Abstract
Alteplase is the only drug licensed for acute ischemic stroke, and in this formulation, the thrombolytic agent recombinant tissue plasminogen activator (rtPA) is stabilized in a solution of -arginine. Improved functional outcomes after alteplase administration have been shown in clinical trials, along with improved histological and behavioral measures in experimental models of embolic stroke. However, in animal models of mechanically induced ischemia, alteplase can exacerbate ischemic damage. We have systematically reviewed the literature of both rtPA and -arginine administration in mechanical focal ischemia. The rtPA worsens ischemic damage under certain conditions, whereas -arginine can have both beneficial and deleterious effects dependent on the time of administration. The interaction between rtPA and -arginine may be leading to the production of nitric oxide, which can cause direct neurotoxicity, altered cerebral blood flow, and disruption of the neurovascular unit. We suggest that alternative formulations of rtPA, in the absence of -arginine, would provide new insight into rtPA neurotoxicity, and have the potential to offer more efficacious thrombolytic therapy for ischemic stroke patients.
Keywords: cerebral ischemia, -arginine, neurotoxicity, stroke, tissue plasminogen activator
Introduction
Recombinant tissue plasminogen activator (rtPA) is a thrombolytic agent that converts plasminogen to plasmin, which breaks down fibrin (Korninger and Collen, 1981). The first experiments that indicated rtPA would be beneficial in acute stroke were performed in rabbit models of embolic stroke (Zivin et al, 1985). Infusion of rtPA following introduction of autologous clots into the cerebral circulation of rabbits results in a significant improvement in neurologic outcome at 24 hours. Angiographic evidence from early human trials shows that rtPA recanalizes occluded blood vessels following ischemic stroke (del Zoppo et al, 1992). Thrombolysis for acute ischemic stroke with alteplase, a preparation of rtPA, improves patient outcomes in selected patients up to 4.5 hours following the onset of symptoms (Bluhmki et al, 2009). Alteplase has become widely used in patients with acute ischemic stroke following large-scale studies, which showed improved outcome even outside of clinical trials (Hill and Buchan, 2005; Wahlgren et al, 2007). Currently, only one formulation of rtPA has been licensed for clinical use, which is alteplase (Activase, Genentech Inc. (South San Francisco, CA, USA); Actilyse, Boehringer Ingelheim International (Ingelheim, Germany); Activacin, Kyowa Hakko Kogyo Ltd. (Tokyo, Japan); GRTPA, Mitsubishi Tanabe Pharma Corporation (Osaka, Japan)). To allow solubility of this formulation, rtPA is dissolved in a solution of -arginine at a concentration of 3.5 g/100 mg of rtPA. Patients currently receive up to 90 mg of rtPA (0.9 mg/kg) with an equivalent dose of 3.15 g of -arginine (31.5 mg/kg) during thrombolytic therapy.
The functional recovery in patients following thrombolysis is dependent on recanalization and the return of oxygen and glucose to the ischemic brain. However, concerns have been raised about the potential neurotoxicity of rtPA in the context of cerebral ischemia independent of its thrombolytic effect. The rtPA neurotoxicity was first highlighted in models of stroke in mice (Wang et al, 1998). In these experiments, mice underwent a mechanical middle cerebral artery occlusion (MCAO), which removes the confounding effect of thrombolysis, and were shown to have greater volume of infarct following rtPA infusion compared with control animals. Some subsequent studies have failed to replicate this (Klein et al, 1999; Meng et al, 1999), but it seems likely that there is a detrimental effect seen with rtPA given that data from tPA knockout mice show a reduction in susceptibility to mechanical, focal cerebral ischemia (Wang et al, 1998), which is reversed in the presence of exogenous rtPA.
-arginine is a substrate for nitric oxide (NO) synthesis from one of the following three enzymes: neuronal NO synthase (nNOS), endothelial NO synthase (eNOS), and inducible NO synthase (iNOS). Nitric oxide can have either neurotoxic or neuroprotective properties following cerebral ischemia (Iadecola, 1997; Moro et al, 2004), and independent of rtPA, -arginine has been the subject of investigation into its neurotoxic and neuroprotective effects in the setting of mechanical cerebral ischemia (Willmot et al, 2005). Given the potential neurotoxicity of rtPA, the effect of -arginine in alteplase needs to be determined and whether it is contributing to the neurotoxicity observed with rtPA following mechanical cerebral ischemia.
We have systematically reviewed the evidence separately for both rtPA and -arginine in mechanical cerebral ischemia to assess neurotoxicity independent of the thrombolytic effects of rtPA. This is followed by a discussion of the possible interaction between rtPA and -arginine.
Systematic Review of Recombinant Tissue Plasminogen Activator and -Arginine in Mechanical Cerebral Ischemia
Search Strategy
MEDLINE (1966 to January 2010) and EMBASE (1988 to January 2010) databases were searched using Pubmed and Ovid, respectively, by two independent investigators (GWJH and BAS). The search terms for the rtPA studies included ‘tissue plasminogen activator,' ‘tPA,' ‘thrombolysis,' ‘stroke,' ‘cerebral ischemia,' and the medical subject heading (MeSH) expansions of these terms. The specific search terms for the -arginine studies were ‘-arginine,' ‘arginine,' ‘stroke,' ‘cerebral ischemia,' and the MeSH expansions of these terms. The reference lists of articles and reviews were also searched as were the personal reference lists of the authors. Studies were included if the experiments involved mechanical models of focal cerebral ischemia in animals, either transient or permanent models, and when rtPA intravenously or -arginine by any method was administered. Protocols that used thrombotic or embolic models of stroke, or that did not measure infarct volume were excluded. Additional information recorded for the rtPA studies was species and model used, dose of rtPA, the control with which rtPA was compared, duration of ischemia, timing of rtPA administration, and timing and method of infarct measurement. Features extracted from the -arginine studies were species and model used, timing and route of administration, and timing and method of infarct volume measurement. We performed subgroup analyses on the basis of these a priori factors to identify the cause of any heterogeneity in the results.
Data Extraction
Means and s.d. of infarct volumes from the studies identified were extracted from the text where possible or by use of a screen grab tool when they were represented in diagrammatic form. The Stroke Therapy Academic Industry Roundtable score was calculated for each study according to criteria described previously (Horn and Limburg, 2001). The data sets were compiled using Cochrane Review Manager 5.0 (Review Manager, 2008). The data were analyzed in a continuous, random effects model of the standard mean differences, and have been displayed using summary tables and forest plots.
Results of Recombinant Tissue Plasminogen Activator Systematic Review
The search yielded 852 articles within which 25 articles met the inclusion criteria. In all, 20 articles were included for further analysis, which provided 29 sets of data (see Table 1 for a summary of the characteristics). Of the remaining articles, three were excluded because the numbers of animals or the s.d. were not presented (Liu et al, 2004; Nagai et al, 1999; Yamashita et al, 2009) and two were excluded due to timing of administration of rtPA at 6 hours following ischemia, which is not representative of clinical use (Gautier et al, 2003; Thiyagarajan et al, 2008).
Table 1. Summary of the studies included in the systematic review investigating the effect of rtPA administration on mechanical focal cerebral ischemia.
| Study | Species | Model | Duration of ischemia (minutes) | Dose tPA (mg/kg) administration (minutes) | Timing of tPA (minutes) | STAIR score | Source of rtPA |
|---|---|---|---|---|---|---|---|
| Armstead et al (2006) | SD rats | Filament | 120 | 6 | 240 | 2 | Genentech |
| Armugam et al (2009) | SD rats | Filament | 60 | 10 | 90 | 2 | B-I |
| Burggraf et al (2007) | Wistar rats | Filament | 180 | 0.9, 9, 18 | 150 | 3 | B-I |
| Crome et al (2007) | C57BL/6 mice | Filament | Permanent | 10 | 90 | 3 | B-I |
| Kilic et al (2001) | C57BL/6j mice | Filament | 90 | 0.2, 1, 2, 10 | 90 | 3 | B-I |
| Kilic et al (2005a) | C57BL/6 mice | Filament | 90 | 10 | 90 | 3 | B-I |
| Kilic et al (2005b) | C57BL/6 mice | Filament | 90 | 10 | 90 | 2 | B-I |
| Kilic et al (2005c) | C57BL/6j mice | Filament | 90 | 10 | 90 | 2 | B-I |
| Klein et al (1999) | Wistar rats | Ligation | 120 | 10 | 120 | 4 | Genentech |
| Lu et al (2009) | SD rats | Filament | 300 | 1 | 300 | 4 | Genentech |
| Machado et al (2009) | Wistar rats | Filament | 180 | 10 | 180 | 3 | Genentech |
| Meng et al (1999) | SD rats | Filament | 120 | 10 | 120 | 3 | Genentech |
| Oka et al (2009) | SD rats | Filament | 60 | 10 | 60 | 5 | TMP Co |
| Tang et al (2009) | SD rats | Filament | 120 | 10 | 120 | 5 | B-I |
| Tsuji et al (2005) | SH rats | Filament | 120 | 10 | 120 | 2 | Genentech |
| Wang et al (1998) | SV129 and C57BL/6 mice | Filament | 120 | 0.9 | 120 | 3 | Genentech |
| Wiegler et al (2008) | ICR-CD1 mice | Filament | 30 | 0.9 | 30 | 4 | Calbiochem |
| Yagi et al (2009) | Wistar rats | Filament | 180 | 10 | 180 | 4 | TMP Co |
| Yang et al (2007) | SD rats | Ligation | 90 | 2.5, 5, 7.5, 10 | 90 | 3 | B-I |
| Zhang et al (2004b) | Wistar rats | Filament | 90 | 5 | 90 | 4 | Not specified |
B-I, Boehringer-Ingelheim; rtPA, recombinant tissue plasminogen activator; SD, Sprague Dawley; SH, spontaneously hypertensive; STAIR, Stroke Therapy Academic Industry Roundtable; TMP Co, Tanabe Mitsubishi Pharma Co.
In all studies, animals were killed at 24 hours after ischemia. Timings are reported relative to the onset of ischemia.
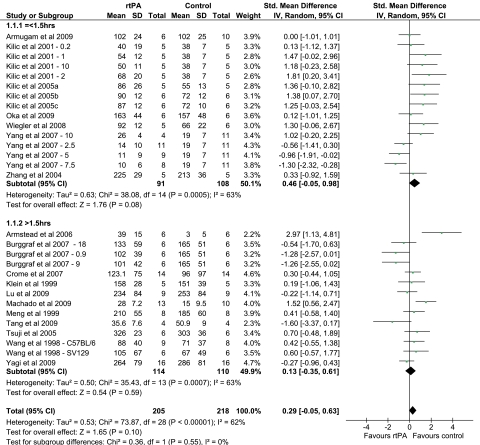
The majority of the experiments show that rtPA administration increases infarct volume following mechanical ischemia, and the combined analysis favors the control group in terms of infarct volume (Figures 1 and 2). However, there is heterogeneity within the results (I2=62%), partly explained by the varied experimental protocols that have been used (Table 1). The a priori subgroup analyses examined the effect of the model used (Supplementary Figure 1), species (Supplementary Figure 2), dose of rtPA administered (Supplementary Figure 3), duration of ischemia (Figure 1), and the control solution used for comparison (Supplementary Figure 4). In addition, we performed a post hoc analysis combining subgroups for model and species (Figure 2). All groups report using a preparation of rtPA solubilized in -arginine with the exception of Zhang et al (2004b). This article does not specify the source of the rtPA, although other work by the same group does report using a preparation of alteplase and this does contain -arginine (Yamashita et al, 2009).
Figure 1.
Forest plot summarizing the data for the effect of recombinant tissue plasminogen activator (rtPA) administration on infarct volume in models of mechanical focal cerebral ischemia. Subgroups are divided on the basis of duration of ischemia. Numbers after study title reflect the dose of rtPA (mg/kg). Numbers and letters after the study title reflect the strain of mice used. CI, confidence interval.
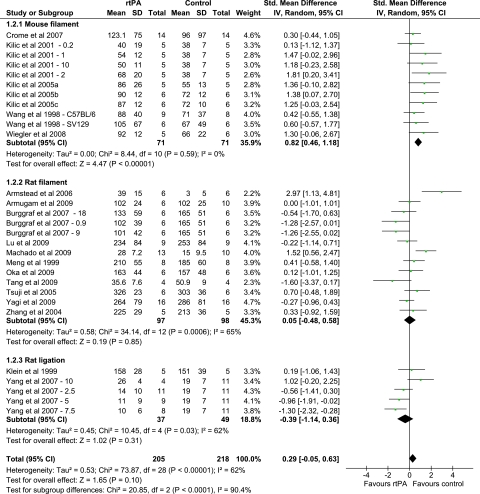
Figure 2.
Forest plot summarizing the data for the effect of recombinant tissue plasminogen activator (rtPA) administration on infarct volume in models of mechanical focal cerebral ischemia. Subgroups are divided on the basis of species and model of ischemia. Numbers after study title reflect the dose of rtPA (mg/kg). Numbers and letters after the study title reflect the strain of mice used. CI, confidence interval.
The models used for mechanical focal ischemia of the MCA were broadly divided into two groups: the intraluminal filament model, used by the majority of groups, and a method of external ligation. The filament model involves introduction of an intraluminal siliconized filament into the common carotid artery or external carotid artery, which is advanced until the origin of the MCA is occluded (Longa et al, 1989). For external ligation of the MCA, a craniotomy is performed followed by external occlusion of the MCA, using a microaneurysm clip (Buchan et al, 1992b; Klein et al, 1999) or a ligature (Yang et al, 2007), in combination with common carotid artery occlusion to reduce the effect of collateral circulation. Neither model explained the heterogeneity seen (Supplementary Figure 1).
The mouse model shows homogeneity showing an increase in infarct volume following rtPA administration (Supplementary Figure 2). Although this may be a phenomenon of the species, it was noted that all the mouse models use a filament model of ischemia so tend to have more homogeneous protocols.
The dose of rtPA used in the experiments also does not explain the differences between experimental groups (Supplementary Figure 3). We divided the doses of rtPA used into three subgroups: <1.1 mg/kg, 1.1 to 8.9 mg/kg, and >8.9 mg/kg. The dose of rtPA administered to patients for acute stroke treatment is 0.9 mg/kg and three of the articles use this dose (Table 1). The rationale for using higher doses of rtPA is that the equivalent thrombolytic effect in rats and mice compared with humans was initially observed at the higher dose of 10 mg/kg in vitro (Korninger and Collen, 1981). However, this need for higher doses of rtPA in rodents has recently been challenged following in vivo assessment (Haelewyn et al, 2010). The rationale for using the intermediate doses was unclear, but most likely it was to show a dose-dependent effect. There is no direct correlation between the dose of rtPA used and the effect on infarct volume (r=−0.097, P=0.61), suggesting the doses of rtPA alone cannot explain the heterogeneity of the effect of rtPA on infarct volume.
The duration of ischemia varied from 30 minutes to permanent, and this did not affect the outcome following rtPA administration (Figure 1). Combined analysis of those studies that use a duration of ischemia, which is ⩽1.5 hours, suggests that early administration of rtPA increases infarct volume. One explanation for this is that after a given period of ischemia, the infarct size may be close to maximal and therefore there is limited potential for rtPA to exacerbate the ischemic injury. Only in a model with a shorter duration of ischemia, where there is potentially a larger ischemic penumbra can the neurotoxic effects of rtPA be seen.
The subgroup analyzed according to the control solution used for comparison highlighted the scarcity of protocols using the -arginine carrier solution of rtPA as a control, with 24 of 29 sets of data opting for saline instead of the vehicle of rtPA (Supplementary Figure 4). It is therefore difficult to draw conclusions from the small number in the subgroup that use alternative controls such as the carrier solution for rtPA containing -arginine. Those studies using saline as a control have heterogeneity, and it is unclear whether any effects seen are due to rtPA or the -arginine in the carrier solution.
No individual subgroup analysis sufficiently explains the heterogeneity seen, which suggests that a combination of factors is responsible. A combined analysis of both species and model showed the homogeneous effect of rtPA in the mouse filament model in increasing the infarct volume, but in the rat models, no such consistency is found (Figure 2).
Results of -Arginine Systematic Review
The search yielded 868 articles within which 12 articles met the inclusion criteria. In all, 10 articles were included in further analysis, which provided 16 sets of data (see Table 2 for a summary of the characteristics). Of the remaining articles, two were excluded due to using a model involving significant hemorrhage (Chiou and Hong, 1997; Hong and Hwang, 2000).
Table 2. Summary of the studies included in the systematic review investigating the effect of -arginine administration on mechanical focal cerebral ischemia.
| Study | Species | Model | Duration of ischemia (minutes) | Dose -arginine (mg/kg) | Timing of -arginine | Route | Timing of death (hours) | STAIR score |
|---|---|---|---|---|---|---|---|---|
| Buisson et al (1993) | SD rats | Cautery | Permanent | 300 | +5 minutes to +3 hours | IP | 48 | 2 |
| Escott et al (1998) | SD rats | Filament | 120 | 300 | +5 minutes | IP | 24 | 4 |
| He et al (1995) | SH rats | Ligation | Permanent | 300 IP then 200 IV | −20 minutes to +1 hour | IP and IV | 24 | 2 |
| Iadecola et al (1995a, 1995b) | SH rats | Cautery | Permanent | 300 | +24 hours to +96 hours | IP | 96 | 3 |
| Morikawa et al (1992a, 1992b) | SH rats | Ligation and cautery | Permanent | 300 | −16 hours to +2 hours | IP | 24 | 2 |
| Morikawa et al (1994) | SD and SH rats | Cautery | Permanent | 300 | +5 minutes to 1 hour | IV and IP | 24 | 2 |
| Temiz et al (2003) | NZ rabbits | Ligation | Permanent | 2.5, 7.5, 12.5 | 0 hour | IV | 6 | 5 |
| Zhang et al (1996a) | SD rats | Filament | 120 | 300 | +24 hours to +96 hours | IP | 96 | 3 |
| Zhang et al (2004a) | SD rats | Filament | 60, 180, 360 | 500 | + 2 hours or +3 hours | IP | Not stated | 3 |
| Zhao et al (2003) | C57BL/6 mice | Cautery | Permanent | 300 | +12 hours to +96 hours | IP | 96 | 2 |
IP, intraperitoneal; IV, intravenous; SD, Sprague Dawley; SH, spontaneously hypertensive; STAIR, Stroke Therapy Academic Industry Roundtable; NZ, New Zealand.
Timings are reported relative to the onset of ischemia.
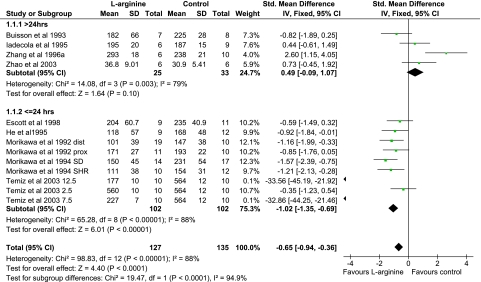
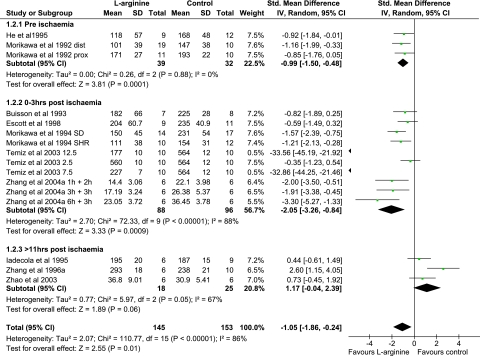
Overall, the effect of -arginine seems to reduce infarct volume following mechanical ischemia (Figures 3 and 4). However, there is significant heterogeneity (I2=88%) of the results. The following a priori subgroup analyses were performed: duration of ischemia (Supplementary Figure 5), species (Supplementary Figure 6), model (Supplementary Figure 7), timing of histological examination (Figure 3), and timing of -arginine administration (Figure 4).
Figure 3.
Forest plot summarizing the data for the effect of -arginine administration on infarct volume in models of mechanical focal cerebral ischemia. Subgroups are divided on the basis of timing of histological evaluation relative to the onset of ischemia. Single numbers after study title reflect the dose of -arginine (mg/kg), and in the Zhang et al (2004a) experiments, the first number is the duration of ischemia and the second number is the timing of administration of -arginine. Letters after the study title reflect the strain of rats or model used. CI, confidence interval; dist, distal middle cerebral artery occlusion; prox, proximal middle cerebral artery occlusion; SD, Sprague Dawley; SHR, spontaneously hypertensive rat.
Figure 4.
Forest plot summarizing the data for the effect of -arginine administration on infarct volume in models of mechanical focal cerebral ischemia. Subgroups are divided on the basis of timing of administration of -arginine relative to the onset of ischemia. Single numbers after study title reflect the dose of -arginine (mg/kg), and in the Zhang et al (2004a) experiments, the first number is the duration of ischemia and the second number is the timing of administration of -arginine. Letters after the study title reflect the strain of rats or model used. CI, confidence interval; dist, distal middle cerebral artery occlusion; prox, proximal middle cerebral artery occlusion; SD, Sprague Dawley; SHR, spontaneously hypertensive rat.
In contrast to the rtPA experiments, 11 of 16 -arginine sets of data use permanent ischemia models. Only three articles use transient ischemia (1, 2, or 6 hours) (Escott et al, 1998; Zhang et al, 1996a, 2004a), and although they show a beneficial effect of -arginine, there is significant heterogeneity (Supplementary Figure 5). The results using permanent models also suggest a significant reduction in infarct volume following -arginine administration, but there is still heterogeneity.
Within the species subgroups, one study uses a mouse model, one uses a rabbit model, and the remainder use rat models (Table 2). This means that species alone cannot account for the variable effects of -arginine on infarct volume (Supplementary Figure 6). The models of ischemia used include the intraluminal filament model and cautery or ligation of the MCA with or without CCA occlusion. When subgroups were created on this basis, no clear homogeneity emerged (Supplementary Figure 7).
A pattern became apparent when the data were examined by timing of histological examination after kill (Figure 3) and -arginine administration (Figure 4). Animals that were killed at or before 24 hours following ischemia had a reduction in infarct volume. Those that were killed after 24 hours following ischemia show a much more heterogeneous response to -arginine (Figure 3). This tendency toward a beneficial effect of -arginine when death is at 24 hours following ischemia is most likely explained by the timing of -arginine administration, which is earlier in these animals (Figure 4).
Early administration of -arginine is beneficial following ischemia (Figure 4). In this situation, -arginine is thought to be acting as a substrate for eNOS, which produces NO and causes vasodilatation to augment blood flow during ischemia and thus reduce infarct volume (Huang et al, 1996; Iadecola, 1997). In contrast, delayed administration of -arginine (>11 hours following ischemia onset) seems to exacerbate ischemic damage (Figure 4). In this instance, -arginine may be producing NO, which reacts with free radical species to form cytotoxins such as peroxynitrite. Inhibition of either nNOS with ARL 17,477 or iNOS with aminoguanidine protects from ischemic injury (Iadecola et al, 1995b; Zhang et al, 1996b). Furthermore, experiments examining the temporal protective effect of aminoguanidine show neuroprotection only from 24 hours following ischemia (Zhang and Iadecola, 1998) and correlates with the upregulation of iNOS expression in the brain following ischemia (Iadecola et al, 1995a).
In the studies identified, no experiment used the dose of -arginine clinically administered in alteplase (31.5 mg/kg). In some experimental models of rtPA administration, a 10-fold higher dose of alteplase is used, because this dose of rtPA has been shown to thrombolyze rodent clots (Korninger and Collen, 1981). This 10-fold higher dose of alteplase contains an equivalent dose of 315 mg/kg -arginine, which is similar to the dose of 300 mg/kg used by the majority of -arginine studies (Table 2). Temiz et al use much lower doses of -arginine ranging from 2.5 to 12.5 mg/kg (Table 2). This group show markedly beneficial effects of 7.5 and 12.5 mg/kg -arginine following ischemia, but this has not been reproduced in other experiments.
Summary
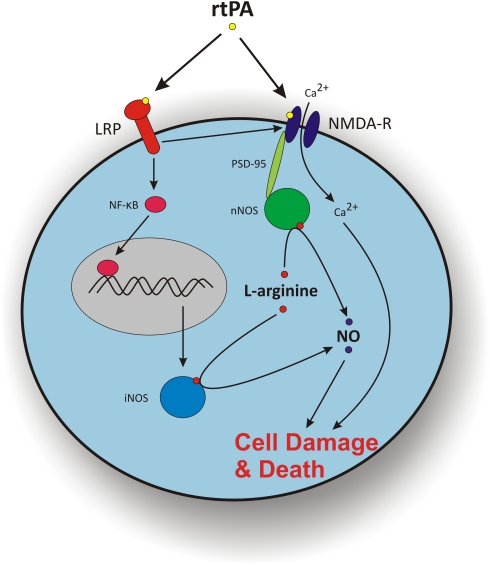
In certain circumstances, rtPA seems to worsen ischemic injury; however, the conditions required for this effect are not clear. -arginine has a neuroprotective role when administered early following ischemia, but delayed administration worsens ischemic damage. It might seem that because in clinical practice, rtPA is always administered within 4.5 hours of ischemia, the -arginine in alteplase would be beneficial. However, some mechanisms by which rtPA acts in combination with -arginine may potentiate neurotoxicity during ischemia. To ascertain whether rtPA neurotoxicity is mediated by NO synthesis, one needs to examine the direct targets of rtPA (Figure 5).
Figure 5.
Potential mechanisms by which recombinant tissue plasminogen activator (rtPA) confers neurotoxicity during ischemia and its interaction with -arginine via nNOS and iNOS. The rtPA cleaves the NR1 subunit of the NMDA receptor increasing the flux of Ca2+ into the cell. This increase in intracellular Ca2+ concentration can lead to cell damage and death. The NMDA receptors, through the scaffolding protein PSD-95, can interact with nNOS, metabolizing -arginine to NO, which can also lead to cell damage and death. The rtPA can also activate LRP, which can facilitate the action of rtPA on NMDA receptors previously described. The LRP can also activate the transcription factor NF-κB, which upregulates the expression of iNOS. The iNOS metabolizes -arginine to NO leading to cell damage and death. iNOS, inducible nitric oxide synthase; LRP, low-density lipoprotein receptor-related protein; NF-κB, nuclear factor κ light-chain enhancer of activated B cells; NMDA-R, N-methyl--aspartate receptor; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; PSD-95, postsynaptic density-95.
Mechanisms of Recombinant Tissue Plasminogen Activator Neurotoxicity in the Presence of -Arginine
The mechanisms of rtPA neurotoxicity have been extensively reviewed elsewhere (Kaur et al, 2004; Yepes et al, 2009). The following discussion only refers to mechanisms of rtPA neurotoxicity that may be interacting with the -arginine component of alteplase.
In order for rtPA to cause neurotoxicity it needs to reach the brain parenchyma. During both ischemic and nonischemic conditions, rtPA has been shown to cross the blood–brain barrier (Benchenane et al, 2005; Harada et al, 2005). Once in the parenchyma, the mechanisms by which rtPA causes direct neurotoxicity may include pathways acting via N-methyl--aspartic acid (NMDA) receptors, low-density lipoprotein receptor-related protein (LRP), and matrix metalloproteinases (MMP). The relative contributions of these pathways have yet to be established.
N-Methyl--Aspartic Acid Receptor-Mediated Excitotoxicity
During ischemia, widespread neuronal depolarization releases glutamate, which activates α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate and NMDA receptors. Prolonged activation of the NMDA receptor results in an influx of calcium and consequent cell death (Figure 5). Indeed, inhibition of the NMDA receptor with MK-801 reduces infarct volume following ischemia (Buchan et al, 1992a), and importantly it also reduces rtPA-related ischemic neurotoxicity (Kilic et al, 2005a). The NMDA-mediated excitotoxic injury is exacerbated by exogenous rtPA (Nicole et al, 2001), which is thought to be due to cleavage of the Kringle 2 region of the NR1 subunit of the NMDA receptor (Lopez-Atalaya et al, 2008). The tPA knockout mice are protected from excitotoxicity (Yepes et al, 2002) as well as ischemic damage (Wang et al, 1998) lending weight to the mechanism of rtPA neurotoxicity in ischemia acting via the NMDA receptor.
The NMDA receptor is known to influence the production of NO by modulating the activity of nNOS, and for this endogenous tPA is required (Park et al, 2008). The nNOS activity requires -arginine but also calmodulin, which is dependent on calcium (Bredt and Snyder, 1990). However, the tPA-mediated NO release following NMDA administration is not thought to rely on an intracellular rise in calcium, but rather phosphorylation of nNOS (Park et al, 2008). The NMDA receptor and nNOS activity have also been linked by the scaffolding protein postsynaptic density-95 (Brenman et al, 1996) and interruption of the association between the NMDA receptor and postsynaptic density-95 removes any effects of tPA on NO production (Sattler et al, 1999).
Nitric oxide is directly implicated in mediating excitotoxicity (Dawson et al, 1991) and is also integral to tPA-dependent excitotoxicity (Parathath et al, 2006). Excitotoxicity induced by kainic acid treatment increases NOS activity, which is reduced in both tPA knockout mice and following MK-801 administration in wild-type mice. The tPA knockout mice have increased neuronal survival following excitotoxic insult; however, that increase in survival was removed following exogenous rtPA administration. Application of a NO scavenging compound or a NOS inhibitor protected from the damaging effect of rtPA in these animals (Parathath et al, 2006). This links tPA-mediated excitotoxicity via the NMDA receptor with NOS activity directly.
Low-Density Lipoprotein Receptor-Related Protein-Mediated Pathway
The LRP is a cell surface receptor expressed in many types of brain cells and is implicated in a number of physiological and pathophysiological neuronal functions. Pertinent to this discussion, LRP activation upregulates nuclear factor-κB (Zhang et al, 2007), which is a transcription factor for iNOS (Xie et al, 1994) and the LRP pathway may mediate the action of tPA on the NMDA receptor in neurons directly. Ischemia causes an increase in the level of LRP expression following MCAO, but tPA knockout mice do not show this rise (Zhang et al, 2009), indicating that tPA is responsible for the increase in LRP expression. This effect seems to be mediated by microglial activation, which then upregulates iNOS activity. In neuronal culture, calcium influx attributable to the effect of rtPA is reduced via inhibition of LRP with receptor-associated protein (Martin et al, 2008). This suggests that the neurotoxic effects of rtPA via LRP and NMDA receptors are intrinsically linked and one of the key downstream mediators is iNOS (Figure 5).
In addition to these effects, LRP is also thought to mediate rtPA activation of MMP-9 (Wang et al, 2003). Upregulation of MMPs is associated with increased vascular permeability following degradation of the extracellular matrix (Aoki et al, 2002) and in neuronal cultures exogenous rtPA upregulates both MMP-2 and MMP-9 (Lee et al, 2007). The integrity of the blood–brain barrier is affected by the administration of rtPA (Yepes et al, 2003), and this leads to parenchymal edema and hemorrhage in excess of the risk from thrombolysis (Yepes et al, 2009). This is thought to contribute to symptomatic intracranial hemorrhage, which occurs in about 6% of patients treated with alteplase (NINDS, 1995). When alteplase is administered in combination with a MMP-9 inhibitor in a rat embolic stroke model, no reduction in infarct volume is observed, but hemorrhagic transformation is decreased (Sumii and Lo, 2002). This suggests that rtPA-induced MMP upregulation contributes more to edema and hemorrhage than to direct neurotoxicity. We hypothesize that coadministration of -arginine with rtPA may worsen this by increasing blood flow as a consequence of the NO produced from eNOS.
Nitric Oxide Synthase Isoforms and Cerebral Ischemia
Each of the NOS isoforms produces different effects on the ischemic brain and has different temporal profiles of upregulation following ischemia, as has been reviewed elsewhere (Iadecola, 1997; Moro et al, 2004; Samdani et al, 1997). Although the effects of eNOS protect the ischemic brain, the effects of iNOS or nNOS are detrimental. The effect of alteplase on NOS isoform expression varies. The eNOS expression is diminished after alteplase administration following ischemia, which corresponds to an increase in infarct volume (Kilic et al, 2005b). The nNOS levels are not increased by alteplase administration (Kilic et al, 2005a), but cerebral ischemia does increase iNOS expression, which is further augmented by administration of alteplase (Kilic et al, 2005a,). This iNOS upregulation by alteplase correlates with an increase in the volume of infarction supporting the hypothesis that iNOS activity is mediating rtPA neurotoxicity following ischemia.
An alternative explanation is that the change of NOS expression levels by alteplase is due to the -arginine administered in combination with the rtPA. Until rtPA can be administered in the absence of -arginine, their independent roles are difficult to distinguish. From the results of the meta-analysis it seems that -arginine administered early following ischemia reduces infarct volume (Figure 4), but given the effect of rtPA on the relative levels of the NOS isoforms described above, the benefit of -arginine following ischemia may not be observed in the presence of rtPA.
Effects of Recombinant Tissue Plasminogen Activator on Cerebral Blood Flow in the Presence of -Arginine
Aside from its thrombolytic properties, it is not understood how rtPA interacts with the signaling pathways that control cerebral blood flow (CBF). The effect of rtPA on the vasculature following ischemia may involve interaction with the NO pathway. Nitric oxide is one of many mediators by which neurovascular coupling is controlled (Dirnagl et al, 1994; Harder et al, 1998), but the precise role NO plays has yet to be fully characterized. Endogenous tPA is integral to the function of the neurovascular unit by controlling the NMDA receptor-mediated NO production through phosphorylation of nNOS (Park et al, 2008). Following a stroke, the properties of the neurovascular unit alter (del Zoppo, 2009) and autoregulation of CBF is disturbed. An increased production of NO may contribute to this and alteplase administration may further disrupt this process.
The rtPA administration leads to dysfunctional vascular tone and abnormal reactivity to vasoactive mediators, which results in edema and hemorrhagic transformation (Cipolla et al, 2000; Nassar et al, 2004). During reperfusion after 90 minutes of mechanical MCAO in the rat, rtPA treatment leads to an initial hyperperfusion when compared with ischemia alone (Kilic et al, 2001). However, this is followed by a selective hypoperfusion of the ischemic penumbra contributing to an increased infarct volume (Kilic et al, 2001), suggesting that alteplase is worsening the dysfunctional regulation of blood flow. This may be explained by the downregulation of eNOS by alteplase administration following ischemia (Kilic et al, 2005b). In pig models, rtPA induced pial small artery and arteriole dilatation, which was blocked by the NOS inhibitor -NG-nitroarginine, suggesting that NO contributes to the vascular responses of rtPA (Armstead et al, 2004). Initially, this response was thought to be due to the -arginine present in the rtPA formulation, but when -arginine was administered alone, no such arterial dilatation was observed. Following ischemia, it is still not known how the presence of -arginine contributes to the vascular effects of rtPA.
In the absence of rtPA, -arginine has independent effects on the CBF following ischemia. In healthy human volunteers, -arginine increases CBF, although this response is diminished in patients with a history of lacunar infarct (Pretnar-Oblak et al, 2006). In animal models of permanent cerebral ischemia, -arginine increases cortical CBF compared with saline in a dose-dependent manner (Morikawa et al, 1992b; Willmot et al, 2005), probably because of the vasodilator properties of NO from eNOS modulating cyclic guanosine monophosphate production. It has been suggested that -arginine produces hemodynamic changes in the cortex, but not the striatum (Caramia et al, 1998), but these differing responses have not been reproduced following ischemia (He et al, 1995). However, there is some evidence that the ischemic vessels preferentially dilate in response to -arginine administration compared with those vessels in nonischemic tissue (He et al, 1995).
Conclusions
Clinical trials have shown benefit from alteplase for selected patients with acute ischemic stroke. Early administration of -arginine alone seems to be beneficial in experimental cerebral ischemia and coadministration with rtPA may contribute to the improvement seen after alteplase treatment. Alteplase treatment independent of thrombolysis can exacerbate ischemic injury. This may be due to an interaction with -arginine, which might be blunting the effect seen in clinical trials. Mechanistically, it seems that rtPA neurotoxicity is related to NO production through iNOS and nNOS activity. Given that the only current formulation of rtPA contains high levels of -arginine, this carrier may be fuelling rtPA neurotoxicity. Whatever the combined effect is, it is important to dissociate the individual effects of -arginine and rtPA, which has not been performed to date.
We suggest that alternative carriers of rtPA are identified to more accurately assess the effects of rtPA administration following ischemia. -arginine has a biologically inert enantiomer, -arginine, which could potentially be used as a carrier of rtPA. -arginine is not a substrate for NOS (Palmer et al, 1988), and it has no effect on infarct volume following cerebral ischemia (Zhang et al, 1996a). However, -arginine may possess pharmacological effects such as central nervous system stimulant properties (Navarro et al, 2005), and therefore may not be an appropriate vehicle to use for stabilizing rtPA. Other amino acids that could be used to solubilize rtPA include lysine, ɛ-amino caproic acid, and glycylglycine (Cleary et al, 1989). Experiments with alternative carriers of rtPA will resolve uncertainty around the confounding effect of -arginine in alteplase. Using this information, novel preparations of rtPA could be formulated and trialed in experimental models of both mechanical and embolic stroke. It is not known whether these alternative carriers could provide a more efficacious and less neurotoxic formulation of rtPA than with -arginine translating into improved functional outcomes for patients after acute stroke.
Acknowledgments
The authors acknowledge the support of the Oxford University Clinical Academic Graduate School (GWJH), Fondation Leducq (BAS and AMB), Medical Research Council UK (AMB), and the National Institute for Health Research Biomedical Research Centre (JK and AMB).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Cines DB, Al-Roof Higazi A. Altered NO function contributes to impairment of uPA and tPA cerebrovasodilation after brain injury. J Neurotrauma. 2004;21:1204–1211. doi: 10.1089/neu.2004.21.1204. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Nassar T, Akkawi S, Smith DH, Chen XH, Cines DB, Higazi AA. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nat Neurosci. 2006;9:1150–1155. doi: 10.1038/nn1757. [DOI] [PubMed] [Google Scholar]
- Armugam A, Cher CD, Lim K, Koh DC, Howells DW, Jeyaseelan K. A secretory phospholipase A2-mediated neuroprotection and anti-apoptosis. BMC Neurosci. 2009;10:120. doi: 10.1186/1471-2202-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Berezowski V, Ali C, Fernandez-Monreal M, Lopez-Atalaya JP, Brillault J, Chuquet J, Nouvelot A, MacKenzie ET, Bu G, Cecchelli R, Touzani O, Vivien D. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation. 2005;111:2241–2249. doi: 10.1161/01.CIR.0000163542.48611.A2. [DOI] [PubMed] [Google Scholar]
- Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, Wardlaw J, Hacke W. Stroke treatment with alteplase given 3.0-4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8:1095–1102. doi: 10.1016/S1474-4422(09)70264-9. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Slivka A, Xue D. The effect of the NMDA receptor antagonist MK-801 on cerebral blood flow and infarct volume in experimental focal stroke. Brain Res. 1992a;574:171–177. doi: 10.1016/0006-8993(92)90814-p. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Xue D, Slivka A. A new model of temporary focal neocortical ischemia in the rat. Stroke. 1992b;23:273–279. doi: 10.1161/01.str.23.2.273. [DOI] [PubMed] [Google Scholar]
- Buisson A, Margaill I, Callebert J, Plotkine M, Boulu RG. Mechanisms involved in the neuroprotective activity of a nitric oxide synthase inhibitor during focal cerebral ischemia. J Neurochem. 1993;61:690–696. doi: 10.1111/j.1471-4159.1993.tb02174.x. [DOI] [PubMed] [Google Scholar]
- Burggraf D, Martens HK, Dichgans M, Hamann GF. rt-PA causes a dose-dependent increase in the extravasation of cellular and non-cellular blood elements after focal cerebral ischemia. Brain Res. 2007;1164:55–62. doi: 10.1016/j.brainres.2007.05.066. [DOI] [PubMed] [Google Scholar]
- Caramia F, Yoshida T, Hamberg LM, Huang Z, Hunter G, Wanke I, Zaharchuk G, Moskowitz MA, Rosen BR. Measurement of changes in cerebral blood volume in spontaneously hypertensive rats following -arginine infusion using dynamic susceptibility contrast MRI. Magn Reson Med. 1998;39:160–163. doi: 10.1002/mrm.1910390123. [DOI] [PubMed] [Google Scholar]
- Chiou GCY, Hong SJ. Prevention and/or treatment of ischemic stroke with nitric oxide synthase substrates. Drug Develop Res. 1997;40:88–93. [Google Scholar]
- Cipolla MJ, Lessov N, Clark WM, Haley EC., Jr Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 2000;31:940–945. doi: 10.1161/01.str.31.4.940. [DOI] [PubMed] [Google Scholar]
- Cleary S, Mulkerrin MG, Kelley RF. Purification and characterization of tissue plasminogen activator kringle-2 domain expressed in Escherichia coli. Biochemistry. 1989;28:1884–1891. doi: 10.1021/bi00430a068. [DOI] [PubMed] [Google Scholar]
- Crome O, Doeppner TR, Schwarting S, Muller B, Bahr M, Weise J. Enhanced poly(ADP-ribose) polymerase-1 activation contributes to recombinant tissue plasminogen activator-induced aggravation of ischemic brain injury in vivo. J Neurosci Res. 2007;85:1734–1743. doi: 10.1002/jnr.21305. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Poeck K, Pessin MS, Wolpert SM, Furlan AJ, Ferbert A, Alberts MJ, Zivin JA, Wechsler L, Busse O, Greentree R, Jr, Brass L, Mohr JP, Feldman E, Hacke W, Kase CS, Biller J, Gress D, Otis SM. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–982. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Niwa K, Lindauer U, Villringer A. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am J Physiol. 1994;267:H296–H301. doi: 10.1152/ajpheart.1994.267.1.H296. [DOI] [PubMed] [Google Scholar]
- Escott KJ, Beech JS, Haga KK, Williams SC, Meldrum BS, Bath PM. Cerebroprotective effect of the nitric oxide synthase inhibitors, 1-(2-trifluoromethylphenyl) imidazole and 7-nitro indazole, after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:281–287. doi: 10.1097/00004647-199803000-00006. [DOI] [PubMed] [Google Scholar]
- Gautier S, Petrault O, Gele P, Laprais M, Bastide M, Bauters A, Deplanque D, Jude B, Caron J, Bordet R. Involvement of thrombolysis in recombinant tissue plasminogen activator-induced cerebral hemorrhages and effect on infarct volume and postischemic endothelial function. Stroke. 2003;34:2975–2979. doi: 10.1161/01.STR.0000101914.62066.7B. [DOI] [PubMed] [Google Scholar]
- Haelewyn B, Risso J-J, Abraini JH. Human recombinant tissue-plasminogen activator (alteplase): why not use the /‘human/' dose for stroke studies in rats[quest] J Cereb Blood Flow Metab. 2010;30:900–903. doi: 10.1038/jcbfm.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Kano T, Katayama Y, Matsuzaki T, Tejima E, Koshinaga M. Tissue plasminogen activator extravasated through the cerebral vessels: evaluation using a rat thromboembolic stroke model. Thromb Haemost. 2005;94:791–796. doi: 10.1160/TH05-03-0164. [DOI] [PubMed] [Google Scholar]
- Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- He Z, Ibayashi S, Nagao T, Fujii K, Sadoshima S, Fujishima M. -arginine ameliorates cerebral blood flow and metabolism and decreases infarct volume in rats with cerebral ischemia. Brain Res. 1995;699:208–213. doi: 10.1016/0006-8993(95)00907-8. [DOI] [PubMed] [Google Scholar]
- Hill MD, Buchan AM. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ. 2005;172:1307–1312. doi: 10.1503/cmaj.1041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Hwang JH. Reduction of neuronal damage in ischemic stroke using a combination therapy of TMB-8 with -arginine. Kaohsiung J Med Sci. 2000;16:170–180. [PubMed] [Google Scholar]
- Horn J, Limburg M. Calcium antagonists for ischemic stroke: a systematic review. Stroke. 2001;32:570–576. doi: 10.1161/01.str.32.2.570. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro--arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Xu S, Casey R, Ross ME. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J Cereb Blood Flow Metab. 1995a;15:378–384. doi: 10.1038/jcbfm.1995.47. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J Physiol. 1995b;268:R286–R292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator. J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- Kilic E, Bahr M, Hermann DM. Effects of recombinant tissue plasminogen activator after intraluminal thread occlusion in mice: role of hemodynamic alterations. Stroke. 2001;32:2641–2647. doi: 10.1161/hs1101.097381. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Bahr M, Hermann DM. Tissue plasminogen activator-induced ischemic injury is reversed by NMDA antagonist MK-801 in vivo. Neurodegener Dis. 2005a;2:49–55. doi: 10.1159/000089283. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Matter CM, Luscher TF, Bassetti CL, Hermann DM. Aggravation of focal cerebral ischemia by tissue plasminogen activator is reversed by 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor but does not depend on endothelial NO synthase. Stroke. 2005b;36:332–336. doi: 10.1161/01.STR.0000152273.24063.f7. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Reiter RJ, Bassetti CL, Hermann DM. Tissue-plasminogen activator-induced ischemic brain injury is reversed by melatonin: role of iNOS and Akt. J Pineal Res. 2005c;39:151–155. doi: 10.1111/j.1600-079X.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- Klein GM, Li H, Sun P, Buchan AM. Tissue plasminogen activator does not increase neuronal damage in rat models of global and focal ischemia. Neurology. 1999;52:1381–1384. doi: 10.1212/wnl.52.7.1381. [DOI] [PubMed] [Google Scholar]
- Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–565. [PubMed] [Google Scholar]
- Lee SR, Guo SZ, Scannevin RH, Magliaro BC, Rhodes KJ, Wang X, Lo EH. Induction of matrix metalloproteinase, cytokines and chemokines in rat cortical astrocytes exposed to plasminogen activators. Neurosci Lett. 2007;417:1–5. doi: 10.1016/j.neulet.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lopez-Atalaya JP, Roussel BD, Levrat D, Parcq J, Nicole O, Hommet Y, Benchenane K, Castel H, Leprince J, To Van D, Bureau R, Rault S, Vaudry H, Petersen KU, Santos JS, Ali C, Vivien D. Toward safer thrombolytic agents in stroke: molecular requirements for NMDA receptor-mediated neurotoxicity. J Cereb Blood Flow Metab. 2008;28:1212–1221. doi: 10.1038/jcbfm.2008.14. [DOI] [PubMed] [Google Scholar]
- Lu A, Kurosawa Y, Luskey K, Pyne-Geithman G, Caudell D, Clark J. Hemorrhagic profile of the fibrinolytic alfimeprase after ischemia and reperfusion. Neurol Res. 2009;31:209–214. doi: 10.1179/174313209X393933. [DOI] [PubMed] [Google Scholar]
- Machado LS, Sazonova IY, Kozak A, Wiley DC, El-Remessy AB, Ergul A, Hess DC, Waller JL, Fagan SC. Minocycline and tissue-type plasminogen activator for stroke: assessment of interaction potential. Stroke. 2009;40:3028–3033. doi: 10.1161/STROKEAHA.109.556852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AM, Kuhlmann C, Trossbach S, Jaeger S, Waldron E, Roebroek A, Luhmann HJ, Laatsch A, Weggen S, Lessmann V, Pietrzik CU. The functional role of the second NPXY motif of the LRP1 beta-chain in tissue-type plasminogen activator-mediated activation of N-methyl-D-aspartate receptors. J Biol Chem. 2008;283:12004–12013. doi: 10.1074/jbc.M707607200. [DOI] [PubMed] [Google Scholar]
- Meng W, Wang X, Asahi M, Kano T, Asahi K, Ackerman RH, Lo EH. Effects of tissue type plasminogen activator in embolic versus mechanical models of focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1316–1321. doi: 10.1097/00004647-199912000-00004. [DOI] [PubMed] [Google Scholar]
- Morikawa E, Huang Z, Moskowitz MA. -arginine decreases infarct size caused by middle cerebral arterial occlusion in SHR. Am J Physiol. 1992a;263:H1632–H1635. doi: 10.1152/ajpheart.1992.263.5.H1632. [DOI] [PubMed] [Google Scholar]
- Morikawa E, Rosenblatt S, Moskowitz MA. -arginine dilates rat pial arterioles by nitric oxide-dependent mechanisms and increases blood flow during focal cerebral ischaemia. Br J Pharmacol. 1992b;107:905–907. doi: 10.1111/j.1476-5381.1992.tb13382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa E, Moskowitz MA, Huang Z, Yoshida T, Irikura K, Dalkara T. -arginine infusion promotes nitric oxide-dependent vasodilation, increases regional cerebral blood flow, and reduces infarction volume in the rat. Stroke. 1994;25:429–435. doi: 10.1161/01.str.25.2.429. [DOI] [PubMed] [Google Scholar]
- Moro MA, Cardenas A, Hurtado O, Leza JC, Lizasoain I. Role of nitric oxide after brain ischaemia. Cell Calcium. 2004;36:265–275. doi: 10.1016/j.ceca.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- Nassar T, Akkawi S, Shina A, Haj-Yehia A, Bdeir K, Tarshis M, Heyman SN, Higazi AA. In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone. Blood. 2004;103:897–902. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- Navarro E, Alonso SJ, Martin FA, Castellano MA. Toxicological and pharmacological effects of -arginine. Basic Clin Pharmacol Toxicol. 2005;97:149–154. doi: 10.1111/j.1742-7843.2005.pto_973110.x. [DOI] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- NINDS Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Oka F, Fujisawa H, Nomura S, Kajiwara K, Kato S, Fujii M, Izuma H, Uozumi K, Gondo T, Suzuki M. Mechanistic insight into neurotoxicity of tissue plasminogen activator-induced thrombolysis products in a rat intraluminal middle cerebral artery occlusion model. J Neurotrauma. 2009;26:1577–1584. doi: 10.1089/neu.2008.0768. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Rees DD, Ashton DS, Moncada S. -arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988;153:1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Parathath SR, Parathath S, Tsirka SE. Nitric oxide mediates neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. J Cell Sci. 2006;119:339–349. doi: 10.1242/jcs.02734. [DOI] [PubMed] [Google Scholar]
- Park L, Gallo EF, Anrather J, Wang G, Norris EH, Paul J, Strickland S, Iadecola C. Key role of tissue plasminogen activator in neurovascular coupling. Proc Natl Acad Sci USA. 2008;105:1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretnar-Oblak J, Zaletel M, Zvan B, Sabovic M, Pogacnik T. Cerebrovascular reactivity to -arginine in patients with lacunar infarctions. Cerebrovasc Dis. 2006;21:180–186. doi: 10.1159/000090530. [DOI] [PubMed] [Google Scholar]
- Review Manager 2008(The Nordic Cochrane Centre TCC, ed), 5.0 ed.: Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration
- Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- Tang J, Li YJ, Mu J, Li Q, Yang DY, Xie P. Albumin ameliorates tissue plasminogen activator-mediated blood-brain barrier permeability and ischemic brain injury in rats. Neurol Res. 2009;31:189–194. doi: 10.1179/174313209X393898. [DOI] [PubMed] [Google Scholar]
- Temiz C, Tun K, Ugur HC, Dempsey RJ, Egemen N. -arginine in focal cerebral ischemia. Neurol Res. 2003;25:465–470. doi: 10.1179/016164103101201869. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan M, Fernandez JA, Lane SM, Griffin JH, Zlokovic BV. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J Neurosci. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, Huang PL, Wang X, Montaner J, Lo EH. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36:1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- Wiegler K, Bonny C, Coquoz D, Hirt L. The JNK inhibitor XG-102 protects from ischemic damage with delayed intravenous administration also in the presence of recombinant tissue plasminogen activator. Cerebrovasc Dis. 2008;26:360–366. doi: 10.1159/000151639. [DOI] [PubMed] [Google Scholar]
- Willmot M, Gray L, Gibson C, Murphy S, Bath PM. A systematic review of nitric oxide donors and -arginine in experimental stroke; effects on infarct size and cerebral blood flow. Nitric Oxide. 2005;12:141–149. doi: 10.1016/j.niox.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Yagi K, Kitazato KT, Uno M, Tada Y, Kinouchi T, Shimada K, Nagahiro S. Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke. 2009;40:626–631. doi: 10.1161/STROKEAHA.108.520262. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kamiya T, Deguchi K, Inaba T, Zhang H, Shang J, Miyazaki K, Ohtsuka A, Katayama Y, Abe K. Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J Cereb Blood Flow Metab. 2009;29:715–725. doi: 10.1038/jcbfm.2008.164. [DOI] [PubMed] [Google Scholar]
- Yang DY, Pan HC, Chen CJ, Cheng FC, Wang YC. Effects of tissue plasminogen activator on cerebral microvessels of rats during focal cerebral ischemia and reperfusion. Neurol Res. 2007;29:274–282. doi: 10.1179/016164107X159171. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Coleman TA, Moore E, Wu JY, Mitola D, Bugge TH, Lawrence DA. Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent. J Clin Invest. 2002;109:1571–1578. doi: 10.1172/JCI14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang C, An J, Strickland DK, Yepes M. The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain. Am J Pathol. 2009;174:586–594. doi: 10.2353/ajpath.2009.080661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Casey RM, Ross ME, Iadecola C. Aminoguanidine ameliorates and -arginine worsens brain damage from intraluminal middle cerebral artery occlusion. Stroke. 1996a;27:317–323. doi: 10.1161/01.str.27.2.317. [DOI] [PubMed] [Google Scholar]
- Zhang F, Iadecola C. Temporal characteristics of the protective effect of aminoguanidine on cerebral ischemic damage. Brain Res. 1998;802:104–110. doi: 10.1016/s0006-8993(98)00557-5. [DOI] [PubMed] [Google Scholar]
- Zhang J-X, Zhang H-X, Li L-F, Li G-F. Effects of -arginine and aminoguanidine on focal cerebral ischemic injury in rat. Chin J Clin Rehab. 2004a;8:2559–2561. [Google Scholar]
- Zhang W, Sato K, Hayashi T, Omori N, Nagano I, Kato S, Horiuchi S, Abe K. Extension of ischemic therapeutic time window by a free radical scavenger, Edaravone, reperfused with tPA in rat brain. Neurol Res. 2004b;26:342–348. doi: 10.1179/016164104225014058. [DOI] [PubMed] [Google Scholar]
- Zhang X, Polavarapu R, She H, Mao Z, Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation. Am J Pathol. 2007;171:1281–1290. doi: 10.2353/ajpath.2007.070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Reif D, Macdonald J, Tang WX, Kamp DK, Gentile RJ, Shakespeare WC, Murray RJ, Chopp M. ARL 17,477, a potent and selective neuronal NOS inhibitor decreases infarct volume after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1996b;16:599–604. doi: 10.1097/00004647-199607000-00009. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ross ME, Iadecola C. L-Arginine increases ischemic injury in wild-type mice but not in iNOS-deficient mice. Brain Res. 2003;966:308–311. doi: 10.1016/s0006-8993(02)04223-3. [DOI] [PubMed] [Google Scholar]
- Zivin JA, Fisher M, DeGirolami U, Hemenway CC, Stashak JA. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 1985;230:1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.