Abstract
Mammalian electron transfer flavoproteins comprise a mitochondrial matrix heterodimer, and an electron transfer flavoprotein dehydrogenase localized in the mitochondrial inner membrane. Electrons from primary acyl-CoA dehydrogenases, of mitochondrial metabolism of fatty acids and amino acids, are transferred to the matricial heterodimer and, subsequently, to the electron transfer flavoprotein dehydrogenase, which transfers electrons to ubiquinone of the mitochondrial electron transport chain. Several evidences suggest that these proteins may convey electrons directly to molecular oxygen, yielding reactive oxygen species. In this work, we investigated phenotypes of the yeast mutants affected in the orthologous genes of the matrix heterodimer (AIM45 and YGR207c/CIR1) and of the electron transfer flavoprotein dehydrogenase (YOR356w/CIR2). The mutant strains aim45 and yor356w/cir2 displayed better growth on several non-fermentable carbon sources, which depended on the component of the electron transport chain that accepts the electrons resulting from its mitochondrial oxidation. Furthermore, upon heat shock, the mutant strains presented decreased intracellular oxidation, suggesting that these flavoproteins are a source of reactive oxygen species. Other phenotypes identified suggest that AIM45, YGR207c/CIR1 and YOR356w/CIR2 can protect cells from oxidative and heat stress, which encompass increased heat stress sensitivity, superoxide sensitivity, both only on non-fermentable carbon sources.
Keywords: Electron transfer flavoproteins (ETF), mitochondrion, intracellular oxidation under stress conditions.
INTRODUCTION
Mitochondria are essential organelles; they perform fundamental functions in the energetic metabolism by providing ATP for energy-requiring cellular processes, in the anabolism of amino acids, and even in programmed cell death. To understand how the organelle functions we need to determine the function of all its proteins, which are encoded by the nuclear and the mitochondrial genomes. The simple eukaryotic organism Saccharomyces cerevisiae has been proven to be a fundamental model for the research of many aspects of cell biology, including the study of mitochondria at physiological, genetic and molecular levels. In fact, most of its mitochondrial proteins are similar to those found in human mitochondria but investigation on yeast mitochondrial proteome has shown the existence of proteins with unknown function [1, 2]. It is the case of the putative electron transfer flavoprotein (ETF) and the electron transfer flavoprotein dehydrogenase (ETF-dH), where the corresponding genes are still functionally unknown, in contrast with the human orthologs [3].
In mammals, the ETF is a heterodimer composed of subunits ETFα and ETFβ, involved in electron transfer from primary acyl-CoA dehydrogenases to ETF-dehydrogenase (ETF-dH), associated to the mitochondrial inner membrane, which transfers electrons to ubiquinone (CoQ) of the mitochondrial electron transport chain (ETC). Electron-donor metabolic reactions in which acyl-CoA dehydrogenases are catalysts are β-oxidation, amino acid catabolism [4], choline catabolism [5] and sarcosine and dimethylglycine catabolism [6]. In the case of β-oxidation, ETF and ETF-dH transfer electrons from the first reaction catalyzed by acyl-CoA dehydrogenases (with specificity of action depending on the length of the fatty acid) to CoQ [7]. The ETF heterodimer becomes reduced (ETFH2) and transfers electrons to the mitochondrial inner membrane-bound ETF-dH that, in turn, becomes reduced and able to transfer electrons to CoQ. In the catabolism of the amino acids lysine, hydroxylysine and tryptophan, reports have suggested that ETF is coupled to oxidative decarboxylation of glutaric acid, catalyzed by the flavo-enzyme glutarylCoA dehydrogenase, in the metabolic pathway leading to acetyl-CoA [6, 4]. For the catabolism of the amino acids leucine and valine/isoleucine, acyl-CoA dehydrogenases catalyzing one reaction in leucine oxidation (isovaleryl- CoA dehydrogenase) and valine/isoleucine oxidation are known to transfer electrons to ETF [8]. Choline catabolism involves conversion to glycine betaine through two dehydrogenation reactions catalyzed by mitochondrial choline oxidase, which, subsequently transfer electrons to ETF [9]. Catabolism of sarcosine and dimethylglycine is also coupled to the mitochondrial electron transport system by the transfer of electrons from, respectively, the flavo-enzymes sarcosine dehydrogenase and dimethylglycine dehydrogenase to the ETF [6].
The dehydrogenation of the heterodimer ETFH2 to ETFH and ETF is catalyzed by ETF-dH and the rate of this reaction is decreased by mutations in ETFα. This causes a decrease in the rate of reduction of CoQ and β-oxidation impairment. These mutations, together with mutations in ETFβ and ETF-dH have been demonstrated to cause the disease glutaric aciduria type II [10]. In mammalian cells, β-oxidation takes place in both mitochondria and peroxisomes, depending on fatty acid length and number and position of double bonds. Indirect evidence suggests the existence of a β-oxidation metabolon in mitochondria [11], associating β-oxidation enzymes together and with other proteins like ETF and ETF-dH. On the other hand, in yeast, β-oxidation is exclusively peroxisomal and, hence, not directly coupled to ATP generation. The yeast orthologues of ETFα, ETFβ and ETF-dH were reported to be mitochondrial proteins [12, 1] and are encoded by, respectively, AIM45, YGR207c/CIR1 and YOR356w/CIR2. Several evidence suggest that yeast ETF and ETF-dH could play additional roles in mitochondrial metabolism. In an approach involving analysis of yeast cell extracts by two-dimensional electrophoresis and identification by mass spectrometry, it was proposed that Ypr004c/Aim45 and Yor356w/Cir2 are associated in a mitochondrial dehydrogenases supramolecular complex [12]. This complex associates mitochondrial dehydrogenases of the tricarboxylic acid (TCA) cycle, dehydrogenases bound to the mitochondrial inner membrane involved in oxidation of NADH and glycerol 3-phosphate and the aldehyde dehydrogenase of ethanol metabolism. Furthermore, genetic interaction of YPR004c/AIM45 with SDH1, encoding succinate dehydrogenase, suggests yeasts may use both succinate dehydrogenase and ETF with ETF-dH to convey electrons to ubiquinone [13]. Interestingly, macromolecular complexes involving ETF and ETF-dH have been reported in mammalian cells [14]. These complexes associate ETF and ETF-dH with ubiquinone, complex III and succinate dehydrogenase or medium-chain acyl-CoA dehydrogenase. Another physical interaction involves Ypr004c/Aim45 and Ygr207c/Cir1 with frataxin, a protein involved in mitochondrial iron homeostasis and in mitochondrial energy conversion and oxidative phosphorylation [15]. Together, these results point to an activity of ETF and ETF-dH close to succinate dehydrogenase complex and frataxin in transferring electrons to ubiquinone [13].
The close interaction of ETF and ETF-dH with mitochondrial dehydrogenases and their role in electron transfer from metabolic reactions to the ETC, suggest that they can be part of the oxidative stress response and to be a source of reactive oxygen species (ROS) under impairment of mitochondrial function. In this study, we used aim45, ygr207c/cir1 and yor356w/cir2 mutants to assess the growth performance, intracellular redox state and oxidative and heat stress sensitivity to show that the yeast putative ETF and ETF-dH can be a source of reactive oxygen species and can participate in the cellular response against oxidative stress under stress conditions.
MATERIALS AND METHODOLOGY
Strains and Media
Saccharomyces cerevisiae strains used in this study are BY4741 (MATa; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0) and the derived mutants in AIM45 (BY4741 AIM45::kanMX4), YGR207c/CIR1 (BY4741 YGR207c/CIR1::kanMX4) and YOR356w/CIR2 (BY4741 YOR356w/CIR2::kanMX4), all supplied by Euroscarf [16]. The BY4741-derived mutant strains affected in AIM45, YGR207c/CIR1 and YOR356w/CIR2 are hereafter referred as aim45, ygr207c/cir1 and yor356w/cir2, respectively. For all experiments, strains were grown on YPD (yeast extract, 1% w/v; peptone, 2% w/v and glucose, 2% w/v), YNBDUra- (yeast nitrogen base, 0.67% w/v; glucose, 2% w/v; and auxotrophic requirements, 40mg mL-1), YNBMethUra- (yeast nitrogen base, 0.67% w/v; methanol, 0.5% w/v; and auxotrophic requirements, 40 mg mL-1) or YNBRUra- (yeast nitrogen base, 0.67% w/v; raffinose, 2% w/v; and auxotrophic requirements, 40 mg mL-1) at 30ºC or 39ºC, 200r.p.m. and monitored for growth spectrofotometrically at 600nm. For growth plate assays, cells were grown overnight on YPD, diluted to OD600=1, serially diluted with sterilized deionized water and 3μL of each dilution were spotted on each culture medium. Media tested were based on YPD supplemented with agar (2% w/v) and replacement of the carbon source by ethanol (YPEth), acetate (YPAcet), lactate (YPLact), pyruvate (YPPyr), malate (YPMal) and succinate (YPSucc), all at 2% (w/v) concentration, and methanol (YPMeth) at 0.5% (v/v). For oxidative stress resistance tests, 3.5mM hydrogen peroxide or 0.025mM menadione sodium bisulfite were added to YPD or YPEth. Escherichia coli strain DH5α (F- endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR nupG Φ80dlacZΔM15 Δ(lacZYA-argF)U169, hsdR17(rK- mK+), λ–) was used for Pro41 plasmid construction and LB medium (tryptone, 1% w/v; yeast extract, 0.5% w/v; NaCl, 1% w/v; pH 7.0) was used for bacterial cultures with incubation at 37ºC, 200r.p.m.
Oxidative Fluorescence
The methodology for intracellular oxidation measurement was based on the method described previously [17]. Briefly, cells were grown overnight on YPD, centrifuged to discard the medium, resuspended in fresh YPD to OD600=0.1 and incubated under the same conditions to allow further growth for two generations. For each assay, 20mL of this culture were further incubated with the oxidant-sensitive probe 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA, 50μM final concentration) for 60 minutes at 30ºC and 200r.p.m., to load the cells with the fluorochrome. Cells were washed twice with ice-cold 50mM phosphate buffer, pH6.0 and resuspended in 1mL of the same buffer in a 1.5mL microtube. Subsequently, cells were heat shocked in a water bath at 50ºC for 30 minutes and immediately placed on ice before further manipulation. Then, cells were washed twice with ice-cold phosphate buffer, mixed with an equal volume of glass beads (0.5mm diameter), resuspended on 500μL of the same buffer and disrupted by vortexing for 1 minute 5 times with 1 minute interval on ice. Cell debris and glass beads were sedimented by centrifugation and the supernatant was collected and maintained on ice until fluorescence measurement. Fluorescence measurements were made in 200μL of cell-free extract mixed with 2mL phosphate buffer using a Perkin-Elmer LS-50 spectrofluorimeter with excitation wavelength at 504nm and emission wavelength at 524nm. Fluorescence values were divided by the total protein of each supernatant determined with the Bio-Rad Protein Assay kit.
Plasmid Construction and S. cerevisiae Transformation
Plasmid Pro41 containing a gene fusion encoding the green fluorescent protein (GFP) fused in frame with the peroxisome-targeting sequence serine-lysine-leucine (GFP:skl) in its C-terminal end and the auxotrophic marker URA3 was constructed from plasmid Pca41, kindly supplied by Ronald Wanders [18]. The centromeric Pca41 plasmid contains the mentioned construct downstream the catalase (CTA1) promoter and, additionally, a TRP1 auxotrophic marker. In order to be able to select transformants and to maintain the plasmid in the strains used in this study, the TRP1 marker was replaced by the URA3 marker as follows. A ligation reaction was set up with the products of Pca41 double digestion with BglI and BglII (yielding a BglI-BglI 3550-bp fragment containing the 3’ end of ampR, the CTA1 promoter, the insert GFP:skl and the 5’ end of lacZ; a BglI-BglII fragment of 1736-bp containing the 5’ end of ampR, the TRP1 marker and part of the ARS1 sequence; and a BglII-BglI fragment of 1550-bp containing part of the ARS1 sequence and the 3’ end of lacZ) and of the YCplac33 digestion with BglI (yielding a 1568-bp fragment containing the 3’ end of ampR and the 5’ end of lacZ and a 4035-bp fragment containing the 5’ end of ampR, the URA3 marker, the ARS1 sequence and the 3’ end of lacZ). After E. coli transformation with the reaction mixture, colonies were screened for Pro41 plasmid yielding BglI-BglI 3550-bp, BglI-BglII 2485-bp and BglII-BglI 1550-bp fragments upon BglI and BglII double digestion. After selection of the E. coli transformant harboring the correct plasmid, plasmid DNA was purified by the alkaline lysis method and DNA was kept at 4ºC until use. Ligation reactions, restriction enzyme digestion reactions, E. coli transformation and plasmid extraction were performed according to manufacturer’s instructions and to standard protocols [19].
Plasmid Pro41 containing a gene fusion encoding the green fluorescent protein (GFP) fused in frame with the peroxisome-targeting sequence serine-lysine-leucine (GFP:skl) in its C-terminal end and the auxotrophic marker URA3 was constructed from plasmid Pca41, kindly supplied by Ronald Wanders [18]. The centromeric Pca41 plasmid contains the mentioned construct downstream the catalase (CTA1) promoter and, additionally, a TRP1 auxotrophic marker. In order to be able to select transformants and to maintain the plasmid in the strains used in this study, the TRP1 marker was replaced by the URA3 marker as follows. A ligation reaction was set up with the products of Pca41 double digestion with BglI and BglII (yielding a BglI-BglI 3550-bp fragment containing the 3’ end of ampR, the CTA1 promoter, the insert GFP:skl and the 5’ end of lacZ; a BglI-BglII fragment of 1736-bp containing the 5’ end of ampR, the TRP1 marker and part of the ARS1 sequence; and a BglII-BglI fragment of 1550-bp containing part of the ARS1 sequence and the 3’ end of lacZ) and of the YCplac33 digestion with BglI (yielding a 1568-bp fragment containing the 3’ end of ampR and the 5’ end of lacZ and a 4035-bp fragment containing the 5’ end of ampR, the URA3 marker, the ARS1 sequence and the 3’ end of lacZ). After E. coli transformation with the reaction mixture, colonies were screened for Pro41 plasmid yielding BglI-BglI 3550-bp, BglI-BglII 2485-bp and BglII-BglI 1550-bp fragments upon BglI and BglII double digestion. After selection of the E. coli transformant harboring the correct plasmid, plasmid DNA was purified by the alkaline lysis method and DNA was kept at 4ºC until use. Ligation reactions, restriction enzyme digestion reactions, E. coli transformation and plasmid extraction were performed according to manufacturer’s instructions and to standard protocols [19].
Peroxisome Proliferation
Plasmid Pro41 was used to transform BY4741, aim45, ygr207c/cir1 and yor356w/cir2 strains by the lithium acetate method [19]. Cells were plated on minimal medium with auxotrophic requirements except uracil (YNBDUra-) to select transformants. For each strain, three independent transformants were isolated, grown on methanol as sole carbon and energy source and checked for GFP fluorescence with a Leica Microsystems DM fluorescence microscope. The presence of punctuated green fluorescence inside the cells was indicative of the presence of peroxisomes, successful transformation with the Pro41 plasmid and expression of the GFP:skl reporter. For peroxisome proliferation experiments, cells were grown overnight on YNBDUra- (30ºC, 200r.p.m.), washed once with sterilized deionized water, resuspended in the same volume of fresh YNBDUra- or YNBMethUra- media and incubated for 4h or 6h, respectively, at the same conditions (YNBMethUra- and YNBDUra-) or at 39ºC, 200r.p.m. (YNBDUra-) before observation. For antimycin A induction of peroxisome proliferation, cells were grown overnight on YNBRUra- at 30ºC, 200r.p.m., antimycin A was added at 1μg mL-1 final concentration and, after 28h incubation, cells were observed. For fluorescence microscopy observation, an aliquot of the culture was used and the number of cells with punctuated fluorescence was recorded. A minimum of 100 cells was counted and results were expressed as percentage of cells with peroxisomes referred to the total number of cells.
Statistical Analysis
Data are expressed as mean values ±SD of at least three independent assays. Values were compared by t test and p < 0.05 was considered as statistically significant.
RESULTS
Mutants Affected in AIM45, YGR207c/CIR1 and YOR356w/CIR2 Display Higher Growth Rate on Non-fermentable Carbon Sources
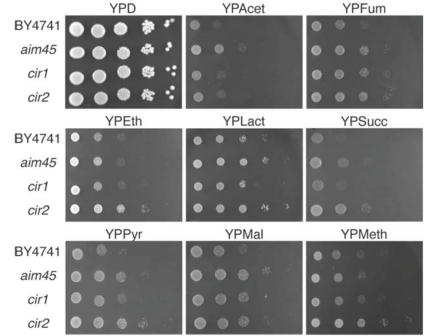
Alignment of the predicted amino acid sequences of Aim45, Ygr207c/Cir1 and Yor356w/Cir2 with human ETFα, ETFβ and ETF-dH, respectively, shows significant homology, the identity ranging from 46% in Ygr207c/Cir1 and ETFβ to 50% in Aim45 and ETFα and 52% in Yor356w/Cir2 and ETF-dH. This homology might correspond to similar functions in yeast cells, however, fundamental differences between mitochondria from mammalians and yeasts, like the absence of β-oxidation in yeast mitochondria, suggest that these proteins could have other functions. As a first approach, we investigated the growth performance of the mutant strains on glucose and on different non-fermentable carbon sources, including metabolites that feed electrons to the mitochondrial external NADH dehydrogenases Nde1 and Nde2 (ethanol, pyruvate and acetate), the mitochondrial internal NADH dehydrogenase Ndi1 (ethanol, pyruvate, lactate, malate and, indirectly, fumarate), complex II from the electron transport chain (ETC) (succinate) and a peroxisome-metabolizable substrate (methanol) (Fig. 1). Generally, mutants affected in AIM45 and YOR356w/CIR2 were the best growth-performing strains on substrates that feed electrons to the ETC (all substrates except methanol). The ygr207c/cir1 mutant displayed clear better growth than the wild type only on fumarate, a substrate that feeds electrons to the internal NADH dehydrogenase. Besides pyruvate, malate and lactate, this substrate conveys electrons exclusively to this dehydrogenase, which is not the case for ethanol. Only the yor356w/cir2 mutant grew better than the other strains when methanol was used. This substrate is metabolized in peroxisomes yielding dihydroxyacetone phosphate, which is subsequently metabolized in the cytosol and mitochondria.
Fig. (1).
Mutants affected in genes encoding ETF and ETF-dH display higher growth rate on non-fermentable carbon sources. Cells of the parental strain (BY4741) and of mutants affected in AIM45 (aim45), YGR207c/CIR1 (cir1) and YOR356w/CIR2 (cir2) were grown overnight and diluted to OD600=1. From this suspension, 3µL of 10-1-10-5 dilutions were spotted on rich media containing glucose (YPD), acetate (YPAcet), fumarate (YPFum), ethanol (YPEth), lactate (YPLact), succinate (YPSucc), pyruvate (YPPyr), malate (YPMal) or methanol (YPMeth) as sole carbon source. After 48h incubation at 30ºC, plates were photographed. Photographs represent typical results from at least three independent experiments.
AIM45, YGR207c/CIR1 and YOR356w/CIR2 Protect Against Superoxide and Heat Stress Only when Cells are Respiring
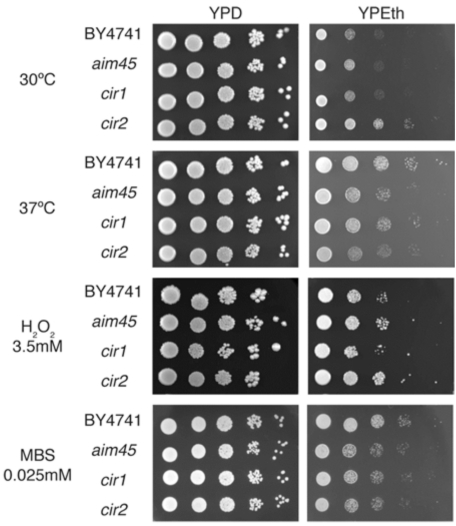
The putative role of Aim45, Ygr207c/Cir1 and Yor356w/Cir2 in electron transfer reactions could provide a target for oxidative stress agents and, hence, protect cells. To investigate this hypothesis, we assayed all strains for growth in the presence of two oxidative agents: hydrogen peroxide and menadione, a superoxide-generating compound (Fig. 2). Growth on glucose was similar in all strains under all conditions. In the presence of hydrogen peroxide, the growth differences between the four strains tested have remained constant when compared with growth on ethanol as sole carbon and energy source at 30ºC. On the other hand, mutants displayed a slightly higher sensitivity to menadione on ethanol as sole carbon and energy source. As the yor356w/cir2 mutant grows better, on ethanol at 30ºC, than the other strains (Fig. 1), our results suggest that this mutant may be considerably more sensitive than aim45 and ygr207c/cir1 to oxidative stress by menadione, when compared to the parental strain. It is well known that heat stress causes perturbation to mitochondria, which results in higher ROS production [20]. We wanted to investigate whether high temperature could mimic oxidative stress results by incubating all strains at 37ºC on YPD and YPEth (Fig. 2). Mutant strains displayed higher sensitivity than the parental strain under 37ºC incubation on YPEth in a similar pattern when compared to menadione as stress agent (Fig. 2), suggesting that AIM45, YOR356w/CIR2 and, to a lesser extent, YGR207c/CIR1 are involved in protection against superoxide.
Fig. (2).
Mutants affected in genes encoding ETF and ETF-dH are more sensitive to heat stress and menadione when respiring ethanol. Cells of the parental strain (BY4741) and of mutants affected in AIM45 (aim45), YGR207c/CIR1 (cir1) and YOR356w/CIR2 (cir2) were grown overnight and diluted to OD600=1. From this suspension, 3µL of 10-1-10-5 dilutions were spotted on rich media containing glucose (YPD) or ethanol (YPEth) as sole carbon source andsupplemented with 3.5mM H2O2 or 0.025mM menadione sodium bisulfite (MBS). After 48h incubation at 30ºC or 37ºC, plates were photographed. Photographs represent typical results from at least three independent experiments.
Intracellular Oxidation upon Heat Shock is Decreased in aim45, ygr207c/cir1 and yor356w/cir2 Mutants
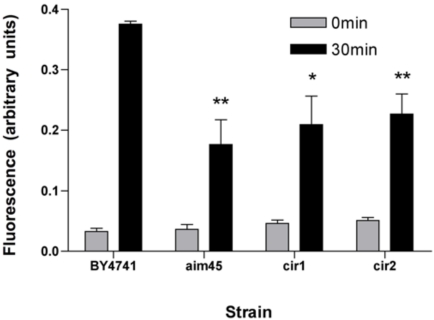
The unusual phenotype of better growth performance when genes are deleted (Fig. 1) can be explained, among other causes, by affected redox state in mitochondria during respiratory metabolism, for instance by decreased ROS production in the mutant strains or by a better performance of the ETC in these mutants. In an attempt to understand these growth phenotypes and the ones of heat and oxidative stress (Fig. 2) displayed by the mutant strains, we investigated the involvement of AIM45, YGR207c/CIR1 and YOR356w/CIR2 in the oxidation state of the cell when mitochondria are perturbed by heat shock. To measure the intracellular oxidation, we used the fluorescent oxidant-sensitive probe H2DCFDA. This compound can diffuse freely through plasma membranes to the cytosol where it is deacetylated by cellular esterases, becoming unable to diffuse through the plasma membrane. After loading with the fluorescent probe, cells were heat-shocked by incubation at 50ºC for 30min and fluorescence was recorded in the cell lysate. As expected, upon heat shock, higher levels of fluorescence from the oxidized probe were measured as compared to incubation at 30ºC, indicating an increase in intracellular oxidation state (Fig. 3). Intracellular oxidation is similar among all strains at 30ºC; however, after incubation at 50ºC, all mutants presented nearly half fluorescence in relation to the parental strain. These results suggest that, upon heat shock conditions, Aim45, Ygr207c/Cir1 and Yor356w/Cir2 contribute to the increase in intracellular oxidation state of yeast cells.
Fig. (3).
Mutants affected in genes encoding ETF and ETF-dH display decreased intracellular oxidation upon heat shock. Cells of the parental strain (BY4741) and of mutants affected in AIM45(aim45), YGR207c/CIR1 (cir1) and YOR356w/CIR2 (cir2) were loaded with the oxidant-sensitive fluorescent probe 2’,7’-dichlorodihydrofluorescein diacetate and exposed to 50ºC for 30min. Fluorescence was recorded in each cell lysate and compared to loaded cells without exposition to 50ºC. *P < 0.05 and **P < 0.01 when compared with strain BY4741 with the same time incubation..
Under Conditions of Mitochondrial Perturbation by Antimycin A, the ygr207c/cir1 Mutant Displays Increased Peroxisomal Proliferation
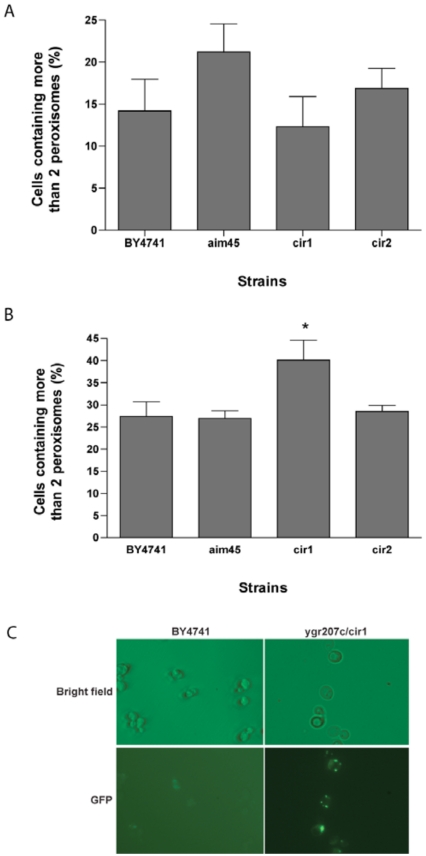
Phenotypes of mutants affected in AIM45, YGR207c/CIR1 and YOR356w/CIR2, suggest a significant involvement of these genes in mitochondrial function, which is particularly highlighted under stress conditions. An important adaptation to mitochondrial dysfunction is the retrograde regulation, which involves altered gene expression, metabolism and organelle homeostasis [21]. One of the remarkable adaptations is peroxisome proliferation that, together with an increased expression of genes encoding enzymes of the glyoxylate cycle (CIT2) and of first three reactions of the tricarboxylic acid cycle (CIT1, ACO1 and IDH1/2), allows efficient use of two carbon compounds, like ethanol and acetate, and the maintenance of anabolic reactions for nitrogen-based compounds like glutamate and lysine. Therefore, we reasoned that the aim45, ygr207c/cir1 and yor356w/cir2 mutants could have an activated retrograde regulation pathway due to altered mitochondrial function, particularly under stress conditions. A simple method to assess this is to measure peroxisome proliferation by fluorescence microscopy in cells expressing GFP fused with the skl peroxisome-targeting peptide [22]. To test heat stress, we used a lower temperature (39ºC) than the one from the intracellular oxidation assay in order to allow survival of cells long enough to induce peroxisome proliferation. Under these conditions, strains did not display significant differences. The strain affected in AIM45 presented a reproducible slightly higher percentage of cells with more than 2 peroxisomes suggesting that this gene could be important for normal functioning of mitochondria under heat stress (Fig. 4A). We also tested the complex III-inhibitor antimycin A in cultures of all strains and determined the percentage of cells with peroxisome proliferation [23] (Fig. 4B). Only the ygr207c/cir1 mutant displayed statistically significant increased peroxisome proliferation. These results suggest that ETFα, ETFβ and ETF-dH act differently upon the action of different mitochondria-perturbing agents and that they may be important for mitochondrial function under stress conditions. This conclusion is supported by the lack of difference in peroxisome proliferation between all strains when the assay was performed on cells grown on the peroxisome metabolizable substrate methanol in the absence of stress (not shown).
Fig. (4).
Altered peroxisome proliferation in cells affected in AIM45 and YGR207c/CIR1 upon heat stress and antimycin A treatment, respectively. Mutant cells affected in AIM45 (aim45), YGR207c/CIR1 (cir1) and YOR356w/CIR2 (cir2) and the correspondent parental strain (BY4741), harboring a plasmid containing the green fluorescent protein gene fused with a peroxisome-targeting signal peptide (skl), were grown overnight on YPD, ressuspended in fresh medium, and incubated at 39ºC for 6h (A) or incubated in the presence of antimycin A at 30ºC for 28h (B) and cells containing more than 2 peroxisomes were counted on a fluorescence microscope. C: bright field and GFP fluorescence representative micrographs of strains BY4741 and ygr207c/cir1 from the antimycin A incubation experiment. *P < 0.05 when compared with strain BY4741.
DISCUSSION
Mammalian ETF and ETF-dH are fairly well studied, namely their protein structure, interaction with substrates, kinetics of electron transfer and metabolism (for a recent review see [3]). Function-inactivating mutations cause a metabolic disorder known as glutaric aciduria II, characterized by a dysfunction in acyl-CoA dehydrogenases of fatty acids, amino acids metabolism and renal excretion of glutaric and lactic acids. The yeast orthologs are still poorly characterized. Several observations suggest that yeast ETF and ETF-dH orthologs may have additional roles besides those identified for the mammalian counterparts: β-oxidation does not take place in mitochondria, regulation of genes encoding mitochondrial proteins is subject to catabolite repression by glucose, Ypr004c/Aim45 and Ygr207c interact with the frataxin-homolog iron chaperone Yfh1 [13] and microarray analysis indicate that the genes encoding ETF and ETF-dH have altered expression upon environmental changes such as heat, cold and oxidative shocks [24]. Our results suggest that the yeast orthologous genes encoding ETFα, ETFβ and ETF-dH are involved in cellular protection against superoxide and are involved in intracellular oxidation, particularly under mitochondrial perturbation conditions such as heat shock.
The better growth effect on non-fermentable carbon sources by the knockout mutations on AIM45, YGR207c/CIR1 and YOR356w/CIR2 can be explained by an indirect effect like less production of ROS during respiratory metabolism. Our results indicating decreased intracellular oxidation under heat shock in all mutants (Fig. 3) partially support this assumption. Altered growth rate was specific according to mutation and carbon source (Fig. 1), that is, each gene mutation only caused altered growth on carbon sources according to the ETC component functioning as acceptor of electrons. This could be a consequence of the spatial arrangement of mitochondrial dehydrogenases in the supramolecular complexes [12]. As some of these dehydrogenases are TCA cycle enzymes, the supramolecular dehydrogenase complex could have a different activity depending on the type of carbon source. However, in all cases, the increase in growth rate observed is not very pronounced, suggesting that the energetic metabolism might be unchanged or only slightly increased. Accordingly, oxygen consumption rates were nearly the same on isolated mitochondria from each strain with several of the substrates tested in growth experiments (our unpublished results). The notion of competition between dehydrogenases for oxidized ubiquinone has been reported [25] to explain different levels of hydrogen peroxide production by rat isolated mitochondria when respiring on substrates that feed electrons to different ETC complexes. A similar competition between ETF and ETF-dH with complex II (succinate dehydrogenase, Sdh1), the internal NADH dehydrogenase (Ndi1) and the external NADH dehydrogenases (Nde1 and Nde2), would explain the aim45, ygr207c/cir1 and yor356w/cir2 mutants better growth by a more efficient oxidation of ubiquinone by these dehydrogenases.
Different sensitivities observed with the aim45, ygr207c/cir1 and yor356w/cir2 mutants to oxidative stress agents hydrogen peroxide and menadione can be interpreted in terms of the different mechanisms of action of these compounds in the cellular context. While peroxide toxicity is mediated by the Fenton reaction, which releases hydroxyl radicals upon reaction with Fe2+, menadione generates the highly oxidized radical superoxide, which causes more pronounced inhibitory effects on mitochondrial enzymes than peroxide [26]. Some of these enzymes are dehydrogenases from the TCA cycle that could become more sensitive or more exposed to superoxide activity when one of the supramolecular complex dehydrogenases is missing, namely, Aim45, Ygr207c/Cir1 or Yor356w/Cir2.
In the model proposed by Davidson and Schiestl [20], the cofactor flavin is a source of ROS, whose amount depends on mutations (coq7, affecting ubiquinone synthesis and nde1nde2, affecting the external NADH dehydrogenases) and heat stress. Our results of intracellular oxidation state (Fig. 3) can be explained assuming that ETF and ETF-dH are flavoproteins that contribute to the bulk of superoxide production. In fact, the possibility of ROS production by ETF and ETF-dH has been proposed earlier for rat skeletal muscle and heart mitochondria respiring on any substrate [25]. In addition, expression of AIM45 is repressed at 37ºC, as are the genes involved in the synthesis and assembly of complexes II, III, IV and V [27], suggesting that the ETC activity is decreased, presumably as an adaptation preventing excessive ROS production. This similar expression regulation and the observed lower intracellular oxidation in the mutant affected in AIM45 (Fig. 3) are compatible with its involvement in the ETC.
An association of YGR207c/CIR1 to the mitochondrial function in non-physiological conditions is suggested by the peroxisome proliferation observed in the correspondent mutants (Fig. 4). This is a cellular response to mitochondrial dysfunction by ETC impairment or mitochondrial genome loss, involving the so-called retrograde pathway, allowing circumventing insufficiency of mitochondrial metabolism by providing nitrogen-based compounds precursors of several amino acids like glutamate and lysine. Recently, a model explaining the ETF specific interactions with different proteins, in its role of electron transfer to the ETC, has been proposed, which differs from the classical induced fit interaction mechanism [3]. In this new proposed mechanism, the FAD domain of ETF becomes mobile upon ETF-protein complex formation, allowing random motion so that, eventually, an optimal geometry is established leading to efficient interprotein electron transfer in a process called “conformational sampling”. This suggests a wide interaction of ETF and ETF-dH with substrates and other dehydrogenases compatible with the phenotypes associated with the mitochondrial functions of the mutants observed in this work. In addition, a phenotype of higher frequency of mitochondrial genome loss in the aim45 mutant was observed in an approach combining computational prediction methods with quantitative experiments [28]. The induction of peroxisome proliferation reported in this work is in accordance with this finding and suggests interplay with mechanisms of genome integrity maintenance.
CONCLUSION
This work presents evidence pointing to a role of AIM45, CIR1 and CIR2 in the redox state of the cell under non-physiological conditions. In addition, results suggest that Aim45, Cir1 and Cir2 can be a source of reactive oxygen species in conditions of mitochondrial perturbation, in particular under extreme heat stress.
ACKNOWLEDGEMENTS
The authors would like to thank the Scientific Editing Programme of Universidade do Minho for revising the English text of the manuscript.
CONFLICT OF INTEREST
The authors declare there are no competing financial interests.
REFERENCES
- 1.Sickmann A, Reinders J, Wagner Y, et al. The proteome of Saccharomyces cerevisiae mitochondria. Proc Natl Acad Sci USA. 2003;100:13207–12. doi: 10.1073/pnas.2135385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinders J, Zahedi RP, Pfanner N, Meisinger C, Sickmann A. Toward the complete yeast mitochondrial proteome: multidimensional separation techniques for mitochondrial proteomics. J Proteome Res. 2006;5:1543–54. doi: 10.1021/pr050477f. [DOI] [PubMed] [Google Scholar]
- 3.Toogood HS, Leys D, Scrutton NS. Dynamics driving function: new insights from electron transferring flavoproteins and partner complexes. FEBS J. 2007;274:5481–504. doi: 10.1111/j.1742-4658.2007.06107.x. [DOI] [PubMed] [Google Scholar]
- 4.Mattoon JR, Haight RD. Glutaric acid accumulation by a lysine-requiring yeast mutant. J Biol Chem. 1962;237:3486–90. [PubMed] [Google Scholar]
- 5.Spaan AN, Ijlst L, van Roermund CWT, Wijburg FA, Wanders RJA, Waterham HR. Identification of the human mitochondrial FAD transporter and its potential role in multiple acyl-CoA dehydrogenase deficiency. Mol Genet Metab. 2005;86:441–7. doi: 10.1016/j.ymgme.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Frisell WR, Cronin JR, Mackenzie CG. Coupled flavoenzymes in mitochondrial oxidation of N-methyl groups. J Biol Chem. 1962;237:2975–80. [PubMed] [Google Scholar]
- 7.Thorpe C, Kim JJ. Structure and mechanism of action of the acyl-CoA dehydrogenases. FASEB J. 1995;9:718–25. doi: 10.1096/fasebj.9.9.7601336. [DOI] [PubMed] [Google Scholar]
- 8.Finocchiaro G, Ito M, Tanaka K. Purification and properties of short chain acyl-CoA, medium chain acyl-CoA, and isovaleryl-CoA dehydrogenases from human liver. J Biol Chem. 1987;262:7982–9. [PubMed] [Google Scholar]
- 9.Fan F, Germann MW, Gadda G. Mechanistic studies of choline oxidase with betaine aldehyde and its isosteric analogue 3,3-dimethylbutyraldehyde. Biochemistry. 2006;45:1979–86. doi: 10.1021/bi0517537. [DOI] [PubMed] [Google Scholar]
- 10.Salazar D, Zhang L, deGala GD, Frerman FE. Expression and characterization of two pathogenic mutations in human electron transfer flavoprotein. J Biol Chem. 1997;272:26425–33. doi: 10.1074/jbc.272.42.26425. [DOI] [PubMed] [Google Scholar]
- 11.Eaton S. Control of mitochondrial beta-oxidation flux. Prog Lipid Res. 2002;41:197–239. doi: 10.1016/s0163-7827(01)00024-8. [DOI] [PubMed] [Google Scholar]
- 12.Grandier-Vazeille X, Bathany K, Chaignepain S, Camougrand N, Manon S, Schmitter JM. Yeast mitochondrial dehydrogenases are associated in a supramolecular complex. Biochemistry. 2001;40:9758–69. doi: 10.1021/bi010277r. [DOI] [PubMed] [Google Scholar]
- 13.González-Cabo P, Vazquez-Manrique RP, Garcia-Gimeno MA, Sanz P, Palau F. Frataxin interacts functionally with mitochondrial electron transport chain proteins. Hum Mol Genet. 2005;14:2091–8. doi: 10.1093/hmg/ddi214. [DOI] [PubMed] [Google Scholar]
- 14.Parker A, Engel PC. Preliminary evidence for the existence of specific functional assemblies between enzymes of the beta-oxidation pathway and the respiratory chain. Biochem J. 2000;345:429–35. [PMC free article] [PubMed] [Google Scholar]
- 15.Ristow M, Pfister MF, Yee AJ, et al. Frataxin activates mitochondrial energy conversion and oxidative phosphorylation. Proc Natl Acad Sci U S A. 2000;97:12239–43. doi: 10.1073/pnas.220403797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brachmann CB, Davies A, Cost GJ, et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–32. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Almeida T, Marques M, Mojzita D, et al. Isc1p plays a key role in hydrogen peroxide resistance and chronological lifespan through modulation of iron levels and apoptosis. Mol Biol Cell. 2008;19:865–76. doi: 10.1091/mbc.E07-06-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elgersma Y, van den Berg M, Tabak HF, Distel B. An efficient positive selection procedure for the isolation of peroxisomal import and peroxisome assembly mutants of Saccharomyces cerevisiae. Genetics. 1993;135:731–40. doi: 10.1093/genetics/135.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ausubel F, Brent R, Kingston RE, et al., editors. Current protocols in molecular biology. New York: John Wiley & Sons; 1989. [Google Scholar]
- 20.Davidson JF, Schiestl RH. Mitochondrial respiratory electron carriers are involved in oxidative stress during heat stress in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:8483–9. doi: 10.1128/MCB.21.24.8483-8489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Butow RA. Mitochondrial retrograde signaling. Annu Rev Genet. 2006;40:159–85. doi: 10.1146/annurev.genet.40.110405.090613. [DOI] [PubMed] [Google Scholar]
- 22.Van Roermund CWT, Drissen R, van den Berg M, et al. Identification of a peroxisomal ATP carrier required for medium-chain fatty acid -oxidation and normal peroxisome proliferation in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:4321–9. doi: 10.1128/MCB.21.13.4321-4329.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein CB, Waddle JA, Hale IV W, et al. Genome-wide responses to mitochondrial dysfunction. Mol Biol Cell. 2001;12:297–308. doi: 10.1091/mbc.12.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasch AP, Spellman PT, Kao CM, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–57. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–90. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 26.Cabiscol E, Piulats E, Echave P, Herrero E, Ros J. Oxidative stress promotes specific protein damage in Saccharomyces cerevisiae. J Biol Chem. 2000;275:27393–8. doi: 10.1074/jbc.M003140200. [DOI] [PubMed] [Google Scholar]
- 27.Sakaki K, Tashiro K, Kuhara S, Mihara K. Response of genes associated with mitochondrial function to mild heat stress in yeast Saccharomyces cerevisiae. J Biochem. 2003;134:373–84. doi: 10.1093/jb/mvg155. [DOI] [PubMed] [Google Scholar]
- 28.Hess DC, Myers CL, Huttenhower C, et al. Computationally driven, quantitative experiments discover genes required for mitochondrial biogenesis. PLoS Genet. 2009;5:e1000407. doi: 10.1371/journal.pgen.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]