Abstract
Vascular risk factors contribute to the progression of dementia in Alzheimer's disease (AD) and influence platelet activation. However, the degree of platelet activation as a possible underlying mechanism of this progression has not been studied till now. Significantly higher baseline expression of both platelet activation biomarkers, activated glycoprotein IIb–IIIa complex and P-selectin, was observed in patients with AD with fast cognitive decline compared with AD patients with slow cognitive decline during a 1-year follow-up period. These results suggest that platelet activation could be a putative prognostic biomarker for the rate of cognitive decline and a potential new treatment target in AD patients.
Keywords: Alzheimer's disease, cognitive decline, dementia, platelet activation, P-selectin.
Introduction
Alzheimer's disease (AD) is the most common cause of dementia in the elderly, affecting ∼30 million individuals worldwide. Some patients with AD experience rapid cognitive decline, whereas others decline much slower. The biologic basis of the different progression is not well understood. Both AD and atherosclerosis have been found to share similar vascular risk factors. In addition, several epidemiologic studies have shown that vascular risk factors such as arterial hypertension and hypercholesterolemia also contribute to the progression of AD (Helzner et al, 2009; Roselli et al, 2009). Moreover, treatment of vascular risk factors has been associated with reduced risk of developing AD (Forette et al, 2002) and with a slower cognitive decline in AD patients (Deschaintre et al, 2009). In accordance with these findings, the amount of cerebrovascular degeneration with consecutive hypoperfusion of the brain seems to influence clinical manifestation and severity of cognitive decline in AD patients. However, the underlying mechanisms of this association between vascular risk factors and AD are not yet understood.
Platelet activation can be assessed by the detection of surface activation markers through flow cytometry. Activation of glycoprotein (GP)IIb–IIIa measured by an activation-specific antibody (PAC-1) indicates platelet activation. Persistent elevated platelet activation (PAC-1) 3 months after stroke is associated with increased incidence of recurrent stroke and with progression of carotid artery disease (Fateh-Moghadam et al, 2007). P-selectin is an adhesion molecule stored in platelet α-granules that becomes expressed on the surface of activated platelets (Stellos et al, 2010). Increased P-selectin expression on platelets is associated with silent cerebral infarction in patients with atrial fibrillation, acute cerebrovascular ischemia and extent of myocardial necrosis and inflammation in patients with acute myocardial infarction.
Taken together, as platelet activation predicts recurrent stroke and previous stroke is the most important predictor of incidence of dementia in stroke survivors in a large stroke cohort (Tatemichi et al, 1990), we investigated the expression of activated fibrinogen receptor and P-selectin on circulating platelets in patients with AD and evaluated their association with cognitive decline in patients with AD after a 1-year follow-up period.
Materials and methods
A detailed description of the methods used is included in Supplementary Materials available on the JCBFM website.
Subjects
A total of 40 outpatients with AD and 30 healthy elderly controls were included in the study. The clinical severity of cognitive impairment was assessed by the Mini-Mental State Examination (MMSE). All patients were followed up prospectively and clinically reexamined estimating the cognitive status with the help of MMSE 1 year later (range 11 to 13 months). The ethics committee of the University of Tuebingen approved the study and written informed consent was obtained.
Whole-Blood Flow Cytometry
Platelets were studied for surface expression of activated fibrinogen receptor (platelet membrane GPIIb–IIIa complex activation) and P-selectin (CD62P) by flow cytometric analysis, as described previously (Stellos et al, 2010). Specific monoclonal antibody binding was expressed as mean fluorescence intensity and was used as a quantitative measurement of platelet proteins' surface expression.
Data Analysis
All statistical analyses were carried out using the statistical analysis software package SPSS 17 (SPSS, Munich, Germany). The data are presented as mean±s.d. Significance for the results was set at P<0.05.
Results
The baseline demographic and laboratory parameters of AD patients and healthy controls are displayed in Supplementary Table 1. At the baseline time point, no significant difference on the expression of platelet membrane GPIIb–IIIa complex (P=0.451) and P-selectin (P=0.950) was observed between AD patients and healthy controls.
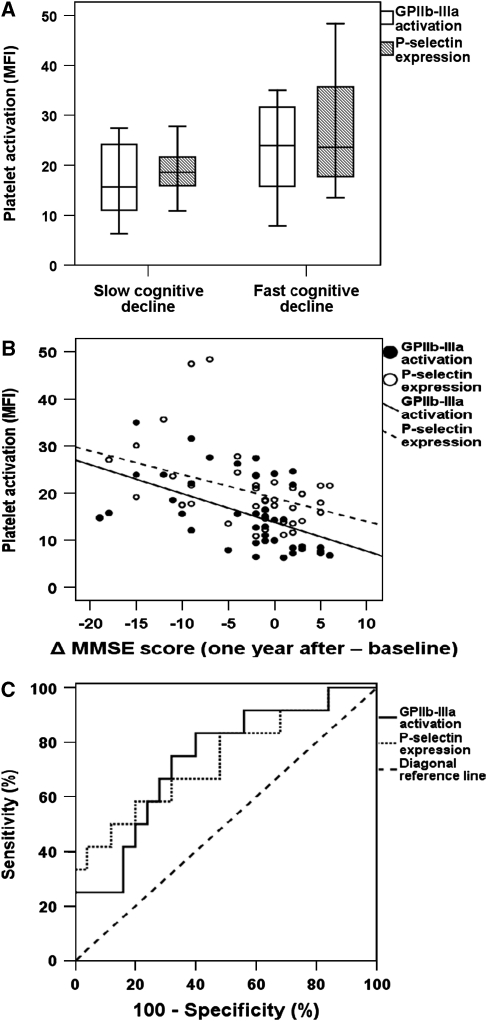
All patients were prospectively followed up and clinically reexamined estimating the cognitive status with the help of MMSE 1 year later (range 11 to 13 months). Among AD patients, 28 presented with a slow cognitive decline (decrease of MMSE score ⩽4 per year) and 12 patients displayed a fast cognitive decline (decrease of MMSE score >4 per year; Supplementary Table 2). Patients with AD with fast cognitive decline showed significantly higher baseline expression of both platelet activation biomarkers, activated GPIIb–IIIa complex and P-selectin, than AD patients with slow cognitive decline did during the 1-year follow-up period (mean fluorescence intensity: mean±s.d.: fast versus slow cognitive decline: GPIIb–IIIa: 20.7±8.1 versus 13.6±6.6; P<0.001; P-selectin: 26.4±11.9 versus 17.6±4.4; P<0.001; Figure 1A).
Figure 1.
Platelet activation associates with cognitive decline in Alzheimer's disease (AD). (A) Box plot showing platelet GPIIb–IIIa complex activation and P-selectin expression levels in AD patients with fast versus slow cognitive decline defined after a 1-year follow-up period. (B) Correlation between change of MMSE score (one year after baseline) and platelet GPIIb–IIIa activation (n=40; r=−0.573; P<0.001) and P-selectin expression (n=40; r=−0.411; P=0.009) in AD patients. (C) Receiver operating characteristic curve for platelet GPIIb–IIIa activation and P-selectin expression for fast cognitive decline after 1-year follow-up in AD patients. GPIIb–IIIa, glycoprotein IIb–IIIa; MMSE, Mini-Mental State Examination.
The rate of cognitive decline (defined as the change Δ of MMSE scores) was significantly associated with age (r=0.315, P=0.040), baseline MMSE scores (r=−0.378, P=0.012), platelet activated GPIIb–IIIa (r=−0.573, P<0.001; Figure 1B), and P-selectin expression (r=−0.411, P=0.009; Figure 1B) and showed a trend of association with history of myocardial infarction (r=−0.292; P=0.058). Applying a multiple logistic regression analysis for either GPIIb–IIIa or P-selectin and baseline MMSE scores, the age and history of myocardial infarction revealed that platelet activation was the only significant independent predictor of cognitive decline in AD patients (P⩽0.05 for both).
The baseline expression of platelet GPIIb–IIIa or P-selectin was increased in patients with fast cognitive decline independent of age, gender, years of education, baseline MMSE, and cardiovascular risk factors, as evaluated by a univariate analysis of variance (Table 1). Antihypertensive treatment seems to influence platelet GPIIb–IIIa activation in patients with fast cognitive decline, whereas intake of aspirin or cholinesterase inhibitors (ChEIs) showed at least a trend.
Table 1. Univariate analysis of variance of platelet GPIIb–IIIa complex activation and P-selectin expression in AD patients with fast versus slow cognitive decline.
| GPIIb–IIIa activation P-value | P-selectin P-value | |
|---|---|---|
| Characteristics | ||
| Gender | 0.752 | 0.408 |
| Age (years) | 0.280 | 0.437 |
| Education (years) | 0.391 | 0.450 |
| MMSE | 0.503 | 0.466 |
| CVRFs | ||
| Arterial hypertension | 0.187 | 0.724 |
| Hypercholesterinemia | 0.522 | 0.348 |
| Diabetes mellitus | 0.861 | 0.424 |
| Smoking | 0.417 | 0.361 |
| Alcohol | 0.847 | 0.959 |
| CAD | 0.123 | 0.712 |
| History of myocardial infarction | 0.573 | 0.320 |
| History of stroke | 0.447 | 0.074 |
| Medication | ||
| Aspirin | 0.084 | 0.476 |
| Statin | 0.763 | 0.983 |
| Antihypertensives | 0.002 | 0.757 |
| ChEI | 0.055 | 0.899 |
AD, Alzheimer's disease; CAD, coronary artery disease; ChEI, cholinesterase inhibitor; CVRFs, cardiovascular risk factors; GPIIb–IIIa, glycoprotein IIb–IIIa; MMSE, Mini-Mental State Examination.
Next, we performed a receiver operating characteristic analysis to evaluate the ability of platelet GPIIb–IIIa activation or P-selectin levels to predict a fast cognitive decline. GPIIb-IIIa: area under the curve 0.762 (P=0.009), 75% sensitivity, 68% specificity, 86% negative predictive value, and an odds ratio of 6.3 at a level of ⩾15.6; P-selectin: area under the curve 0.737 (P=0.021), 67% sensitivity, 71% specificity, 83% negative predictive value and an odds ratio of 5.0 at a level of ⩾18.9 (Figure 1C).
Discussion
In this study, we report that baseline platelet activation is significantly higher in AD patients with later fast cognitive decline than in AD patients with later slow cognitive decline independent of age, gender, years of education, baseline MMSE, and cardiovascular risk factors and is significantly associated with the rate of cognitive decline during 1-year follow-up. Thus, platelet activity could be the missing link for the known association between vascular risk factors and progression of AD (Helzner et al, 2009).
Increased platelet activity could contribute to dementia progression through several mechanisms: (1) Platelet-bound GPIIb–IIIa and P-selectin are involved in the adhesion of platelets on endothelial cells at the sites of vascular lesions and in the further recruitment of leukocytes on vascular wall regulating vascular inflammation. This mechanism could trigger perivascular inflammation in the brain contributing to dementia progression (Akiyama et al, 2000). (2) Platelet activation is associated with progression of carotid artery disease, while aggregating platelets contribute to vasoconstriction with consecutive hypoperfusion (Houston et al, 1986). In this context, a recent study has shown reduced cerebral blood flow using transcranial Doppler in early AD patients, suggesting cerebral vasoconstriction (Claassen et al, 2009). Thus, platelet-mediated cerebrovascular dysfunction may trigger AD pathology and disease progression caused by brain hypoperfusion, creating a neuronal energy crisis (de la Torre, 2009). (3) Platelets contain and secrete amyloid-β peptides on stimulation into the peripheral blood, which constitute the main component of amyloid plaques in the brain from AD patients and also seem to be deposited in the vasculature. Cognitive decline in AD patients has been associated with an AD-specific reduction in the platelet amyloid precursor protein isoform ratio (Baskin et al, 2000). Moreover, in accordance with our present findings, Prodan et al (2008) have recently reported a significant linear correlation between the initial coated-platelet levels, which is a fraction of activated platelets, and AD progression measured by MMSE scores in a 2-year follow-up period.
Interestingly, comparing baseline and 1-year follow-up MMSE scores, we identified 12 AD patients who presented with cognitive improvement 1 year after the baseline examination. This finding is in accordance with previous studies reporting that up to 34% of the patients with untreated AD may present with a better or unchanged MMSE score after a 1-year follow-up period (Clark et al, 1999). Moreover, patients treated with a ChEI may score higher in MMSE after 1 year compared with placebo (Lyle et al, 2008). For instance, in the Penn Hospital cohort, 72% of survivors treated with a ChEI showed a cognitive improvement after 1-year follow-up and even 47% at the end of the second year (Lyle et al, 2008). Nevertheless, the differential mechanism leading to a cognitive decline or improvement in patients with AD is not fully understood. In this study, 75% of the patients with cognitive improvement 1 year later were under ChEI treatment, and ChEI treatment showed at least a slight influence on the association between platelet activity measured by GPIIb–IIIa activation and cognitive decline.
Few of our AD patients showed an extraordinary large decline in MMSE scores after the 1-year follow-up period. This finding is in accordance with previous studies reporting that the rate of cognitive decline in AD patients considerably varies after the 1-year follow-up period (Carcaillon et al, 2007). For example, Carcaillon et al (2007) have previously reported that the annual change in the MMSE score in AD patients ranged from +3.3 to −16.1 points.
Taken together, platelet activation, measured by GPIIb–IIIa complex activation or P-selectin expression, could be a putative prognostic biomarker for the rate of cognitive decline and a potential new treatment target in AD patients.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin F, Rosenberg RN, Iyer L, Hynan L, Cullum CM. Platelet APP isoform ratios correlate with declining cognition in AD. Neurology. 2000;54:1907–1909. doi: 10.1212/wnl.54.10.1907. [DOI] [PubMed] [Google Scholar]
- Carcaillon L, Pérès K, Péré JJ, Helmer C, Orgogozo JM, Dartigues JF. Fast cognitive decline at the time of dementia diagnosis: a major prognostic factor for survival in the community. Dement Geriatr Cogn Disord. 2007;23:439–445. doi: 10.1159/000102017. [DOI] [PubMed] [Google Scholar]
- Claassen JA, Diaz-Arrastia R, Martin-Cook K, Levine BD, Zhang R. Altered cerebral hemodynamics in early Alzheimer disease: a pilot study using transcranial Doppler. J Alzheimers Dis. 2009;17:621–629. doi: 10.3233/JAD-2009-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Sheppard L, Fillenbaum GG, Galasko D, Morris JC, Koss E, Mohs R, Heyman A. Variability in annual Mini-Mental State Examination score in patients with probable Alzheimer disease: a clinical perspective of data from the Consortium to Establish a Registry for Alzheimer's Disease. Arch Neurol. 1999;56:857–862. doi: 10.1001/archneur.56.7.857. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Cerebrovascular and cardiovascular pathology in Alzheimer's disease. Int Rev Neurobiol. 2009;84:35–48. doi: 10.1016/S0074-7742(09)00403-6. [DOI] [PubMed] [Google Scholar]
- Deschaintre Y, Richard F, Leys D, Pasquier F. Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology. 2009;73:674–680. doi: 10.1212/WNL.0b013e3181b59bf3. [DOI] [PubMed] [Google Scholar]
- Fateh-Moghadam S, Htun P, Tomandl B, Sander D, Stellos K, Geisler T, Langer H, Walton K, Handschu R, Garlichs C, Daniel WG, Gawaz M. Hyperresponsiveness of platelets in ischemic stroke. Thromb Haemost. 2007;97:974–978. [PubMed] [Google Scholar]
- Forette F, Seux ML, Staessen JA, Thijs L, Babarskiene MR, Babeanu S, Bossini A, Fagard R, Gil-Extremera B, Laks T, Kobalava Z, Sarti C, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Birkenhager WH. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002;162:2046–2052. doi: 10.1001/archinte.162.18.2046. [DOI] [PubMed] [Google Scholar]
- Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DS, Shepherd JT, Vanhoutte PM. Aggregating human platelets cause direct contraction and endothelium-dependent relaxation of isolated canine coronary arteries. Role of serotonin, thromboxane A2, and adenine nucleotides. J Clin Invest. 1986;78:539–544. doi: 10.1172/JCI112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle S, Grizzell M, Willmott S, Benbow S, Clark M, Jolley D. Treatment of a whole population sample of Alzheimer's disease with donepezil over a 4-year period: lessons learned. Dement Geriatr Cogn Disord. 2008;25:226–231. doi: 10.1159/000114450. [DOI] [PubMed] [Google Scholar]
- Prodan CI, Ross ED, Vincent AS, Dale GL. Rate of progression in Alzheimer's disease correlates with coated-platelet levels—a longitudinal study. Transl Res. 2008;152:99–102. doi: 10.1016/j.trsl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Roselli F, Tartaglione B, Federico F, Lepore V, Defazio G, Livrea P. Rate of MMSE score change in Alzheimer's disease: influence of education and vascular risk factors. Clin Neurol Neurosurg. 2009;111:327–330. doi: 10.1016/j.clineuro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Stellos K, Bigalke B, Stakos D, Henkelmann N, Gawaz M. Platelet-bound P-selectin expression in patients with coronary artery disease: impact on clinical presentation and myocardial necrosis, and effect of diabetes mellitus and anti-platelet medication. J Thromb Haemost. 2010;8:205–207. doi: 10.1111/j.1538-7836.2009.03659.x. [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Foulkes MA, Mohr JP, Hewitt JR, Hier DB, Price TR, Wolf PA. Dementia in stroke survivors in the Stroke Data Bank cohort. Prevalence, incidence, risk factors, and computed tomographic findings. Stroke. 1990;21:858–866. doi: 10.1161/01.str.21.6.858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.