Abstract
Neuroglobin (Ngb) is a recently discovered globin that affords protection against hypoxic/ischemic-induced cell injury in brain. Hypoxic/ischemic injury is associated with accumulation of reactive oxygen species (ROS) and/or reactive nitrogen species (RNS). In previous studies, we found that Ngb has antioxidative properties, and protects PC-12 cells against hypoxia- and β-amyloid-induced cell death. To further delineate the potential role of Ngb in protection against cerebral ischemia–reperfusion injury in vivo, we developed a transgenic mouse line that overexpresses Ngb. Hippocampal ischemia–reperfusion injury was induced by a 10-minute bilateral occlusion of the common carotid arteries, and the animal brains were assessed 3 days later. CA1 neural injury was determined by cresyl violet staining. Lipid peroxidation was assessed using a malonyldialdehyde assay kit, whereas ROS/RNS accumulation was determined by Het staining in the CA1 hippocampal region. Hippocampal Ngb mRNA and protein expressions were assessed by reverse transcriptase-PCR and western blotting, respectively. Neuroglobin was successfully overexpressed in the hippocampus of Ngb transgenic mice. After ischemia–reperfusion, CA1 ROS/RNS production and lipid peroxidation were markedly decreased in Ngb transgenic mice compared with wild-type mice. Furthermore, CA1 neuronal injury was also markedly reduced. Thus, Ngb may confer protection against ischemia–reperfusion injury in the brain through its intrinsic antioxidant properties.
Keywords: global ischemia, hippocampus, neuroglobin, neuron, oxidative stress, transgenic mouse
Introduction
Neuroglobin (Ngb) is a recently discovered globin across the mammalian and nonmammalian kingdom, which shows a high affinity for oxygen (Burmester et al, 2000; Moens and Dewilde, 2000). Neuroglobin expression is preferentially if not exclusively restricted to neurons, is widely and heterotopically expressed in the central nervous system, and is more particularly abundant in the cerebral cortex, hippocampus, thalamus, hypothalamus, and cerebellum of the rodent brain (Geuens et al, 2003; Hundahl et al, 2008; Moens and Dewilde, 2000; Reuss et al, 2002; Wystub et al, 2003). Increasing evidence indicates that Ngb has a role in neuronal protection after hypoxic and ischemic insults (Greenberg et al, 2008; Khan et al, 2006; Liu et al, 2009; Sun et al, 2001, 2003; Wang et al, 2008). Administration of antisense oligodeoxynucleotides directed against Ngb led to more severe stroke in vivo (Sun et al, 2003). Furthermore, overexpression of Ngb in a transgenic mouse model seemed to reduce cerebral infarct size after middle cerebral artery occlusion (MCAO) (Khan et al, 2006; Wang et al, 2008). These findings support the notion that Ngb may protect neurons against hypoxic-ischemic insults. However, the mechanisms underlying such Ngb-mediated neuronal protection during hypoxic/ischemic stress remain largely unknown.
A number of protective mechanisms have been proposed for Ngb, and these include acting as an oxygen sensor and storage molecule (Schmidt et al, 2003; Trent et al, 2001), operating as a guanine nucleotide dissociation inhibitor (Wakasugi and Morishima, 2005; Watanabe and Wakasugi, 2008), interacting with Na+-K+-ATPase (Xu et al, 2003), and even possibly by improving mitochondrial function (Liu et al, 2009) and inhibiting apoptosis (Raychaudhuri et al, 2010). In addition to those putative roles, it has also been suggested that Ngb may act as a scavenger of toxic reactive species, such as nitrogen monoxide, peroxynitrite, and hydrogen peroxide (Herold et al, 2004). Considering the critical role of oxidative stress in cerebral ischemia–reperfusion injury, Ngb could serve as an intrinsic reactive oxygen species (ROS)/reactive nitrogen species (RNS) scavenger during the reperfusion phase, rather than serve in a less likely cellular oxygen-delivery function. Indeed, we have previously shown that Ngb overexpression protects neuronal cells from hydrogen peroxide- and β-amyloid-induced cell injury, and that such protection seems to be primarily mediated through Ngb antioxidant properties (Li et al, 2008a, 2008b). These results provide further support to the hypothesis that Ngb may have a protective role during ischemia–reperfusion injury through ROS/RNS scavenging.
It has now been well established that oxidative stress has an important role in the pathophysiology of stroke (Clemens, 2000) and that excessive production of both ROS and/or RNS occurs during ischemia–reperfusion of neural tissues (Sugawara and Chan, 2003). The increased production of ROS/RNS can lead to cellular damage and promote cell death because ROS and RNS will not only oxidize vital cellular components such as lipids, proteins, and DNA (Sugawara and Chan, 2003) but will also alter several signaling pathways that ultimately promote cellular damage and death during cerebral ischemia and reperfusion (Chan, 2001). For example, remarkable decreases in infarct volume were observed after permanent MCAO in superoxide dismutase (SOD)1-overexpressing transgenic mice (Chan et al, 1994), and conversely, SOD1-deficient mice show increased cell death and brain edema after transient MCAO and global cerebral ischemia (Kondo et al, 1997). Therefore, an intrinsically expressed and inducible molecule such as Ngb in neurons that shows antioxidant properties could afford protection against ischemia–reperfusion injury. Indeed, Ngb overexpression attenuated oxidative stress markers and infarct size in a mouse MCAO model (Wang et al, 2008).
On the basis of the aforementioned considerations, we hypothesized that overexpression of Ngb may prevent or at least markedly reduce ischemia–reperfusion-induced brain injury through attenuation of ROS/RNS generation.
Materials and methods
Generation of Neuroglobin Overexpression in Transgenic Mice
A complementary DNA (cDNA) encoded with human wild-type Ngb was synthesized using a modification of recursive PCR strategy. Neuroglobin cDNA was subcloned into an expression vector pcDNA3.1 (pcDNA3.1-Ngb). A BamH1-Xho1 fragment from pcDNA3.1-Ngb was further subcloned into pUB6 plasmid with the human ubiquitin C promoter. Neuroglobin transgenic mice were generated in the transgenic core facility at the University of Louisville. Several founder lines of Ngb transgenic mice were generated. All offspring mice were genotyped. F1 Ngb transgenic mice (FVB) were further backcrossed into the C57/B6 strain for at least 10 generations. Neuroglobin mRNA and protein expressions in the brain were assessed at the age of 2 months. All the handling for transgenic mice strictly followed institutional and National Institute of Health guidelines for the care and use of laboratory animals.
Induction of Global Ischemia–Reperfusion
Neuroglobin transgenic and wild-type mice (10 weeks of age) were anesthetized with halothane, intubated, and mechanically ventilated. As required, blood (0.5 to 0.6 mL) was withdrawn from an implanted venous catheter to reduce mean arterial pressure (MAP) to 30 to 35 mm Hg. The common carotid arteries on both sides were then temporarily occluded for 10 minutes after which carotid perfusion was reallowed, and any blood that was withdrawn was reinfused back to the animal through the venous catheter. Body temperature was carefully and tightly maintained at homeothermic levels during the ischemic and postischemic phases. Sham-operated mice served as controls. Mice were allowed to recover for either 1 day (Het staining) or 3 days (histology analysis) after the intervention. Neuronal oxidation induced by ischemia–reperfusion was determined by oxidized Het staining. Ischemic neuronal injury in the hippocampus was assessed by cresyl violet and hematoxylin and eosin staining.
Histologic Analysis of Neuronal Loss in the Hippocampus
Mice were killed by deep anesthesia. The brain was perfused with heparinized physiologic saline, followed by 4% paraformaldehyde. The brains were removed and postfixed in 4% paraformaldehyde for 2 hours and then embedded in paraffin. Sections were cut and stained with cresyl violet (for visualization) and hematoxylin and eosin (for cell count). Numbers of morphologically normal neurons and neurons showing the features of ischemic cell change (shrunken cell bodies, triangulated, pyknotic nuclei, and eosinophilic cytoplasm) were counted by an observer who was blinded to the experimental condition using a 100-mm2 grid at × 40 magnification. Neurons were counted in 10 different fields in each region at × 40 magnification using defined areas (equivalent to 0.00625 mm2) in the CA1 hippocampus. The percentage of ischemic neurons was calculated by adding together the number of ischemic and normal neurons counted in the defined area in the 10 different fields.
Measurement of Reactive Oxygen Species Production in the Hippocampus
Reactive oxygen species production in the hippocampal tissue in the postischemic phase oxidizes Het to ethidium, generating a fluorescence signal that can be visualized and quantified in hippocampus sections. Het (Molecular Probes, Carlsbad, CA, USA) 0.5 mg in 200 mL saline was administered by tail vein injection 23 hours after global ischemia. Mice were killed by deep anesthesia 1 hour later. The brain was perfused with heparinized physiologic saline, followed by 4% paraformaldehyde. The brains were removed and postfixed in 4% paraformaldehyde for 2 hours. Sections of 50 mm thickness were prepared, and Het fluorescence intensity was determined. To quantify levels of lipid oxidation, Het reactivity was visualized and the average fluorescence intensity was measured in the CA1 region of the hippocampus. At least six areas of 0.05 mm2 were quantified in the CA1 region of the hippocampus.
Immunohistochemistry
Neuroglobin trangenic mice were deeply anesthetized and perfused intracardially with 4% phosphate-buffered paraformaldehyde. Serial sections were cut on a microtome. The free floating sections were incubated with primary anti-Ngb (1:500 dilution, Biovendor, Candler, NC, USA). For double staining, brain sections were incubated with Ngb and neuronal nuclei (1:1,000) antibodies. Immunostained sections were further visualized with fluorescein isothiocyanate- or rhodamine-conjugated secondary antibody. Sections were assessed using a Nikon Ellipse E800 microscope (Nikon, Melville, NY, USA), and images were acquired using a SPOT digital camera (Belmont, CA, USA).
Assessment of Lipid Peroxidation and Nitrotyrosine Formation
Malonyldialdehyde (MDA), an index of lipid peroxidation, and nitrotyrosine formation were measured using commercial assay kits (OxisResearch, Portland, OR, USA). In brief, after experimental treatments, tissues were washed three times with phosphate-buffered saline and homogenized in 20 mmol/L phosphate buffer (pH 7.4) containing 0.5 mmol/L butylated hydroxytoluene to prevent sample oxidation. Lysates were centrifuged at 1,000 g for 10 minutes, and 200 μL aliquots of the supernatants were used according to the instructions of the manufacturer. A standard curve was used to determine the absolute concentration. Values were standardized to micrograms of protein for each sample.
Measurement of SOD, Glutathione Peroxidase, and Catalase Activity
Brain tissues were homogenized using a homogenizer in 10 volumes of a 50 mmol/L sodium phosphate buffer (pH 7.4) at 4°C. Homogenates were centrifuged at 15,000 g for 10 minutes, and the supernatant obtained was used for the following antioxidant enzyme measurements. The SOD, GPX, and catalase activity was determined spectrophotometrically using commercially available assay kits obtained from Cayman Chemicals (Ann Arbor, MI, USA). Sample protein content was measured by using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). Enzyme activities were then normalized to the corresponding protein concentration for each sample.
Quantitative Reverse Transcriptase-PCR
Total RNA was extracted from tissues of Ngb transgenic mice using the RNeasy kit (Qiagen, Valencia, CA, USA). Aliquots of total RNA (1 μg) were reverse transcribed to cDNA using random primers and Superscript II Reverse Transcriptase. cDNA equivalent to 100 ng of total RNA were subjected to real-time PCR analysis. Cycling conditions consisted of one cycle at 95°C for 10 minutes, and 40 three-segment cycles (95°C for 15 seconds, 59°C for 1 minute, and 72°C for 30 seconds).
Western Blotting
Brain tissues were homogenized by standard procedures. Homogenate proteins (50 μg) were heated for 10 minutes at 90°C, loaded onto 18% PAGE gels, then transferred electrophoretically onto nitrocellulose membranes. Membranes were incubated overnight at 4°C with the primary antibody (anti-Ngb, diluted 1:1,000, Biovendor). Neuroglobin protein bands were detected with secondary antibodies and visualized by enhanced chemiluminescence reagents. The same membranes were also blotted with β-actin antibody (Sigma, St Louis, MO, USA), and Ngb blots were then normalized to β-actin. Densitometric analysis was performed using a gel scanning densitometer (Molecular Dynamics, Sunnyvale, CA, USA). Data were expressed as fold increase of corresponding controls.
Data Analysis
All results are reported as mean±s.e.m. unless otherwise indicated. The Mann–Whitney U-test was used to compare neuronal damage between sham and ischemia–reperfusion-treated groups. Comparisons between the wild-type and Ngb overexpression groups in sham and ischemia–reperfusion conditions were made by using ANOVA (analysis of variance) procedures, followed by one-way multiple comparison post hoc tests. Statistical significance was assumed at P≤0.05.
Results
Neuroglobin is Overexpressed in Hippocampal Neurons in Neuroglobin Transgenic Mice
To assess whether the Ngb transgene was able to induce Ngb overexpression in the hippocampus, Ngb mRNA and protein expressions were examined in hippocampal tissues using real-time reverse transcriptase-PCR and western blotting, respectively, and subsequently confirmed by immunohistochemistry. Neuroglobin mRNA expression was significantly increased in the hippocampal region in Ngb transgenic mice (*P<0.01 versus wild type, Figure 1A), and protein expression was also markedly enhanced (*P<0.01 versus wild type, Figure 1B). Furthermore, increases in Ngb protein expression were further confirmed in the hippocampus, and colocalized with neuronal nuclei, a neuronal cell marker, suggesting that Ngb-expressing cells were primarily and almost exclusively neurons. Although the subcellular distribution of Ngb was not thoroughly assessed in this study, it seemed that overexpressed Ngb was mainly localized in the cytoplasm, a finding that is consistent with the distribution pattern previously observed in cortical neurons (Li et al, 2006) (Figure 1C).
Figure 1.
Ngb mRNA and protein expressions in the hippocampus of Ngb transgenic mice. The hippocampal tissues of Ngb transgenic mice or wild-type mice (male, 10 weeks old) were harvested and subjected to either Ngb mRNA and protein analyses or to immunohistochemistry. (A) Ngb mRNA expression in the hippocampus of Ngb transgenic and wild-type mice. Data were expressed as a percentage of wild type (n=8 per group, *P<0.01 versus wild type). (B) Ngb protein expression in the hippocampal tissue of Ngb transgenic and wild-type mice. Data are expressed as a percentage of wild type (n=8 per group). (C) Western blot images of Ngb and β-actin protein expressions in the hippocampal tissues of Ngb transgenic and wild-type mice. (D and E) Immunohistochemical staining of Ngb and NeuN in the hippocampus of Ngb transgenic and wild type mouse. NeuN, neuronal nuclei; NgB, neuroglobin.
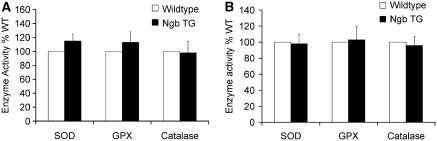
Neuroglobin Overexpression Does Not Alter SOD, GPX, and Catalase Activity in the Brain of Neuroglobin Transgenic Mice
To investigate potential effects of Ngb overexpression on the endogenous antioxidant system in the hippocampus, the hippocampal levels of enzymatic activity of SOD, GPX, and catalase were measured before (Figure 2A) or 1 day after surgery (Figure 2B). No significant changes in the activity of SOD, GPX, and catalase were found in Ngb-overexpressing mice compared with wild-type mice (*P>0.05 versus wild type, Figure 2).
Figure 2.
Effect of Ngb overexpression on the SOD, GPX, and catalase activity in the hippocampus. Hippocampal tissues from Ngb transgenic mice or wild-type mice (male, 10 weeks, n=8 in each group) were harvested (A) before or (B) 1 day after surgery and subjected to assessment of the enzymatic activities of SOD, GPX, and catalase. Data are expressed as the percentage of wild type (n=8 per group, P>0.05). NgB, neuroglobin; SOD, superoxide dismutase.
Neuroglobin Overexpression Decreases Ischemia–Reperfusion-Induced Lipid Peroxidation and Nitrotyrosine Formation
To determine the effects of Ngb overexpression on ischemia–reperfusion-induced lipid peroxidation and nitrotyrosine formation, mice were subjected to either ischemia–reperfusion or sham procedures. Ischemia–reperfusion was associated with marked increases in MDA and nitrotyrosine formation in wild-type mice (n=8, *P<0.01 versus sham, Figures 3A and 3B). However, ischemia–reperfusion-induced MDA formation was significantly attenuated in Ngb transgenic mice (n=8, *P<0.005 versus sham, #P<0.01 versus wild type, Figure 3A), and similar findings emerged for nitrotyrosine formation (n=8, *P<0.005 versus sham, #P<0.01 versus wild type, Figure 3B).
Figure 3.
Effect of Ngb overexpression on ischemia–reperfusion-induced lipid peroxidation and nitrotyrosine formation. After 10 minutes of ischemia and 3-day recovery, the hippocampal tissues were harvested in Ngb transgenic mice or wild-type mice (male, 10 weeks, n=8 in each group), and subjected to assessment of lipid peroxidation and nitrotyrosine formation. (A) Effect of Ngb overexpression on ischemia–reperfusion induced MDA formation. Data were expressed as a percentage of sham control (n=8 per group, *P<0.01 versus sham control, #P<0.01 versus wild type). (B) Effect of Ngb overexpression on ischemia–reperfusion induced nitrotyrosine formation. Data are expressed as a percentage of sham control (n=6 per group). MDA, malonyldialdehyde; NgB, neuroglobin.
Neuroglobin Overexpression Attenuates Ischemia–Reperfusion-Induced Reactive Oxygen Species Production and Protects Neurons from Ischemia–Reperfusion Injury
Overall ROS production in the hippocampus tissue was measured at day 1 of reperfusion after Het administration. A marked increase in Het staining occurred after the ischemia–reperfusion procedure in wild-type mice (*P<0.01 versus sham, Figure 4A), and was reduced in Ngb transgenic mice (n=9, #P<0.05 versus wild type, Figure 4A). To further examine whether Ngb overexpression could attenuate ischemia–reperfusion-induced neuronal injury and improve cell survival under ischemic insults, cresyl violet staining was conducted. In wild-type mice, evidence of increased cell injury was apparent after ischemia–reperfusion, and was attenuated in Ngb transgenic mice (Figure 4B). In addition, the staining pattern of Het matched that of cresyl violet staining in all experimental groups (Figure 4C), thereby suggesting that Ngb overexpression may protect neurons from ischemia–reperfusion-induced cell injury and reduce oxidative stress. To further quantitatively assess neuronal loss elicited by ischemia–reperfusion, total and damaged neuronal cell counts were performed in the CA1 region after 3 days from surgery. In the wild-type group, the number of damaged CA1 neurons was significantly increased after ischemia–reperfusion (n=9, *P<0.05 versus Ngb trangenic mice, Figure 4D). However, ischemia–reperfusion-induced neuronal loss was significantly reduced in Ngb transgenic mice (n=9, #P<0.05 versus wild mice, Figure 4D).
Figure 4.
Effect of Ngb overexpression on ischemia–reperfusion-induced ROS/RNS production and neuronal injury. After 10 minutes ischemia and 1- or 3-day recovery periods, Ngb transgenic mice or wild-type mice (male, 10 weeks old) were deeply anesthetized and perfused intracardially with 4% phosphate-buffered paraformaldehyde. Serial hippocampal sections were obtained and subjected to Het staining and to quantitative analysis of neuronal damage. (A) Het fluorescence in the hippocampus after ischemia–reperfusion. Data are expressed as a percentage of sham control (n=9 per group, *P<0.01 versus sham control, #P<0.01 versus wild type). (B) Cresyl violet staining of the hippocampus after ischemia–reperfusion. (C) Quantitative analysis of neuronal damage in the CA1 region of the hippocampus after ischemia–reperfusion. Data are expressed as the percentage of damaged neurons from the total number of neurons counted (n=9 per group, Mann–Whitney U-test). NgB, neuroglobin; RNS, reactive nitrogen species; ROS, reacticve oxygen species.
Discussion
Neuroglobin was initially discovered in the human and murine brains (Burmester et al, 2000) and further found to be expressed in almost all vertebrates within neuronal tissues, thereby gaining its name (Fuchs et al, 2004). However, Ngb is heterotopically found across different brain regions, suggesting functional roles that transcend its putative oxygen-carrying capacity (Geuens et al, 2003; Reuss et al, 2002; Wystub et al, 2003). Recent studies have suggested that Ngb has a role in neuronal protection in response to hypoxia and ischemia (Greenberg et al, 2008). Furthermore, Ngb overexpression protects neuronal cells from oxidant stress such as that induced by hydrogen peroxide or β-amyloid peptides (Li et al, 2008a, 2008b). Therefore, it is conceivable to hypothesize that Ngb's neuronal protection in vivo is also operational by similar mechanisms. To further explore the physiologic function in vivo, we generated a transgenic mouse line that overexpresses Ngb under a human ubiquitin C promoter; such Ngb would be theoretically expressed in both neuronal and nonneuronal tissues. In preliminary studies on these transgenic mice, we confirmed that Ngb is expressed not only in the brain but also in the heart and kidneys. For this study, both Ngb mRNA and protein expressions were increased in the hippocampus, and in the other brain regions (data not shown). We should emphasize that we initially generated several mouse lines but that only a line with a moderate level of Ngb protein overexpression (approximately threefold) was used in this study, because these levels resembled the magnitude of hypoxia-induced increases in Ngb expression in the hippocampus (Li et al, 2006). As can be readily seen in the current study, overexpressed Ngb was clearly associated with neurons. However, the subcellular distribution of the Ngb protein was not further examined because it does not appear to be altered by hypoxia (Li et al, 2006).
As endogenous antioxidative defense systems will scavenge ROS/RNS induced by cerebral ischemia–reperfusion, it was important to examine whether overexpression of Ngb altered the endogenous antioxidative defense system, i.e., SOD, GPX, and catalase activities (Chan, 2001; Sugawara and Chan, 2003). This is particularly important as we have shown that Ngb overexpression decreased the excess ROS/RNS induced by H2O2 treatment in PC-12 cells (Li et al, 2008a) and therefore, it is possible that Ngb scavenging of ROS/RNS may have occurred by recruitment of the endogenous antioxidative system in the brain. However, our findings do not support this assumption, as we found that although Ngb was overexpressed in hippocampal neurons, there were no differences in the enzymatic activities of the antioxidant enzymes among transgenic and wild-type mice before or after ischemic surgery. Thus, the ROS-scavenging capacity of Ngb seems to be independent of the endogenous ROS/RNS scavenging system, and allows for intrinsic scavenging properties in the neuronal tissue that appears to be beneficial for brain regions subjected to ischemia–reperfusion insults.
Indeed, considering that oxidative stress has a mechanistic role in the pathophysiology of many neurologic diseases including ischemic stroke (Clemens, 2000), the excessive production of ROS/RNS will lead to oxidation of vital cellular components, such as lipids, proteins, and DNA, and eventually, either directly or indirectly, lead to mitochondrial dysfunction, apoptosis, and cell death (Sugawara and Chan, 2003). In this study, we have shown that global ischemia and reperfusion not only elicited increased lipid peroxidation, as shown as increased MDA tissue levels but was also associated with increased nitrotyrosine formation. Furthermore, Ngb overexpression favorably attenuated ROS production induced by a 10-minute ischemia, followed by 3 days of reperfusion. This is in contrast with previous studies showing that the silencing of Ngb enhances the susceptibility to oxidative stress through a 14-3-3-gamma pathway (Ye et al, 2009). Thus, the putative scavenging of ROS/RNS by intrinsic Ngb may confer resistance against ischemia–reperfusion injury in the brain. However, we should also point out that although the detection of nitrotyrosine was assumed to specifically indicate the presence of peroxynitrite from the reaction of nitric oxide and superoxide anion radical, such that Ngb-associated reduction of ROS/RNS species would yield the observed attenuation of nitrotyrosine, the recruitment of myeloperoxidase activity and heme proteins following the ischemic period could also be involved (Baldus et al, 2002; Thomas et al, 2002; Rane et al, 2005). Furthermore, peroxynitrite scavenging by Ngb could also modify heme protein nitration, and thus alter the overall nitrotyrosine formation. Alternatively, the possibility that nitration of amino-acid residues within the heme binding pocket of Ngb alters the reaction kinetics with other ROS/RNS cannot be excluded.
The mounting evidence suggests that Ngb has a role in neuronal protection after hypoxic and ischemic insults. For example, Ngb is upregulated in the margins of ischemic stroke (Jin et al, 2010), and intracerebral administration of a Ngb expressing adenovirus construct will reduce infarct size and improved functional outcomes in an experimental stroke model (Sun et al, 2003). Neuroglobin transgenic mice display reduced cerebral infarct size after MCAO (Khan et al, 2006; Wang et al, 2008). However, the mechanisms underlying such Ngb-mediated neuronal protection during hypoxic/ischemic stress remain largely unknown. One recently proposed mechanism suggested that Ngb inhibits the intrinsic pathway of apoptosis and modulates the activation of procaspase 9 by interaction with cytochrome c (Raychaudhuri et al, 2010). In addition, Ngb was able to scavenge nitrogen monoxide, peroxynitrite, and hydrogen peroxide (Herold et al, 2004). More recently, hydrogen peroxide concentration was found to be inversely correlated with the level of Ngb protein expression in a hypoxia–reoxygenation cell model (Fordel et al, 2007b). Furthermore, the overexpression of Ngb was associated with improved cell survival after hydrogen peroxide (Fordel et al, 2006) or nitric oxide exposures (Jin et al, 2008). Thus, Ngb could be operating as a ROS scavenger, by reduction reactions leading to ferric Ngb conversion to ferrous Ngb by endogenous reducing enzyme systems (Nicolis et al, 2007; Trandafir et al, 2007). Additional evidence has emerged whereby the overexpression of Ngb was associated with modulation of hypoxic gene response in neurons (Yu et al, 2009), such that it is highly likely that multiple pathways may be involved in the beneficial effects of Ngb during hypoxia or hypoxia–reoxygenation conditions.
It is now quite well established that Ngb overexpression leads to significant reductions in infarct size in stroke models (Greenberg et al, 2008; Khan et al, 2006, 2007; Sun et al, 2003; Wang et al, 2008). Furthermore, in vitro studies have documented the antioxidant properties of Ngb (Fordel et al, 2006, 2007a, 2007b; Jin et al, 2008; Lardinois et al, 2008; Li et al, 2008a, 2008b; Liu et al, 2009; Nicolis et al, 2007; Petersen et al, 2008; Ye et al, 2009; Yu et al, 2009). However, no direct evidence has been obtained thus far to show that Ngb overexpression in vivo can attenuate ischemia–reperfusion-associated ROS production in the brain tissue, as reported herein. Therefore, this study provides more direct evidence linking Ngb to ROS scavenging, and that such properties have clear beneficial effects on the outcome of hypoxia–ischemia insults.
In summary, although the initial observations on Ngb pointed toward its intrinsic affinity for low-molecular-weight diatomic gases as seen with other globins, the relatively low level of Ngb expression in cerebral neurons casts doubts on Ngb function as a reservoir for oxygen, especially during periods of acute ischemia. The neuroprotective role of Ngb may reside in its ability to scavenge ROS/RNS species, but could also be accounted for Ngb being part of a signaling pathway that transmits the redox state of the cell, conferring protection against oxidative stress or inhibiting apoptosis. In this study, Ngb decreased ischemia–reperfusion-induced ROS/RNS overproduction and lipid peroxidation in vivo, thereby improving neuronal cell survival. Therefore, Ngb, by its intrinsic intracellular ROS/RNS scavenger properties, seems to have a protective role against ischemic cell injury. Further exploration of Ngb antioxidant function may provide opportunities for novel pharmacological interventions aiming at preventing or palliating cerebral ischemic injury.
Acknowledgments
We thank Kenneth R Brittian for his technical assistance in immunohistochemistry.
The authors declare no conflict of interest.
References
- Baldus S, Eiserich JP, Brennan ML, Jackson RM, Alexander CB, Freeman BA. Spatial mapping of pulmonary and vascular nitrotyrosine reveals the pivotal role of myeloperoxidase as a catalyst for tyrosine nitration in inflammatory diseases. Free Radic Biol Med. 2002;33:1010–1019. doi: 10.1016/s0891-5849(02)00993-0. [DOI] [PubMed] [Google Scholar]
- Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chan PH, Epstein CJ, Kinouchi H, Kamii H, Imaizumi S, Yang G, Chen SF, Gafni J, Carlson E. SOD-1 transgenic mice as a model for studies of neuroprotection in stroke and brain trauma. Ann NY Acad Sci. 1994;738:93–103. doi: 10.1111/j.1749-6632.1994.tb21794.x. [DOI] [PubMed] [Google Scholar]
- Clemens JA. Cerebral ischemia: gene activation, neuronal injury, and the protective role of antioxidants. Free Radic Biol Med. 2000;28:1526–1531. doi: 10.1016/s0891-5849(00)00258-6. [DOI] [PubMed] [Google Scholar]
- Fordel E, Thijs L, Martinet W, Lenjou M, Laufs T, Van Bockstaele D, Moens L, Dewilde S. Neuroglobin and cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death. Neurosci Lett. 2006;410:146–151. doi: 10.1016/j.neulet.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Fordel E, Thijs L, Martinet W, Schrijvers D, Moens L, Dewilde S. Anoxia or oxygen and glucose deprivation in SH-SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions. Gene. 2007a;398:114–122. doi: 10.1016/j.gene.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Fordel E, Thijs L, Moens L, Dewilde S. Neuroglobin and cytoglobin expression in mice. Evidence for a correlation with reactive oxygen species scavenging. FEBS J. 2007b;274:1312–1317. doi: 10.1111/j.1742-4658.2007.05679.x. [DOI] [PubMed] [Google Scholar]
- Fuchs C, Heib V, Kiger L, Haberkamp M, Roesner A, Schmidt M, Hamdane D, Marden MC, Hankeln T, Burmester T. Zebrafish reveals different and conserved features of vertebrate neuroglobin gene structure, expression pattern, and ligand binding. J Biol Chem. 2004;279:24116–24122. doi: 10.1074/jbc.M402011200. [DOI] [PubMed] [Google Scholar]
- Geuens E, Brouns I, Flamez D, Dewilde S, Timmermans JP, Moens L. A globin in the nucleus! J Biol Chem. 2003;278:30417–30420. doi: 10.1074/jbc.C300203200. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K, Khan AA. Neuroglobin: an endogenous neuroprotectant. Curr Opin Pharmacol. 2008;8:20–24. doi: 10.1016/j.coph.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S, Fago A, Weber RE, Dewilde S, Moens L. Reactivity studies of the Fe(III) and Fe(II)NO forms of human neuroglobin reveal a potential role against oxidative stress. J Biol Chem. 2004;279:22841–22847. doi: 10.1074/jbc.M313732200. [DOI] [PubMed] [Google Scholar]
- Hundahl CA, Allen GC, Nyengaard JR, Dewilde S, Carter BD, Kelsen J, Hay-Schmidt A. Neuroglobin in the rat brain: localization. Neuroendocrinology. 2008;88:173–182. doi: 10.1159/000129698. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Xie L, Khan AA, Greenberg DA. Neuroglobin protects against nitric oxide toxicity. Neurosci Lett. 2008;430:135–137. doi: 10.1016/j.neulet.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao Y, Mao X, Xie L, Greenberg DA. Neuroglobin expression in ischemic stroke. Stroke. 2010;41:557–559. doi: 10.1161/STROKEAHA.109.567149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Sun Y, Jin K, Mao XO, Chen S, Ellerby LM, Greenberg DA. A neuroglobin-overexpressing transgenic mouse. Gene. 2007;398:172–176. doi: 10.1016/j.gene.2007.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci USA. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardinois OM, Tomer KB, Mason RP, Deterding LJ. Identification of protein radicals formed in the human neuroglobin-H2O2 reaction using immuno-spin trapping and mass spectrometry. Biochemistry. 2008;47:10440–10448. doi: 10.1021/bi800771k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Lee SK, Pouranfar F, Brittian KR, Clair HB, Row BW, Wang Y, Gozal D. Hypoxia differentially regulates the expression of neuroglobin and cytoglobin in rat brain. Brain Res. 2006;1096:173–179. doi: 10.1016/j.brainres.2006.04.063. [DOI] [PubMed] [Google Scholar]
- Li RC, Morris MW, Lee SK, Pouranfar F, Wang Y, Gozal D. Neuroglobin protects PC12 cells against oxidative stress. Brain Res. 2008a;1190:159–166. doi: 10.1016/j.brainres.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Pouranfar F, Lee SK, Morris MW, Wang Y, Gozal D. Neuroglobin protects PC12 cells against beta-amyloid-induced cell injury. Neurobiol Aging. 2008b;29:1815–1822. doi: 10.1016/j.neurobiolaging.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu Z, Guo S, Lee SR, Xing C, Zhang C, Gao Y, Nicholls DG, Lo EH, Wang X. Effects of neuroglobin overexpression on mitochondrial function and oxidative stress following hypoxia/reoxygenation in cultured neurons. J Neurosci Res. 2009;87:164–170. doi: 10.1002/jnr.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens L, Dewilde S. Globins in the brain. Nature. 2000;407:461–462. doi: 10.1038/35035181. [DOI] [PubMed] [Google Scholar]
- Nicolis S, Monzani E, Ciaccio C, Ascenzi P, Moens L, Casella L. Does human neuroglobin act only as a scavenger? Reactivity and endogenous modification by nitrite and hydrogen peroxide. Biochem J. 2007;407:89–99. doi: 10.1042/BJ20070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MG, Dewilde S, Fago A. Reactions of ferrous neuroglobin and cytoglobin with nitrite under anaerobic conditions. J Inorg Biochem. 2008;102:1777–1782. doi: 10.1016/j.jinorgbio.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Rane MJ, Gozal D, Butt W, Gozal E, Pierce WM, Jr, Guo SZ, Wu R, Goldbart AD, Thongboonkerd V, McLeish KR, Klein JB. Gamma-amino butyric acid type B receptors stimulate neutrophil chemotaxis during ischemia-reperfusion. J Immunol. 2005;174:7242–7249. doi: 10.4049/jimmunol.174.11.7242. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri S, Skommer J, Henty K, Birch N, Brittain T. Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death. Apoptosis. 2010;15:401–411. doi: 10.1007/s10495-009-0436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss S, Saaler-Reinhardt S, Weich B, Wystub S, Reuss MH, Burmester T, Hankeln T. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience. 2002;115:645–656. doi: 10.1016/s0306-4522(02)00536-5. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Giessl A, Laufs T, Hankeln T, Wolfrum U, Burmester T. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem. 2003;278:1932–1935. doi: 10.1074/jbc.M209909200. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci USA. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci USA. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DD, Espey MG, Vitek MP, Miranda KM, Wink DA. Protein nitration is mediated by heme and free metals through Fenton-type chemistry: an alternative to the NO/O2- reaction. Proc Natl Acad Sci USA. 2002;99:12691–12696. doi: 10.1073/pnas.202312699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trandafir F, Hoogewijs D, Altieri F, Rivetti di Val Cervo P, Ramser K, Van Doorslaer S, Vanfleteren JR, Moens L, Dewilde S. Neuroglobin and cytoglobin as potential enzyme or substrate. Gene. 2007;398:103–113. doi: 10.1016/j.gene.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Trent JT, III, Watts RA, Hargrove MS. Human neuroglobin, a hexacoordinate hemoglobin that reversibly binds oxygen. J Biol Chem. 2001;276:30106–30110. doi: 10.1074/jbc.C100300200. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Morishima I. Identification of residues in human neuroglobin crucial for Guanine nucleotide dissociation inhibitor activity. Biochemistry. 2005;44:2943–2948. doi: 10.1021/bi0477539. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu J, Zhu H, Tejima E, Tsuji K, Murata Y, Atochin DN, Huang PL, Zhang C, Lo EH. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–1874. doi: 10.1161/STROKEAHA.107.506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Wakasugi K. Neuroprotective function of human neuroglobin is correlated with its guanine nucleotide dissociation inhibitor activity. Biochem Biophys Res Commun. 2008;369:695–700. doi: 10.1016/j.bbrc.2008.02.089. [DOI] [PubMed] [Google Scholar]
- Wystub S, Laufs T, Schmidt M, Burmester T, Maas U, Saaler-Reinhardt S, Hankeln T, Reuss S. Localization of neuroglobin protein in the mouse brain. Neurosci Lett. 2003;346:114–116. doi: 10.1016/s0304-3940(03)00563-9. [DOI] [PubMed] [Google Scholar]
- Xu WL, Wang CL, Liao ZY, Zhang YL, Yu LH, Meng FW, Wang XX, Meng FW, Yin ZY, Qian LJ, Zhang CG. [Identification of interaction and interaction domains between neuroglobin and Na(+), K(+)-ATPase beta2 subunit] Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35:823–828. [PubMed] [Google Scholar]
- Ye SQ, Zhou XY, Lai XJ, Zheng L, Chen XQ. Silencing neuroglobin enhances neuronal vulnerability to oxidative injury by down-regulating 14-3-3gamma. Acta Pharmacol Sin. 2009;30:913–918. doi: 10.1038/aps.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Liu J, Guo S, Xing C, Fan X, Ning M, Yuan JC, Lo EH, Wang X. Neuroglobin-overexpression alters hypoxic response gene expression in primary neuron culture following oxygen glucose deprivation. Neuroscience. 2009;162:396–403. doi: 10.1016/j.neuroscience.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]