Abstract
Objective
Intra-amniotic infection/inflammation (IAI) is one of the most important mechanisms of disease in preterm birth. Triggering receptor expressed on myeloid cells (TREM)-1 is a transmembrane glycoprotein expressed by neutrophils, macrophages and mature monocytes. TREM-1 is upregulated in biological fluids and tissues infected by Gram (+) and Gram (-) bacteria and fungi, amplifies the production of pro-inflammatory cytokines and chemokines, and its soluble form (sTREM-1) is released in the presence of infection. The aim of this study was to determine the effect of gestational age, parturition (term and preterm) and intra-amniotic infection/inflammation in the amniotic fluid (AF) concentrations of sTREM-1.
Study design
This cross-sectional study included 434 patients in the following groups: 1) mid-trimester of pregnancy (14-18 weeks, n=38); 2) normal pregnant women at term with (n=39) and without (n=39) labor; 3) patients with spontaneous preterm labor (PTL) and intact membranes classified into: a) PTL who delivered at term (n=99); b) PTL who delivered preterm (<37 weeks gestation) without IAI (n=80); and c) PTL with IAI (n=59); and 4) women with preterm prelabor rupture of membranes (PROM) with (n=40) and without (n=40) IAI. The AF concentration of sTREM-1 was determined by enzyme-linked immunoassay. Non-parametric statistics were used for analyses.
Results
1) sTREM-1 was detected in all AF samples; 2) the median AF sTREM-1 concentration at term was higher than in the mid-trimester (4277.6 pg/mL vs. 1140.4 pg/mL; p<0.001); 3) among patients with PTL, the median AF sTREM-1 concentration was significantly higher in patients with IAI than in those without IAI (6154.4 pg/mL vs. 3282.8 pg/mL; p<0.001) and those with PTL who delivered at term (6154.4 pg/mL vs. 2794 pg/mL; p<0.001); 4) patients with preterm PROM with IAI had a higher median AF sTREM-1 concentration than those without IAI (7893.1 pg/mL vs. 3386.6 pg/mL; p<0.001); 5) no differences were observed in the median AF sTREM-1 concentration between patients with spontaneous labor at term and those at term not in labor (4712.4 pg/mL vs. 4277.6 pg/mL repectively; p=0.4); and 6) an AF sTREM-1 concentration ≥6,416 pg/mL (derived from a ROC curve) had a sensitivity of 72% and a specificity of 89% for the diagnosis of intra-amniotic infection.
Conclusions
sTREM-1 is a physiologic constituent of the AF, and its concentration: 1) is significantly elevated in the presence of IAI; 2) increases with advancing gestation; and 3) does not change in the presence of spontaneous labor at term. We propose that sTREM-1 play a role in the innate immune response against intra-amniotic infection.
Keywords: preterm labor, preterm delivery, preterm prelabor rupture of membranes, PPROM, pregnancy, amniocentesis, microbial invasion of the amniotic cavity, MIAC, cytokines, chorioamnionitis
Introduction
Intrauterine infection is one of the most important mechanisms of disease in preterm birth.[1-11] Intra-amniotic infection and/or inflammation (IAI) is present in approximately one third of the patients with spontaneous preterm labor (PTL)[10,12,13] and in almost half of patients with preterm prelabor rupture of membranes (PROM),[14] and it is associated with increased perinatal morbidity, mortality and long-term sequelae such as cerebral palsy and chronic lung disease.[15-21]
Host defense against microbial invasion involves the participation of the innate and adaptive immune response. The first line of defense of the innate immunity against infection are the epithelial surfaces that act as mechanical barriers, and host commensal bacteria that compete with pathogenic microorganisms and produce mucus to impaired microbial adhesion as well as enzymes and antimicrobial peptides. If this line of defense is surpassed, pathogens are recognized by pattern recognition receptors such as mannose-binding lectin, macrophage mannose receptor, scavenger receptors and toll-like receptors that identify patterns of molecular structures on the surface of microorganisms which are ingested and killed by neutrophils and tissue macrophages.[22] The interaction between pathogens and macrophages initiates an inflammatory response by activation of macrophages, release of cytokines and chemokines, and attraction of neutrophils and monocytes to the site of infection.[22]
Triggering receptor expressed on myeloid cells (TREM)-1 is a transmembrane glycoprotein that belongs to the immunoglobulin superfamily receptors and that is mainly expressed and up-regulated in monocytes and neutrophils after stimulation with lipopolysaccharide (LPS), heat-inactivated Gram (+) bacteria, Gram (-) bacteria, or fungi.[23,24] TREM-1 expression is significantly increased in neutrophils isolated from the peritoneal cavity of patients with septic shock due to bacterial peritonitis, while expression of TREM-1 is normal in neutrophils obtained in peritoneal lavage from patients with systemic inflammatory response syndrome (SIRS) due to non-microbial peritoneal inflammation.[25] In addition, a solid body of evidence in patients with sepsis,[26-34] pneumonia,[35-37] and other infections[38-42] have demonstrated that TREM-1 expression and concentrations of its soluble form (sTREM-1) in different biological fluids are significantly higher in patients with bacterial infection than in those with a non-microbial inflammatory process.
Soluble TREM-1 concentrations have been reported in cervicovaginal fluid and serum from asymptomatic patients at risk for preterm delivery[43] as well as in the amniotic fluid of patients with spontaneous preterm delivery with and without microbial infection of the amniotic cavity (MIAC). The objective of this study was to determine if sTREM-1 concentration changes with advancing gestation, spontaneous labor at term, and in the presence of intra-amniotic infection/inflammation in patients with PTL with intact membranes and in those with PROM.
Materials and Methods
Study design and population
A cross-sectional study was designed by searching our clinical database and bank of biological samples, and included 434 patients in the following groups: 1) women in the mid-trimester of pregnancy (14-18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=38); 2) normal pregnant women at term with (n=39) and without (n=39) spontaneous labor; 3) patients with an episode of PTL and intact membranes who were classified into: a) PTL who delivered at term (n=99); b) PTL who delivered preterm (<37 weeks gestation) without IAI (n=80); and c) PTL with IAI (n=59); and 4) women with PROM with (n=40) and without (n=40) IAI.
All women provided written informed consent prior to the collection of amniotic fluid. The collection and utilization of amniotic fluid for research purposes was approved by the Institutional Review Boards of the participant institutions and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Many of these samples have been previously used to study the biology of inflammation, hemostasis, and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Definitions
Patients were considered to have a normal pregnancy outcome if they did not have any medical, obstetrical, or surgical complication, and delivered a term neonate (≥37 weeks) of appropriate birth weight for gestational age[44,45] without complications. Spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes associated with cervical change before 37 completed weeks of gestation that required hospitalization. Preterm PROM was diagnosed by sterile speculum examination confirming pooling of amniotic fluid in the vagina in association with nitrazine and ferning tests when necessary, before 37 weeks of gestation and in the absence of labor. Intra-amniotic infection was defined as a positive amniotic fluid culture for micro-organisms. Intra-amniotic inflammation was diagnosed by an amniotic fluid interleukin (IL)-6 concentration ≥2.6 ng/mL.[13] Histologic chorioamnionitis was diagnosed based on the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes.[46] Acute funisitis was diagnosed by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton's jelly using the criteria previously described.[47]
Sample collection
Amniotic fluid samples were obtained from transabdominal amniocentesis performed for genetic indication, evaluation of microbial status of the amniotic cavity and/or assessment of fetal lung maturity in patients approaching term. Women at term in labor consisted of women who were admitted for suspected preterm labor because of uncertain dates and had an amniocentesis for the assessment of fetal lung maturity. The criteria for considering that these patients were at term in labor was derived retrospectively, if the following criteria were met: 1) spontaneous labor; 2) delivery within 24 hours from amniocentesis; 3) analysis of amniotic fluid consistent with maturity; 4) birthweight >2500 grams; 5) absence of respiratory distress syndrome or other complications of prematurity; and 6) physical examination of the newborn by pediatricians consistent with a term neonate. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell (WBC) count, glucose concentration and Gram-stain were also performed shortly after collection as previously described.[48-50] The results of these tests were used for clinical management. Amniotic fluid IL-6 concentrations were used only for research purposes. Amniotic fluid not required for clinical assessment was centrifuged for 10 minutes at 4°C and the supernatant was aliquoted and stored at −70°C until analysis. Among patients with spontaneous preterm labor with intact membranes who delivered within 72 hours of amniocentesis, placenta, umbilical cord, and chorioamniotic membranes were collected and the presence or absence of histologic chorioamnionitis and/or funisitis was assessed. The 72 hours interval was chosen to preserve a meaningful temporal relationship between amniotic fluid sTREM-1 concentration and placental histopathologic findings.
Determination of soluble TREM-1 immunoassay in amniotic fluid
Specific and sensitive enzyme-linked immunoassays were used to determine TREM-1 concentrations in human amniotic fluid. Immunoassays for human TREM-1 were purchased from R&D Systems (Minneapolis, MN) and were validated for using human amniotic fluid prior to the conduction of this study. Validation included spike and recovery experiments, which produced parallel curves indicating that amniotic fluid constituents did not interfere with antigen-antibody binding in this assay system. Standards or amniotic fluid samples were incubated in 96-well micro titer plates pre-coated with monoclonal antibodies against human TREM-1. During this incubation any TREM-1 present in the standards or amniotic fluid samples is bound by the immobilized antibodies. After washing away any remaining unbound substances, an enzyme conjugated polyclonal antibody specific for TREM-1 is added to the wells of the micro titer plates. Repeated washing and aspiration removed all unbound substances; a substrate solution was added to the wells and color developed in proportion to the amount of TREM-1 bound in the initial step. The color development was stopped with the addition of an acid solution and the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA). The concentrations of TREM-1 in amniotic fluid samples were determined by interpolation from individual standard curves composed of human TREM-1. The calculated inter- and intra-assay coefficients of variation for soluble TREM-1 immunoassays in our laboratory were 4.9% and 4.2%, respectively, and the sensitivity was calculated to be 35.1 pg/mL.
Statistical analysis
The normality of the data was tested using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Because amniotic fluid sTREM-1 concentrations were not normally distributed, non-parametric tests were used for analyses. Comparisons between proportions were performed with the Chi-square test. Kruskal-Wallis with post-hoc analysis and Mann-Whitney U tests were used for continuous variables. Adjustment for multiple comparisons was performed using the Bonferroni method.[51] Analysis of covariance (ANCOVA) was used to investigate the association between the preterm labor and PROM subgroups, amniotic fluid sTREM-1 concentration and storage time. Spearman rank correlation was utilized to assess correlations between amniotic fluid concentration of sTREM-1, WBC count and IL-6. Among patients with preterm labor and intact membranes, receiver-operating characteristic (ROC) curve analyses were performed to determine amniotic fluid sTREM-1 concentration cutoffs for the identification of patients who had MIAC and intra-amniotic inflammation. A Kaplan-Meier survival analysis was conducted to assess the amniocentesis-to-delivery interval, using an amniotic fluid sTREM-1 concentration cutoff derived from the ROC curve for the presence of intra-amniotic infection. Spontaneous labor was entered in the analysis as the event of interest, and patients who delivered due to fetal or maternal indications were treated as censored observations with a censoring time equal to the amniocentesis-to-delivery interval. Cox proportional hazard modeling was performed to examine the differences in amniocentesis-to-delivery interval, according to sTREM-1 concentration in amniotic fluid while controlling for other confounding factors (amniotic fluid culture result, cervical dilatation and gestational age at amniocentesis). A p-value of <0.05 was considered statistically significant. The statistical package used was SPSS v.15.0 (SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinical characteristics of the study population
Table I presents the demographic and clinical characteristics of patients in the midtrimester, term not in labor and term in labor groups. Tables II and III display the demographic and clinical characteristics of patients with spontaneous PTL and intact membranes and those with PROM, respectively. Among patients with PTL with intact membranes, those with IAI had a significantly lower median gestational age at amniocentesis than those without IAI who delivered preterm and those who delivered at term (Table II). Similar results were observed between patients with PROM with IAI than in those without IAI (Table III).
Table I. Demographic and clinical characteristics of patients in the midtrimester and those at term with and without spontaneous labor.
| Midtrimester (n=38) |
pa | Term No Labor (n=39) |
Term In Labor (n=39) |
pb | |
|---|---|---|---|---|---|
| Maternal age (years) | 36 (35-38) |
<0.01 | 28 (22-33) |
22 (19-26) |
<0.01 |
| GA at amniocentesis (weeks) | 16 (16-17) |
<0.01 | 38.3 (37.9-39.1) |
38.5 (37.6-39.4) |
NS |
| GA at delivery (weeks) | 39 (38-40) |
0.03 | 38.3 (37.9-39.1) |
38.5 (37.6-39.4) |
NS |
| Birthweight (grams) | 3,332 (3,160-3,624) |
NS | 3,250 (3,095-3,563) |
3,390 (3,100-3,540) |
NS |
Values are expressed as median (interquartile range).
NS: not significant.
GA: Gestational Age
pa: comparison between patients in the mid-trimester and those at term not in labor
pb: comparison between patients at term not in labor and those at term in labor
Table II. Demographic and clinical characteristics of patients presenting with spontaneous preterm labor with intact membranes.
| PTL without IAI Term delivery (n=99) |
p | PTL without IAI Preterm delivery (n=80) |
pa | PTL with IAI Preterm delivery (n=59) |
pb | |
|---|---|---|---|---|---|---|
| Maternal age (years) | 23 (18-31) |
NS | 22 (19-30) |
NS | 22 (19-28) |
NS |
| GA at amniocentesis (weeks) | 31.6 (29.4-33.4) |
NS | 32.1 (30.4-33.3) |
0.02 | 29.7 (25.3-33.3) |
0.01 |
| GA at delivery (weeks) | 38.7 (38-39.9) |
<0.01 | 34.7 (33.3-35.7) |
<0.01 | 31.1 (25.9-33.3) |
<0.01 |
| Birthweight (grams) | 3,230 (2,970-3,550) |
<0.01 | 2,377 (1,982-2,740) |
<0.01 | 1,640 (850-2,200) |
<0.01 |
Values expressed as median (interquartile range)
p: comparison between PTL who delivered at term and PTL without IAI
pa: comparison between PTL who delivered preterm without IAI and PTL with IAI
pb: comparison between PTL who delivered at term and PTL with IAI
PTL: preterm labor; GA: gestational age; IAI: intra-amniotic infection/inflammation NS: not significant
Table III. Demographic and clinical characteristics of patients presenting with preterm prelabor rupture of membranes.
| Preterm PROM without IAI (n=44) |
Preterm PROM with IAI (n=47) |
p | |
|---|---|---|---|
| Maternal age (years) | 24.5 (20-34.5) |
30.5 (24-38.8) |
0.01 |
| GA at amniocentesis (weeks) | 32.3 (29-33.5) |
30.1 (27.3-32.3) |
0.02 |
| GA at delivery (weeks) | 33.1 (31.2-34.6) |
30.7 (28.6-32.9) |
<0.001 |
| Birthweight (grams) | 2,015 (1,678-2,315) |
1,645 (1,340-2,033) |
0.02 |
Values expressed as median (interquartile range)
PROM: prelabor rupture of membranes; GA: gestational age; IAI: intra-amniotic infection/inflammation NS: not significant
Amniotic fluid sTREM-1 concentrations in normal pregnancy and term parturition
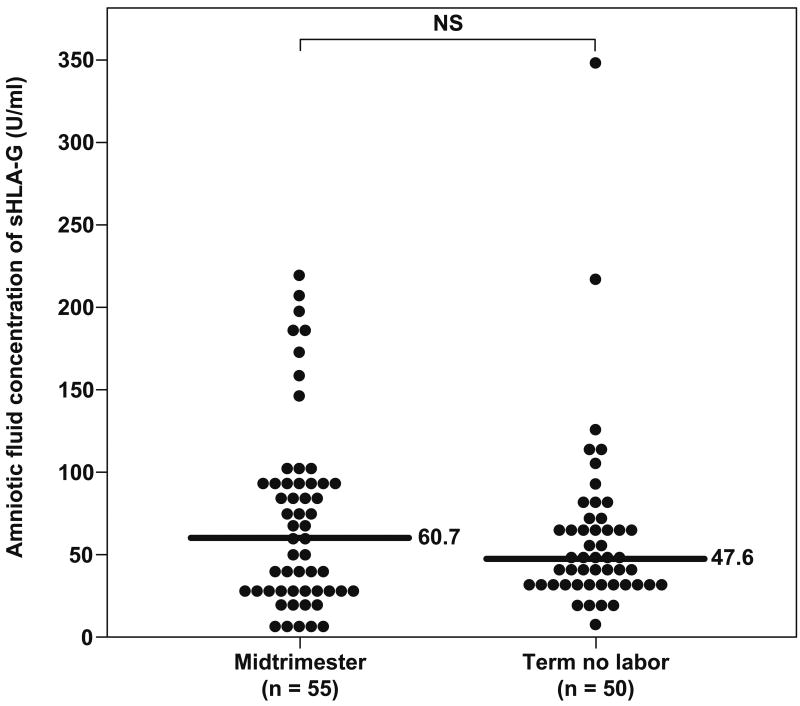
sTREM-1 was detected in all amniotic fluid samples included in this study. Women with a normal pregnancy at term not in labor had a significantly higher median sTREM-1 concentration in amniotic fluid than women in the mid-trimester [term not in labor: 4277.6 pg/mL, interquartile range (IQR) 2046.5-5900.6 vs. mid-trimester: 1140.4 pg/mL, IQR 631.7-1401.8; p<0.001] (Figure 1). In contrast, no significant differences were observed in the median amniotic fluid sTREM-1 concentration between patients with spontaneous labor at term and those at term not in labor (term in labor: 4712.4 pg/mL, IQR 3368.4-6008.8 vs. term not in labor: 4277.6 pg/mL, IQR 2046.5-5900.6; p=0.4) (Figure 1).
Figure 1. Amniotic fluid concentrations of sTREM-1 in normal pregnancies at mid-trimester and in those at term with and without labor.
The median amniotic fluid sTREM-1concentration was significantly higher in pregnancies at term not in labor than in those in the mid-trimester (4277.6 pg/mL, IQR 2046.5-5900.6 vs. 1140.4 pg/mL, IQR 631.7-1401.8; p<0.001). No significant differences were found in the median amniotic fluid concentration of sTREM-1 between women with spontaneous labor at term and those at term not in labor (4712.4 pg/mL, IQR 3368.4-6008.8 vs. 4277.6 pg/mL, IQR 2046.5-5900.6; p=0.4).
sTREM-1 concentrations in amniotic fluid from patients with spontaneous preterm labor and intact membranes
Patients with PTL with IAI had a significantly higher median amniotic fluid concentration of sTREM-1 than those who delivered preterm without IAI (PTL with IAI: 6154.4 pg/mL, IQR 3973.9–8987.4 vs. PTL without IAI: 3282.8 pg/mL, IQR 2265–4535.6; p<0.001), and than those who delivered at term (PTL delivered at term: 2794 pg/mL, IQR 1845.4-4321; p<0.001) (Figure 2). No differences were found in the median amniotic fluid sTREM-1 concentrations between patients with PTL without IAI who delivered preterm and those who delivered at term (p=0.2, Figure 2). These results did not change after adjusting for storage time (ANCOVA, p<0.001).
Figure 2. Amniotic fluid concentrations of sTREM-1 among women with spontaneous preterm labor (PTL) and intact membranes.
The median amniotic fluid concentration of sTREM-1 was significantly higher in patients with intra-amniotic infection/inflammation (IAI) than in women without IAI (6154.4 pg/mL, IQR 3973.9–8987.4 vs. 3282.8 pg/mL, IQR 2265–4535.6; p<0.001) as well as in those who delivered at term (6154.4 pg/mL, IQR 3973.9–8987.4 vs. 2794 pg/mL, IQR 1845.4-4321; p<0.001). There was no significant difference in the median amniotic fluid concentration of sTREM-1 between those who delivered preterm and those who delivered at term.
Amniotic fluid sTREM-1 concentrations in preterm PROM
Among patients with PROM, those with IAI had a significantly higher median amniotic fluid sTREM-1 concentration than patients with PROM without IAI (PROM with IAI: 7893.1 pg/mL, IQR 4085.5-13670.1 vs. PROM without IAI: 3386.6 pg/mL, IQR 2330.5–4027.5; p<0.001) (Figure 3). These results did not change after adjusting for storage time (ANCOVA, p<0.001). In the absence of IAI, no differences were observed in the median amniotic fluid sTREM-1 concentration between patients with PTL who delivered at term and those with preterm PROM (PTL without IAI: 2794.1 pg/mL, IQR 1845.4-4321 vs. preterm PROM without IAI: 3386.6 pg/mL, IQR 2330.5–4027.5; p=0.3).
Figure 3. Amniotic fluid concentrations of sTREM-1 in women with preterm prelabor rupture of the membranes (PROM).
(a) The median amniotic fluid sTREM-1 concentration was significantly higher in patients with intra-amniotic infection/inflammation (IAI) than in those without IAI (7893.1 pg/mL, IQR 4085.5-13670.1 vs. 3386.6 pg/mL, IQR 2330.5–4027.5; p<0.001).
Amniotic fluid sTREM-1 concentrations and its association with histologic chorioamnionitis
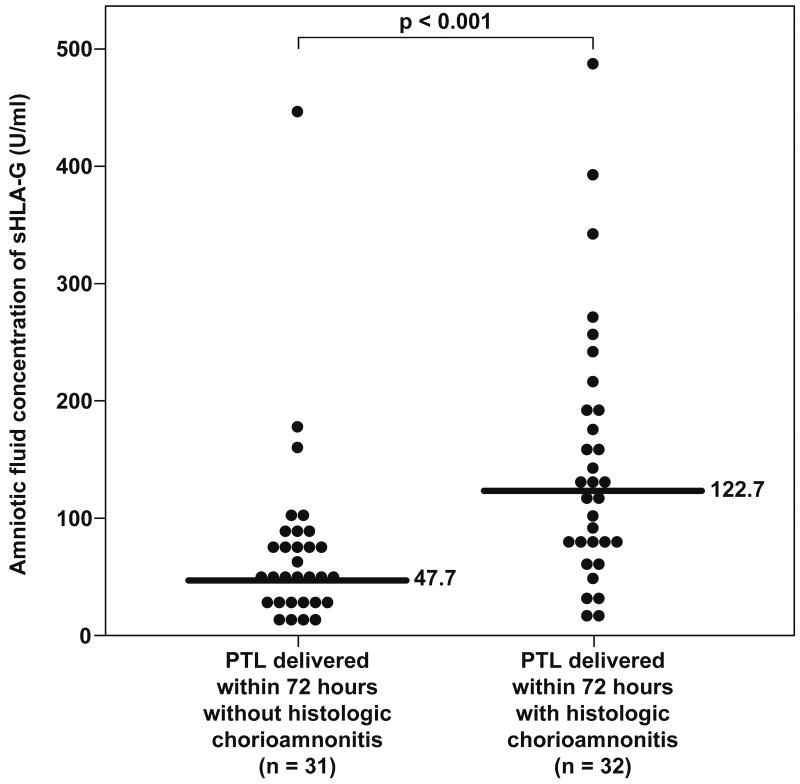
Placental histopathologic diagnoses were available in 75% (47/63) of patients with spontaneous preterm labor who delivered within 72 hours of amniocentesis, and in 49% (23/47) of cases there was evidence of placental inflammation. Patients with histologic chorioamnionitis and/or funisitis had a significantly higher median sTREM-1 concentration in amniotic fluid than those without histologic inflammation (placental inflammation: 7936.9 pg/mL, IQR 4134.8-11740.5 vs. no inflammation: 4180.5 pg/mL, IQR 3125.7-4935.4, respectively; p=0.003) (Figure 4).
Figure 4. Amniotic fluid concentrations of sTREM-1 in patients with spontaneous preterm labor with and without histologic chorioamnionitis who delivered within 72 hours from amniocentesis.
Patients with histologic chorioamnionitis and/or funisitis had a significantly higher median sTREM-1 concentration in amniotic fluid than those without histologic inflammation (7936.9 pg/mL, IQR 4134.8-11740.5 vs. 4180.5 pg/mL, IQR 3125.7-4935.4, respectively; p=0.003).
sTREM-1 concentrations in amniotic fluid in the presence of intra-amniotic infection/inflammation
A significant correlation was observed between amniotic fluid concentrations of sTREM-1 and WBC count as well as glucose and IL-6 concentrations in patients with spontaneous preterm labor and those with PROM (Spearman rho coefficient: glucose: -0.34, p<0.001; IL-6: 0.48, p<0.001; and WBC count: 0.35; p<0.001). Figures 5a and 5b illustrate receiver operating characteristic (ROC) curves for the identification of MIAC and intra-amniotic inflammation, respectively, in patients with spontaneous preterm labor and intact membranes. The diagnostic performance of sTREM-1 concentrations in amniotic fluid, for the identification of MIAC and intra-amniotic inflammation, was calculated using cutoff values derived from these ROC curves (Table IV). Using an amniotic fluid sTREM-1 concentration cutoff ≥6,416 pg/mL, a survival analysis was conducted to determine the relationship between intra-amniotic infection and the duration of the amniocentesis-to-delivery interval. Patients delivered due to fetal or maternal indications were censored. The median amniocentesis-to-delivery interval was significantly shorter in patients with an amniotic fluid sTREM-1 concentration ≥6,416 pg/mL compared to those with a concentration <6,416 pg/mL [2 days (95% CI 0.8-3) vs. 37 days (95% CI 30-44), respectively; p<0.001] (Figure 6). These results remained significant after adjusting for the results of the amniotic fluid culture, storage time, as well as cervical dilatation and gestational age at amniocentesis, (Cox proportional-hazards regression: 1.9, 95% CI 1.1-3.2; p=0.013).
Figure 5. Receiver operating characteristic (ROC) curves of amniotic fluid sTREM-1 concentration in patients with spontaneous preterm labor and intact membranes.
(a) ROC curve for the identification of positive amniotic fluid culture for microorganisms [area under the curve (AUC) for amniotic fluid sTREM-1: 82.3%; p<0.001]. (b) ROC curve for the identification of intra-amniotic inflammation, defined as amniotic fluid IL-6 concentration ≥2.6 ng/mL (AUC for amniotic fluid sTREM-1: 80.3%; p<0.001).
Table IV. Diagnostic indices and likelihood ratios of amniotic fluid sTREM-1 concentration for the detection of intra-amniotic infection and intra-amniotic inflammation in patients presenting with spontaneous preterm labor with intact membranes.
| Cutoff (pg/mL) |
Area under the ROC curve | Sensitivity* (%) |
Specificity* (%) |
Positive likelihood ratio (95% CI) |
Negative likelihood ratio (95% CI) |
|
|---|---|---|---|---|---|---|
| Intra-amniotic infection | ≥ 6,416 | 0.823 | 72 (18/25) |
89.2 (190/213) |
6.7 (4.3-9.1) |
0.3 (0.2-0.5) |
| Intra-amniotic inflammation | ≥ 4,367 | 0.803 | 72.9 (43/59) |
75.4 (135/179) |
3.0 (2.2-3.8) |
0.4 (0.2-0.5) |
ROC: receiver-operating characteristic; CI: confidence interval
Intra-amniotic infection: positive amniotic fluid culture for microorganisms
Intra-amniotic inflammation: amniotic fluid IL-6 concentration ≥ 2.6 ng/mL
Numbers in parentheses represent proportions
Discussion
Principal findings of the study
Soluble TREM-1 is a physiologic constituent of amniotic fluid. Of interest, sTREM-1 concentrations in amniotic fluid: 1) are significantly elevated in the presence of intra-amniotic infection/inflammation in patients with spontaneous preterm labor with intact membranes and in those with PROM; 2) significantly correlate with indirect amniotic fluid markers for intra-amniotic infection/inflammation, such as WBC count and IL-6 concentrations; and 3) are significantly higher in patients with histologic chorioamnionitis and/or funisitis than that of those without histologic markers of placental inflammation. Finally, amniotic fluid sTREM-1 concentrations increase with advancing gestation and do not change in the presence of labor at term.
What is sTREM-1?
TREM-1 is a transmembrane glycoprotein of 234 aminoacids and approximately 30 kDa that belongs to the immunoglobulin superfamily receptors and that is mainly expressed in monocytes and neutrophils, as well as in alveolar and hepatic macrophages, and endothelial cells.[23,24,52] TREM-1 was recently discovered by Bouchon et al,[23] who characterized this receptor as a single extracellular immunoglobulin-like domain of the V-type, a transmembrane region with a charged lysine residue, and a short cytoplasmatic tail without signaling motifs. Because of the latter, the charged residue in the transmembrane region facilitates the association with the transmembrane adaptor protein DAP12, which is a signal transduction molecule. Activation of TREM-1 with DAP12 induces calcium mobilization, tyrosine phosphorylation and activation of transcription factors such as ELK1, nuclear factor of activated T cells (NFAT), activator protein 1 (AP1) and nuclear factor κB (NF -κB), which are associated with transcription of genes encoding for pro-inflammatory cytokines and chemokines as well as cell-surface molecules.[23,53]
In addition to TREM-1, Bouchon et al discovered a TREM-1-homologue called TREM-2, which is also associated with DAP12 for intracellular signaling.[54] In contrast to TREM-1, TREM-2 is expressed in immature monocyte-derived dendritic cells as well as in osteoclasts,[55] brain[56] and microglia,[57] orchestrating the differentiation of myeloid precursors towards mature dendritic cells, osteoclast, microglia and possibly oligodendrocytes. This evidence suggests that TREM-2 plays a role in bone modeling and brain myelination.[53] Both TREM-1 and -2 are coded by genes located on chromosome 6p21 and are closely linked to the major histocompatibility complex and to NKp44, a triggering NK-cell receptor.[23,58,59]
Soluble TREM-1
The origin of the soluble form for sTREM-1 is subject of debate. It has been proposed that sTREM-1 results from an alternative splice variant of TREM-1 (TREM-1sv) that lacks the transmembrane and cytoplasmatic domain and has a molecular weight of 17.5 kDa.[60] However, Gibot et al[61] demonstrated that cultured human monocytes stimulated with LPS released a soluble form of TREM-1 that has a molecular weight of 27 kDa. Recently, Gomez-Piña et al[62] demonstrated in cultured monocytes that stimulation with LPS induces surface overexpression of TREM-1 and that production of sTREM-1 was associated with TREM-1 removal from the cell surface. Indeed, sTREM-1 was recovered from the supernatant of LPS-treated monocytes, while was not present in that of untreated monocytes. Moreover, addition of a general matrix metalloproteinase (MMP) inhibitor (GM6001) to cultured monocytes challenged with LPS resulted in prolonged cell surface TREM-1 expression and marked decrease in sTREM-1 concentration in the supernatant of LPS-stimulated monocytes. The authors did not find alternative splicing of TREM-1 in LPS-stimulated monocytes but, in contrast, Western Blot analysis of supernatants using an antibody that recognizes the extracellular domain of TREM-1 showed a band of approximately 27 kDa after LPS challenge, which is in agreement with a study reported before.[61] These findings support the view that sTREM-1 is the result of shedding of the TREM-1 ectodomain through proteolytic cleavage by metalloproteinases.[62]
Soluble TREM-1 in amniotic fluid
The results reported herein showed that sTREM-1 is a physiologic constituent of the amniotic fluid, since it was found in all samples included in this study. These results are in agreement with those recently reported by Menon and Fortunato.[63] Furthermore, we demonstrated that the amniotic fluid concentration of sTREM-1 increased with advancing gestational age and that did not change in the presence of labor at term. These findings are novel and suggest that this may be of value to the host in preparation for parturition at term. Although labor at term is considered an inflammatory process,[64-67] it is possible that sTREM-1 may play a specific role as a mechanism of defense against intra-amniotic infection.
TREM-1 expression in response to infection
A solid body of evidence support a role for TREM-1 in the inflammatory response associated to infection. In vitro studies[23,25] have demonstrated that TREM-1 is selectively up-regulated on neutrophils and monocytes after stimulation with LPS, heat-inactivated Gram (+) bacteria, Gram (-) bacteria, or fungi. This effect is limited to extracellular bacteria or bacterial cell-wall products, but not to intracellular bacteria.[25] Gibot et al[61,68-70] reported a series of interesting observations in different mice models of sepsis and pneumonia. Sepsis induced a significantly higher expression of surface TREM-1 in monocytes and peritoneal macrophages as well as up-regulation of TREM-1 gene expression compared to sham-operated animals. Soluble TREM-1 was detected in plasma and peritoneal fluid of septic animals, but it was almost absent in controls.
In vivo TREM-1 overexpression determined by flow cytometry has been observed in neutrophils present in skin lesions and lymphadenopathies caused by bacterial infections. Interestingly, minimal TREM-1 expression was observed in conditions of non-microbial inflammation characterized by infiltration of monocytes and neutrophils, such as psoriasis and ulcerative colitis.[25] Also, TREM-1 expression was markedly increased in neutrophils isolated from the peritoneal cavity of patients with septic shock caused by bacterial peritonitis, while expression of TREM-1 was normal in neutrophils obtained in peritoneal lavage from patients with SIRS due to non-microbial peritoneal inflammation.[25] These findings led to suggest that TREM-1 may play a role in the acute inflammatory response associated with microbial infections.
In addition to myeloid cells, fetal membranes have been proposed as a source for sTREM-1 in amniotic fluid. RT-PCR studies[63] on cultured fetal membranes from patients at term with or without labor did not find expression of TREM-1; however, TREM-1 expression was induced by LPS. Interestingly, TREM-1 was expressed in fetal membranes from patients with preterm delivery, regardless of the presence or absence of intra-amniotic infection.
sTREM-1 as a diagnostic tool in bacterial infections
Compelling evidence in patients with sepsis,[26-34] pneumonia,[35-37] pleural effusion,[38-40] pulmonary aspiration syndrome[41] and septic arthritis[42] have demonstrated that TREM-1 expression and/or sTREM-1 concentrations in different biological fluids are significantly higher in patients with bacterial infection that that of those with a non-microbial inflammatory process, implying that sTREM-1 may be a useful diagnostic tool. Indeed, plasma concentrations of sTREM-1, C-reactive protein and procalcitonin were significantly elevated in patients with sepsis compared to that of those with SIRS, and sTREM-1 appears to be a better marker for infection than C-reactive protein and procalcitonin.[26]
The present study demonstrated that patients with spontaneous preterm labor with intact membranes had a significantly higher amniotic fluid concentration of sTREM-1 in the presence of IAI compared to those without IAI who delivered preterm or at term. Menon and Fortunato[63] reported similar results in amniotic fluid obtained transvaginally from patients with spontaneous preterm delivery with MIAC (defined as a positive PCR for microorganisms). We further determined the amniotic fluid concentration of sTREM-1 in patients with PROM as well as in those with placental inflammation. The results showed that the presence of IAI in patients with PROM was associated with a significantly higher concentration of sTREM-1 compared to those without IAI, a finding that was also observed among patients with histologic chorioamnionitis/funisitis versus those without placental inflammation.
Among asymptomatic patients at risk for preterm delivery, Vogel et al[43] determined cervicovaginal and serum concentrations of multiple inflammatory markers between 12 and 25 weeks of gestation. The authors found that cervicovaginal concentrations of nine inflammatory markers (including sTREM-1) were undetectable in more than 50% of the samples. In contrast, high serum sTREM-1 concentrations (≥1000 pg/mL) were associated with a significantly higher risk for spontaneous preterm delivery before 35 weeks [relative risk: 4.6 (95% CI 2.1-10.1)]. Using cutoff values derived from ROC curves, we determined that the amniotic fluid concentration of sTREM-1 had a sensitivity and specificity of 72% and 89% for the diagnosis of intra-amniotic infection, and 73% and 75% for intra-amniotic inflammation. Moreover, the amniocentesis-to-delivery interval was significantly shorter among patients with spontaneous preterm labor and an elevated amniotic fluid sTREM-1 concentration (≥6,416 pg/mL) compared to those with a low concentration (2 days vs. 37 days). Further studies are needed to determine the diagnostic performance of sTREM-1 for prediction of preterm delivery.
TREM-1 and sTREM-1 as modulators of the inflammatory response
TREM-1 is considered to play a role in acute inflammatory responses.[24] Here, we found a significant correlation between amniotic fluid concentrations of sTREM-1 and indirect markers of intra-amniotic inflammation such as WBC count and IL-6 concentrations. mRNA TREM-1 expression and/or sTREM-1 concentrations have been observed to be elevated in tissue and biological fluids from patients with inflammatory conditions such as rheumatoid arthritis,[42] acute pancreatitis,[71,72] peptic ulcer,[73] Helicobacter pylori gastritis,[74] and inflammatory bowel disease.[75] In vitro studies[25,76] have demonstrated that in neutrophils and monocytes incubated with LPS and other toll-like receptors ligands, TREM-1 triggers and amplify the release of pro-inflammatory cytokines and chemokines such as interleukin (IL)-6, IL-8, monocyte chemotactic protein-1 and -3 (MCP-1 and MCP-3), macrophage inflammatory protein-1 alpha (MIP-1α) and granulocyte-macrophage colony-stimulating factor (GM-CSF), while inhibiting production of IL-10 in monocytes. Triggering of TREM-1 also induces neutrophils to release myeloperoxidase[25] and enhances both oxidative burst and phagocytosis.[77]
Several reports in septic mice have[25,61,69,70,78] demonstrated that synthetic peptides (LP17) used to inhibit the recognition of TREM-1 by its ligand and small interference RNA (siRNA) suppress TREM-1 mRNA and protein expression, modulate the production of pro-inflammatory cytokines such as TNF-α and IL-β, decrease bacterial clearance by impairing neutrophils oxidative burst, and improve the hemodynamic status and promotes survival. In humans, among patients with sepsis, plasma sTREM-1 concentrations remain stable or increase in non-surviving patients and decrease in survivors.[28] The traditional view is that persistent and exaggerated systemic inflammation accounts for the increased morbidity and mortality in patients with sepsis. It is possible that sTREM-1 may compete with TREM-1 ligand(s) down-regulating the TREM-1 pathway in order to modulate the inflammatory response.[79] These observations suggest that TREM-1 in vivo modulation may be a suitable therapeutic tool in sepsis.[68,69,78]
In conclusion, sTREM-1 concentrations in amniotic fluid are significantly elevated in the presence of intra-amniotic infection/inflammation in patients with spontaneous preterm labor with intact membranes and also in those with PROM. We propose that sTREM-1 play a role in the innate immune response against intra-amniotic infection.
Acknowledgments
This research was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Naeye RL, Ross SM. Amniotic fluid infection syndrome. Clin Obstet Gynaecol. 1982;9:593–607. [PubMed] [Google Scholar]
- 2.Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 3.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 5.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 6.Ledger WJ. Infection and premature labor. Am J Perinatol. 1989;6:234–236. doi: 10.1055/s-2007-999583. [DOI] [PubMed] [Google Scholar]
- 7.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 8.Brocklehurst P. Infection and preterm delivery. BMJ. 1999;318:548–549. doi: 10.1136/bmj.318.7183.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 10.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12:145–155. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, Bracken MB. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166:1382–1388. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 13.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 14.Kim KW, Romero R, Park HS, Park CW, Shim SS, Jun JK, Yoon BH. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197:292–295. doi: 10.1016/j.ajog.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 15.Romero R, Munoz H, Gomez R, Sherer DM, Ghezzi F, Gibbs RS, Alfi O, DeVore GR, Randolph L. Two thirds of spontaneous abortion/fetal deaths after genetic amniocentesis are the result of a pre-existing subclinical inflammatory process of the amniotic cavity. Am J Obstet Gynecol. 1995;172:S261. [Google Scholar]
- 16.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 17.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 18.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, Jun JK. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–779. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 20.Blackwell S, Romero R, Chaiworapongsa T, Kim YM, Bujold E, Espinoza J, Camacho N, Hassan S, Yoon BH, Refuerzo JS. Maternal and fetal inflammatory responses in unexplained fetal death. J Matern Fetal Neonatal Med. 2003;14:151–157. doi: 10.1080/jmf.14.3.151.157. [DOI] [PubMed] [Google Scholar]
- 21.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110(Suppl 20):124–127. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 22.Murphy K, Travers P, Walport M. Innate immunity. 2008:39–108. [Google Scholar]
- 23.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 24.Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis. 2003;187(Suppl 2):S397–S401. doi: 10.1086/374754. [DOI] [PubMed] [Google Scholar]
- 25.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 26.Gibot S, Kolopp-Sarda MN, Bene MC, Cravoisy A, Levy B, Faure GC, Bollaert PE. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med. 2004;141:9–15. doi: 10.7326/0003-4819-141-1-200407060-00009. [DOI] [PubMed] [Google Scholar]
- 27.Knapp S, Gibot S, de VA, Versteeg HH, Colonna M, van der PT. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J Immunol. 2004;173:7131–7134. doi: 10.4049/jimmunol.173.12.7131. [DOI] [PubMed] [Google Scholar]
- 28.Gibot S, Cravoisy A, Kolopp-Sarda MN, Bene MC, Faure G, Bollaert PE, Levy B. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med. 2005;33:792–796. doi: 10.1097/01.ccm.0000159089.16462.4a. [DOI] [PubMed] [Google Scholar]
- 29.Gibot S, Le Renard PE, Bollaert PE, Kolopp-Sarda MN, Bene MC, Faure GC, Levy B. Surface triggering receptor expressed on myeloid cells 1 expression patterns in septic shock. Intensive Care Med. 2005;31:594–597. doi: 10.1007/s00134-005-2572-x. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Roldan N, Ferat-Osorio E, duna-Vicente R, Wong-Baeza I, Esquivel-Callejas N, studillo-de lV, Sanchez-Fernandez P, rriaga-Pizano L, Villasis-Keever MA, Lopez-Macias C, et al. Expression of triggering receptor on myeloid cell 1 and histocompatibility complex molecules in sepsis and major abdominal surgery. World J Gastroenterol. 2005;11:7473–7479. doi: 10.3748/wjg.v11.i47.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong-Baeza I, Gonzalez-Roldan N, Ferat-Osorio E, Esquivel-Callejas N, duna-Vicente R, rriaga-Pizano L, studillo-de l V, Villasis-Keever MA, Torres-Gonzalez R, Estrada-Garcia I, et al. Triggering receptor expressed on myeloid cells (TREM-1) is regulated post-transcriptionally and its ligand is present in the sera of some septic patients. Clin Exp Immunol. 2006;145:448–455. doi: 10.1111/j.1365-2249.2006.03158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibot S, Cravoisy A, Dupays R, Barraud D, Nace L, Levy B, Bollaert PE. Combined measurement of procalcitonin and soluble TREM-1 in the diagnosis of nosocomial sepsis. Scand J Infect Dis. 2007;39:604–608. doi: 10.1080/00365540701199832. [DOI] [PubMed] [Google Scholar]
- 33.Wiersinga WJ, Veer CT, Wieland CW, Gibot S, Hooibrink B, Day NP, Peacock SJ, van der PT. Expression profile and function of triggering receptor expressed on myeloid cells-1 during melioidosis. J Infect Dis. 2007;196:1707–1716. doi: 10.1086/522141. [DOI] [PubMed] [Google Scholar]
- 34.How CK, Chern CH, Wu MF, Wang LM, Huang CI, Lee CH, Hsieh SL. Expression of the triggering receptor expressed on myeloid cells-1 mRNA in a heterogeneous infected population. Int J Clin Pract. 2009;63:126–133. doi: 10.1111/j.1742-1241.2006.01193.x. [DOI] [PubMed] [Google Scholar]
- 35.Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–458. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 36.Richeldi L, Mariani M, Losi M, Maselli F, Corbetta L, Buonsanti C, Colonna M, Sinigaglia F, Panina-Bordignon P, Fabbri LM. Triggering receptor expressed on myeloid cells: role in the diagnosis of lung infections. Eur Respir J. 2004;24:247–250. doi: 10.1183/09031936.04.00014204. [DOI] [PubMed] [Google Scholar]
- 37.Determann RM, Millo JL, Gibot S, Korevaar JC, Vroom MB, van der PT, Garrard CS, Schultz MJ. Serial changes in soluble triggering receptor expressed on myeloid cells in the lung during development of ventilator-associated pneumonia. Intensive Care Med. 2005;31:1495–1500. doi: 10.1007/s00134-005-2818-7. [DOI] [PubMed] [Google Scholar]
- 38.Chan MC, Chang KM, Chao WC, Lin LY, Kuo BI, Hsu JY, Wu CL. Evaluation of a new inflammatory molecule (triggering receptor expressed on myeloid cells-1) in the diagnosis of pleural effusion. Respirology. 2007;12:333–338. doi: 10.1111/j.1440-1843.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu CL, Hsieh WY, Wu CL, Kuo HT, Lu YT. Triggering receptor expressed on myeloid cells-1 in pleural effusions: a marker of inflammatory disease. Respir Med. 2007;101:903–909. doi: 10.1016/j.rmed.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Huang LY, Shi HZ, Liang QL, Wu YB, Qin XJ, Chen YQ. Expression of soluble triggering receptor expression on myeloid cells-1 in pleural effusion. Chin Med J (Engl) 2008;121:1656–1661. [PubMed] [Google Scholar]
- 41.El Solh AA, Akinnusi ME, Peter M, Berim I, Schultz MJ, Pineda L. Triggering receptors expressed on myeloid cells in pulmonary aspiration syndromes. Intensive Care Med. 2008;34:1012–1019. doi: 10.1007/s00134-008-1087-7. [DOI] [PubMed] [Google Scholar]
- 42.Collins CE, La DT, Yang HT, Massin F, Gibot S, Faure G, Stohl W. Elevated synovial expression of triggering receptor expressed on myeloid cells-1 (TREM-1) in patients with septic arthritis or rheumatoid arthritis. Ann Rheum Dis. 2009;68(11):1768–1774. doi: 10.1136/ard.2008.089557. [DOI] [PubMed] [Google Scholar]
- 43.Vogel I, Goepfert AR, Thorsen P, Skogstrand K, Hougaard DM, Curry AH, Cliver S, Andrews WW. Early second-trimester inflammatory markers and short cervical length and the risk of recurrent preterm birth. J Reprod Immunol. 2007;75:133–140. doi: 10.1016/j.jri.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, Carstens MR, Medina LH, Viviani PG, Rojas IT. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev Med Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 46.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26(Suppl A):S114–S117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 48.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 49.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 50.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 51.Bonferroni C. Studi inOnore del Professore Salvatore Ortu Carboni. Rome; Italy: 1935. Il calcolo delle assicurazioni su gruppi di teste; pp. 13–60. [Google Scholar]
- 52.Chen LC, Laskin JD, Gordon MK, Laskin DL. Regulation of TREM expression in hepatic macrophages and endothelial cells during acute endotoxemia. Exp Mol Pathol. 2008;84:145–155. doi: 10.1016/j.yexmp.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3:445–453. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- 54.Bouchon A, Hernandez-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194:1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, Sugahara A, Koito H, Ujike-Asai A, Nakamura A, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest. 2003;111:323–332. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmid CD, Sautkulis LN, Danielson PE, Cooper J, Hasel KW, Hilbush BS, Sutcliffe JG, Carson MJ. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J Neurochem. 2002;83:1309–1320. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cantoni C, Bottino C, Vitale M, Pessino A, Augugliaro R, Malaspina A, Parolini S, Moretta L, Moretta A, Biassoni R. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J Exp Med. 1999;189:787–796. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allcock RJ, Barrow AD, Forbes S, Beck S, Trowsdale J. The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur J Immunol. 2003;33:567–577. doi: 10.1002/immu.200310033. [DOI] [PubMed] [Google Scholar]
- 60.Gingras MC, Lapillonne H, Margolin JF. TREM-1, MDL-1, and DAP12 expression is associated with a mature stage of myeloid development. Mol Immunol. 2002;38:817–824. doi: 10.1016/s0161-5890(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 61.Gibot S, Kolopp-Sarda MN, Bene MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200:1419–1426. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomez-Pina V, Soares-Schanoski A, Rodriguez-Rojas A, del FC, Garcia F, Vallejo-Cremades MT, Fernandez-Ruiz I, Arnalich F, Fuentes-Prior P, Lopez-Collazo E. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol. 2007;179:4065–4073. doi: 10.4049/jimmunol.179.6.4065. [DOI] [PubMed] [Google Scholar]
- 63.Menon R, Fortunato SJ. Induction of triggering receptors of myeloid cell (TREM-1) expression in fetal membranes and higher concentration of soluble TREM-1 in amniotic fluid with spontaneous preterm birth. Reprod Sci. 2008;15:825–830. doi: 10.1177/1933719108320090. [DOI] [PubMed] [Google Scholar]
- 64.Peltier MR. Immunology of term and preterm labor. Reprod Biol Endocrinol. 2003;1:122. doi: 10.1186/1477-7827-1-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130:569–581. doi: 10.1530/rep.1.00197. [DOI] [PubMed] [Google Scholar]
- 66.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394–24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gibot S, Massin F, Le RP, Bene MC, Faure GC, Bollaert PE, Levy B. Surface and soluble triggering receptor expressed on myeloid cells-1: expression patterns in murine sepsis. Crit Care Med. 2005;33:1787–1793. doi: 10.1097/01.ccm.0000172614.36571.75. [DOI] [PubMed] [Google Scholar]
- 69.Gibot S, Alauzet C, Massin F, Sennoune N, Faure GC, Bene MC, Lozniewski A, Bollaert PE, Levy B. Modulation of the triggering receptor expressed on myeloid cells-1 pathway during pneumonia in rats. J Infect Dis. 2006;194:975–983. doi: 10.1086/506950. [DOI] [PubMed] [Google Scholar]
- 70.Gibot S, Buonsanti C, Massin F, Romano M, Kolopp-Sarda MN, Benigni F, Faure GC, Bene MC, Panina-Bordignon P, Passini N, et al. Modulation of the triggering receptor expressed on the myeloid cell type 1 pathway in murine septic shock. Infect Immun. 2006;74:2823–2830. doi: 10.1128/IAI.74.5.2823-2830.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang DY, Qin RY, Liu ZR, Gupta MK, Chang Q. Expression of TREM-1 mRNA in acute pancreatitis. World J Gastroenterol. 2004;10:2744–2746. doi: 10.3748/wjg.v10.i18.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yasuda T, Takeyama Y, Ueda T, Shinzeki M, Sawa H, Takahiro N, Kamei K, Ku Y, Kuroda Y, Ohyanagi H. Increased levels of soluble triggering receptor expressed on myeloid cells-1 in patients with acute pancreatitis. Crit Care Med. 2008;36:2048–2053. doi: 10.1097/CCM.0b013e31817b8824. [DOI] [PubMed] [Google Scholar]
- 73.Koussoulas V, Vassiliou S, Demonakou M, Tassias G, Giamarellos-Bourboulis EJ, Mouktaroudi M, Giamarellou H, Barbatzas C. Soluble triggering receptor expressed on myeloid cells (sTREM-1): a new mediator involved in the pathogenesis of peptic ulcer disease. Eur J Gastroenterol Hepatol. 2006;18:375–379. doi: 10.1097/00042737-200604000-00010. [DOI] [PubMed] [Google Scholar]
- 74.Schmausser B, Endrich S, Beier D, Moran AP, Burek CJ, Rosenwald A, Rieckmann P, Muller-Hermelink HK, Eck M. Triggering receptor expressed on myeloid cells-1 (TREM-1) expression on gastric epithelium: implication for a role of TREM-1 in Helicobacter pylori infection. Clin Exp Immunol. 2008;152:88–94. doi: 10.1111/j.1365-2249.2008.03608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park JJ, Cheon JH, Kim BY, Kim DH, Kim ES, Kim TI, Lee KR, Kim WH. Correlation of Serum-Soluble Triggering Receptor Expressed on Myeloid Cells-1 with Clinical Disease Activity in Inflammatory Bowel Disease. Dig Dis Sci. 2008 doi: 10.1007/s10620-008-0514-5. [DOI] [PubMed] [Google Scholar]
- 76.Bleharski JR, Kiessler V, Buonsanti C, Sieling PA, Stenger S, Colonna M, Modlin RL. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. 2003;170:3812–3818. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- 77.Radsak MP, Salih HR, Rammensee HG, Schild H. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J Immunol. 2004;172:4956–4963. doi: 10.4049/jimmunol.172.8.4956. [DOI] [PubMed] [Google Scholar]
- 78.Gibot S, Massin F, Marcou M, Taylor V, Stidwill R, Wilson P, Singer M, Bellingan G. TREM-1 promotes survival during septic shock in mice. Eur J Immunol. 2007;37:456–466. doi: 10.1002/eji.200636387. [DOI] [PubMed] [Google Scholar]
- 79.Sharif O, Knapp S. From expression to signaling: roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008;213:701–713. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]