SUMMARY
Viruses, including retroviruses like human immunodeficiency virus (HIV) and mouse mammary tumor virus (MMTV), are transmitted from mother to infants through milk. Lymphoid cells and antibodies are thought to provide mammary gland and milk-borne immunity. In contrast, little is known about the role of mammary epithelial cells (MECs). The APOBEC3 family of retroviral restriction factors is highly expressed in macrophages, lymphoid and dendritic cells. We now show that APOBEC3 proteins are also expressed in mouse and human MECs. Lymphoid cell expressed APOBEC3 restricts in vivo spread of MMTV to lymphoid and mammary tissue. In contrast, mammary gland expressed APOBEC3 is packaged into MMTV virions and decreases the infectivity of milk-borne viruses. Moreover, APOBEC3G and other APOBEC3 genes are expressed in human mammary cells and have the potential to restrict viruses produced in this cell type. These data point to a role for APOBEC3 proteins in limiting infectivity of milk-transmitted viruses.
INTRODUCTION
A number of viruses are transmitted through milk from mother to infants, including cytomegalovirus (Meier et al., 2005), vaccinia virus (Abrahao et al., 2009), papillomaviruses (Lindsey et al., 2009; Sarkola et al., 2008) and the retroviruses HIV, simian immunodeficiency virus (SIV), feline immunodeficiency virus (FIV), human T cell leukemia virus (HTLV) I/II and MMTV (Allison and Hoover, 2003; Li et al., 2004; Nandi and McGrath, 1973; O’Neil et al., 1995; Rychert et al., 2006; Sellon et al., 1994; Seltzer and Benjamin, 1990; Ziegler et al., 1985). The best-studied milk-transmitted virus is the murine retrovirus, MMTV, which causes breast cancer in mice (Nandi and McGrath, 1973). MMTV in vivo infection occurs first in dendritic cells (DCs) of the gut, which then transfer the virus to lymphocytes (Ross, 2008). The infected lymphocytes traffic to the mammary gland, resulting in the infection of mammary epithelial cells (MECs) during puberty and pregnancy. Lactating mice shed high levels of cell-free virus into milk, which is then acquired by nursing offspring during the 1st week of life (Ross, 2008).
In addition to being a source of virus that can be transmitted via milk, lymphoid cells in the mammary gland and milk provide passive immunity to the neonate against pathogenic infections (Walker, 2004). Indeed, strong neutralizing anti-MMTV humoral responses in mice result in antibody-coated milk-borne virions that are rendered non-infectious and are not transmitted to newborns (Case et al., 2005). Moreover, it has been suggested that anti-HIV antibodies in human milk block milk-borne transmission (Bouhlal et al., 2005) and that CD8+ T lymphocytes control SIV replication in breast milk lymphocytes (Permar et al., 2008), thereby potentially limiting milk-transmitted infection (John-Stewart et al., 2009). In contrast to the important role that lymphoid cells play in providing mammary gland and milk-borne immunity, little is known how MECs participate in this process.
Recently, several cellular genes have been identified that function in addition to the innate and adaptive immune response to limit virus infection and spread. These include members of the APOBEC3 family of restriction factors, which encode DNA-editing enzymes characterized by the presence of at least one cytidine deaminase domain (LaRue et al., 2009). The number of APOBEC3 genes varies between species, from 1 gene in rodents to 7 genes in humans (LaRue et al., 2009). APOBEC3G and APOBEC3F confer intrinsic immunity to HIV-1 lacking the vif gene (Chiu and Greene, 2008; Cullen, 2006; Malim, 2009). In vif-deficient-HIV-1 producer cells, APOBEC3G and 3F are packaged into progeny virions while in cells infected with vif+ virus, Vif binds APOBEC3F and 3G and targets them for degradation in the proteasome, thereby preventing their packaging and overcoming the anti-viral activity. Virion-packaged APOBEC3 proteins inhibit infection in target cells by deaminating deoxycytidine residues on the DNA minus strand following reverse transcription, inducing mutations in newly synthesized HIV-1 DNA; this leads to both degradation of reversed transcribed DNA prior to integration and to mutation of viral genes in the integrated provirus (Cullen, 2006; Malim, 2009). There is also evidence that APOBEC3 proteins can restrict HIV infection by non-CDA-dependent mechanisms (Malim, 2009).
APOBEC3 proteins also restrict infection by a number of mouse retroviruses. Mice with targeted deletion of the Apobec3 gene are more susceptible to infection by MMTV and a number of murine leukemia viruses (MLV), all of which target cells of hematopoietic origin in vivo (Langlois et al., 2009; Low et al., 2009; Okeoma et al., 2007; Santiago et al., 2008; Takeda et al., 2008). APOBEC3-mediated restriction of MMTV and Friend MLV, but not AK virus infection is independent of cytidine deamination (Langlois et al., 2009; Okeoma et al., 2007; Takeda et al., 2008). Because mothers can transfer viruses to their neonates via milk, it is also possible that intrinsic immune factors like APOBEC3 proteins could limit milk-borne transmission. Here we demonstrate that APOBEC3 proteins are expressed in mouse and human MECs and mammary tissue. We also show that APOBEC3 protein made in MECs is packaged into retrovirus particles and affects viral infectivity. These data highlight the potential importance of MEC-expressed APOBEC3 on the transmission of milk-borne retroviruses.
RESULTS
APOBEC3 knockout mice have increased mammary gland infection
APOBEC3 RNA can be detected in cultured murine mammary epithelial cells and primary mammary tissue (Okeoma et al., 2007; Pauklin et al., 2009). To determine if APOBEC3 in mammary tissue restricts in vivo virus infection, we foster-nursed APOBEC3 wild type (+/+) and knockout (−/−) mice on the same MMTV-infected mothers and monitored infection of mammary gland from 3 – 10 weeks after birth. MMTV infection of spleens served as a positive control, since this tissue was more highly infected in MMTV-inoculated mA3−/− mice than in mA3+/+ mice (Okeoma et al., 2007). In addition, a cohort of MMTV-infected mA3 +/+ and −/− mice was monitored for mammary tumor induction.
Mice lacking APOBEC3 had higher levels of milk-borne MMTV infection of both splenic and mammary tissue at all times examined (Figure 1A and 1B). The difference in infection in the wild type and knockout mice changed with age, from 130-fold to 2-fold and from 17-fold to 3 – fold from 3 to 10 weeks in spleen and mammary tissue, respectively. The higher level of infection in mA3 −/− mice also resulted in increased tumor incidence and decreased time to tumor formation (Figure 1C); at the conclusion of the analysis, 16/26 mA3 −/− developed mammary tumors (average time to tumor = 382 days; median time to tumor = 307 days) in contrast to 4/29 +/+ mice that developed tumors (average time to tumor = 476 days; median time to tumor = 483 days) while no uninfected mA3 +/+ or −/− mice developed mammary tumors (not shown). Thus, loss of APOBEC3-mediated restriction of MMTV infection resulted in higher levels of mammary gland infection and thus caused the virus to be more pathogenic in vivo.
Figure 1.
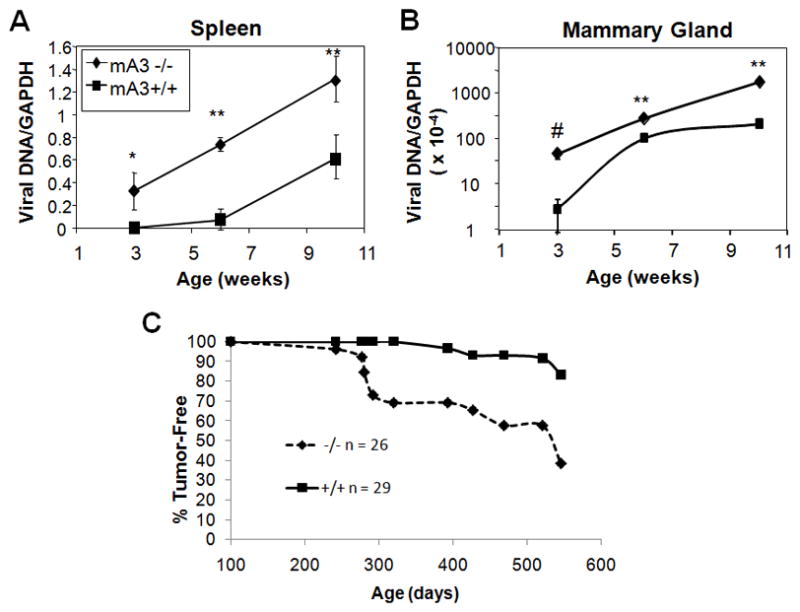
Infection of tissues of mA3+/+ and −/− mice after milk-borne transmission of MMTV. A) and B) RT-qPCR analysis of DNA isolated from spleen and non-lactating mammary gland, respectively, using MMTV- and GAPDH-specific primers; shown are the MMTV values normalized to GAPDH. All error bars represent SD from the averages of the mice analyzed at each time point. From 6 to 19 mice were analyzed for each time point. *, p ≤ 0.02; ** p ≤ 0.001; #, p ≤ 0.01; comparison is between mA3 +/+ and −/− at each time point. C) Mammary tumor incidence in MMTV-infected mA3 −/− and +/+ mice after milk-borne infection. Shown are Kaplan-Meier survival curves that were computed for age at tumor onset. The p-value for the test that these two curves are significantly different (null hypothesis of equality) was <0.0001. N = 26 mA3 −/− mice, 29 mA3 +/+ mice.
Mammary gland infection is restricted by lymphocyte but not mammary cell APOBEC3
The higher level of mammary gland infection leading to increased mammary tumor formation in mA3 −/− mice could result from lack of APOBEC3-mediated restriction of MMTV spread within lymphocytes, mammary epithelial cells or both. To determine in which compartment APOBEC3-mediated restriction occurred, we lethally irradiated mA3 −/− and +/+ mice, reconstituted them with bone marrow from mA3 +/+ mice and then infected them with MMTV. Reconstitution was monitored by FACS analysis of peripheral blood mononuclear cells for CD8+ and CD4+ T cells, CD11c+ DCs and B220+ B cells; in all cases, no differences in reconstitution were seen between the mA3 +/+ and −/− mice (Figure 2A). In addition, RNA was isolated from the spleens, lymph nodes and mammary tissue of the reconstituted animals and examined by reverse-transcribed PCR for APOBEC3. All lymphoid tissue, but not the mammary gland of the mA3 −/− mice expressed APOBEC3 RNA at levels similar to that seen in the reconstituted mA3 +/+ mice (Figure 2B).
Figure 2.
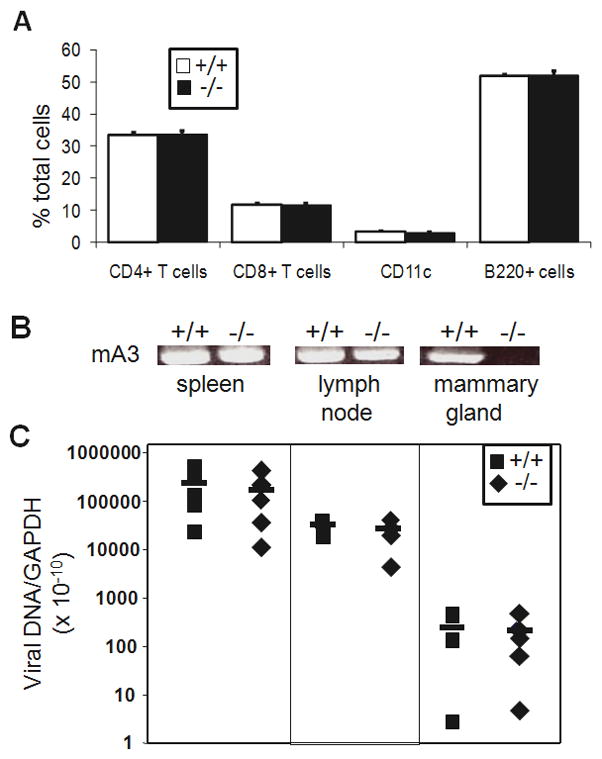
Adoptive transfer of lymphocytes into mA3 +/+ and −/− mice. Five mice of each genotype were lethally irradiated, reconstituted with bone marrow mA3+/+ mice, infected with MMTV and then sacrificed 9 weeks after irradiation. A) FACS analysis of reconstituted mice. PBLs were isolated from the individual mice at sacrifice and stained with the indicated antibodies. Error bars represent SD from the average for the 5 mice of each genotype. B) RNA was isolated from the spleen, lymph nodes (LN) and mammary gland (MG) and subjected to reverse-transcribed PCR with primers specific for APOBEC3. C) DNA was isolated from the indicated tissue and subjected to RT-qPCR specific for exogenous MMTV(RIII) sequences. Values were normalized to GAPDH. This experiment was repeated 2 additional times with similar results.
Not surprisingly, given that the lymphoid tissues of the mA3 +/+ and −/− mice were equally reconstituted with the mA3 +/+ bone marrow, the level of MMTV infection in spleen and lymph node was not significantly different between the two sets of mice (Figure 2C). However, although no APOBEC3 RNA was detected in the mammary tissue of the reconstituted mA3 −/− mice, infection levels were equal to that seen in the reconstituted mA3 +/+ mice, which did express APOBEC3 (Figure 2C). These data strongly suggested that MMTV infection of mammary epithelial cells was determined by lymphocyte virus load and that restriction by APOBEC3 occurred in lymphocytes but not epithelial cells.
APOBEC3 expressed in mammary tissue is packaged into milk-borne virions and restricts infection
MECs are the only source of cell-free MMTV in vivo, which is believed to be the major form of milk-transmitted virus (Nandi and McGrath, 1973). If APOBEC3 made in mammary epithelial cells was packaged into MMTV virions, it would limit virus transmission of milk-borne virus. We next tested the infectivity of virus produced in MMTV-infected mA3−/− and mA3+/+ mammary glands; in both strains, MMTV was transmitted through milk for 5 generations or more. Virions were purified from the milk of the mA3 +/+ and −/− mice and RNA isolated from these virions was quantified by reverse transcribed RT-qPCR. There was about 10-fold more virus in the milk produced by mA3 −/− mice compared to mA3 +/+ mice (Figure 3A), which reflects the higher infection levels in the former (Figure 1B).
Figure 3.
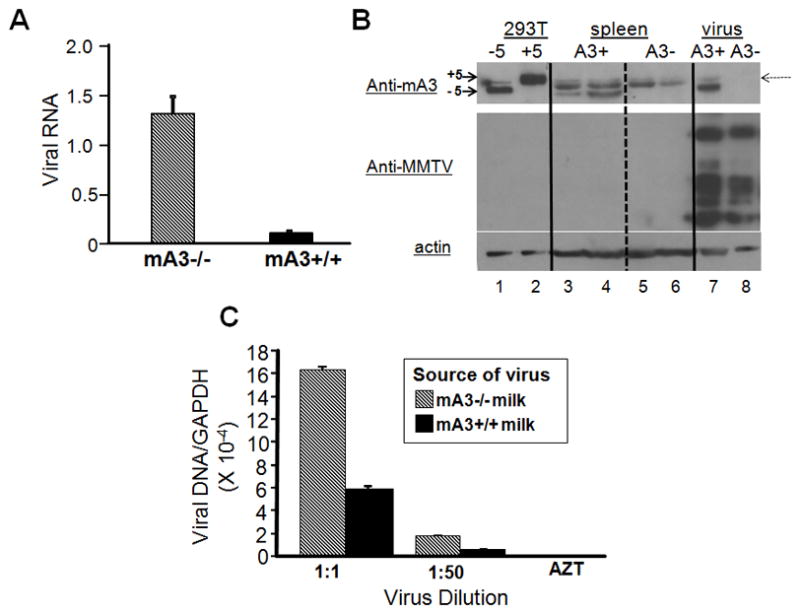
Virus isolated from mA3 −/− milk is more infectious than that isolated from mA3 +/+ milk. A) Virions were isolated from the milk of MMTV-infected mA3 −/− and +/+ mice and RNA isolated from equal amounts of milk was subjected to reverse-transcribed RT-qPCR. Error bars represent the SD of 11 mA3−/− and 9 mA3+/+ milk samples. B) Western blot analysis of APOBEC3 in tissues and virions. Lanes 1 and 2, 293T cells transfected with expression vectors containing the BL/6 delta exon 5 (−5) or BALB/c + exon 5 (+5) cDNAs; lanes 3 and 4, splenic extracts from mA3 +/+ mice; lanes 5 and 6, splenic extracts from mA3 −/− mice; lane 7 and 8, virions isolated from the mammary tissue of mA3 +/+ (A3+, lane 7) or −/− (A3−, lane 8) mice. The top panel was probed with rabbit anti-mouse APOBEC3 antisera then stripped and re-probed with goat anti-MMTV antisera (middle panel); the bottom panel is a duplicate blot of the same extracts probed with anti-β-actin. A cross-reacting protein that is present in the tissue extracts of both +/+ and −/− mice is indicated by a dotted arrow; this protein was detected in some, but not all virus preparations irrespective of the presence of APOBEC3 (not shown). C) Various dilutions of virus isolated from mA3 −/− and +/+ milk were used to infect 293-mTFR1 cells in triplicate, DNA was isolated from the infected cells and subjected to RT-qPCR for MMTV and GAPDH. The numbers presented were normalized to the amount of virion RNA as measured in A). Error bars represent SD from 3 replicate wells. p ≤ 0.001 for both dilutions.
To show that APOBEC3 was packaged into virions made in mammary tissue, virus was isolated from the mammary tumors of mA3 +/+ and −/− mice; mammary tumor tissue has long been used as a source of MMTV (Golovkina et al., 1994). We also analyzed splenic extracts from mA3 +/+ and −/− mice, as well as 293T cells transduced with plasmids encoding HA-tagged BL/6 and BALB/c APOBEC3 proteins (Takeda et al., 2008). Because of allelic differences that cause differential splicing of the RNA in BL/6 vs. BALB/c mice, the predominant APOBEC3 RNA produced in C57BL/6 mice lacks exon 5 and is ~ 3.6 kDa smaller than the full-length BALB/c 51 kDa protein (Okeoma et al., 2009; Takeda et al., 2008). No APOBEC3 protein was found in either the spleen or virus from mA3 −/− mice. In contrast, delta exon 5 APOBEC3 protein was detected in both the spleen and virions isolated from mA3 +/+ mammary glands (Figure 3B).
To examine the infectivity of the milk-borne mA3 +/+ and −/− virus, serial dilutions were used to infect 293T-mTfR1 cells (293T cells stably expressing the MMTV entry receptor, transferrin receptor 1) and DNA was isolated from the infected cells and subjected to RT-qPCR to determine the level of infection. Because of the difference in the amount of virus produced in the two strains of mice, the infection levels are presented after normalization to virion RNA levels. Even after normalization, we found that the virus from mA3−/− mice infected cells at higher levels than that isolated from mA3+/+ mice (Figure 3C). Taken together with the protein analysis in Figure 3B, these data indicate that murine APOBEC3 is packaged into milk-borne virions and limits infectivity.
APOBEC3G is expressed in human mammary epithelial cells
We next examined human MECs, to determine whether any of the human APOBEC3 genes were also expressed these cells. We performed reverse-transcribed RT-qPCR with APOBEC3G-, 3F-, 3A-, 3B- and 3H-specific primers with RNA isolated from four different cultured MEC lines (MCF 7, MCF10, HS578T, T47D) and primary human mammary tissue. APOBEC3G RNA was detected in HS578T and MCF10 cells, as well as all the primary tissue RNAs (Figure 4A) at approximately 10-fold lower levels than the H9 T cell line (Figure S1). In contrast, APOBEC3G RNA was almost undetectable in MCF7 and T47D cells. Very low levels of APOBEC3A, 3F and 3H RNA were found in all four cell lines, only MCF7 cells produced APOBEC3A RNA and T47D, and HS578T and MCF7 but not MCF10 cells contained 3B RNA (Figure S1 and not shown). All of the tested APOBEC3 RNAs were detected in primary mammary tissue, with the exception of APOBEC3H (Figure S2).
Figure 4.

APOBEC3G is made in human mammary epithelial cells. A) RNA was isolated from MCF7, MCF10, HS578T and T47D mammary cell lines, and from seven different primary mammary gland samples from normal, non-lactating females (P1 – P7) and subjected to reverse transcribed RT-qPCR, using primers to APOBEC3G. All values were normalized to GAPDH. The values for the HS578T cell line were set at 1.0 and the results from the other cells and tissues are normalized to this value. B) Reverse transcribed RT-qPCR analysis of RNA isolated from the indicated cell lines after 48 hr treatment with estrogen (E), progesterone (P), or dexamethasone (D); - indicates untreated cells. Error bars represent the SD of 3 replicate wells of each cell line/treatment. *, NS; **, p ≤ 0.04; #, p ≤ 0.01; +, p ≤ 0.02. See also Figures S1, S2 and S3.
A recent study showed that estrogen induces the transcription of several human APOBEC3 genes (Pauklin et al., 2009). We also tested whether progesterone or the synthetic glucocorticoid dexamethasone induced expression of APOBEC3G in HS578T and T47D cells. Hs578T are estrogen receptor (ER) and progesterone receptor (PR) negative, whereas T47D cells are both ER and PR positive; both cell lines have the glucocorticoid receptor (GR) (Buser et al., 2007; Keydar et al., 1979; Small et al., 1999). No hormones significantly changed the level of APOBEC3G RNA in HS578T cells (Figure 4B). In contrast, glucocorticoids, estrogen and progesterone all induced expression of APOBEC3G in T47D cells. APOBEC3A was induced by dexamethasone in both HS578T and T47D cells, there was no effect by any hormone on APOBEC3B or 3F in either cell line and APOBEC3H levels were low even in the presence of hormones (Figure S3).
We also examined APOBEC3G protein expression in the human cell lines and tissue. Using immunoprecipitation followed by Western blot analysis, APOBEC3G protein was detected in untreated HS578T but not the other MEC lines (Figure 5A and not shown), in agreement with the higher RNA levels in HS578T cells (Figure 4A). APOBEC3G protein was also detected in progesterone-induced T47D cells (Figure 5A). In both HS578T and T47D cells, APOBEC3G protein expression was at least 10-fold lower than that detected in H9 cells, consistent with the relative RNA levels in the different cell lines (Figure S1). We also performed indirect immunohistochemistry on HS578T and T47D cells. Abundant cytoplasmic staining, with occasional punctuate structures were detected in HS578T but not T47D cells (Figure 5B). This pattern of staining is similar to that previously reported for APOBEC3G (Gallois-Montbrun et al., 2008; Gallois-Montbrun et al., 2007; Mangeat et al., 2003; Wichroski et al., 2006). Although the rabbit antisera we used also recognizes APOBEC3A (Thielen et al., 2010), both HS578T and T47D cells had very low levels of APOBEC3A RNA (Figures S1 and S3) and we did not see a band of the appropriate molecular weight in the immunoprecipitation/Western blot analyses of extracts from these cells (not shown).
Figure 5.
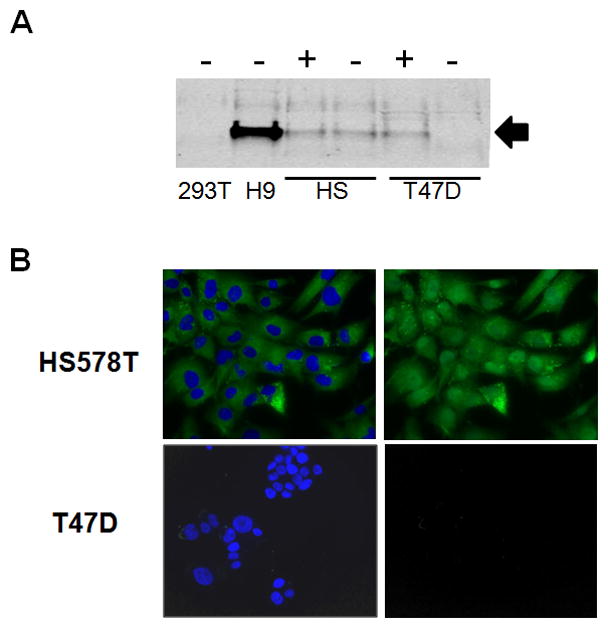
APOBEC3G protein is made in human MECs. A) Western blots of lysates made from the different MECs following 48 hr treatment with (+) or without (−) progesterone. Also included are lysates prepared from 293T (APOBEC3G-) and H9 (APOBEC3+) cells. Arrow points to APOBEC3G. B) Indirect immunofluorescence of HS578T and T47D cells, using anti-APOBEC3G antisera. Cells were counterstained with DAPI. Left panel: IF plus DAPI; right panel, IF alone.
Additionally, three different primary human mammary gland samples were stained with anti-APOBEC3G antisera (Newman et al., 2005); Figure 6 shows a representative section from one of these samples. APOBEC3G protein was detected in the luminal epithelial cells of the intralobular mammary duct. This staining was not seen in sections incubated with normal rabbit serum and or with anti-ABOBEC3G antisera in the presence of recombinant APOBEC3G (r3G) protein. In addition, occasional stromal cells showed reactivity with anti-APOBEC3G antisera which was also blocked by r3G protein. The immunostaining both in cultured mammary cells and primary tissue was predominantly cytoplasmic, indicating that the antisera were recognizing APOBEC3G. However, since we did detect APOBEC3A RNA in primary human mammary tissue (Figure S2), it is also possible that the epithelial or stromal cell staining in mammary tissue was due at least in part to APOBEC3A protein, which has predominantly been found in the nucleus (Chen et al., 2006).
Figure 6.
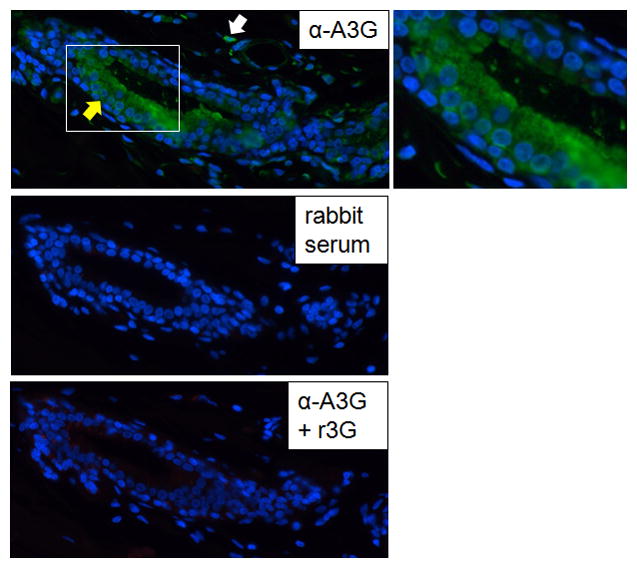
APOBEC3G protein is made in primary human mammary tissue. Indirect IF of primary mammary tissue was performed, using rabbit anti-APOBEC3G antisera in the absence (α-A3G) or presence (α-A3G +r3G) of recombinant APOBEC3G protein. An enlargement of the outlined area of the lumen is shown to the right of the top panel. Yellow arrow, luminal epithelial cells; white arrow, stromal cell.
Vif increases infectivity of HIV-1 virions produced in MECs
We next performed a proof-of-principle experiment to test whether MEC-expressed APOBEC3 proteins could restrict virus infection. HS578T and T47D cells were co-tranfected with the vectors PNL-luc+vif and PNL-luc−vif and a plasmid bearing the vesicular stomatitis virus (VSV) G protein. The transfected cells were grown in the presence and absence of progesterone to induce APOBEC3G expression. If APOBEC3G was packaged into HIV-1 virions in MECs, then vif+ virions should be more infectious than vif− virus when produced by these cells. Additionally, HIV produced by progesterone-treated T47D but not HS578T cells should be less infectious, since APOBEC3G levels were increased in response to hormone in the former and not the latter cell line. Virus was isolated from the supernatants of these cells, analyzed by Western blot analysis and ELISA for Gag protein and then used to infect NP2 cells.
First, we found that PNL-Luc+vif virus was more infectious than PNL-Luc−vif virus, when produced in HS578T cells (Figure 7A), indicating that endogenously-produced APOBEC3G may be packaged into virions and that this was counteracted by Vif. No difference in infectivity was seen when virus was produced from HS578T cells grown in the presence or absence of progesterone (Figure 7A), consistent with the observation that progesterone does not induce APOBEC3G expression in this cell line (Figure 5A). However, virus produced by progesterone-treated T47D cells was less infectious than that produced in untreated cells (Figure 7A). The presence of vif in the vector did not alter the infectivity of virus produced in T47D cells that received no progesterone (Figure 7A), most likely because there was little or no APOBEC3G expression in untreated cells (Figure 5A, B). However, PNL-Luc+vif virus was more infectious than PNL-Luc−vif virus when produced in T47D cells treated with progesterone (Figure 7A), indicating that the increased APOBECG produced in hormone-treated cells was counteracted by Vif. We also performed Western blots on the virions produced by MECs, but although we could detect Gag protein (Figure 7B) and APOBEC3G protein was detectable in cell extracts (Figure 5A), the low levels of virus produced precluded detection of APOBEC3G in virions. Although both T47D and HS578T cells both also expressed APOBEC3B RNA, which could play a role in restricting HIV produced in these cells, expression of this factor was not induced by progesterone. Moreover, APOBEC3B has weaker anti-HIV activity than APOBEC3G (Rose et al., 2005) and it is not highly inhibited by Vif (Doehle et al., 2005; Marin et al., 2008). Thus, our data indicate that APOBEC3G expressed in MECs may be packaged in HIV-1 virions and has anti-viral activity that can be counter-acted by Vif.
Figure 7.
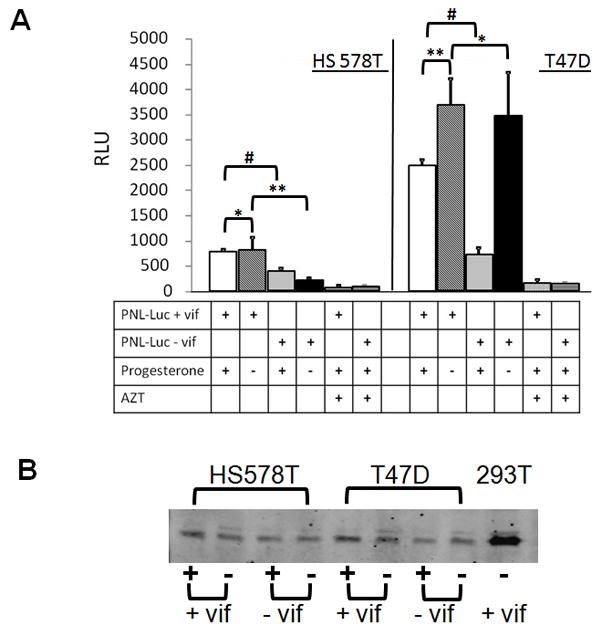
APOBEC3G made in MECs is packaged into HIV. A) HS578T (left) and T47D (right) cells were transduced with PNL-Luc that contained (+ vif) or lacked (− vif) the vif gene and a vector bearing the VSV G protein. Twenty-four hrs post-transduction, progesterone was added to the media, as indicated (+) or left untreated (−) and 24 hr later, virus was harvested from the cell supernatants and used to infect NP2 cells, in the absence or presence of AZT, as indicated. In addition, p24 levels were measured by ELISA. Twenty-four after infection, luciferase assays were performed on lysates prepared from the NP2 cells. RLUs were normalized to the p24 levels in the virus preparations. Error bars represent SD from 3 replicate infections. *, NS; **, p ≤ 0.05; #, p ≤ 0.001. This experiment was performed 3 times with similar results. B) The virion preparations were analyzed by Western blots, using anti-p24 antisera. Virions prepared in PNL-Luc+vif-transduced 293T cells were included as positive controls.
DISCUSSION
A large number of intrinsic immune factors that restrict virus infection in a variety of cell types have been identified in the past few years, in large part through study of viral proteins such as the HIV-1 Vif protein that counteract their action (Goff, 2004). Although APOBEC3G was first identified as an anti-viral factor in HIV-1 infection, APOBEC3 proteins have been shown to function as anti-viral factors against not only retroviruses but also against diverse viruses such as HBV, foamy viruses, HPV and parvoviruses and there is much interest in developing anti-viral therapies that increase the ability of APOBEC3 to inhibit viral infection. However, while much has been learned about how this family of anti-viral factors functions using cell culture models, less is known about how APOBEC3 proteins function in vivo. Recent studies in which mice with targeted deletion of the Apobec3 gene were infected with different mouse retroviruses have begun to increase our understanding of how APOBEC3 proteins decrease the pathogenic effects of in vivo infection.
Here we show that MECs express APOBEC3 that is packaged into virions and restricts infectivity. In mice, APOBEC3 is expressed in lymph nodes (T, B and DCs) at the highest levels, followed by adipose and epithelial tissues such as the mammary gland (Mikl et al., 2005; Okeoma et al., 2007; Su et al., 2004). Our studies demonstrate that APOBEC3 limits MMTV infection of the lymphoid compartment in vivo and that this restriction results in lower levels of virus infection in mammary tissue, accompanied by a decreased incidence in mammary tumorigenesis. These data are consistent with our previous studies showing that MEC infection in mammary tissue depends on the level of lymphoid cell infection (Golovkina et al., 1998). Although APOBEC3 is made in MECs, its absence in this cell type in knockout mice reconstituted with wild type bone marrow did not alter in vivo mammary gland infection.
Although virus infection levels in MECs themselves were not affected by the loss of APOBEC3, APOBEC3 protein produced in mammary tissue was packaged into virions. While there are numerous studies using transfected and primary cells as well as knockout mice to study the effect of APOBEC3 proteins on virus infection and spread, the current study provides direct demonstration that endogenous APOBEC3 protein is packaged into virions in vivo. The presence of APOBEC3 in MMTV virions resulted in a significant decrease in the relative infectivity of MMTV shed in the milk of wild type vs. knockout mice. Our data thus suggest that APOBEC3 expression in MECs is retained in mice as a way of limiting milk-borne transmission of MMTV, and perhaps other mouse viruses; several studies have indicated that the natural transmission route of MLV, which is also restricted by APOBEC3, is through milk (Duggan et al., 2006; Jenson et al., 1976). Milk-borne virus transmission in mice may also be affected by genetic variations in Apobec3. C57BL/6-derived and BALB/c mice have a number of polymorphic differences in both the coding and non-coding regions of Apobec3 and the BL/6 allele has been shown to be expressed at higher levels in vivo, as well as more effective at restricting infection by F-MLV, AK virus and MMTV (Langlois et al., 2009; Okeoma et al., 2009; Takeda et al., 2008). Indeed, we showed previously that MMTV virions isolated from the mammary tumors or milk of C57BL/6 mice were less infectious than those produced in BALB/c mice (Okeoma et al., 2009).
We also show here that APOBEC proteins expressed in human MECs have the potential to restrict virus infectivity. Human APOBEC3F, 3G and 3C are expressed at high levels in spleen, lymphocytes, DCs and monocytes, while APOBEC3B is highly expressed in cancer cells, B lymphoblasts and endothelial cells and APOBEC3A in immature monocytes (Aguiar and Peterlin, 2008; Koning et al., 2009; Refsland et al., 2010; Su et al., 2004). We detected APOBEC3A, 3B, 3F and 3G RNA in primary mammary tissue (Figure S3), but only APOBEC3B and APOBEC3G were highly expressed in cultured MECs (Figure 4A and Figure S1). This difference may be due to the presence of diverse cell types in the primary tissue (MECs, stromal cells, vascular cells, lymphoid cells, etc.); indeed, we detected APOBEC3G protein in both ductal epithelial and stromal cells in primary tissue (Figure 6). This differential expression may play a role in how APOBEC3 proteins restrict infection by diverse viruses in different tissues. The most potent inhibitors of HIV infection, APOBEC3G and 3F, are highly expressed in HIV-target lymphoid cells, while APOBEC3A, 3B, and 3H, are also expressed in keratinocytes and skin, could play a role in restricting human papilloma virus infection (Vartanian et al., 2008).
In contrast to MMTV, it is unclear whether milk-borne transmission of lenti- and delta-viruses is cell-free or cell-associated and whether lymphoid or mammary cells or both are the virus reservoir (Becquart et al., 2002; Bobat et al., 1997; Henderson et al., 2004; Hino et al., 1994; Koulinska et al., 2006; Lawrence and Lawrence, 2004; Leroy et al., 1998; Lewis et al., 1998; Li et al., 2004; Miotti et al., 1999; Pandrea et al., 2008; Rousseau et al., 2004; Rousseau et al., 2003; Rychert et al., 2006; Ziegler et al., 1985). Cultured human MECs can be productively infected with HTLVI (LeVasseur et al., 1998) and several studies have shown that human MECs express low levels of CXCR4 and can be infected by CXCR4-tropic HIV-1; at least in tissue culture cells, these cells produce infectious virus (El Messaoudi et al., 2000; Moriuchi and Moriuchi, 2004; Su et al., 2004; Toniolo et al., 1995). A more recent study has suggested that while primary MECs express CD4, CCR5 and CXCR4, they are not productively infected with HIV-1, but instead sequester the virus in endosomal compartments which could then facilitate infection of CD4+ T cells in the mammary gland (Dorosko and Connor, 2010).
Although it is unclear whether APOBEC3G in mammary tissue plays a role in restricting milk-borne HIV infection, it is interesting that this factor, the major anti-HIV family member, as well as APOBEC3A and 3B, were all highly expressed in primary mammary tissue. It may be that these factors are important for restricting milk-borne transmission of other viruses. For example, APOBEC3A has also been implicated in the restriction of papillomavirus infection, which some studies have shown to be present in human MECs and mammary tumors (de Villiers et al., 2005). That APOBEC3 RNA and protein are found in human mammary tissue suggests that this intrinsic anti-viral factor may have been retained as a means of limiting milk-borne virus transmission in humans as well as in mice.
EXPERIMENTAL PROCEDURES
Ethics Statement
All human tissues were received as unidentified archived biopsied tissue from the Tumor Tissue and Biospecimen bank from the U. of Pennsylvania. The use of these specimens received exemption from the University of Pennsylvania Institutional Research Board. All mice were housed according to the policies of the Institutional Animal Care and Use Committee of the University of Pennsylvania and all experiments performed with mice were approved by this committee.
Cell lines and human tissue
293T, HS578T, MCF 7, MCF 10A (non-tumorigenic mammary epithelial cell line), and T47D cell lines were purchased from American Type Culture Collection. H9 and NP2 cells were obtained from Robert Doms and Jim Hoxie, respectively. 293T-mTfR1 cells have been previously described (Zhang et al., 2003); MMTV infection of these cells was analyzed by RT-qPCR for viral DNA sequences (Okeoma et al., 2009). All cells were maintained according to the suppliers’ instructions. Primary human mammary tissues were obtained from the University of Pennsylvania’s tissue bank in accordance with IRB guidelines and after pathologic review.
Foster nursing and tumorigenesis studies
The mA3-knockout (mA3−/−) and matched controls (mA3+/+) mice were previously described (Okeoma et al., 2007). Age-matched pups from mA3−/− and mA3+/+ MMTV− females were foster-nursed on C57BL/6 MMTV(RIII)+ females. The foster-nursed pups were sacrificed at the indicated times. Different tissues were harvested for DNA isolation and analysis of infection by real time quantitative polymerase chain reaction (RT-qPCR) for integrated MMTV proviruses; all values were normalized to GAPDH, as previously described (Courreges et al., 2007). Foster-nursed mA3+/+ and −/− female mice were kept in constant mating and palpated weekly for mammary tumors; all mice were sacrificed at the time of tumor detection.
Adoptive transfers
Five MMTV− age-matched (6 weeks old) mA3 +/+ and −/− female mice each were lethally irradiated using a Cs-137 irradiator (Gamma-cell 40 Extractor; MDS Nordion). Four hours after irradiation, the mice were reconstituted with bone marrow stem cells from MMTV− mA3+/+ mice. One week after reconstitution, the mice were inoculated with MMTV(RIII) isolated from milk and then sacrificed 9 weeks after irradiation. Prior to sacrifice, peripheral blood lymphocytes from each mouse were subjected to FACs analysis with conjugated anti-mouse CD8, CD4, CD11c and B220 (BD-Pharmingen), to test for uniform reconstitution. At sacrifice, RNA was isolated from lymph nodes, spleens, and mammary glands and subjected to mouse reverse transcribed-PCR with primers specific to mouse APOBEC3 and DNA was isolated and analyzed by RT-qPCR with primers specific to MMTV and GAPDH (Okeoma et al., 2007).
Mouse APOBEC3 RNA and protein analysis
RNA was isolated from milk-borne virions and subjected to reverse transcribed RT-qPCR as previously described (Okeoma et al., 2009). Virions, as well extracts from tissues and 293T cells transiently transfected with mouse APOBEC3 expression vectors (+ and − exon 5; (Takeda et al., 2008) were subjected to western blot analysis with the indicated antibodies. Rabbit anti-mouse APOBEC3 antisera were generated by immunizing rabbits with a hapten-coupled peptide unique to the mouse protein (CSHRKCYSPIRNLISQE) (Lampire Biological Laboratories) followed by affinity purification of the antisera. The goat anti-MMTV antisera were originally obtained from the National Cancer Institute Biochemical Carcinogenesis Branch Repository, Bethesda, MD. Detection was accomplished using the species-appropriate horseradish peroxidase-conjugated secondary antibody followed by ECL (Amersham Biosciences, Inc.).
Human APOBEC3 RNA and protein analysis
Cells were cultured for 48 hr in the presence or absence of 1μM 17-β-estradiol, dexamethasone or progesterone (all purchased from Sigma-Aldrich). RNA was isolated from MCF7, MCF10, HS578T and T47D cells and primary tissues using RNAEasy kits (Qiagen, Inc.) and subjected to reverse transcribed RT-qPCR, with primers to APOBEC3A, –B, –F, –G, and –H (Table S1). To ensure that the reverse transcribed RT-qPCR products were specific to the corresponding Apobec3 gene, the cDNA amplicons were sequenced and the identity of the transcript was confirmed (not shown). APOBEC3G protein expression was analyzed by immunoprecipitation of extracts prepared from HS578T and T47D cells with anti-APOBEC3G monoclonal antibody (murine anti-human APOBEC3G (CEM15) #10069), followed by Western blot analysis with a rabbit anti-APOBEC3G antisera (#10201; (Thielen et al., 2007)); both were obtained from the AIDS Research and Reference Reagent Program.
Packaging of hA3G into HIV-1 virions produced in human MECs
The PNL LUC-vif and PNL LUC-Δvif plasmids were kind gifts from Matija Peterlin (Connor et al., 1995). The PNL-Luc vectors (+/− vif) viruses pseudotyped with VSV-G were generated by plasmid co-transfection of 293T, HS578T, and T47D cells using lipofectamine 2000 (Invitrogen, Inc.). Twenty-four hr after transfection, progesterone was added to the media and 48 hr later virus was harvested from the supernatants. Virions were purified from culture supernatant by centrifugation at 500 × g, followed by filtration through a 0.45μ sterile membrane filter (Millex-HV; Millipore), and pelleting through a 20% sucrose cushion by centrifugation at 105,000 × g for 1 hr. Purified virions were used to infect NP2 cells in the absence or presence of AZT. Forty-eight hrs after infection, cells were harvested and extracts subjected to luciferase assays. Luciferase relative light units (RLUs) were normalized to the p24 levels in the virus preparations. The p24 ELISAs were performed by the University of Pennsylvania Center for AIDS Research Immunology Core. The virions were also analyzed by Western blot analysis with anti-p24 antisera, a kind gift from Robert Doms.
Immunofluorescence
HS578T and T47D cells were cultured on poly-L-lysine (Sigma)-coated cover slips. Twenty four hr later, cells were fixed with paraformaldehyde. Mammary specimens (formalin-fixed and paraffin-embedded) were cut into 4 micron thick sections using a microtome and mounted on charged slides. Sections were deparafinized and rehydrated, then the antigen binding sites were unmasked (antigen unmasking solution; Vector Labs) at 98°C for 20 min. All samples were permeabilized with 0.1% Triton X-100, and non-specific binding sites blocked with normal goat serum in the presence of avidin (Vector Labs) for 1 hr at room temperature. Samples were washed and reacted with polyclonal rabbit anti-APOBEC3G antibody in the absence and presence of recombinant APOBEC3G (NIH AIDS Research and Reference Reagent Program) or normal rabbit serum (Santa Cruz Biotechnology) in the presence of biotin (Vector Labs) for 1 hr at room temperature, washed and then incubated with biotin-labeled goat anti-rabbit IgG for 30 min at 37°C. The APOBEC3G positive signal was detected with FITC-streptavidin. Images were taken with a LEICA DM1000 fluorescent microscope using a Nuance multispectral imaging camera and Nuance software (Cambridge Research Instrumentation Inc, Woburn, MA).
Statistical analysis
Statistical analysis of significant differences between experimental groups was tested using paired two-tailed Student’s t test. Error bars represent standard deviations. For the Kaplan-Meier survival curve (Figure 1D), statistical analyses were done using SAS/STAT software, Version [9] of the SAS System for Windows.
HIGHLIGHTS.
Mouse and human mammary epithelial cells (MECs) express APOBEC3 protein
Mouse mammary tumor virus (MMTV) assembled in mammary tissue packages APOBEC3 protein
APOBEC3 packaged into MMTV limits virus milk-borne transmission
APOBEC3 proteins may also reduce infectivity of viruses like HIV-1 made in human MECs
Supplementary Material
Acknowledgments
We thank Audrey Low, Matija Peterlin, James Hoxie, Masaaki Miyazawa and Robert Doms for reagents used in this study and Peter Kaplan for help with statistical analysis. This research was supported by PHS grants R01-AI-085015 and R01-CA-73746. C.M.O. was supported by a supplement to PHS grant R01-CA-73746. This publication was made possible through core services from the Penn Center for AIDS Research (CFAR), a NIH-funded program (P30 AI 045008). The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: Cat#10067, human APOBEC3G (CEM15) from Immunodiagnostics; Cat#10069, murine anti-human APOBEC3G (CEM15) monoclonal antibody from Immunodiagnostics; Cat#10201, rabbit anti-APOBEC3G-C terminal from Dr. Jaisri Lingappa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahao JS, Oliveira TM, Campos RK, Madureira MC, Kroon EG, Lobato ZI. Bovine vaccinia outbreaks: detection and isolation of vaccinia virus in milk samples. Foodborne Pathog Dis. 2009;6:1141–1146. doi: 10.1089/fpd.2009.0324. [DOI] [PubMed] [Google Scholar]
- Aguiar RS, Peterlin BM. APOBEC3 proteins and reverse transcription. Virus Res. 2008;134:74–85. doi: 10.1016/j.virusres.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Allison RW, Hoover EA. Feline immunodeficiency virus is concentrated in milk early in lactation. AIDS Res Hum Retroviruses. 2003;19:245–253. doi: 10.1089/088922203763315759. [DOI] [PubMed] [Google Scholar]
- Becquart P, Chomont N, Roques P, Ayouba A, Kazatchkine MD, Belec L, Hocini H. Compartmentalization of HIV-1 between breast milk and blood of HIV-infected mothers. Virology. 2002;300:109–117. doi: 10.1006/viro.2002.1537. [DOI] [PubMed] [Google Scholar]
- Bobat R, Moodley D, Coutsoudis A, Coovadia H. Breastfeeding by HIV-1-infected women and outcome in their infants: a cohort study from Durban, South Africa. AIDS. 1997;11:1627–1633. doi: 10.1097/00002030-199713000-00012. [DOI] [PubMed] [Google Scholar]
- Bouhlal H, Latry V, Requena M, Aubry S, Kaveri SV, Kazatchkine MD, Belec L, Hocini H. Natural antibodies to CCR5 from breast milk block infection of macrophages and dendritic cells with primary R5-tropic HIV-1. J Immunol. 2005;174:7202–7209. doi: 10.4049/jimmunol.174.11.7202. [DOI] [PubMed] [Google Scholar]
- Buser AC, Gass-Handel EK, Wyszomierski SL, Doppler W, Leonhardt SA, Schaack J, Rosen JM, Watkin H, Anderson SM, Edwards DP. Progesterone receptor repression of prolactin/signal transducer and activator of transcription 5-mediated transcription of the beta-casein gene in mammary epithelial cells. Mol Endocrinol. 2007;21:106–125. doi: 10.1210/me.2006-0297. [DOI] [PubMed] [Google Scholar]
- Case LK, Purdy A, Golovkina TV. Molecular and cellular basis of the retrovirus resistance in I/LnJ mice. J Immunol. 2005;175:7543–7549. doi: 10.4049/jimmunol.175.11.7543. [DOI] [PubMed] [Google Scholar]
- Chen H, Lilley CE, Yu Q, Lee DV, Chou J, Narvaiza I, Landau NR, Weitzman MD. APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr Biol. 2006;16:480–485. doi: 10.1016/j.cub.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Chiu YL, Greene WC. The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu Rev Immunol. 2008;26:317–353. doi: 10.1146/annurev.immunol.26.021607.090350. [DOI] [PubMed] [Google Scholar]
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Courreges MC, Burzyn D, Nepomnaschy I, Piazzon I, Ross SR. Critical role of dendritic cells in mouse mammary tumor virus in vivo infection. J Virol. 2007;81:3769–3777. doi: 10.1128/JVI.02728-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR. Role and mechanism of action of the APOBEC3 family of antiretroviral resistance factors. J Virol. 2006;80:1067–1076. doi: 10.1128/JVI.80.3.1067-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers EM, Sandstrom RE, zur Hausen H, Buck CE. Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res. 2005;7:R1–11. doi: 10.1186/bcr940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehle BP, Schafer A, Wiegand HL, Bogerd HP, Cullen BR. Differential sensitivity of murine leukemia virus to APOBEC3-mediated inhibition is governed by virion exclusion. J Virol. 2005;79:8201–8207. doi: 10.1128/JVI.79.13.8201-8207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorosko SM, Connor RI. Primary human mammary epithelial cells endocytose human immunodeficiency virus (HIV)-1 and facilitate viral infection of CD4+ T lymphocytes. Journal of virology. 2010 doi: 10.1128/JVI.01263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan J, Okonta H, Chakraborty J. Transmission of Moloney murine leukemia virus (ts-1) by breast milk. J Gen Virol. 2006;87:2679–2684. doi: 10.1099/vir.0.82015-0. [DOI] [PubMed] [Google Scholar]
- El Messaoudi K, Thiry LF, Liesnard C, Van Tieghem N, Bollen A, Moguilevsky N. A human milk factor susceptible to cathepsin D inhibitors enhances human immunodeficiency virus type 1 infectivity and allows virus entry into a mammary epithelial cell line. J Virol. 2000;74:1004–1007. doi: 10.1128/jvi.74.2.1004-1007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallois-Montbrun S, Holmes RK, Swanson CM, Fernandez-Ocana M, Byers HL, Ward MA, Malim MH. Comparison of cellular ribonucleoprotein complexes associated with the APOBEC3F and APOBEC3G antiviral proteins. J Virol. 2008;82:5636–5642. doi: 10.1128/JVI.00287-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallois-Montbrun S, Kramer B, Swanson CM, Byers H, Lynham S, Ward M, Malim MH. Antiviral protein APOBEC3G localizes to ribonucleoprotein complexes found in P bodies and stress granules. J Virol. 2007;81:2165–2178. doi: 10.1128/JVI.02287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SP. Retrovirus restriction factors. Mol Cell. 2004;16:849–859. doi: 10.1016/j.molcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Golovkina TV, Dudley JP, Ross SR. Superantigen activity is need for mouse mammary tumor virus spread within the mammary gland. J Immunol. 1998;161:2375–2382. [PubMed] [Google Scholar]
- Golovkina TV, Jaffe AB, Ross SR. Coexpression of exogenous and endogenous mouse mammary tumor virus RNA in vivo results in viral recombination and broadens the virus host range. J Virol. 1994;68:5019–5026. doi: 10.1128/jvi.68.8.5019-5026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GJ, Hoffman NG, Ping LH, Fiscus SA, Hoffman IF, Kitrinos KM, Banda T, Martinson FE, Kazembe PN, Chilongozi DA, et al. HIV-1 populations in blood and breast milk are similar. Virology. 2004;330:295–303. doi: 10.1016/j.virol.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Hino S, Katamine S, Kawase K, Miyamoto T, Doi H, Tsuji Y, Yamabe T. Intervention of maternal transmission of HTLV-1 in Nagasaki, Japan. Leukemia. 1994;8(Suppl 1):S68–70. [PubMed] [Google Scholar]
- Jenson AB, Groff DE, McConahey PJ, Dixon FJ. Transmission of murine leukemia virus (Scripps) from parent to progeny mice as determined by p30 antigenemia. Cancer Res. 1976;36:1228–1232. [PubMed] [Google Scholar]
- John-Stewart GC, Mbori-Ngacha D, Payne BL, Farquhar C, Richardson BA, Emery S, Otieno P, Obimbo E, Dong T, Slyker J, et al. HV-1-specific cytotoxic T lymphocytes and breast milk HIV-1 transmission. J Infect Dis. 2009;199:889–898. doi: 10.1086/597120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keydar I, Chen L, Karby S, Weiss FR, Delarea J, Radu M, Chaitcik S, Brenner HJ. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979;15:659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- Koning FA, Newman EN, Kim EY, Kunstman KJ, Wolinsky SM, Malim MH. Defining APOBEC3 expression patterns in human tissues and hematopoietic cell subsets. J Virol. 2009;83:9474–9485. doi: 10.1128/JVI.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulinska IN, Villamor E, Chaplin B, Msamanga G, Fawzi W, Renjifo B, Essex M. Transmission of cell-free and cell-associated HIV-1 through breast-feeding. J Acquir Immune Defic Syndr. 2006;41:93–99. doi: 10.1097/01.qai.0000179424.19413.24. [DOI] [PubMed] [Google Scholar]
- Langlois MA, Kemmerich K, Rada C, Neuberger MS. The AKV murine leukemia virus is restricted and hypermutated by mouse APOBEC3. J Virol. 2009;83:11550–11559. doi: 10.1128/JVI.01430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRue RS, Andresdottir V, Blanchard Y, Conticello SG, Derse D, Emerman M, Greene WC, Jonsson SR, Landau NR, Lochelt M, et al. Guidelines for naming nonprimate APOBEC3 genes and proteins. J Virol. 2009;83:494–497. doi: 10.1128/JVI.01976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RM, Lawrence RA. Breast milk and infection. Clin Perinatol. 2004;31:501–528. doi: 10.1016/j.clp.2004.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy V, Newell ML, Dabis F, Peckham C, Van de Perre P, Bulterys M, Kind C, Simonds RJ, Wiktor S, Msellati P. International multicentre pooled analysis of late postnatal mother-to-child transmission of HIV-1 infection. Ghent International Working Group on Mother-to-Child Transmission of HIV. Lancet. 1998;352:597–600. doi: 10.1016/s0140-6736(98)01419-6. [DOI] [PubMed] [Google Scholar]
- LeVasseur RJ, Southern SO, Southern PJ. Mammary epithelial cells support and transfer productive human T-cell lymphotropic virus infections. J Human Virol. 1998;1:214–223. [PubMed] [Google Scholar]
- Lewis P, Nduati R, Kreiss JK, John GC, Richardson BA, MboriNgacha D, Ndinya-Achola J, Overbaugh J. Cell-free human immunodeficiency virus type 1 in breast milk. J Inf Dis. 1998;177:34–39. doi: 10.1086/513816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HC, Biggar RJ, Miley WJ, Maloney EM, Cranston B, Hanchard B, Hisada M. Provirus load in breast milk and risk of mother-to-child transmission of human T lymphotropic virus type I. J Infect Dis. 2004;190:1275–1278. doi: 10.1086/423941. [DOI] [PubMed] [Google Scholar]
- Lindsey CL, Almeida ME, Vicari CF, Carvalho C, Yaguiu A, Freitas AC, Becak W, Stocco RC. Bovine papillomavirus DNA in milk, blood, urine, semen, and spermatozoa of bovine papillomavirus-infected animals. Genet Mol Res. 2009;8:310–318. doi: 10.4238/vol8-1gmr573. [DOI] [PubMed] [Google Scholar]
- Low A, Okeoma CM, Lovsin N, de las Heras M, Taylor TH, Peterlin BM, Ross SR, Fan H. Moloney murine leukemia virus infection in mice lacking the murine APOBEC3 gene. Virology. 2009;385:455–463. doi: 10.1016/j.virol.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci. 2009;364:675–687. doi: 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Marin M, Golem S, Rose KM, Kozak SL, Kabat D. Human immunodeficiency virus type 1 Vif functionally interacts with diverse APOBEC3 cytidine deaminases and moves with them between cytoplasmic sites of mRNA metabolism. J Virol. 2008;82:987–998. doi: 10.1128/JVI.01078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J, Lienicke U, Tschirch E, Kruger DH, Wauer RR, Prosch S. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J Clin Microbiol. 2005;43:1318–1324. doi: 10.1128/JCM.43.3.1318-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikl MC, Watt IN, Lu M, Reik W, Davies SL, Neuberger MS, Rada C. Mice deficient in APOBEC2 and APOBEC3. Mol Cell Biol. 2005;25:7270–7277. doi: 10.1128/MCB.25.16.7270-7277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotti PG, Taha TE, Kumwenda NI, Broadhead R, Mtimavalye LA, Van der Hoeven L, Chiphangwi JD, Liomba G, Biggar RJ. HIV transmission through breastfeeding: a study in Malawi. JAMA. 1999;282:744–749. doi: 10.1001/jama.282.8.744. [DOI] [PubMed] [Google Scholar]
- Moriuchi M, Moriuchi H. Cell-type-dependent effect of transforming growth factor beta, a major cytokine in breast milk, on human immunodeficiency virus type 1 infection of mammary epithelial MCF-7 cells or macrophages. J Virol. 2004;78:13046–13052. doi: 10.1128/JVI.78.23.13046-13052.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S, McGrath CM. Mammary neoplasia in mice. Adv Canc Res. 1973;17:353–414. [Google Scholar]
- Newman EN, Holmes RK, Craig HM, Klein KC, Lingappa JR, Malim MH, Sheehy AM. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Curr Biol. 2005;15:166–170. doi: 10.1016/j.cub.2004.12.068. [DOI] [PubMed] [Google Scholar]
- O’Neil LL, Burkhard MJ, Diehl LJ, Hoover EA. Vertical transmission of feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1995;11:171–182. doi: 10.1089/aid.1995.11.171. [DOI] [PubMed] [Google Scholar]
- Okeoma CM, Lovsin N, Peterlin BM, Ross SR. APOBEC3 inhibits mouse mammary tumor virus replication in vivo. Nature. 2007;445:927–930. doi: 10.1038/nature05540. [DOI] [PubMed] [Google Scholar]
- Okeoma CM, Petersen J, Ross SR. Expression of murine APOBEC3 alleles in different mouse strains and their effect on mouse mammary tumor virus infection. J Virol. 2009;83:3029–3038. doi: 10.1128/JVI.02536-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Onanga R, Souquiere S, Mouinga-Ondeme A, Bourry O, Makuwa M, Rouquet P, Silvestri G, Simon F, Roques P, et al. Paucity of CD4+ CCR5+ T cells may prevent transmission of simian immunodeficiency virus in natural nonhuman primate hosts by breast-feeding. J Virol. 2008;82:5501–5509. doi: 10.1128/JVI.02555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauklin S, Sernandez IV, Bachmann G, Ramiro AR, Petersen-Mahrt SK. Estrogen directly activates AID transcription and function. J Exp Med. 2009;206:99–111. doi: 10.1084/jem.20080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permar SR, Kang HH, Carville A, Mansfield KG, Gelman RS, Rao SS, Whitney JB, Letvin NL. Potent simian immunodeficiency virus-specific cellular immune responses in the breast milk of simian immunodeficiency virus-infected, lactating rhesus monkeys. J Immunol. 2008;181:3643–3650. doi: 10.4049/jimmunol.181.5.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refsland EW, Stenglein MD, Shindo K, Albin JS, Brown WL, Harris RS. Quantitative profiling of the full APOBEC3 mRNA repertoire in lymphocytes and tissues: implications for HIV-1 restriction. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose KM, Marin M, Kozak SL, Kabat D. Regulated production and anti-HIV type 1 activities of cytidine deaminases APOBEC3B, 3F, and 3G. AIDS Res Hum Retroviruses. 2005;21:611–619. doi: 10.1089/aid.2005.21.611. [DOI] [PubMed] [Google Scholar]
- Ross SR. MMTV infectious cycle and the contribution of virus-encoded proteins to transformation of mammary tissue. J Mammary Gland Biol Neoplasia. 2008;13:299–307. doi: 10.1007/s10911-008-9090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau CM, Nduati RW, Richardson BA, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK, Overbaugh J. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis. 2004;190:1880–1888. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau CM, Nduati RW, Richardson BA, Steele MS, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK, Overbaugh J. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J Infect Dis. 2003;187:741–747. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychert J, Lacour N, Amedee AM. Genetic analysis of simian immunodeficiency virus expressed in milk and selectively transmitted through breastfeeding. J Virol. 2006;80:3721–3731. doi: 10.1128/JVI.80.8.3721-3731.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago ML, Montano M, Benitez R, Messer RJ, Yonemoto W, Chesebro B, Hasenkrug KJ, Greene WC. Apobec3 encodes Rfv3, a gene influencing neutralizing antibody control of retrovirus infection. Science. 2008;321:1343–1346. doi: 10.1126/science.1161121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkola M, Rintala M, Grenman S, Syrjanen S. Human papillomavirus DNA detected in breast milk. Pediatr Infect Dis J. 2008;27:557–558. doi: 10.1097/INF.0b013e318169ef47. [DOI] [PubMed] [Google Scholar]
- Sellon RK, Jordan HL, KennedyStoskopf S, Tompkins MB, Tompkins WAF. Feline immunodeficiency virus can be experimentally transmitted via milk during acute maternal infection. J Virol. 1994;68:3380–3385. doi: 10.1128/jvi.68.5.3380-3385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer V, Benjamin F. Breast-feeding and the potential for human immunodeficiency virus transmission. Obstet Gynecol. 1990;75:713–715. [PubMed] [Google Scholar]
- Small W, Jr, Molteni A, Kim YT, Taylor JM, Ts’ao CH, Ward WF. Mechanism of captopril toxicity to a human mammary ductal carcinoma cell line in the presence of copper. Breast Cancer Res Treat. 1999;55:223–229. doi: 10.1023/a:1006233521325. [DOI] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda E, Tsuji-Kawahara S, Sakamoto M, Langlois MA, Neuberger MS, Rada C, Miyazawa M. Mouse APOBEC3 restricts Friend leukemia virus infection and pathogenesis in vivo. Journal of Virology. 2008;82:10998–11008. doi: 10.1128/JVI.01311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen BK, Klein KC, Walker LW, Rieck M, Buckner JH, Tomblingson GW, Lingappa JR. T cells contain an RNase-insensitive inhibitor of APOBEC3G deaminase activity. PLoS Pathog. 2007;3:1320–1334. doi: 10.1371/journal.ppat.0030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen BK, McNevin JP, McElrath JM, Vander Stoep Hunt B, Klein KC, Lingappa JR. Innate immune signaling induces high levels of TC-specific deaminase activity in primary monocyte-derived cells through exression of APOBEC3A isoforms. J Biol Chem. 2010 doi: 10.1074/jbc.M110.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniolo A, Serra C, Conaldi PG, Basolo F, Falcone V, Dolei A. Productive HIV-1 infection of normal human mammary epithelial cells. AIDS. 1995;9:859–866. doi: 10.1097/00002030-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Vartanian JP, Guetard D, Henry M, Wain-Hobson S. Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science. 2008;320:230–233. doi: 10.1126/science.1153201. [DOI] [PubMed] [Google Scholar]
- Walker WA. The dynamic effects of breastfeeding on intestinal development and host defense. Adv Exp Med Biol. 2004;554:155–170. doi: 10.1007/978-1-4757-4242-8_15. [DOI] [PubMed] [Google Scholar]
- Wichroski MJ, Robb GB, Rana TM. Human retroviral host restriction factors APOBEC3G and APOBEC3F localize to mRNA processing bodies. PLoS Pathog. 2006;2:e41. doi: 10.1371/journal.ppat.0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rassa JC, deObaldia EM, Albritton L, Ross SR. Identification of the mouse mammary tumor virus envelope receptor-binding domain. J Virol. 2003;77:10468–10478. doi: 10.1128/JVI.77.19.10468-10478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler RB, Johnson RO, Cooper DA, Gold J. Postnatal transmission of aids-associated retrovirus from mother to infant. Lancet. 1985;i:896–898. doi: 10.1016/s0140-6736(85)91673-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


