Abstract
TORC1 is a central regulator of ribosomal RNA synthesis. Here we report that Sch9 partially mediates TORC1 signaling to regulate pol I- and pol III-dependent transcription. Mechanistically, Sch9 is involved in hyperphosphorylation and cytoplasmic localization of Maf1, and for optimal synthesis of rRNAs and tRNAs. Interestingly, sch9Δ does not affect Maf1 basal phosphorylation and nucleolar localization. In addition, TORC1 is still capable of regulating rRNAs and tRNAs in the absence of Sch9. Moreover, the hyperactive Sch9(2D3E) mutant does not confer significant rapamycin resistance in cell growth. Together, these observations indicate that Sch9 is involved in optimal regulation of ribosome biogenesis by TORC1, but is dispensable for the essential aspects of ribosome biogenesis and cell growth, suggesting that TORC1 controls cell growth through Sch9-dependent and independent mechanisms.
Keywords: TOR (target of rapamycin), RNA polymerase III, Sch9, Maf1, nucleolus
Introduction
The TOR (target of rapamycin) protein kinase belongs to the kinase family of phosphatidylinositol-3 kinase related kinase (PIKK) that phosphorylates Serine/Threonine residue on protein rather than lipid.1 TOR proteins are evolutionally conserved from yeast to mammal. TOR proteins form two functional complexes in all eukaryotic cells.2 In yeast, TOR complex 1 (TORC1) consists of Kog1, Lst8, and either Tor1 or Tor2, while TOR complex 2 (TORC2) consists of Avo1, Avo2, Avo3, Lst8 and Tor2.2 TORC1 but not TORC2 is sensitive to rapamycin. Rapamycin first forms a complex with its intracellular receptor FKBP12 (FK506-binding protein 12 kDa), then targets to FRB (FKBP12-rapamycin binding) domain of Tor1/2, resulting in specific inhibition of TORC1.3 In recent years, TOR has been established to be the centre controller of cell growth. In response to extracellular stimuli, TORC1 regulates multiple growth–related processes including ribosome biogenesis, protein translation, nutrient import and autophagy.4,5 TORC2 is rapamycin-insensitive and involved in regulation of cytoskeleton organization.4,5
Ribosome biogenesis is the process to produce ribosomes, the machinery for protein synthesis, which tightly coupled to cell growth through TORC1. It requires close coordination of all three RNA polymerases (Pols) to produce individual components in an equal ratio.6,7 In yeast, Pol I transcribes 35S ribosomal RNA (rRNA) that is further processed into the mature forms of 25S, 18S and 5.8S rRNAs; Pol II is responsible for transcription of ribosomal protein (RP) genes; Pol III directs 5S rRNA and tRNA synthesis. Overall, ribosome biogenesis accounts for up to 80% of the nuclear transcription in a eukaryotic cell.6,7 The huge energy expense demands for a tight regulation. Deregulation of ribosome biogenesis is a common phenomenon in cancer cells. Furthermore, overproduction of Pol III-dependent transcription can promote oncogenic transformation.8,9 Thus, it is important to further understand the details of how TORC1 coordinates all three RNA polymerases for an accurate production of rRNAs and RPs.
Rapamycin treatment or nutrient starvation in yeast causes rapid repression of ribosomal genes, including rRNA gene (rDNA) and RP genes.10,11 Interestingly, both yeast and mammalian TORs are found to be localized in the nucleus.12-14 Furthermore, yeast TORC1 is associated with rDNA chromatin, including 35S rDNA promoter and 5S rDNA gene, in a rapamycin-sensitive and nutrient-dependent manner to regulate Pol I- and Pol III-dependent transcription.12,15 The simultaneous association of TORC1 with both Pol I and Pol II genes provides a simple yet highly efficient mechanism to coordinate Pol I and Pol III for ribosome biogenesis.5,15 Maf1 is a Pol III repressor that mediates diverse stress signals to regulate 5S rRNA and tRNAs transcriptions.16 Maf1 is regulated by TORC1 through phosphorylation.17-19 Maf1 phospohrylation is correlative to its cytoplasmic localization, however, several studies later suggest that the nucleus-to-cytoplasm transport of Maf1 is dispensable for Pol III inhibition.15,19,20 A recent study revealed that TORC1 also modulates Maf1 nucleolar localization and chromatin association, which is essential for inhibition of Pol III activity.15
Sch9 is a member of the AGC kinase family that is phospohrylated at multiple sites by TORC1 and mediates TORC1 regulation of RP gene transcription by Pol II.21,22 Sch9 has been proposed to be a functional yeast homolog of the mammalian ribosomal S6 kinase 1 (S6K1), a key downstream effector of mTOR. We therefore asked if Sch9 also mediates TORC1 signaling to Pol I and Pol III. Here we show that Sch9 is partially responsible for TORC1 signaling to regulate ribosomal RNA synthesis. Sch9 directly phosphorylates Maf1 and regulates its cytoplasm-to-nucleus translocation but not nucleoplasm-to-nucleolus transport. In agreement with the above observation, Sch9-dependent phosphorylation has minimal effect on Maf1 activity or rapamycin-sensitive cell growth. During preparation of this manuscript, two other studies were published that also reported Sch9 as a Maf1 kinase,23,24 confirming some of our observations.
Results
Sch9 interacts with and phosphorylates Maf1
We have recently shown that TORC1 interacts with Maf1 and phosphorylates Maf1 in vitro.15 However, TORC1 activity toward Maf1 is relatively weak, raising the possibility of a downstream kinase(s). Therefore, we screened a collection of yeast kinase deletion strains for defects in Maf1 phosphorylation. Figure 1A shows a representative experiment with a panel of mutants lacking kinases known to be involved in TOR signaling. We found that Maf1 hyperphosphorylation was compromised in sch9Δ mutant (Fig. 1B). The defect in Maf1 hyperphoshorylation was suppressed by a plasmid-borne SCH9. In addition, a Sch9 hyperactive form Sch9(2D3E)22 conferred rapamycin resistance in Maf1 hyperphosphorylation (Fig. 1B), indicating that Sch9 is involved in Maf1 phoshorylation.
Figure 1.
Sch9 interacts with Maf1 and phosphorylates Maf1 in vitro. (A) Representative data of kinase screen for Maf1 phosphorylation. Kinase deletion strains expressing Maf1-myc9 was cultured to early log phase then treated without and with rapamycin (200 nM) for 30 min. Maf1 phosphorylation was detected through gel mobility shift by western blot (WB) using the anti-Myc antibody 9E10. The slow-migrating form represents phosphorylated Maf1. (B) Sch9 is involved in Maf1 phosphorylation. Maf1-Myc9 phosphorylation was tested in EG103 wild type (WT) and sch9Δ strains containing vector control or expressing Sch9 or its hyperactive form Sch9(2D3E). (C) Sch9 interacts with Maf1. Co-immunoprecipitation (Co-IP) was carried out with lysate from W303a cells expressing Sch9-HA3 or Maf1-Myc9 or both. The proteins in the lysates (Input) and immunoprecipitates (IP) were detected by WB using Myc- and HA-sepcific antibodies. (D) Sch9 phosphorylates Maf1 in vitro. Sch9 wild type, Sch9 kinase dead (K441A), and Sch9 hyperactive (2D3E) were purified from yeast cells treated with or without rapamycin, and incubated with GST-Maf1 purified from E. coli in presence of [γ-32P]-ATP. Sch9 expression was detected by western blot with HA-specific antibody (12CA5); GST-Maf1 phosphorylation was detected by autoradiography; GST-Maf1 protein input was determined by Coomassie blue staining.
We next investigated whether Sch9 interacts with Maf1. To this end, co-immunoprecipitation (co-IP) experiments were carried out with lysates from yeast cells expressing Maf1-Myc9 and/or Sch9-HA3. Sch9-HA3 was found in the co-IP only in the presence of Maf1-Myc9 but not in its absence (Fig. 1C). Consistently, Maf1-Myc9 was precipitated with Sch9-HA3 but not in the control, indicating that Sch9 and Maf1 interact with each other in vivo. We further tested if Sch9 directly phosphorylates Maf1 in vitro. We found that Sch9 but not the kinase-dead Sch9(K441A)25 phosphorylated bacterially expressed Maf1 in the presence of [γ-32P]-ATP (Fig. 3D). The ability of Sch9 to phosphrylate Maf1 was inhibited by rapamycin. However, Sch9(2D3E) was resistant to rapamycin treatment (Fig. 1D). Together, the above data indicate that Sch9 is a Maf1 kinase.
Figure 3.
TORC1 regulates Pol I- and Pol III-directed transcription through both Sch9-dependent and -independent mechanisms. EG103 sch9Δ cells expressing Sch9, vector control or its hyperactive form (2D3E) were treated without and with rapamycin (100 nM) for 30 min. Cells were then subject to metabolic labeling with [5, 6-3H]-Uracil. Total RNA (stable species) was stained with Ethidium bromide (EtBr) as loading control. Different species of newly synthesized RNA (radio-labeled) was detected by autoradiography after separation on acrylamide gel containing 6 M Urea.
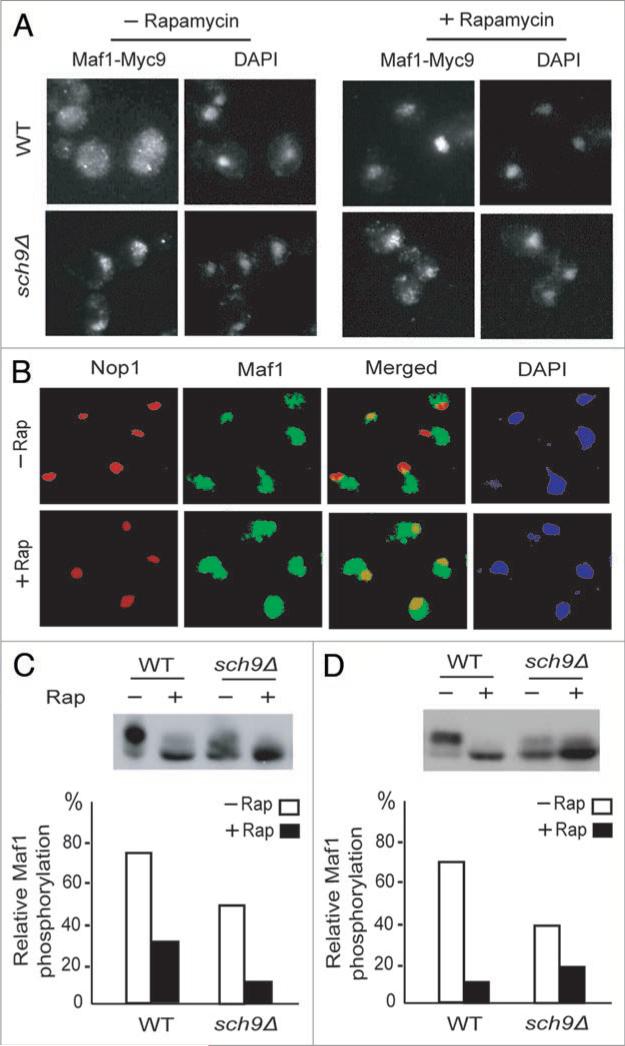
Sch9 regulates Maf1 cytoplasm-to-nucleus transport, but not for Maf1 nucleoplasm-to-nucleolus translocation or basal phosphorylation
Maf1 phosphorylation has been known to regulate both its cytoplasm-to-nucleus translocation17-19 and nucleoplasm-to-nucleolus transport,15 with the latter step being essential for Pol III regulation and the former step being dispensable. Maf1 is present largely in the cytoplasm in absence of rapamycin, but is transported first into the nucleus and then the nucleolus upon rapamycin treatment (Fig. 2A and B).15 We found that the sch9Δ mutation was sufficient to cause Maf1 to accumulate in the nucleus (Fig. 2A). However, regulation of Maf1's nucleoplasm-to-nucleolus transport remained normal, as indicated by the absence of Maf1 in the nucleolus without rapamycin but accumulation of Maf1 in the nucleolus in the presence of rapamycin in sch9Δ mutant strain (Fig. 2B).
Figure 2.
Sch9 regulates Maf1 cytoplasm-to-nucleus, but not nucleoplasm-to-nucleolus translocation or Maf1 basal phosphorylation. (A) Sch9 phosphorylation is required for Maf1 cytoplamic localization. Maf1-Myc9 localization was detected by immuno-fluorescence (IF) in EG103 WT and sch9Δ cells treated without and with 200 nM rapamycin for 60 min. Maf1-Myc9 was stained with Myc-specific antibody (A14) and nucleus was stained with DAPI. (B) EG103 sch9Δ cells were treated without and with 200 nM rapamycin for 60 min. Maf1-Myc9 localization was detected by IF, with Nop1 staining and DAPI staining as markers for the nucleolus and the nucleus, respectively. Maf1-Myc9 was detected by a polyclonal anti-Myc antibody (A14). (C) EG103 WT and sch9Δ cells were treated with and without 200 nM rapamycin for 30 min. Cells were lysed in denaturing buffer. Maf1-Myc9 phosphorylation was detected by WB. The slow-migrating forms show phosphorylated Maf1. Quantifications are percentage of phosphorylated Maf1. (D) EG103 WT and sch9Δ cells were starved with glucose for 1 hour and then restimulated with glucose in the presence or absence of rapamycin. Maf1 phosphorylation was determined as in Fig. 2C.
Because Maf1 phosphorylation is closely correlative with Maf1 regulation, we wondered if Sch9 is only partially responsible for Maf1 phosphorylation. Maf1 phosphorylation is known to be very sensitive to phosphatase.17,18 To improve the detection of Maf1 phosphorylation, we used denaturing lysis buffer and improved the resolution of SDS-PAGE, which allowed us to detect a basal Maf1 phosphorylaiton in sch9Δ cells (Fig. 2C and D). Importantly, Maf1 basal phosphorylation remained sensitive to rapamycin, indicating that TORC1 has the ability to regulate Maf1 phosphorylation independently of Sch9 (Fig. 2C and D), which is likely to be responsible for TORC1 to control Maf1 nucleolus-to-nucleoplasm transport.
TORC1 regulates Pol I- and Pol III-directed transcription by Sch9-dependent and -independent mechanisms
Because TORC1 regulates Maf1 through Sch9-dependent and -independent mechanisms, we asked what the relative role of Sch9 is in Pol III-directed transcription. To this end, we measured rRNA synthesis by metabolically labeling with 3H-Uracil sch9Δ cells expressing Sch9, Sch9(2D3E) or carrying a control vector. In cells lacking Sch9, Pol III-dependent transcription (5S rRNA and tRNAs) was decreased in comparison to cells expressing Sch9, but a basal level remained (Fig. 3). In contrast, Pol III-dependent transcription was moderately elevated in Sch9(2D3E)-expressing cells. Rapamycin still fully repressed Pol III-dependent transcription in SCH9 and sch9Δ cells, but SCH9(2D3E) cells showed considerable rapamycin resistance (Fig. 3). Interestingly, essentially the same results were observed with 35S rRNA synthesis (Fig. 3). Altogether, these data reveal that Sch9 is involved in the rapamycin-sensitive control of both Pol I- and Pol III-dependent transcription. They further indicate that there exists a Sch9-independent mechanism.
Sch9-dependent Maf1 phosphorylation affects Maf1 cytoplamic localization but not its ability to regulate cell growth
To fully understand Maf1 regulation, we have been engaged in characterization of Maf1 phosphorylation sites. In summary of a large body of work, in addition to the previously reported phosphorylation sites (S90, S101, S177/178, S209/210),19 we identified two new sites (S179/S180). Mutation at these sites blocked Maf1 gel mobility (Fig. 4A). To explore the significance of Maf1 phosphorylation, we mutated all eight serine residues to alanine (8SA), which was recently shown to prevent Maf1 phosphorylation by Sch9 in vitro.23,24 We also mutated these serine residues to aspartate (8SD), which potentially mimics the phoshorylation form of Maf1. Consistent with the sch9Δ mutant phenotype, Maf1(8SA) accumulated in the nucleus in the absence of rapamycin (Fig. 4B). However, rapamycin still caused nuclear translocation of the 8SD mutation, consistent with a recent study with GFP-Maf1 in live cell.24 These experiments confirmed that Sch9-dependent phoshorylation is critical for controlling Maf1 cytoplasm-to-nucleus transport.
Figure 4.
Sch9-dependent phosphorylation of Maf1 affects cytoplamic localization but not glycerol-dependent cell growth. (A) FM391 maf1Δ cells expressing Maf1-Myc9 and its mutation forms were treated without and with rapamycin (200 nM) for 30 min. Maf1 phosphorylation was detected by WB. (B) FM391maf1Δ cells expressing Maf1, Maf1(8SA) and Maf1(8SD) were treated without and with 200 nM rapamycin for 60 min. Maf1 localization was detected by IF with anti-Myc antibody (A14). Nucleus was stained with DAPI. (C) FM391maf1Δ cells expressing Maf1, Maf1(8SA) and Maf1(8SD) were 10X serially diluted and spot on YPD and YPG plate and incubated at 37°C for 4 days.
Maf1 is a Pol III repressor and deletion of Maf1 results in overproduction of Pol III-dependent transcription, leading to a unbalanced and defective growth on glycerol media at 37°C.26,27 This glycerol-dependent growth has been used in the field as a convenient readout for Maf1 activity.17-19 In comparison to maf1Δ strain, cells expressing Maf1(8SA) or Maf1(8SD) did not exhibit discernible cell growth defect (Fig. 4C), indicating that Maf1 activity and Pol III-dependent transcription is not significantly changed by Sch9-dependent phosphorylation. These results are in agreement with a recent report that Sch9-dependent phosphorylation of Maf1 only affects Pol III-dependent transcription to a small extent.24
Sch9 does not affect rapamycin-sensitive cell growth
Cell growth is tightly coupled to ribosome biogenesis. Because Sch9 has been proposed to be a major mediator of TORC1 signaling in yeast,24 we asked to what extent Sch9 is involved in cell growth regulation by TORC1. To this end, we tested the rapamycin-sensitive cell growth in cells expressing the rapamycin-resistant and hyperactive Sch9(2D3E). Rapamycin sensitivity assay has been developed that accurately measures the role of other TORC1 signaling components.28 To our surprise, Sch9(2D3E) showed little rapamycin-resistant phenotype (Fig. 5A). In contrast, TOR1-RR that contains the rapamycin-resistant Ser1972→Ile mutation3 conferred strong rapamycin resistance (Fig. 5A). These observations indicate that Sch9 is dispensable in TORC1-dependent growth regulation in yeast.
Figure 5.
Rapamycin-resistant hyperactivation of Sch9 does not confer discernible rapamycin-resistance in cell growth. (A) W303a cells (Wt) expressing Tor1-RR (RR), Sch9 or Sch9(2D3E) were 10X serially diluted and spotted onto YPD plates without and with 10 nM rapamycin, and were incubated at 30°C for 4 days. (B) TORC1 regulates ribosomal RNA synthesis through both Sch9-dependent and -independent mechanisms. TORC1 directly regulates Maf1 basal phosphorylation in the nucleolus, which is critically required for Maf1 nucleoplasm-to-nucleolus transport and Pol III-dependent transcription. TORC1 also regulates the non-essential Maf1 cytoplasm-to-nucleus transport through Sch9-dependent phosphorylation of Maf1. TORC1 also controls Pol I through both Sch9-dependent and Sch9-indendent mechanisms but the Sch9 target(s) is not presently known. Modulation of Pol I and Pol III by Sch9 is dispensable for TORC1 to promote ribosome biogenesis and cell growth.
Discussion
Sch9 is a functional homolog of S6K1 in yeast that is known for mediating TORC1 signaling to regulate Pol II-dependent transcription of RP and Ribi regulon genes.21,22 Since Pols are co-regulated by TORC1, it is not surprising that Sch9 is also involved in Pol I and Pol III control, which is likely to produce a highly efficient mechanism to coordinate ribosome biogenesis. Our data are consistent with that Sch9 is a major kinase that mediates TORC1 signaling to Pol and Pol III because the hyperactive Sch9(2D3E) confers resistance to rapamycin in transcription by these RNA polymerases (Fig. 3). Sch9 facilitates TORC1 regulation of Pol III by phosphorylation of Maf1 (Fig. 1B–D), which controls Maf1 cytoplasm-to-nucleus transport (Fig. 2A). Currently, we do not known precisely how Sch9 is involved in Pol I regulation. Previous studies have indicated that Rrn3 is a major target of TORC1 regulation.29,30 Conceivably, Rrn3 is also a Sch9 substrate. In the absence of Sch9, however, there is still a basal level of ribosomal RNA biosynthesis (Fig. 3), indicating that TORC1 has retained the ability to regulate ribosome biogenesis independently of Sch9. Because TORC1 is a protein kinase that interacts with Maf1 and phosphorylates Maf1 in vitro,15 it appears that TORC1 directly regulates Maf1 and also through Sch9.
We and two other groups have collectively identified up to eight Sch9-dependent phosphorylation sites on Maf1.23,24 The combined mutations at these sites are sufficient to cause Maf1's entry into the nucleus, which has essentially the same phenotype as the sch9Δ mutation (Fig. 4B). However, Maf1 cytoplasmic localization is dispensable for regulating Maf1 activity.15,19,20 Instead, TORC1-dependent regulation of Maf1 nucleoplasm-to-nucleolus transport is the key event for Maf1 activation.15 Here we show that Sch9 is not involved in the control of Maf1 nucleolar localization (Fig. 2B), which is further supported by a recent report that Sch9 inactivation does not lead to significant interaction of Pol III with Maf1.24 We found that in absence of Sch9, Maf1 retains a basal phohsphorylation in a rapamycin-sensitive manner (Fig. 2C and D) and rRNAs and tRNAs transcription remain regulated by TORC1 (Fig. 3). These data is consistent with the model that TORC1 phosphorylates Maf1 directly in the nucleolus in a chromatin-dependent manner and controls Maf1's availability in the nucleolus, which is the key event in Maf1 regulation.15 In summary, we proposed a working model for TORC1 signaling to regulate Pol III-directed transcription. TORC1 phosphorylates Maf1 in the nucleolus, preventing its access to 5S rRNA and tRNA genes, which represents the predominant mechanism. As a complementary mechanism, TORC1 also regulates Maf1 nucleus-to-cytoplasm transport through Sch9. Thus, TORC1 uses both Sch9-dependent and -independent mechanisms to efficiently regulate ribosomal RNA synthesis.
Materials and Methods
Yeast strains and plasmids
Yeast strains used in this study are: W303a (MATa ura3-1 leu2-3,-112 his3-11, -15 trp1-1 ade2-1 can1-100); S288C/FM391 (MATa hisD1 leu2D0 met15D0 ura3D0); snf1, ypk1, mpk1, rim15 and yak1 were replaced by KanMx from S288C/FM391 strains; SZy1701 (W303a MAF1::MYC9-TRP); SZy2009 (S288C/FM391 maf1::KanMX); DBY746 (MATa leu 2-3,112 his3Δ1 trp1-289 ura3-52 GAL+) and SZy2062 (DBY746 sch9::URA3) are kind gifts from Dr Longo;31 SZy2067 (DBY746 bcy1::TRP). Plasmids pRS315-SCH9-HA3 and its kinase dead form (K441A) and hyperactive form (2D3E) containing SCH9 native promoter were derived from pRS416-HA3-SCH9 and pRS416-HA3-SCH9 (K441A), which are kind gifts from Dr. Morano;25 MAF1-MYC9 and its native promoter was inserted into both pRS313 and pRS416. MAF1 mutants were made by site-directed mutagenesis. GST-MAF1 and TOR1-RR were described in our earlier study.15
Cell extracts, western blot, immunoflorescence, metabolic labeling, in vitro kinase assay, and co-immunoprecipitation (co-IP)
Cell extracts, western blot, Immunoflorescence, metabolic labeling and in vitro kinase assay were performed as described before.15 For co-IP, W303a cells expressing Sch9-HA3 or Maf1-Myc9 or both were cultured to early log phase and lysed in IP buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.5 mM EDTA, 0.003% Triton X-100, 2 mM PMSF, Roche Complete protease inhibitor cocktail and phosSTOP tablet). 1 mg/0.5 mL total protein was incubated with either HA (12CA5) or MYC (9E10) antibody for 1 hour with gentle rotation at 4°C. Protein G beads were added into total protein and further incubated for 2 hours. Beads were then washed with IP buffer extensively by spin down and boiled in 2.5X SDS sample buffer.
Acknowledgements
We thank Dr. Morano (University of Texas-Houston Medical School) for kindly sharing the SCH9 plasmids, Dr. Longo (University of Southern California) for sch9Δ strains, and J.H. Cho and H.F. Duan for technical assistance. This work was supported by NIH grant R01-CA123391.
References
- 1.Keith CT, Schreiber SL. PIK-related kinases: DNA repair, recombination and cell cycle checkpoints. Science. 1995;270:50–1. doi: 10.1126/science.270.5233.50. [DOI] [PubMed] [Google Scholar]
- 2.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo J, Bonenfant D, et al. Two TOR Complexes, Only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–68. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 3.Zheng XF, Florentino D, Chen J, Crabtree GR, Schreiber SL. TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell. 1995;82:121–30. doi: 10.1016/0092-8674(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 4.Wullschleger S, Loewith R, Hall MN. TOR Signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Tsang C, Zheng X. TOR-in(g) the nucleus. Cell Cycle. 2007;6:25–9. doi: 10.4161/cc.6.1.3675. [DOI] [PubMed] [Google Scholar]
- 6.Warner JR. The economics of ribosome biosynthesis in yeast. Trends Biochem Sci. 1999;24:437–40. doi: 10.1016/s0968-0004(99)01460-7. [DOI] [PubMed] [Google Scholar]
- 7.Moss T, Stefanovsky VY. At the center of eukaryotic life. Cell. 2002;109:545–8. doi: 10.1016/s0092-8674(02)00761-4. [DOI] [PubMed] [Google Scholar]
- 8.White R. RNA polymerases I and III, growth control and cancer. Nat Rev Mol Cell Biol. 2005;6:69–78. doi: 10.1038/nrm1551. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DL, Johnson SAS. Cell Biology: RNA metabolism and oncogenesis. Science. 2008;320:461–2. doi: 10.1126/science.1158680. [DOI] [PubMed] [Google Scholar]
- 10.Zaragoza D, Ghavidel A, Heitman J, Schultz MC. Rapamycin induces the G0 program of transcriptional repression in yeast by interfering with the TOR signaling pathway. Mol Cell Biol. 1998;18:4463–70. doi: 10.1128/mcb.18.8.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers T, Walter P. Regulation of ribosmome biogenesis by the rapamycin-sensitive TOR-signaling pathway in Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:987–1000. doi: 10.1091/mbc.10.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Tsang C, Watkins M, Bertram P, Zheng X. Nutrient regulates Tor1 nuclear localization and association with rDNA promoter. Nature. 2006;442:1058–61. doi: 10.1038/nature05020. [DOI] [PubMed] [Google Scholar]
- 13.Drenan RM, Liu X, Bertram PG, Zheng XFS. FKBP12-Rapamycin-associated protein or mammalian target of rapamycin (FRAP/mTOR) localization in the Endoplasmic reticulum and the Golgi apparatus. J Biol Chem. 2004;279:772–8. doi: 10.1074/jbc.M305912200. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Shu L, Hosoi H, Murti KG, Houghton PJ. Predominant nuclear localization of mammalian target of rapamycin in normal and malignant cells in culture. J Biol Chem. 2002;277:28127–381. doi: 10.1074/jbc.M202625200. [DOI] [PubMed] [Google Scholar]
- 15.Wei Y, Tsang CK, Zheng XF. Mechanisms of regulation of RNA polymerase III-dependent transcription by TORC1. EMBO J. 2009;28:2220–30. doi: 10.1038/emboj.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Upadhya R, Lee J, Willis I. Maf1 is an essential mediator of diverse signals that repress RNA polymerase III transcription. Mol Cell. 2002;10:1489–94. doi: 10.1016/s1097-2765(02)00787-6. [DOI] [PubMed] [Google Scholar]
- 17.Roberts D, Wilson B, Huff J, Stewart A, Cairns B. Dephosphorylation and genome-wide association of Maf1 with Pol III-transcribed genes during repression. Mol Cell. 2006;22:633–44. doi: 10.1016/j.molcel.2006.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oficjalska-Pham D, Harismendy O, Smagowicz W, Gonzalez de Pered A, Boguta M, Sentenac A, et al. General repression of RNA polymerase III transcription is triggered by protein phosphatase type 2A-mediated dephosphorylation of Maf1. Mol Cell. 2006;22:623–32. doi: 10.1016/j.molcel.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Moir RD, Lee J, Haeusler RA, Desai N, Engelke DR, Willis IM. Protein kinase A regulates RNA polymerase III transcription through the nuclear localization of Maf1. Proc Natl Acad Sci USA. 2006;103:15044–9. doi: 10.1073/pnas.0607129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Towpik J, Graczyk D, Gajda A, Lefebvre O, Boguta M. Derepression of RNA polymerase III transcription by phosphorylation and nuclear export of its negative regulator, Maf1. J Biol Chem. 2008;283:17168–74. doi: 10.1074/jbc.M709157200. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen P, Rupes I, Sharom JR, Schneper L, Broach JR, Tyers M. A dynamic transcriptional network communicates growth potential to ribosome synthesis and critical cell size. Genes Dev. 2004;18:2491–505. doi: 10.1101/gad.1228804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, et al. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell. 2007;26:663–74. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Moir RD, Willis IM. Regulation of RNA polymerase III transcription involves SCH9-dependent and SCH9-independent branches of the target of rapamycin (TOR) pathway. J Biol Chem. 2009;284:12604–8. doi: 10.1074/jbc.C900020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber A, Bodenmiller B, Uotila A, Stahl M, Wanka S, Gerrits B, et al. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev. 2009;23:1929–43. doi: 10.1101/gad.532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morano KA, Thiele DJ. The Sch9 protein kinase regulates Hsp90 chaperone complex signal transduction activity in vivo. EMBO J. 1999;18:5953–62. doi: 10.1093/emboj/18.21.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pluta K, Lefebvre O, Martin NC, Smagowicz WJ, Stanford DR, Ellis SR, et al. Maf1p, a negative effector of RNA polymerase III in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:5031–40. doi: 10.1128/MCB.21.15.5031-5040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciesla M, Towpik J, Graczyk D, Oficjalska-Pham D, Harismendy O, Suleau A, et al. Maf1 is involved in coupling carbon metabolism to RNA Polymerase III transcription. Mol Cell Biol. 2007;27:7693–702. doi: 10.1128/MCB.01051-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan TF, Carvalho J, Riles L, Zheng XFS. A chemical genomics approach toward understanding the global functions of TOR. Proc Natl Acad Sci USA. 2000;97:13227–32. doi: 10.1073/pnas.240444197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer C, Zhao J, Yuan X, Grummt I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 2004;18:423–34. doi: 10.1101/gad.285504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claypool JA, French SL, Johzuka K, Eliason K, Vu L, Dodd JA, et al. Tor pathway regulates Rrn3p-dependent recruitment of Yeast RNA polymerase I to the promoter but does not participate in alteration of the number of active genes. Mol Biol Cell. 2004;15:946–56. doi: 10.1091/mbc.E03-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–90. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]