Abstract
Glucocorticoid administration is known to decrease calcium absorption in vivo and the vitamin D-dependent active transport of calcium by rat duodenum in vitro. The basis for this antivitamin D-like effect of glucocorticoids is unclear.
Previous studies in the rat failed to demonstrate an effect of glucocorticoid treatment on the hepatic conversion of the parent vitamin to 25-hydroxycholecalciferol (25-HCC). Moreover, pharmacologic doses of 25-HCC did not restore intestinal calcium transport to normal. The results of these experiments suggested that if indeed glucocorticoids interfere with the metabolism of vitamin D, the step involved must be subsequent to 25-hydroxylation.
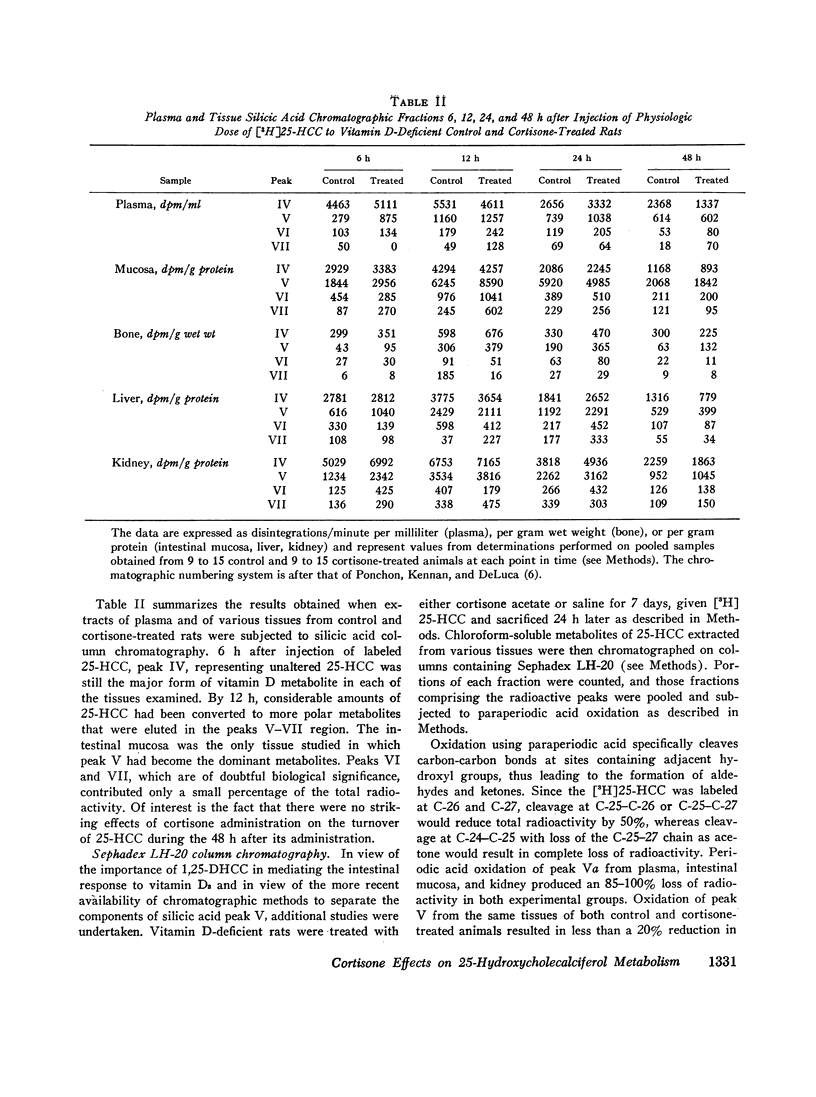
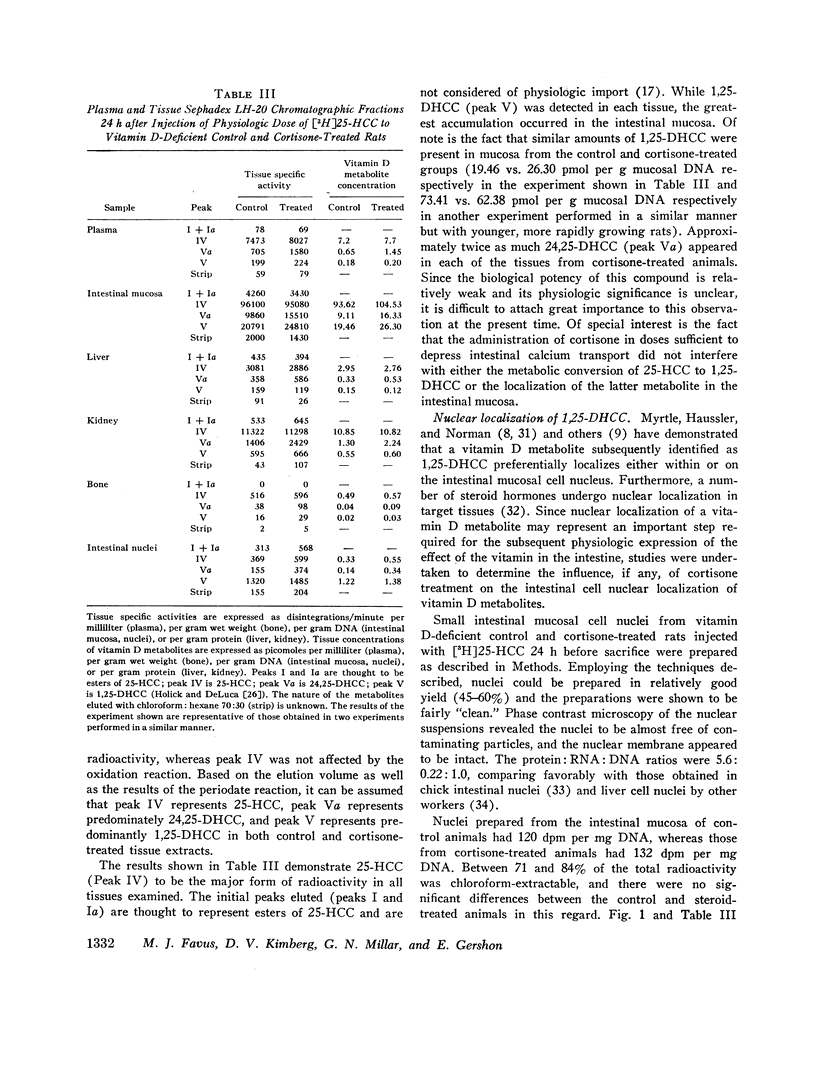
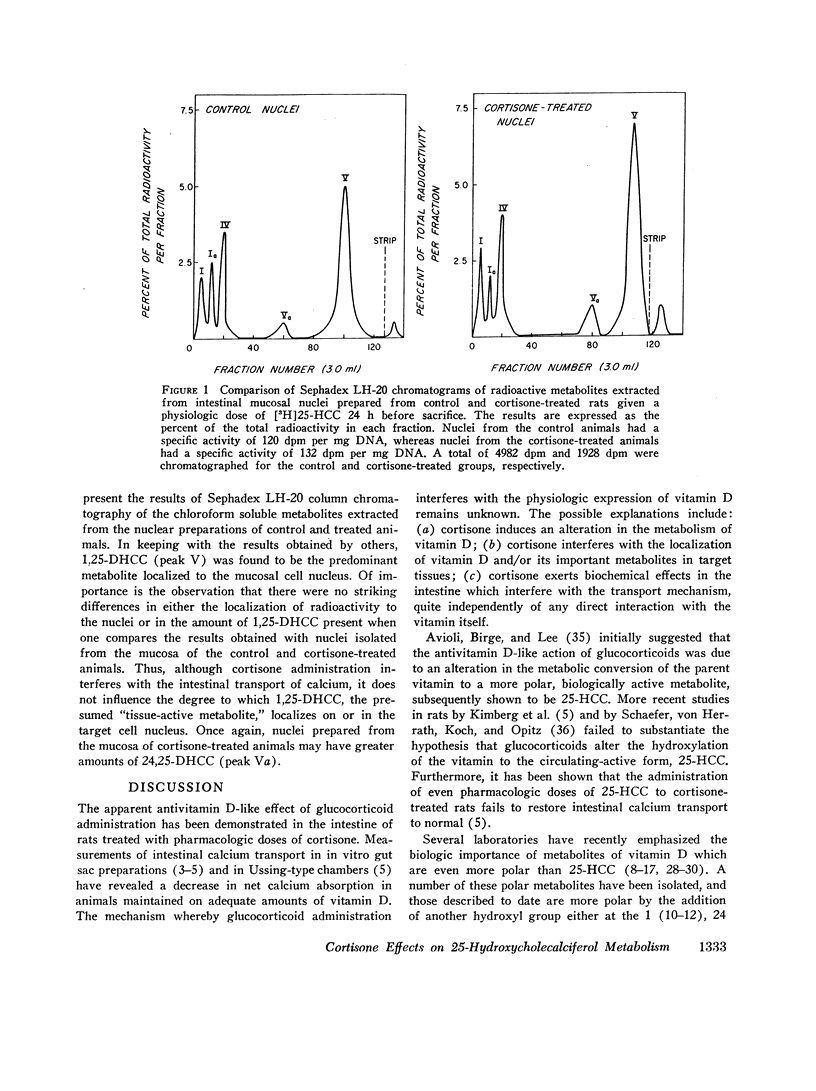
The present studies demonstrate that the administration of cortisone to vitamin D-deficient rats does not affect the rate of conversion of a physiologic dose of [3H]25-HCC to the biologically important metabolite, 1,25-dihydroxycholecalciferol (1,25-DHCC). Furthermore, pretreatment with glucocorticoids affects neither the tissue distribution nor the subcellular localization on or in intestinal mucosal cell nuclei of 1,25-DHCC. Of note is the fact that 1,25-DHCC is currently considered to be the “tissue-active” form of the vitamin in the intestine. Whereas tissues from cortisone-treated animals had increased concentrations of the biologically less active 24,25-DHCC, the physiologic significance of this observation remains unclear.
The results of the present studies strongly support the concept that the antivitamin D-like effects of glucocorticoids in the intestine are due to hormonal influences on the biochemical reactions responsible for calcium transport. While the effects of these hormones are opposite in direction to those of vitamin D, they occur by a mechanism that is independent of a direct interaction with either the vitamin or its biologically active metabolites.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRIGHT F., CARROLL E. L., DEMPSEY E. F., HENNEMAN P. H. The cause of hypercalcuria in sarcoid and its treatment with cortisone and sodium phytate. J Clin Invest. 1956 Nov;35(11):1229–1242. doi: 10.1172/JCI103378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON J., HARPER C., DENT C. E., PHILPOT G. R. Effect of cortisone on calcium metabolism in sarcoidosis with hypercalcaemia; possibly antagonistic actions of cortisone and vitamin D. Lancet. 1954 Oct 9;267(6841):720–724. doi: 10.1016/s0140-6736(54)90492-4. [DOI] [PubMed] [Google Scholar]
- Avioli L. V., Birge S. J., Lee S. W. Effects of prednisone on vitamin D metabolism in man. J Clin Endocrinol Metab. 1968 Sep;28(9):1341–1346. doi: 10.1210/jcem-28-9-1341. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baerg R. D., Kimberg D. V., Gershon E. Effect of renal insufficiency on the active transport of calcium by the small intestine. J Clin Invest. 1970 Jun;49(6):1288–1300. doi: 10.1172/JCI106341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R. J., DeLuca H. F., Gray R. W. Metaboism of 25-hydroxycholecalciferol in target and nontarget tissues. Biochemistry. 1970 Sep 15;9(19):3649–3652. doi: 10.1021/bi00821a001. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Suda T., Schnoes H. K., Tanaka Y., Holick M. F. 25,26-dihydroxycholecalciferol, a metabolite of vitamin D3 with intestinal calcium transport activity. Biochemistry. 1970 Nov 24;9(24):4776–4780. doi: 10.1021/bi00826a022. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Gray R., Boyle I., DeLuca H. F. Vitamin D metabolism: the role of kidney tissue. Science. 1971 Jun 18;172(3989):1232–1234. doi: 10.1126/science.172.3989.1232. [DOI] [PubMed] [Google Scholar]
- HARRISON H. E., HARRISON H. C. Transfer of Ca45 across intestinal wall in vitro in relation to action of vitamin D and cortisol. Am J Physiol. 1960 Aug;199:265–271. doi: 10.1152/ajplegacy.1960.199.2.265. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Myrtle J. F., Norman A. W. The association of a metabolite of vitamin D3 with intestinal mucosa chromatin in vivo. J Biol Chem. 1968 Aug 10;243(15):4055–4064. [PubMed] [Google Scholar]
- Haussler M. R., Norman A. W. Chromosomal receptor for a vitamin D metabolite. Proc Natl Acad Sci U S A. 1969 Jan;62(1):155–162. doi: 10.1073/pnas.62.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M. F., DeLuca H. F. A new chromatographic system for vitamin D3 and its metabolites: resoluation of a new vitamin D3 metabolite. J Lipid Res. 1971 Jul;12(4):460–465. [PubMed] [Google Scholar]
- Holick M. F., Schnoes H. K., DeLuca H. F., Gray R. W., Boyle I. T., Suda T. Isolation and identification of 24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in the kidney. Biochemistry. 1972 Nov 7;11(23):4251–4255. doi: 10.1021/bi00773a009. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Schnoes H. K., DeLuca H. F., Suda T., Cousins R. J. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry. 1971 Jul 6;10(14):2799–2804. doi: 10.1021/bi00790a023. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Baerg R. D., Gershon E., Graudusius R. T. Effect of cortisone treatment on the active transport of calcium by the small intestine. J Clin Invest. 1971 Jun;50(6):1309–1321. doi: 10.1172/JCI106610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawson D. E., Fraser D. R., Kodicek E., Morris H. R., Williams D. H. Identification of 1,25-dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature. 1971 Mar 26;230(5291):228–230. doi: 10.1038/230228a0. [DOI] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Barker D. C., Kodicek E. Isolation of chick intestinal nuclei. Effect of vitamin D3 on nuclear metabolism. Biochem J. 1969 Nov;115(2):263–268. doi: 10.1042/bj1150263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Kodicek E. Metabolism of vitamin D. A new cholecalciferol metabolite, involving loss of hydrogen at C-1, in chick intestinal nuclei. Biochem J. 1969 Nov;115(2):269–277. doi: 10.1042/bj1150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb J. N., Tolentino E. M. Effects of cortisone on ribosomal RNA synthesis in rat liver. Endocrinology. 1970 May;86(5):1033–1040. doi: 10.1210/endo-86-5-1033. [DOI] [PubMed] [Google Scholar]
- Lund J., DeLuca H. F. Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J Lipid Res. 1966 Nov;7(6):739–744. [PubMed] [Google Scholar]
- Myrtle J. F., Haussler M. R., Norman A. W. Evidence for the biologically active form of cholecalciferol in the intestine. J Biol Chem. 1970 Mar 10;245(5):1190–1196. [PubMed] [Google Scholar]
- Norman A. W., Midgett R. J., Myrtle J. F., Nowicki H. G. Studies on calciferol metabolism. I. Production of vitamin D metabolite 4B from 25-OH-cholecalciferol by kidney homogenates. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1082–1087. doi: 10.1016/0006-291x(71)90015-5. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Midgett R. J., Myrtle J. F. Studies on calciferol metabolism. 3. Comparison of species distribution and chromatographic separation of vitamin D metabolites. J Lab Clin Med. 1971 Oct;78(4):561–573. [PubMed] [Google Scholar]
- O'Malley B. W. Mechanisms of action of steroid hormones. N Engl J Med. 1971 Feb 18;284(7):370–377. doi: 10.1056/NEJM197102182840710. [DOI] [PubMed] [Google Scholar]
- Omdahl J., Holick M., Suda T., Tanaka Y., DeLuca H. F. Biological activity of 1,25-dihydroxycholecalciferol. Biochemistry. 1971 Jul 20;10(15):2935–2940. doi: 10.1021/bi00791a022. [DOI] [PubMed] [Google Scholar]
- Ponchon G., Kennan A. L., DeLuca H. F. "Activation" of vitamin D by the liver. J Clin Invest. 1969 Nov;48(11):2032–2037. doi: 10.1172/JCI106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPORN M. B., WANKO T., DINGMAN W. The isolation of cell nuclei from rat brain. J Cell Biol. 1962 Oct;15:109–120. doi: 10.1083/jcb.15.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer K., von Herrath D., Koch H. U., Opitz A. Effect of cortisone on vitamin D metabolism. Isr J Med Sci. 1971 Mar;7(3):533–534. [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Schnoes H. K., Ponchon G., Tanaka Y., Holick M. F. 21,25-dihydroxycholecalciferol. A metabolite of vitamin D3 preferentially active on bone. Biochemistry. 1970 Jul 7;9(14):2917–2922. doi: 10.1021/bi00816a025. [DOI] [PubMed] [Google Scholar]
- WILLIAMS G. A., BOWSER E. N., HENDERSON W. J., UZGIRIES V. Effects of vitamin D and cortisone on intestinal absorption of calcium in the rat. Proc Soc Exp Biol Med. 1961 Mar;106:664–666. doi: 10.3181/00379727-106-26436. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. A procedure for the isolation of enzymically active rat-liver nuclei. Biochem J. 1964 Aug;92(2):313–317. doi: 10.1042/bj0920313. [DOI] [PMC free article] [PubMed] [Google Scholar]


