactive to cTnI, Nkx2.5, and GATA4 (data not shown). Spontaneously contracting clusters were first identified after 2 weeks of differentiation and their number increased over time. In addition, treatment of hESC-derived beating clusters with IBMX led to a concentration-dependent increase of their contractile activity (Fig. 4C).
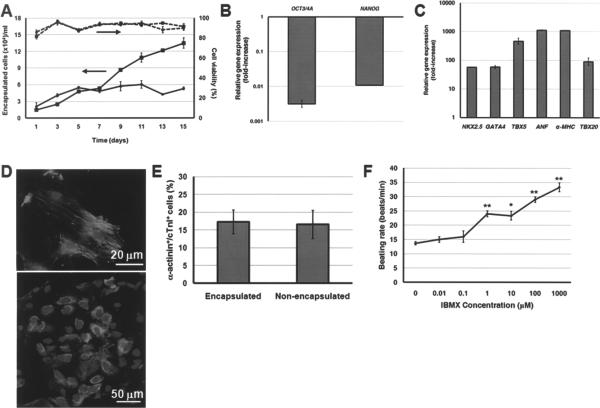
Figure 4.
(A) Relative expression of pluripotency markers OCT3/4A and NANOG after 15 days of hESC differentiation. Gene expression is normalized to that of undifferentiated hESCs. (B) Relative expression of heart cell markers on day 15 of differentiation. Gene expression was normalized to that of hESCs differentiated in the same medium as monolayers (Control; also shown for β-MHC expression). (C) The beating rate of hESC-derived cells was modulated by IBMX. *p < 0.005, **p < 0.0005 compared to no IBMX treatment.
Then, we explored if hESCs in pLL-coated LC and NLC capsules can also be coaxed to cardiac cells in the same serum-based differentiation medium. Cell aggregates were released from the beads at different times and analyzed. In both LC and NLC capsules, cell viability remained high throughout the differentiation. Yet, there was a 9.36 ± 1.42-fold cell expansion in LC beads after 2 weeks compared to only a 2.69 ± 0.78-fold in solid capsules (Fig. 5A).
Figure 5.
(A) Viability and the concentration of live cells (per ml of medium) from hESCs encapsulated in NLC (circles) or LC (squares) beads and cultured in dishes is shown. (B) Expression of OCT3/4A and NANOG after 2 weeks of differentiation in LC capsules is shown normalized to the corresponding gene expression by undifferentiated hESCs. (C) Expression of heart cell genes by hESCs encapsulated in LC beads and differentiated for 15 days. The expression is compared to that of hESCs maintained in the same differentiation medium as monolayers. (D) After a 15-day differentiation, the cells were plated and stained for cTnI (upper panel) or cardiac MHC (lower panel). DAPI costaining is also shown. (E) Cells differentiated in LC capsules or as EBs without encapsulation plated in slides, costained for α-actinin and cTnI, and scored. Values are shown as mean ± SD from at least 10 distinct fields. (F) The contractile activity of differentiated cells was modulated by incubation with IBMX. *p < 0.01, **p < 0.001 compared to no IBMX treatment.
We subsequently assayed differentiated cells in LC capsules for the expression of cardiac cell markers. Like their nonencapsulated counterparts, hESCs differentiated in LC beads exhibited a downregulation of OCT3/4A and NANOG (Fig. 5B) and a marked increase in genes such as α-MHC, ANF, NKX2.5, and TBX20 (Fig. 5C). The induction of cardiogenic differentiation in LC beads was corroborated by immunostaining for markers such as cTnI and cardiac MHC (Fig. 5D). Flow cytometry analysis revealed that ~32% of cells were Nkx2.5+ and a similar fraction was GATA4+ (see Differentiation of Encapsulated ESCs in a Stirred-suspension Bioreactor Culture below).
Comparison of differentiated populations derived from nonencapsulated hESCs and hESCs in pLL-coated LC beads showed 16.57 ± 3.96% and 17.31 ± 3.31% α-actinin+/cTnI+ cells, respectively (Fig. 5E). In addition, EBs released from LC beads organized into clusters displaying contractile activity, which was accelerated upon exposure to increasing concentrations of IBMX (Fig. 5F). Thus, encapsulation in alginate capsules and dissolution of the bead core did not affect markedly the cardiogenic differentiation of hESCs induced by serum-containing medium in dish cultures.
Differentiation of Encapsulated ESCs in a Stirred-Suspension Bioreactor Culture
We showed that the encapsulation of ESCs in pLL-coated LC beads did not hinder their differentiation to heart cells when the same protocols developed for coaxing nonencapsulated mESCs and hESCs in static cultures were implemented. Given the scalability of various encapsulation methods (12,37), one could envision their use for generating suitable amounts of stem cell-laden beads for seeding commercial-scale bioreactors for the production of therapeutically useful cells.
Hence, the question is whether ESCs encapsulated in pLL-coated LC capsules can be differentiated en masse toward heart cells in a stirred-suspension vessel. For this purpose, spinner flasks were inoculated with 2 × 104 cells/ml of mESC- or hESC-laden pLL-coated LC beads. The same differentiation media were used as described above (i.e., DSFM-L + BMP4 for mESCs and medium with FBS for hESCs).
Differentiating mESCs cultured for 1 week grew 5.15 ± 0.11 times while their viability remained above 90% (Fig. 6A). The cumulative LDH activity in the medium, which was also determined as a measure of potential cell lysis, did not exceed 2 U/ml (Fig. 6B). mESCs withdrawn on day 7 from the bioreactor, released from beads, and plated formed beating foci within 2 days, responding to IBMX by increasing their contractile activity in a dose-dependent manner (data not shown). These cells expressed heart cell genes (Fig. 6C) and were immunopositive for cTnT (Fig. 6D) and Nkx2.5 (Fig. 6E). Interestingly, bioreactor cultures yielded a higher fraction of Nkx2.5+ cells compared to encapsulated mESCs differentiated in dishes (Fig. 3C).
Figure 6.
(A) Viability and live cell concentration (per ml of medium) from mESCs encapsulated in LC beads and cultured in spinner flasks. (B) Profile of LDH activity in the spinner flask cultures of encapsulated mESCs. (C) Expression of cardiac genes by LC bead-encapsulated mESCs differentiated in a bioreactor for 7 days. The expression of corresponding genes by mESCs differentiated in LC beads in static culture for the same period was used for comparison. Cells released from the beads at day 7 and plated for 2 days were stained for (D) cTnT (red) and (E) Nkx2.5 (green). Merged micrographs (D) and the last image to the right in (E) show DAPI costaining.
Human ESCs in LC capsules were also coaxed to heart cells while cultured in spinner flasks with serum-containing medium. Encapsulated hESCs cultured in spinner flasks with differentiation medium expanded 8.52 ± 0.89-fold in 15 days with >85% viability (Fig. 7). The slower growth towards the end of the culture could be attributed to the advanced differentiated state of the cells given that the encapsulated aggregates did not overgrow the beads in any of our experiments. The cumulative LDH activity increased over time but (as for mESCs) did not exceed 2 U/ml (Fig. 7B).
Figure 7.
(A) Profiles of viability and live cell concentration for hESCs encapsulated in LC beads and cultured in spinner flasks. (B) Profile of LDH activity in the spinner flask culture of encapsulated hESCs. (C) Expression of cardiac genes by LC bead-encapsulated hESCs differentiated in a bioreactor for 2 weeks. (D) Fractions of Nkx2.5+ and GATA4+ cells resulting from the differentiation of hESCs encapsulated in LC beads and cultured in dishes (open bars) or spinner flasks (gray bars) for 2 weeks. Upon their release from the LC beads, hESC-derived aggregates form beating foci which respond to chronotropic agents such as (E) IBMX and (F) diltiazem. *p < 0.001 compared to baseline response.
Cells expressing human cardiomyocyte genes such as α-MHC and TBX20 (Fig. 7C) emerged at the end of the differentiation. When analyzed by flow cytometry, 36.9 ± 0.5% of cells in stirred suspension expressed NKX2.5 compared to 32.5 ± 0.7% in plates after 14 days of differentiation (Fig. 7D). At the same time, there were 49 ± 0.2% and 32.7 ± 0.4% GATA4+ cells in the spinner flask and dish cultures, respectively. Thus, the fractions of hESC- and mESC-derived cells expressing cardiac cell markers were higher in spinner flasks than in dishes.
Lastly, beating progeny from encapsulated hESCs differentiated in the bioreactor responded to chronotropic agents in an organotypic manner. Incubation of beating bodies with increasing concentrations of IBMX resulted in acceleration of their contractile rate (Fig. 7E), whereas diltiazem, a Ca2+ channel blocker, had the opposite effect (Fig. 7F).
Thus, the cardiogenic differentiation of ESCs in pLL-coated LC capsules cultured in bioreactors is shown for the first time. Cells were coaxed in serum-free medium with a physiologically relevant factor (BMP4; for mESCs) as well as in FBS-containing medium (nonspecific differentiation; for hESCs) yielded higher fractions of cells expressing heart cell-specific markers in a bioreactor than in dishes. Therefore, the system described here is promising for the production of cardiomyocytes from stem cells for therapies.
DISCUSSION
In this study, we investigated a 3D culture system based on alginate microencapsulation for the cardiogenic differentiation of mESCs and hESCs. We provide a first account of ESC differentiation in pLL-coated LC alginate beads towards heart cell progeny. Encapsulation of ESCs in these microcapsules does not affect cell viability, and aggregates of particular size can be obtained by adjusting the seeded ESC density. We showed that stem cells in alginate beads can be directed to heart cells either under serum-free conditions with physiologically relevant factors or in serum-supplemented medium. More importantly, this is the first report of successful cardiogenic differentiation of hESCs and mESCs in pLL-coated LC alginate beads cultured in scalable stirred-suspension vessels. Our findings collectively show that processes combining encapsulation of human stem cells in alginate beads, liquefaction of the capsule core, and culture in bioreactors may be employed for generating cells in therapeutically suitable quantities.
Previously, ESCs were coaxed to heart cell progeny in stirred-suspension bioreactor cultures with the formation of EBs either in low-adhesion surfaces (1,5) or via agitation of single dispersed mESCs (42). While the former method of generating EBs is hardly scalable and results in a wide distribution of EB sizes, the latter raises concerns about the effects of agitation-induced shear on the viability of ESCs and their ability for differentiation. Moreover, our experience has been that control of the ESC aggregate size in spinner flasks via agitation alone is challenging. More recently, hESC differentiation to heart cells was achieved by inoculating spinner flasks with EBs generated after harvesting specific size hESC colonies from micropatterned surfaces (35). There may be practical considerations regarding the preparation of sufficient quantities of EBs/cells on slides for loading bioreactors for commercial production. Moreover, the cost associated with the fabrication of micropatterned surfaces with an extended area for large scale cell cultivation can be considerable.
Nonetheless, results in these studies demonstrate the importance of aggregate size on the cardiogenic differentiation of ESCs. To that end, other methods have also been developed to control the EB size including forced aggregation/centrifugation in 96-well plates and seeding in 3D microwells (20,31,34), which allow for the precise manipulation of the stem cell microenvironment.
We chose to control the aggregate size by seeding ESCs at specific densities in pLL-coated alginate beads with diameters between 500 and 600 μm and liquefying the bead core. This approach is advantageous for generating stem cell aggregates of relatively uniform size intended for culture in stirred-suspension bioreactors. First, large quantities of cells can be processed and microcapsules can be generated in numbers that are sufficient to load large-scale bioreactors. For example, 2 × 106 mouse fibroblasts/ml can be encapsulated in beads with a mean diameter of 320 μm under GMP conditions at a rate of 5,200 beads/s, corresponding to 330 ml of alginate/cell suspension per hour whereas rates of up to 10,000 beads/s are feasible (43). Second, clustering of smaller aggregates due to mixing in the spinner flask is avoided. We did not observe oversized aggregates, which can be detrimental for cell viability and directed commitment due to limitations in nutrient/factor transport. Prevention of excessive clumping also permits tighter control of the bioprocess. Third, the pLL layer provides structural rigidity to low-concentration alginate beads and permits core liquefaction under mild conditions, thus preserving the viability of cells. Within LC beads, cells form aggregates and grow more than in solid capsules. The mechanical stability of the microcapsules makes possible the prolonged culture of cells under continuous agitation (and induced shear) in a bioreactor. Fourth, the cost of employing cell microencapsulation technologies compares favorably to that of other methods requiring, for example, microfabrication. This makes cell encapsulation economically attractive for use in commercial-scale bioprocesses. Fifth, the bead interior provides a microenvironment that is protected from the shear field in the bioreactor while molecules (e.g., nutrients) can be exchanged with the bulk of the medium or circulate (e.g., paracrine factors) within the bead. This may be particularly important for directing the differentiation of stem cells in serum-free media, in which cells are increasingly labile to shear (45).
The permeability of pLL-layered alginate beads can be controlled through parameters such as the incubation time for pLL coating and the number of pLL or alginate layers (23). The pLL-layered beads in this study were not coated with extra layers of alginate, which may reduce the bead permeability. Capsules were sufficiently stable in the bioreactor since we did not observe any broken pLL-coated LC beads in any of the experimental conditions we implemented. Beads in our experiments were permeable to a 106 kDa IgG-FITC. Others (3) showed the diffusion of 300 kDa compounds through pLL-coated alginate capsules. Most differentiation agents such as growth factors have typically a lower molecular weight (e.g., activin A is a homodimer of ~13 kDa monomers) making the pLL-layered alginate matrix an ideal system for directing stem cell fate with physiologically relevant factors.
To demonstrate this, mESCs encapsulated in pLL-coated LC beads were coaxed with BMP4 under serum-free conditions both in static and bioreactor cultures. BMPs are secreted by the anterior mesoderm and layers adjacent to the embryonic heart (46). Deficiency in the expression of one BMP member is compensated by overexpression of other BMPs (14) and most likely not all BMPs are required for in vitro cardiogenic commitment of stem cells. We recognize that BMP4 alone is not sufficient to drive ESCs to mature functional cardiac cells. However, the fractions of beating foci were higher in cultures treated with BMP4 than FBS, even after the addition of RA to the latter. Moreover, BMP4 drives the cardiogenic differentiation of hESCs and cynomolgus monkey ESCs (18,52) besides mESCs. Hence, the serum-free protocol we reported here may be relevant for directing human stem cells to cardiac cells.
We also showed the differentiation of encapsulated human ESCs in static and bioreactor cultures, albeit in serum-containing medium, which is typically used in coaxing hESC-derived EBs to heart cells (49). Cells expressing cardiac markers emerged and organized into beating foci responding to chronotropic agents. Interestingly enough, the fractions of encapsulated hESC-derived Nkx2.5+ (and GATA4+) cells and mESC-derived Nkx2.5+ cells were higher in the bioreactor than in dish cultures. The enhanced differentiation efficiency can be due in large part to the better transport characteristics in the bioreactor, although further studies are warranted to address this issue. The improved cardiogenic commitment of LC bead-encapsulated ESCs in the bioreactor may also explain the somewhat reduced expansion of human and mouse cells compared to those cultured in dishes.
Yet, the difference in the percentage of Nkx2.5+ cells between static and suspension cultures was more pronounced for differentiating mESCs (Fig. 3C) than hESCs (Fig. 7D). This may reflect differences in species and/or differentiation protocols. hESCs were coaxed to cardiomyocyte-like cells in a nonspecific fashion (i.e., in serum-containing medium), whereas the differentiation of mESCs was carried out with BMP4 and without serum. In addition, a higher fraction of Nkx2.5+ cells resulted from cells in LC compared to NLC beads. The more extensive proliferation of cells in the LC capsules may have contributed to this finding. Another explanation may be based on the action of paracrine mechanisms, which are important for the differentiation of ESCs within EBs (4). The increased fluidity in the interior of LC capsules allows paracrine factors to circulate more easily, thus promoting cell differentiation.
In our study, we chose to encapsulate hESCs (and mESCs) at 5 × 106/ml of alginate solution because the aggregates generated (after 1 day) were ~200 μm in diameter (Fig. 1G). This is close to the average size (~210 μm), which was found to be optimal for cardiac induction of H9 cells in spinner flask cultures (35). We acknowledge that the EB size should be considered along with other parameters impacting the differentiation outcome, such as the heterogeneity of the initial ESC population, hypoxia, and agents used for differentiation. Nonetheless, the role of ESC aggregate size has not been explored in conjunction with differentiation protocols utilizing physiologically relevant stimuli in bioreactor cultures. In addition, it appears that the range of EB sizes resulting in optimal cardiogenic differentiation may depend on the ESC line used (9). We also seeded spinner flasks with 2 × 104 cells/ml, although it would interesting to investigate whether the differentiation outcome depends on the initial concentration of ESCs seeded in the bioreactor. These issues can be tackled with a system combining stem cell encapsulation and bioreactor cultivation as described here.
Alginate microencapsulation technology has been employed for the culture of ESCs coaxed towards various lineages, including insulin-producing cells (47), bone cells (19) and hepatocytes (29). We showed for the first time that human and mouse ESCs in pLL-coated LC beads can be coaxed to cardiac (although possibly a mix of atrial, ventricular, and nodal) cells when cultured in dishes or stirred-suspension vessels. Cell encapsulation was carried out by a widely used coaxial airflow droplet generation method. However, methods for mammalian cell encapsulation are constantly improved to achieve smaller size beads (39), better mechanical stability of capsules, lower shear exerted on the cell suspension during bead formation, versatility in the selection of biomaterials, and higher throughput. After differentiation of the encapsulated stem cells, the resulting progeny may be used directly (e.g., for implantation) or after its release and separation from the capsules. The latter requires the development of operations for downstream processing under mild conditions. Moreover, efficient differentiation methods based on the use of defined media free of animal products will also be necessary for obtaining cells suitable for clinical applications.
The simplicity, scalability, and cost-effectiveness of the method presented here in conjunction with the wide use of stirred-suspension bioreactors in industry warrant its further development for use in commercial settings. Mouse and human stem cells encapsulated in pLL-layered LC alginate beads remain viable, proliferate, and can be directed towards cardiac cells in bioreactor cultures. This technology can enable the en masse production of cardiomyocytes from stem cells, including human induced pluripotent stem cells, for heart therapies.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Emily Leitsch for her excellent technical assistance. D.J. and A.P. are recipients of a Mark Diamond Research Fund award. This work was supported by grants from the New York State Stem Cell Science Trust (NYSTEM contract # C024355) and the National Institutes of Health (HL092398) to E.S.T.
REFERENCES
- 1.Akasha AA, Sotiriadou I, Doss MX, Halbach M, Winkler J, Baunach JJ, Katsen-Globa A, Zimmermann H, Choo Y, Hescheler J, Sachinidis A. Entrapment of embryonic stem cells-derived cardiomyocytes in macroporous biodegradable microspheres: Preparation and characterization. Cell. Physiol. Biochem. 2008;22:665–672. doi: 10.1159/000185550. [DOI] [PubMed] [Google Scholar]
- 2.Amir G, Miller L, Shachar M, Feinberg MS, Holbova R, Cohen S, Leor J. Evaluation of a peritoneal-generated cardiac patch in a rat model of heterotopic heart transplantation. Cell Transplant. 2009;18:275–282. doi: 10.3727/096368909788534898. [DOI] [PubMed] [Google Scholar]
- 3.Awrey DE, Tse M, Hortelano G, Chang PL. Permeability of alginate microcapsules to secretory recombinant gene products. Biotechnol. Bioeng. 1996;52:472–484. doi: 10.1002/(SICI)1097-0290(19961120)52:4<472::AID-BIT3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 4.Bader A, Gruss A, Hollrigl A, Al-Dubai H, Capetanaki Y, Weitzer G. Paracrine promotion of cardiomyo-genesis in embryoid bodies by LIF modulated endoderm. Differentiation. 2001;68:31–43. doi: 10.1046/j.1432-0436.2001.068001031.x. [DOI] [PubMed] [Google Scholar]
- 5.Bauwens C, Yin T, Dang S, Peerani R, Zandstra PW. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: Oxygen-mediated enhancement of cardiomyocyte output. Biotechnol. Bioeng. 2005;90:452–461. doi: 10.1002/bit.20445. [DOI] [PubMed] [Google Scholar]
- 6.Bauwens CL, Peerani R, Niebruegge S, Woodhouse KA, Kumacheva E, Husain M, Zandstra PW. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26:2300–2310. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- 7.Borlongan CV, Thanos CG, Skinner SJ, Geaney M, Emerich DF. Transplants of encapsulated rat choroid plexus cells exert neuroprotection in a rodent model of Huntington's disease. Cell Transplant. 2008;16:987–992. [PubMed] [Google Scholar]
- 8.Bugarski B, Smith J, Wu J, Goosen MFA. Methods for animal-cell immobilization using electrostatic droplet generation. Biotechnol. Tech. 1993;7:677–682. [Google Scholar]
- 9.Burridge PW, Anderson D, Priddle H, Barbadillo Munoz MD, Chamberlain S, Allegrucci C, Young LE, Denning C. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25:929–938. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- 10.Claycomb WC, Lanson NA, Jr., Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornolti R, Figliuzzi M, Remuzzi A. Effect of micro- and macroencapsulation on oxygen consumption by pancreatic islets. Cell Transplant. 2009;18:195–201. doi: 10.3727/096368909788341252. [DOI] [PubMed] [Google Scholar]
- 12.De Vos P, De Haan BJ, Van Schilfgaarde R. Upscaling the production of microencapsulated pancreatic islets. Biomaterials. 1997;18:1085–1090. doi: 10.1016/s0142-9612(97)00040-9. [DOI] [PubMed] [Google Scholar]
- 13.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: Formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 14.Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev. Dyn. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials. 2007;28:2706–2717. doi: 10.1016/j.biomaterials.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groot MD, Leuvenink HGD, Keizer PPM, Fekken S, Schuurs TA, Schilfgaarde RV. Effective removal of alginate-poly-L-lysine microcapsules from pancreatic islets by use of trypsin-EDTA. J. Biomed. Mater. Res. A. 2003;67A:679–683. doi: 10.1002/jbm.a.10023. [DOI] [PubMed] [Google Scholar]
- 17.Honda M, Kurisaki A, Ohnuma K, Okochi H, Hamazaki TS, Asashima M. N-cadherin is a useful marker for the progenitor of cardiomyocytes differentiated from mouse ES cells in serum-free condition. Biochem. Biophys. Res. Commun. 2006;351:877–882. doi: 10.1016/j.bbrc.2006.10.126. [DOI] [PubMed] [Google Scholar]
- 18.Hosseinkhani M, Hosseinkhani H, Khademhosseini A, Bolland F, Kobayashi H, Gonzalez SP. Bone morphogenetic protein-4 enhances cardiomyocyte differentiation of cynomolgus monkey ESCs in knockout serum replacement medium. Stem Cells. 2007;25:571–580. doi: 10.1634/stemcells.2006-0225. [DOI] [PubMed] [Google Scholar]
- 19.Hwang YS, Cho J, Tay F, Heng JY, Ho R, Kazarian SG, Williams DR, Boccaccini AR, Polak JM, Mantalaris A. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials. 2009;30:499–507. doi: 10.1016/j.biomaterials.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc. Natl. Acad. Sci. USA. 2009;106:16978–16983. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kehoe DE, Lock LT, Parikh A, Tzanakakis ES. Propagation of embryonic stem cells in stirred suspension without serum. Biotechnol. Prog. 2008;24:1342–1352. doi: 10.1002/btpr.57. [DOI] [PubMed] [Google Scholar]
- 23.King GA, Daugulis AJ, Faulkner P, Goosen MFA. Alginate-polylysine microcapsules of controlled membrane molecular-weight cutoff for mammalian-cell culture engineering. Biotechnol. Prog. 1987;3:231–240. [Google Scholar]
- 24.Koike M, Sakaki S, Amano Y, Kurosawa H. Characterization of embryoid bodies of mouse embryonic stem cells formed under various culture conditions and estimation of differentiation status of such bodies. J. Biosci. Bioeng. 2007;104:294–299. doi: 10.1263/jbb.104.294. [DOI] [PubMed] [Google Scholar]
- 25.Lane WR. A Microburette for producing small liquid drops of known size. J. Sci. Instrum. 1947;24:98–101. [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Lock LT, Tzanakakis ES. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng. Part A. 2009;15:2051–2063. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguire T, Davidovich AE, Wallenstein EJ, Novik E, Sharma N, Pedersen H, Androulakis IP, Schloss R, Yarmush M. Control of hepatic differentiation via cellular aggregation in an alginate microenvironment. Biotechnol. Bioeng. 2007;98:631–644. doi: 10.1002/bit.21435. [DOI] [PubMed] [Google Scholar]
- 29.Maguire T, Novik E, Schloss R, Yarmush M. Alginate-PLL microencapsulation: Effect on the differentiation of embryonic stem cells into hepatocytes. Biotechnol. Bioeng. 2006;93:581–591. doi: 10.1002/bit.20748. [DOI] [PubMed] [Google Scholar]
- 30.Magyar JP, Nemir M, Ehler E, Suter N, Perriard JC, Eppenberger HM. Mass production of embryoid bodies in microbeads. Ann. NY Acad. Sci. 2001;944:135–143. doi: 10.1111/j.1749-6632.2001.tb03828.x. [DOI] [PubMed] [Google Scholar]
- 31.Mohr JC, De Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27:6032–6042. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Moustafa T, Girod S, Tortosa F, Li R, Sol JC, Rodriguez F, Bastide R, Lazorthes Y, Sallerin B. Viability and functionality of bovine chromaffin cells encapsulated into alginate-PLL microcapsules with a liquefied inner core. Cell Transplant. 2006;15:121–133. doi: 10.3727/000000006783982106. [DOI] [PubMed] [Google Scholar]
- 33.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106:1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 35.Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E, Zandstra PW. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol. Bioeng. 2009;102:493–507. doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- 36.Orive G, Hernandez RM, Gascon AR, Calafiore R, Chang TM, De Vos P, Hortelano G, Hunkeler D, Lacik I, Shapiro AM, Pedraz JL. Cell encapsulation: Promise and progress. Nat. Med. 2003;9:104–107. doi: 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- 37.Poncelet D, De Smet BP, Beaulieu C, Neufeld RJ. Scale-up of gel bead and microcapsule production in cell immobilization. In: Goosen MFA, editor. Fundamentals of animal cell encapsulation and immobilization. CRC Press; Boca Raton, FL: 1993. pp. 113–142. [Google Scholar]
- 38.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, Mcdermott M, Meigs J, Moy C, Nichol G, O'donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics—2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 39.Sakai S, Hashimoto I, Kawakami K. Production of cell-enclosing hollow-core agarose microcapsules via jetting in water-immiscible liquid paraffin and formation of embryoid body-like spherical tissues from mouse ES cells enclosed within these microcapsules. Biotechnol. Bioeng. 2008;99:235–243. doi: 10.1002/bit.21624. [DOI] [PubMed] [Google Scholar]
- 40.Sargent CY, Berguig GY, Kinney MA, Hiatt LA, Carpenedo RL, Berson RE, Mcdevitt TC. Hydrodynamic modulation of embryonic stem cell differentiation by rotary orbital suspension culture. Biotechnol. Bioeng. 2010;105:611–626. doi: 10.1002/bit.22578. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki D, Shimizu T, Masuda S, Kobayashi J, Itoga K, Tsuda Y, Yamashita JK, Yamato M, Okano T. Mass preparation of size-controlled mouse embryonic stem cell aggregates and induction of cardiac differentiation by cell patterning method. Biomaterials. 2009;30:4384–4389. doi: 10.1016/j.biomaterials.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Schroeder M, Niebruegge S, Werner A, Willbold E, Burg M, Ruediger M, Field LJ, Lehmann J, Zweigerdt R. Differentiation and lineage selection of mouse embryonic stem cells in a stirred bench scale bioreactor with automated process control. Biotechnol. Bioeng. 2005;92:920–933. doi: 10.1002/bit.20668. [DOI] [PubMed] [Google Scholar]
- 43.Schwinger C, Koch S, Jahnz U, Wittlich P, Rainov NG, Kressler J. High throughput encapsulation of murine fibroblasts in alginate using the JetCutter technology. J. Microencapsul. 2002;19:273–280. doi: 10.1080/02652040110105328. [DOI] [PubMed] [Google Scholar]
- 44.Taha MF, Valojerdi MR, Mowla SJ. Effect of bone morphogenetic protein-4 (BMP-4) on cardiomyocyte differentiation from mouse embryonic stem cell. Int. J. Cardiol. 2007;120:92–101. doi: 10.1016/j.ijcard.2006.08.118. [DOI] [PubMed] [Google Scholar]
- 45.Van Der Pol L, Tramper J. Shear sensitivity of animal cells from a culture-medium perspective. Trends Biotechnol. 1998;16:323–328. doi: 10.1016/s0167-7799(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 46.Van Wijk B, Moorman AF, Van Den Hoff MJ. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc. Res. 2007;74:244–255. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 47.Wang N, Adams G, Buttery L, Falcone FH, Stolnik S. Alginate encapsulation technology supports embryonic stem cells differentiation into insulin-producing cells. J. Biotechnol. 2009;144:304–312. doi: 10.1016/j.jbiotec.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Wobus AM, Kaomei G, Shan J, Wellner MC, Rohwedel J, Ji G, Fleischmann B, Katus HA, Hescheler J, Franz WM. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 1997;29:1525–1539. doi: 10.1006/jmcc.1997.0433. [DOI] [PubMed] [Google Scholar]
- 49.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ. Res. 2002;91:501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 50.Zammaretti P, Jaconi M. Cardiac tissue engineering: Regeneration of the wounded heart. Curr. Opin. Biotechnol. 2004;15:430–434. doi: 10.1016/j.copbio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Zandstra PW, Bauwens C, Yin T, Liu Q, Schiller H, Zweigerdt R, Pasumarthi KB, Field LJ. Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng. 2003;9:767–778. doi: 10.1089/107632703768247449. [DOI] [PubMed] [Google Scholar]
- 52.Zhang P, Li J, Tan Z, Wang C, Liu T, Chen L, Yong J, Jiang W, Sun X, Du L, Ding M, Deng H. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]