Abstract
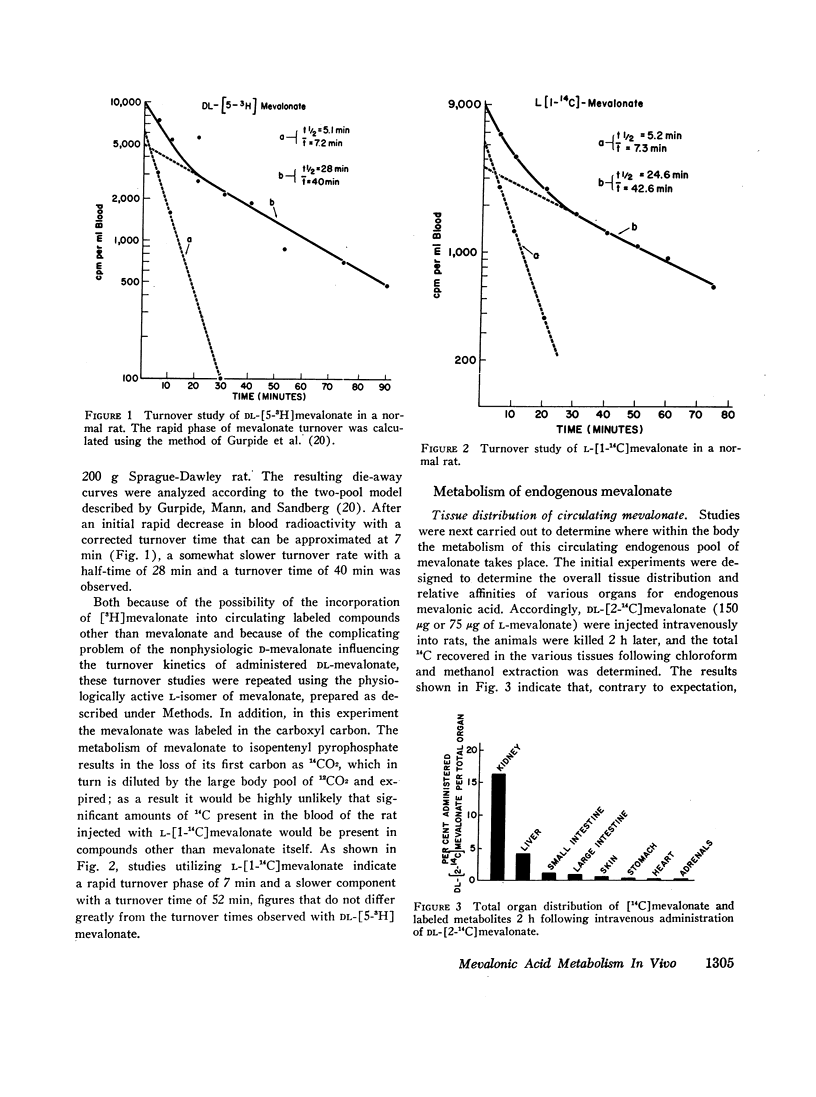
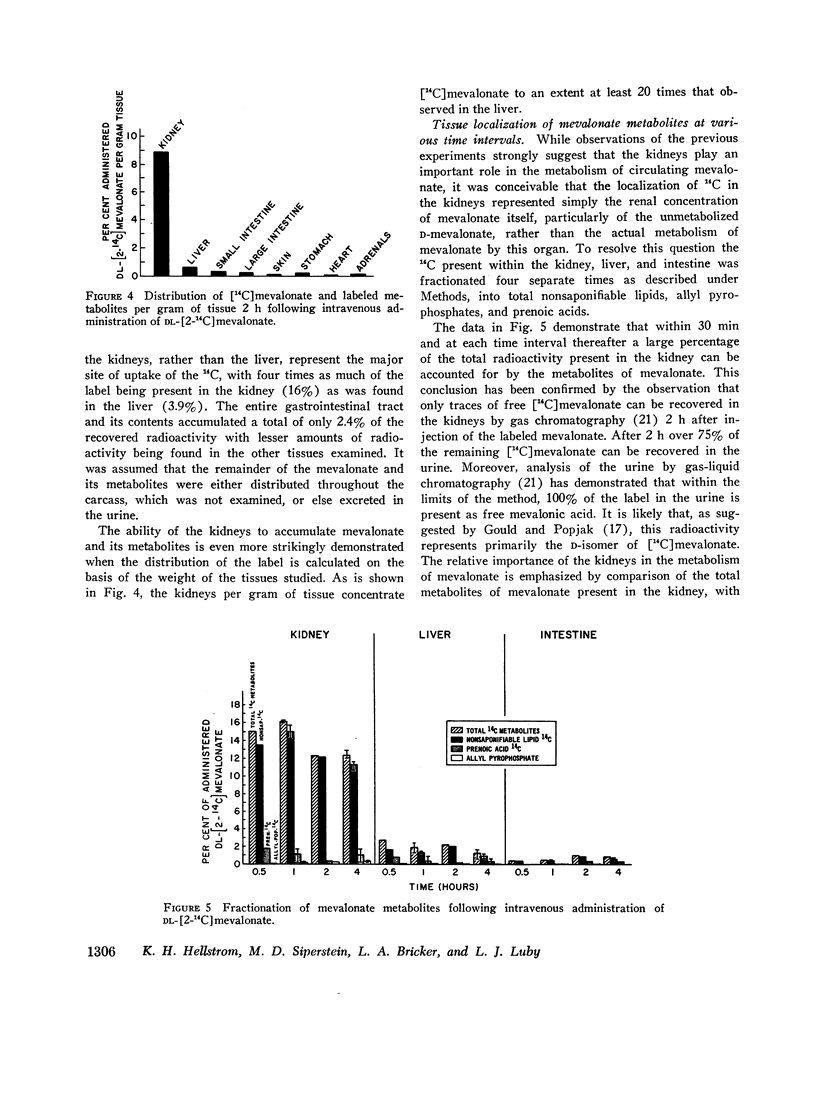
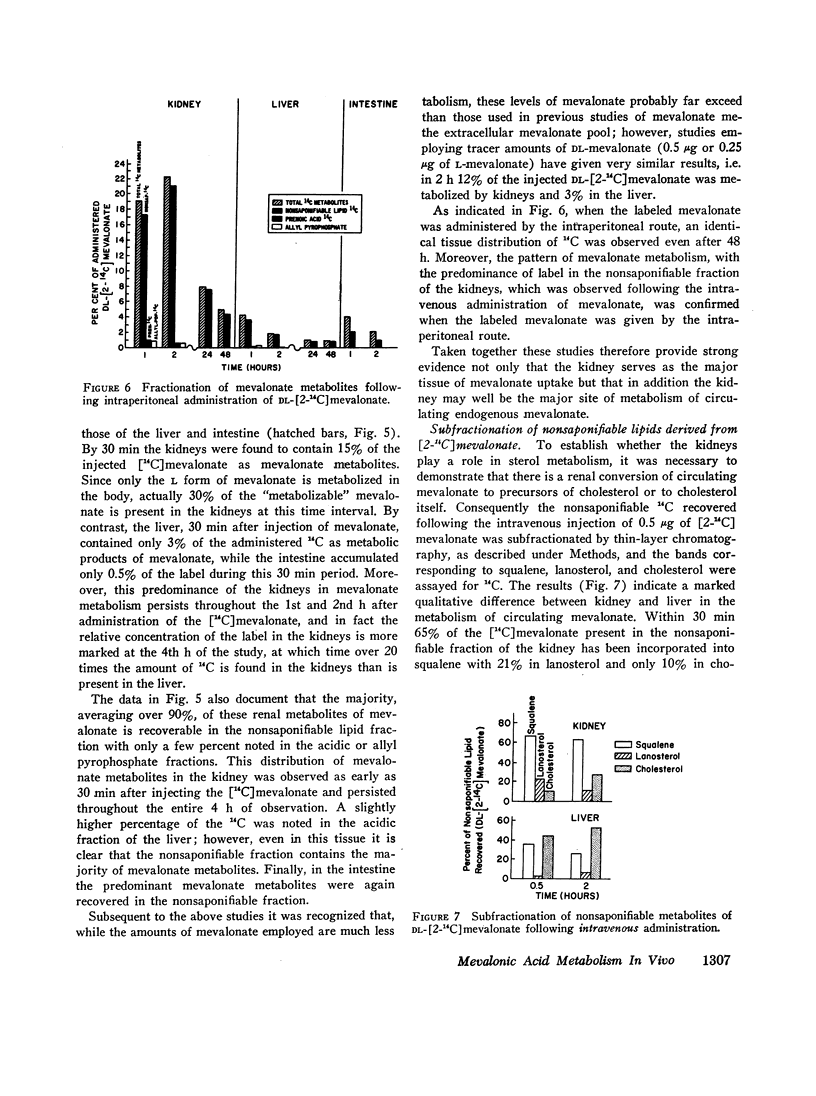
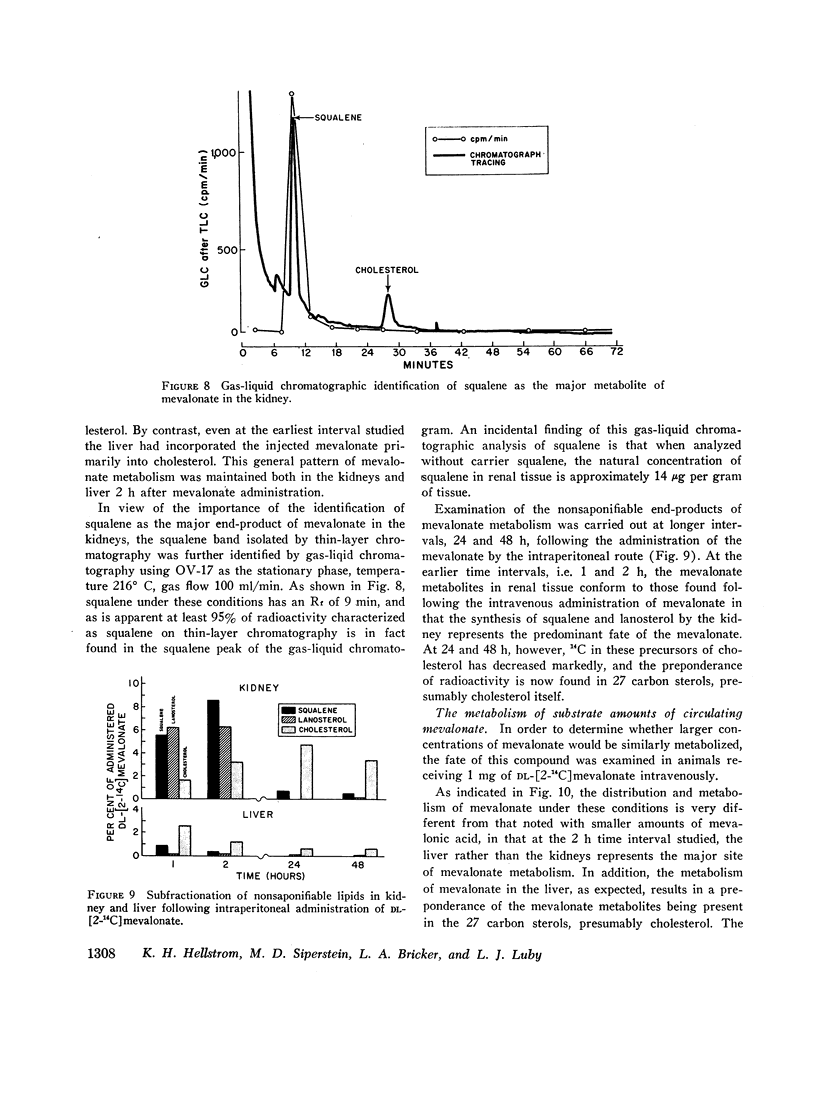
Studies were performed to examine synthesis, tissue localization, and metabolism of mevalonic acid in normal rats. Circulating mevalonate was found to have a rapid turnover phase of 5 min and a slower phase of 40-50 min. Under in vitro conditions the synthesis of mevalonate is carried out most actively by the liver and only to a minor extent by the other tissues studied. The most unexpected finding of this study was that both in vivo and in vitro the kidneys rather than the liver are the primary site of the metabolism of circulating mevalonate. Whereas mevalonate in the liver is rapidly transformed to cholesterol, the major products of mevalonate metabolism in the renal tissues during the same time period are squalene and lanosterol. Exogenous in contrast to circulating mevalonate is metabolized primarily in the intestine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barratt T. M., Walser M. Extracellular fluid in individual tissues and in whole animals: the distribution of radiosulfate and radiobromide. J Clin Invest. 1969 Jan;48(1):56–66. doi: 10.1172/JCI105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker L. A., Morris H. P., Siperstein M. D. Loss of the cholesterol feedback system in the intact hepatoma-bearing rat. J Clin Invest. 1972 Feb;51(2):206–215. doi: 10.1172/JCI106805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARATTINI S., PAOLETTI P., PAOLETTI R. Lipid biosynthesis in vivo from acetate-1-C 14 and 2-C 14 and mevalonic-2-C 14 acid. Arch Biochem Biophys. 1959 Sep;84:253–255. doi: 10.1016/0003-9861(59)90579-x. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S., AVIGAN J., STEINBERG D. Studies of cholesterol biosynthesis. V. The time course and pathway of the later stages of cholesterol biosynthesis in the livers of intact rats. J Biol Chem. 1963 Apr;238:1287–1293. [PubMed] [Google Scholar]
- GOODMAN D. S., POPJAK G. Studies on the biosynthesis of cholesterol. XII. Synthesis of allyl pyrophosphates from mevalonate and their conversion into squalene with liver enzymes. J Lipid Res. 1960 Jul;1:286–300. [PubMed] [Google Scholar]
- GOODMAN D. S. SQUALENE IN HUMAN AND RAT BLOOD PLASMA. J Clin Invest. 1964 Jul;43:1480–1485. doi: 10.1172/JCI105024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GURPIDE E., MANN J., SANDBERG E. DETERMINATION OF KINETIC PARAMETERS INA TWO-POOL SYSTEM BY ADMINISTRATION OF ONE OR MORE TRACERS. Biochemistry. 1964 Sep;3:1250–1255. doi: 10.1021/bi00897a012. [DOI] [PubMed] [Google Scholar]
- Gold P. H., Olson R. E. Studies on coenzyme Q. The biosynthesis of coenzyme Q9 in rat tissue slices. J Biol Chem. 1966 Aug 10;241(15):3507–3516. [PubMed] [Google Scholar]
- Linn T. C. The effect of cholesterol feeding and fasting upon beta-hydroxy-beta-methylglutaryl coenzyme A reductase. J Biol Chem. 1967 Mar 10;242(5):990–993. [PubMed] [Google Scholar]
- POPJAK G., GOSSELIN L., GORE I. Y., GOULD R. G. Studies on the biosynthesis of cholesterol. 6. Coenzyme requirements of liver enzymes for synthesis of squalene and of sterol from DL-3-hydroxy-3-methyl-[2-14C] pentano-5-lactone. Biochem J. 1958 Jun;69(2):238–248. doi: 10.1042/bj0690238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIPERSTEIN M. D., FAGAN V. M. DELETION OF THE CHOLESTEROL-NEGATIVE FEEDBACK SYSTEM IN LIVER TUMORS. Cancer Res. 1964 Aug;24:1108–1115. [PubMed] [Google Scholar]
- SIPERSTEIN M. D., GUEST M. J. Studies on the site of the feedback control of cholesterol synthesis. J Clin Invest. 1960 Apr;39:642–652. doi: 10.1172/JCI104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Rodwell V. W. Diurnal variation and cholesterol regulation of hepatic HMG-CoA reductase activity. Biochem Biophys Res Commun. 1969 Nov 20;37(5):867–872. doi: 10.1016/0006-291x(69)90972-3. [DOI] [PubMed] [Google Scholar]
- Siperstein M. D., Fagan V. M., Dietschy J. M. A gas-liquid chromatographic procedure for the measurement of mevalonic acid synthesis. J Biol Chem. 1966 Feb 10;241(3):597–601. [PubMed] [Google Scholar]
- Siperstein M. D., Fagan V. M., Morris H. P. Further studies on the deletion of the cholesterol feedback system in hepatomas. Cancer Res. 1966 Jan;26(1):7–11. [PubMed] [Google Scholar]
- Siperstein M. D., Gyde A. M., Morris H. P. Loss of feedback control of hydroxymethylglutaryl coenzyme A reductase in hepatomas. Proc Natl Acad Sci U S A. 1971 Feb;68(2):315–317. doi: 10.1073/pnas.68.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCHEN T. T. Mevalonic kinase: purification and properties. J Biol Chem. 1958 Nov;233(5):1100–1103. [PubMed] [Google Scholar]
- White L. W., Rudney H. Regulation of 3-hydroxy-3-methylglutarate and mevalonate biosynthesis by rat liver homogenates. Effects of fasting, cholesterol feeding, and triton administration. Biochemistry. 1970 Jun 23;9(13):2725–2731. doi: 10.1021/bi00815a021. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. The quantification of cholesterol excretion and degradation in the isotopic steady state in the rat: the influence of dietary cholesterol. J Lipid Res. 1964 Jul;5(3):409–417. [PubMed] [Google Scholar]


