Abstract
Aim
Microbial biofilm matrix contains polysaccharides and proteins and can require extracellular nucleic acids for initial formation. Experiments were designed to identify infectious pathogens in human aneurysms and to characterize biofilm formed by calcified human arterial-derived nanoparticles.
Materials & method
A total of 26 different microbial pathogens were isolated from 48 inflammatory aneurysms. Consistent amounts (0.49 McFarland units) of nanoparticles derived from similar tissue were seeded into 24-well plates and cultured for 21 days in the absence (control) or presence of RNase, tetracycline or gentamicin.
Results
Control biofilm developed within 14 days, as detected by concanavalin A and BacLight™ Green staining. The formation of biofilm in wells treated with RNase was not different from the control; however, gentamicin partially inhibited and tetracycline completely inhibited biofilm formation. Therefore, nanoparticle biofilm retains some characteristics of conventional bacterial biofilm and requires protein–calcium interactions, although extracellular RNA is not required.
Conclusion
This model system may also allow study of nanosized vesicles derived from donor tissue, including any microbes present, and could provide a useful tool for in vitro investigation of nanoparticle biofilm formation.
Keywords: biofilm, gentamicin, matrix, tetracycline
In nature, biofilms are surface-associated structures of communal microorganisms encased within a secreted matrix of exopolysaccharides. Biofilms may contribute to human disease and reduce performance of implantable medical devices [1–4]. It is critical, therefore, to better understand the composition and factors leading to their formation.
Atherosclerosis results from inflammatory processes and infectious agents, which include bacteria (i.e., Treponema pallidum, Chlamydia pneumoniae, Helicobacter pylori and Porphyromonas gingivalis) and viruses (i.e., coxsackie B4, herpes simplex virus and cytomegalovirus) [5–11]. Antibiotics, including tetracycline (TCN), are effective in reducing some vascular diseases, including syphilitic aortitis, expansion of aortic aneurysms and a number of adverse events in patients with coronary artery disease [12–18]. Discrepancies among studies regarding the efficacy of antibiotic treatment for cardiovascular conditions may reflect concurrent pathophysiological parameters, such as heterogeneous patient populations, and confounding risk factors, such as metabolic syndrome, which may not necessarily cause atherosclerosis but could predispose select individuals to the risk of chronic infection. Therefore, associations among infection, antibiotics and vascular disease require further investigation.
Nanosized particles (nanoparticles [NPs]) have been identified in diseased human tissues including arterial plaque, kidney stones, gall stones, prostate glands, placenta and ovarian tumors. Those NPs isolated from arterial tissue and kidney stones form calcific films under standard tissue-culture conditions [19–25]. Whether or not these films can rightfully be called biofilms is debatable.
Nanoparticle films contain both organic (proteins, nucleic acids and lipids) and inorganic mineral components [26–31]. A DNA sequence identified as Nanobacterium sanguineum was reported from NPs isolated from human and cow sera [32]. However, only partial DNA sequences of common environmental bacteria were amplified by PCR from film derived from NPs isolated from human saliva. Thus, characterization of a NP film may depend upon the tissue of origin and could be derived from contaminating flora [33] rather than from a unique nanosized bacterium [32,34].
Extracellular nucleic acids participate in the formation of some bacterial biofilm [35]. In addition, some enzymes participating in RNA translation were found in cultures of NPs [27,36]. Other studies provide evidence that the formation of a NP film may result from physical–chemical interactions of proteins in serum with hydroxyapatite or calcium carbonate [29–31,37]. However, formation of a NP film is reduced or limited by inhibitors of aerobic metabolism and antimicrobial agents, suggesting the involvement of enzymatic processes in addition to physical–chemical interactions of proteins with inorganic salts [26–28,38,39].
The extracellular matrix of microbial bio-films contains polysaccharides and enzymes that affect propagation and stability of the biofilm [3]. Depending upon environmental conditions, some types of bacteria precipitate intracellular hydroxyapatite or form cell wall vesicles that could potentially interact with mammalian cells, initiating inflammatory responses [40–42]. Indeed, cultured, human-derived NPs reduce platelet aggregation [43] and modulate vascular remodeling when injected intravenously into animals [44,45], suggesting a pathological potential. Therefore, studies were designed to identify infectious pathogens in diseased arterial tissue and to characterize the film formed by cultured NPs derived from similar arteries. Experiments tested the hypothesis that formation of a NP film is dependent upon extracellular nucleic acids, enzymatic and physical–chemical calcification processes. In this article, the nomenclature biofilm, initially proposed by Cisar et al. to describe a NP-derived film in culture is used, even though culturable NPs may not be a biofilm in the traditional sense [33].
Methods
Identification of microbes in vascular tissue & isolation of NPs
Segments of aneurysms explanted as part of surgical vascular repair were analyzed. Explanted segments were placed in sterile saline, homogenized and cultured for aerobic or anaerobic organisms in the Clinical Microbiology Laboratory at the Mayo Clinic (MN, USA) using standard techniques. Some segments of explanted aneurysms were homogenized and forced through a 0.2 μm filter. The filtrate was inoculated into 10 ml vented tissue-culture flasks containing standard culture medium [28] comprising Dulbecco’s modified Eagle’s medium (DMEM 10–013, Mediatech, Inc., VA, USA), 10% γ-irradiated fetal bovine serum (Atlanta Biologicals, Inc., GA, USA) and 50-μM β-mercaptoethanol, which had been filtered (0.2 μm) prior to use. Flasks then were placed in a humidified incubator at 37°C, 90% O2/10% CO2. Every 4–6 weeks, flasks were scraped with a rubber spatula and divided 1:10 into fresh DMEM and 10% fetal bovine serum. NPs exist as both adherent biofilm and planktonic (floating) forms suspended in the medium. In this study, planktonic forms of NPs were used exclusively. Random NP cultures were screened for Mycoplasma using a rapid PCR test in the Mayo Clinic Microbiology Laboratory; all NP cultures tested negative. The collection of human tissue and review of medical records were performed in compliance with regulations of the State of Minnesota and the USA (Institutional Review Board-approved protocols 291-99, 2126-01 and 1030-03).
Analysis of NP biofilm
The outer wells of 24-well culture plates (Costar®, Corning, Inc., NY, USA) were filled with sterile-filtered water to reduce evaporation from the seeded wells during the course of the experiment. A sterile glass cover slip was placed in the bottom of the remaining inner eight wells of each plate. These wells were filled with 3 ml of standard culture medium, either alone, or with DNase (40 units/ml, three different lots, TURBO™ DNase; Ambion, Inc., TX, USA), RNase (50 units/ml; RNase I; Ambion, Inc., TX, USA), TCN HCl (12μg/ml; Gallipot, Inc., MN, USA) [27] or gentamicin sulfate (GM; 12 μg/ml; Spectrum Chemical MFG CORP, CA, USA) [46] in duplicate (two wells per condition). DNase and RNase were used to determine whether extracellular nucleic acids were required for the formation of the NP biofilm. TCN and GM are antibiotics of the same class but differ in their capacity to chelate calcium and were chosen in order to differentiate between the potential mechanisms of action: mineral–protein interactions versus enzymatic activity [47,48].
A total of 12 sets of plates were prepared for each harvest day: six contained medium with and without treatments (blanks), and six contained identical treatments but were seeded with NPs (density of 0.49 McFarland units). This initial seeding density was chosen based on preliminary experiments that demonstrated measureable changes in turbidity within the 3-week time frame. Plates were incubated (37°C, 90% O2/10% CO2) for up to 21 days. Treatments (diluted in medium) or equal volumes of medium (control) were added to each well every day. At days 1, 7, 14 and 21, a 2-ml aliquot of medium was collected from each well. Care was taken not to agitate the well, thus leaving the biofilm undisturbed. Medium was analyzed for turbidity (Hach 2100N Turbidimeter, CO, USA) in McFarland units, reflecting the density and size of suspended NPs.
The remaining medium in each well was aspirated and discarded. The biofilm remaining on each cover slip was fixed with 2% paraformaldehyde (0.5 ml; 10 min; Electron Microscopy Sciences, PA, USA) and washed with 0.5 ml phosphate-buffered saline (PBS). Cover slips were mounted on flame-cleaned slides with ProLong Gold mounting medium (days 1, 7, 14; Invitrogen, CA, USA) or ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI), a DNA-binding stain (day 21; Invitrogen). Slides were stored overnight away from light, then sealed and kept at 4°C before examination.
Slides were coded so that the observer was blinded to the sampling day and treatment. Using a dark-field microscope (CytoViva, Inc., AL, USA) with a Dage-Maryland Telecommunications, Inc. (MTI) camera (Excel M, Dage-MTI of Michigan City, Inc., IN, USA) and Exponent software (Dage-MTI, Inc.), five fields were selected from each slide. Each 100× field was analyzed for the percent area covered by biofilm using KS400 software (Zeiss, NY, USA).
Staining of NP biofilm
Planktonic NPs were harvested then diluted in fresh standard medium. Aliquots of 2.5 ml were plated onto glass cover slips and incubated, as described earlier. After 14 days, cover slips were removed and examined by confocal laser scanning microscopy.
Concanavalin A (Con-A; Concanavalin A-Alexa Fluor® 488; Molecular Probes, OR, USA), a lectin compound that selectively binds to glucose and mannose residues of cell wall polysaccharide in the form of a fluorescent conjugate, was used to label and visualize the biofilm. Con-A stock solutions were prepared (1 mg/ml in 0.1 M sodium bicarbonate, pH 8.3) and aliquots were stored at −20°C until use. For labeling biofilm, each cover slip was rinsed briefly in PBS, placed into a petri dish containing 25-μg/ml Con-A in 2-ml PBS, incubated for 60 min at 37°C, removed, rinsed briefly and mounted on a microscope slide using ProLong Gold antifade reagent (Molecular Probes). Cover slips with unstained biofilm served as controls. In some experiments, cover slips were labeled with BacLight™ Green (Molecular Probes), a non-nucleic acid-labeling reagent highly specific for bacteria. Stock solutions of 1 mM were prepared in dimethyl sulfoxide and diluted to a final concentration of 2 μM in PBS. The labeling procedure was similar to that used for Con-A, except that each biofilm specimen was labeled for 30 min at 25°C followed by fixation in 2% paraformaldehyde before mounting and imaging. Several control samples were also prepared on cover slips in order to rule out autofluorescence during imaging and to validate the specificity of the stain for bacteria: unstained bio-film, hydroxyapatite crystals exposed to culture medium, Staphylococcus epidermidis and porcine aortic smooth muscle (PASM) cells. S. epidermidis and PASM cells were cultured according to standard techniques [49]. Each cover slip was observed using a Zeiss LSM 510 confocal laser-scanning system (Carl Zeiss, Inc., Oberkochen, Germany) equipped with an argon laser and an Axiovert 200 M inverted microscope fitted with a 100×/1.4 Plan Apochromat oil-immersion objective (Carl Zeiss, Inc.). The emission light was passed through a 505–550 band-pass filter. Images were captured and processed using Zeiss LSM 510 software.
Statistical analysis
Values are shown as the average turbidity or percent area covered by biofilm of each well, corrected for the average value obtained from the corresponding blank well for that day and treatment. To account for correlations and to estimate average responses of observations sharing the same covariates, such as plate effects of repeated images, generalized estimating equation (GEE) models [50] were fitted to both the turbidity and biofilm data. The GEE model with an exchangeable correlation structure was assumed and the identity-link function for a normal random variable was specified. The GEE model was fitted with the R library geepack (R programming language version 2.8.0, open source), which yielded estimates for the treatment effect and time effect. Differences were estimated between each treatment and control group at each time point, as well as for differences within treatments compared with day 1. Statistical significance was accepted at p < 0.05.
Results
Identification of microbes in vascular tissue
Explanted segments of abdominal aortic aneurysms, classified by the attending surgeon as inflammatory without adhesions or anastomoses to the intestines, were obtained from 48 patients. A total of 26 different organisms were cultured from these aneurysms using routine microbiologic techniques (Table 1). Multiple bacteria were cultured from some samples; one sample produced six different organisms. Additional aneurysms (n = 9) not sent for routine microbial culture were processed using conditions to propagate NPs as described in the methods section. Calcific NP biofilm developed in four (44%) of these nine preparations. Subcultures of one of these isolates were used for experiments below.
Table 1.
Bacteria and fungi cultured from human abdominal aortic aneurysms*.
| Male | Female | |
|---|---|---|
| Aspergillus | 1 | |
| Bacillus | 1 | |
| Bacillus (Gram negative) | 1 | 1 |
| Bacillus (Gram positive) | 1 | |
| Bacteroides fragilis | 1 | |
| Beauveria | 1 | |
| Coccus resembeling Staphylococcus | 6 | 1 |
| Corynebacterium | 1 | |
| Escherichia coli | 4 | |
| Enterococcus | 2 | |
| Geotrichum | 1 | |
| Histoplasma capsulatum | 1 | |
| Klebsiella oxytoca | 1 | |
| Klebsiella pneumonia | 2 | |
| Lactobacillus | 1 | |
| Peptostreptococcus saccharolyticus | 1 | |
| Propionibacterium acnes | 3 | |
| Pseudomonas aeruginosa | 1 | |
| Salmonella species | 1 | |
| Serratia marcescens | 1 | |
| Staphylococcus aureus | 1 | 4 |
| Staphylococcus, coagulase negative | 16 | 3 |
| Streptococcus | 3 | |
| Streptococcus pneumoniae | 1 | |
| Streptococcus virdans | 2 | 2 |
| Yeast | 1 |
Numbers represent those showing positive cultures for each type of microbe. Some aneurysms were positive for multiple organisms.
Aneurysms were collected from 48 patients.
Analysis of NP biofilm
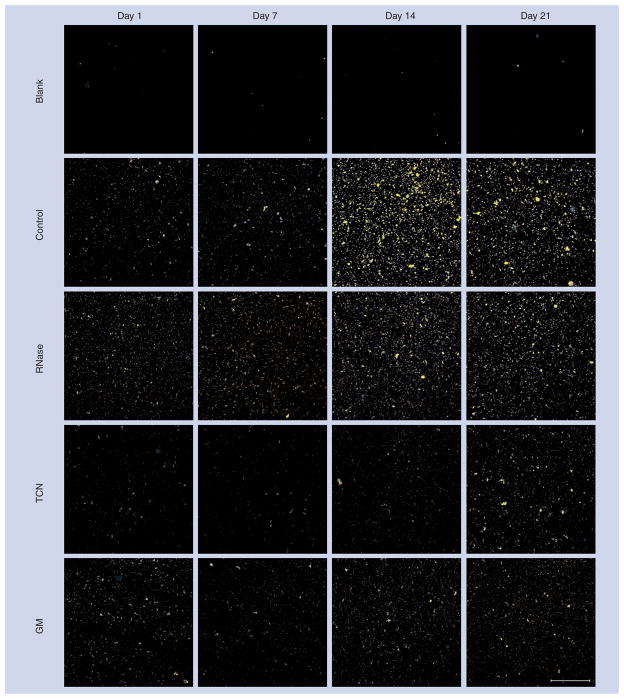
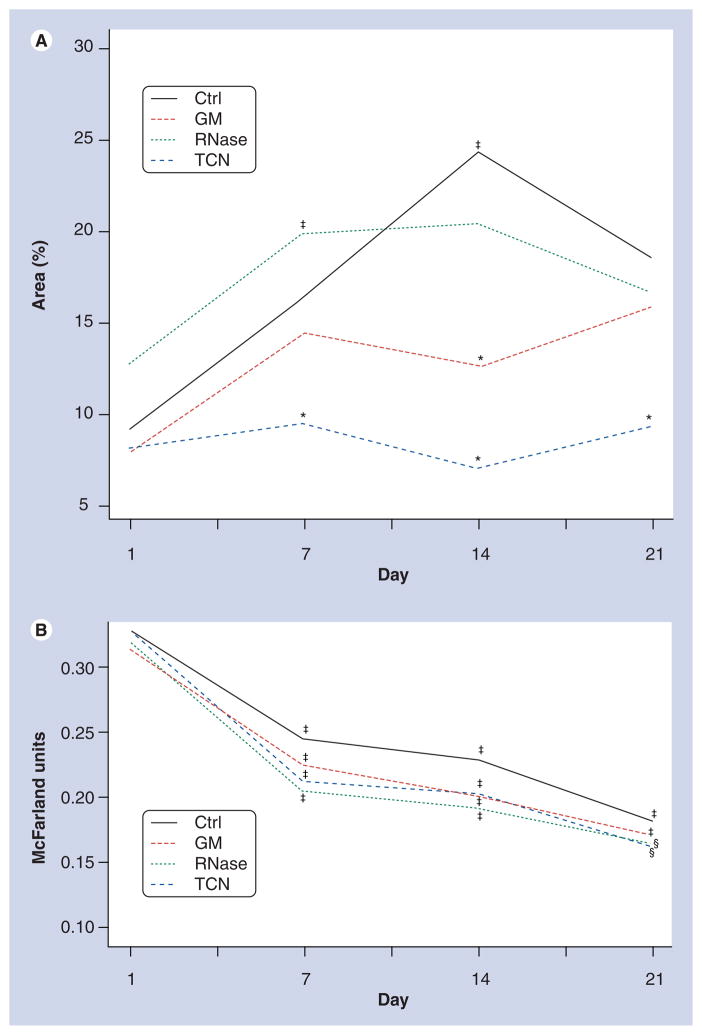
Significant adherent biofilm developed in wells seeded with NPs alone (control), reaching a maximum by day 14 (Figures 1 & 2A). Biofilm also formed in NP-seeded wells treated with RNase, reaching a maximum and plateau at day 7. However, in wells treated with GM, development of NP biofilm was significantly less than that in control wells at day 14. TCN also reduced NP biofilm development compared with controls at all time points and to a greater extent than GM (Figures 1 & 2A). Although images of wells containing TCN appeared to demonstrate an increase in biofilm formation by day 21 (Figure 1), this appearance was caused by increased background values also present in wells containing TCN but no NPs. When the background is taken into account, the actual amount of biofilm present in the TCN wells was less than that of the other day 21 values (Figure 2A). Similar experiments were attempted using commercial DNase. However, wells containing DNase (from multiple lots) demonstrated bacterial overgrowth of Rothia mucilaginosa. These plates were discarded and no data were obtained. No other plates of NP biofilm used in the subsequent analyses showed bacterial overgrowth.
Figure 1. Representative 100× darkfield images of nanoparticle biofilm on cover slips from wells collected on days 1, 7, 14 and 21 postplating.
The blank wells (top row) were not inoculated with nanoparticles (NPs) and received no treatments. Control wells (second row) were inoculated with NPs and received no treatments. All other rows were seeded with NPs and treated with RNase (50 units/ml; third row), TCN (12 μg/ml; fourth row) or GM (12 μg/ml; fifth row). Scale bar is 100 μm.
GM: Gentamicin; TCN: Tetracycline.
Figure 2. Effects of treatments on the formation of (A) nanoparticle biofilm and (B) the turbidity of the media.
Each value represents the mean of n = 120–150 values/day. Values are the average of each measurement corrected for the average value of the corresponding blank wells. Turbidity is measured in McFarland units.
*Statistical difference from control.
‡Statistical difference from day 1.
§Statistical difference from both control and day 1, p < 0.05.
Ctrl: Control; GM: Gentamicin; TCN: Tetracycline.
The turbidity of the medium, a quantitative index of the number of planktonic NPs, decreased significantly over the course of the 21-day treatment period in all groups (Figure 2B). The turbidity of medium from control wells trended higher than that of all other treatments. By day 21, the turbidity of the medium from wells treated with either RNase or TCN was significantly lower than that of control wells (Figure 2B).
Staining of NP biofilm
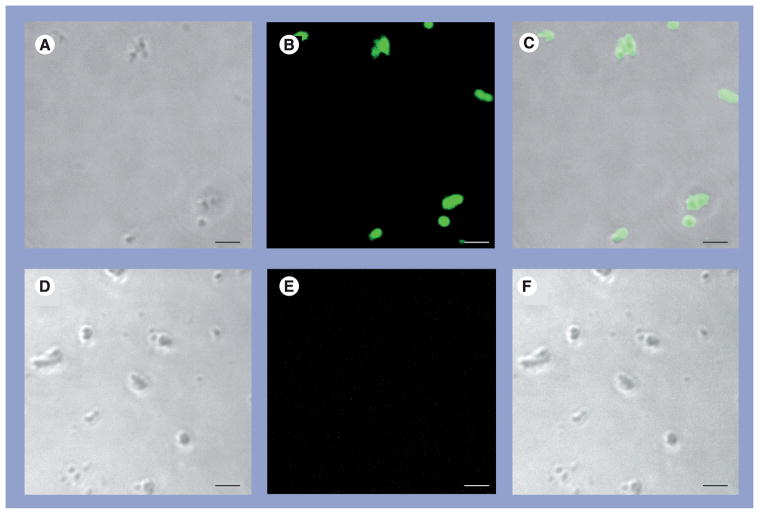
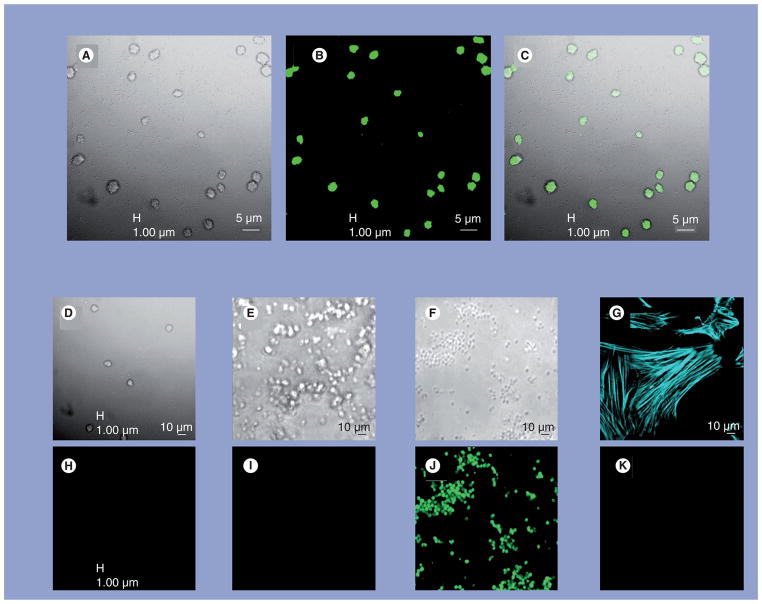
Nanoparticle biofilm (day 14) stained intensely with Con-A (Figure 3) and BacLight Green (Figure 4). S. epidermidis, used as a positive control, also stained intensely with BacLight Green, whereas commercial hydroxyapatite crystals did not (negative control). PASM cells, an additional negative control for the stain, stained appropriately for F-actin but were not stained by BacLight Green (Figure 4). None of the NP biofilm cover slips collected at day 21 stained positive for DAPI.
Figure 3. Nanoparticle biofilm at day 14.
The examples are stained with Con-A Alexa Fluor® 488 (A–C). (D–F) Unstained example. (A & D) are transmitted light. (B & E) are Con-A. (C & F) are the corresponding overlays. Each bar is 5 μm.
Con-A: Concanavalin A.
Figure 4. Nanoparticles stained with BacLight™ Green and examined by confocal laser scanning microscopy.
Two images were taken simultaneously: (A) using transmitted light and (B) using 488-nm laser excitation. The overlay of the two images (C) shows that localization of the fluorophore corresponds to the 3–5 mm structures seen by transmitted light. Images of samples taken under identical acquisition settings are also shown for nanoparticles to which no dye was applied ((D & H) control for nonspecific staining), hydroxyapatite crystals maintained under culture conditions ((E & I) control for the interaction of calcium phosphate with media proteins) and for Staphylococcus epidermidis ((F & J) positive control for bacterial staining). Porcine vascular smooth muscle cells stained for F-actin with Alexa Fluor® 647 phalloidin (G) but did not stain with BacLight Green ((K) negative control for specific bacterial staining).
Discussion
Results of this study establish that microbial pathogens are commonly present in aortic aneurysms and provide evidence that calcifying NPs can be propagated from some, but not all, such aneurysms. Furthermore, mannose and glucose were identified within the NP biofilm, as evidenced by Con-A staining, similar to that observed in a standard microbial biofilm [3]. Therefore, these data provide significant additions to the current pool of knowledge regarding the characterization of the biofilm formed by human-derived NPs.
New information also is provided regarding factors that affect development of NP biofilm. First, a decrease in turbidity of the NP culture medium over time after seeding was inversely related to the development of the biofilm under control conditions. This observation is consistent with the established characteristics of biofilm development, during which planktonic bacteria adhere to a surface, form colonies and eventually release new planktonic bacteria that perpetuate the cycle [3]. In the present study, turbidity of the media did not increase after 21 days under control conditions. Therefore, NP biofilm may not be identical to classical microbial biofilm and it is possible that after seeding into culture wells, NPs form aggregates through purely chemical processes, which then settle and adhere to the cover slips.
Previous work demonstrated that NPs in culture incorporate [3H]uridine [28], suggesting that NP biofilm development might rely upon RNA. However, these results indicate that RNase did not alter the development of NP biofilm. This observation does not preclude the presence of RNA, rather it suggests that biofilm development is not dependent on the presence of extracellular RNA, since RNase does not cross cell membranes. The absence of DAPI staining also suggests that if extracellular nucleic acids are present in NP biofilm, the amounts are below the sensitivity levels of DAPI as used in these experiments. Unfortunately, it was not possible to evaluate the contribution of extracellular DNA to the formation of NP biofilm, owing to contamination of commercial DNases with the common oral flora bacterium [35]. Previous experiments, however, have suggested that DNA is present in NP isolates [27,28,32,33]. The current work suggests the possibility that DNA sequences are derived from the source material, in other words, commensal microbes present in the donor tissue, since numerous varieties of microbes could be cultured from explanted inflammatory aneurysms (Table 1). Further support for this hypothesis is provided by the positive staining of NP cultures with BacLight Green (Figure 4). BacLight Green is a proprietary probe, thus the specific microbial proteins(s) that mediate labeling are unknown. However, enzymatic activity is probably required to activate the dye, since cell fixation is suggested only after labeling [51].
Previous studies evaluated the propagation of NP biofilm in the presence of various antibiotics, including TCN [27,38]. In order to distinguish between the relative importance of calcium chelation [29,30,37] and the more specific biochemical effects involved in the development of NP biofilms, two antibiotics with the same antimicrobial mechanism, but with different calcium-chelating properties, were chosen for this study. Both TCN and GM block tRNA binding in ribosomes [47]. However, TCN chelates calcium while GM does not [38,48]. The complete inhibition of NP biofilm formation by TCN could be owing to its calcium-chelating properties, its ability to block tRNA binding to other proteins or both. It appears that inhibition of nucleic acid–protein binding is an important component in the biofilm process, since GM partially inhibited NP biofilm formation. These results agree with previous studies of NP biofilm derived from bovine sera and human kidney stones [27,38]. Collectively, these results suggest that both an enzymatic process and a chemical–protein interaction are required for NP biofilm development.
The decrease in biofilm formation in the presence of TCN and GM was accompanied by a decrease in turbidity of the media from wells seeded with NPs. Thus, the decrease in biofilm formation was not due to stabilization of the planktonic forms of NPs within the media. Furthermore, since turbidity in the wells treated with both GM and TCN decreased with time, these chemicals in some way disrupted the chemical interactions required for NP formation.
Conclusion
The results of this study provide data that bring together two ostensibly contradictory concepts of NP composition and provide the basis for a hypothesis regarding the production of biologic NPs and their contribution to disease. Consistent with historical cardiovascular literature that relates infectious burden to atherosclerotic disease, pathogens were identified from explanted aneurysms. Processing of explanted, infected material by homogenization and filtration could produce microbial byproducts and perhaps generate membrane vesicles, some of which might retain enzymatic activity. Nucleic acid segments might also result from this processing. These segments may not represent complete sequences, and the variable sequences reported thus far may reflect the heterogeneity of the microbial population within the diseased tissue [27,33,52,53].
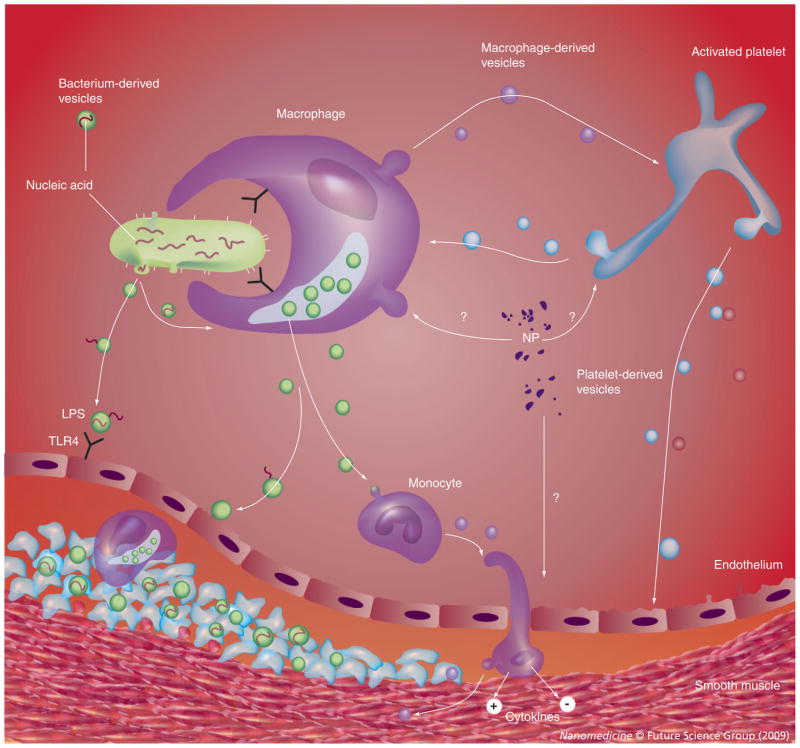
The fact that NPs were propagated from less than half of those aneurysms prepared for culture suggests that the type of commensal microbe and/or total microbial burden may be critical for NP propagation in culture. This concept is similar to one proposed by Ciftcioglu and colleagues, who suggested that NPs may act as a system for delivery of microbial toxins to tissue [38,53]. Indeed, NPs derived from blood may reflect populations of cell-derived, nanosized vesicles (also called microvesicles or microparticles), which vary with disease conditions [54–57], or L-forms of bacteria that are carried by blood elements (Figure 5) [58,59]. This proposed model does not rule out the possibility that proteins and minerals could form NP aggregates independent of intact enzymatic activity, but instead purely driven by physiochemical processes (pH, protein and ion concentrations) [26,29–31,37].
Figure 5. Potential interactions among nanoparticles derived from pathogens, blood and vascular tissue in progression of atherosclerotic plaque.
Infectious burden is known to be associated with accelerated atherosclerotic processes and nano- and/or microsized vesicles from pathogens or native cells could be retained in homogenate preparations of the donor tissue used for NP culture. Pathogens could release substances such as LPS, which activate cellular inflammation through binding to TLRs (TLR4). This model explains the propagation of NPs from homogenates of some, but not all, inflammatory aneurysms and explains the presence of multiple nucleic acid sequences of common microbes in some, but not all, cultures of NPs.
LPS: Lipopolysaccharide; NP: Nanoparticle; TLR: Toll-like receptor.
Future perspective
While the term NP defines entities based on size, it is reasonable to speculate that the chemical composition of NPs and their activity will vary depending on their biological sources. Heterogeneity of biochemical composition using biologically derived NPs should be expected and not be considered as a rationale to stop investigation into their physiological and pathophysiological potential. Rather, the challenge for investigators is to understand how NP entities might develop in disease and whether their formation can be exploited as diagnostic or prognostic biomarkers. Furthermore, standardization of starting material, collection and culture conditions and methods to improve identification of the composition of NPs might help eliminate the confusion over what appear to be contradictory findings and may provide a model system for the investigation of biofilm formation on implantable medical devices.
Executive summary.
Aerobic, anaerobic bacteria and fungi were cultured from explanted human inflammatory aneurysms. Calcifying, self-propagating nanoparticles (NPs) were cultured from some, but not all, aneurysms.
Cultured NPs formed a biofilm, which stained positive for mannose and bacterial proteins.
Formation of NP biofilm was reduced by tetracycline, partially reduced by gentamicin and not affected by RNase. Biofilm formed by NPs may reflect the tissue of origin and, perhaps, retain proteins and enzymatic activity characteristic of that tissue, including those of resident pathogens.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
The authors acknowledge support from the NIH (grant number HL889888), Fetzer Foundation and Mayo Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
- 1.Deva A, Chang L. Bacterial biofilms: a cause for accelerated capsular contracture? Aesthet Surg J. 1999;16:130–133. [Google Scholar]
- 2.Braxton EE, Jr, Ehrlich GD, Hall-Stoodley L, et al. Role of biofilms in neurosurgical device-related infections. Neurosurg Rev. 2005;28(4):249–255. doi: 10.1007/s10143-005-0403-8. [DOI] [PubMed] [Google Scholar]
- 3■.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. One of the original descriptions of biofilm formation. [DOI] [PubMed] [Google Scholar]
- 4.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407(6805):762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 5.Frishman WH, Ismail A. Role of infection in atherosclerosis and coronary artery disease: a new therapeutic target? Cardiol Rev. 2002;10:199–210. doi: 10.1097/00045415-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Kiechl S, Egger G, Mayr M, et al. Chronic infections and the risk of carotid atherosclerosis. Prospective results from a large population study. Circulation. 2001;103:1064–1070. doi: 10.1161/01.cir.103.8.1064. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Shearer GM, Norman JE, et al. Host response to cytomegalovirus infection as a determinant of susceptibility to coronary artery disease. Sex-based differences in inflammation and type of immune response. Circulation. 2000;102:2491–2496. doi: 10.1161/01.cir.102.20.2491. [DOI] [PubMed] [Google Scholar]
- 8■.Epstein SE, Zhu J, Burnett MS, Zhou YF, Vercellotti GM, Hajjar D. Infection and atherosclerosis: potential roles of pathogen burden and molecular mimicry. Arterioscler Thromb Vasc Biol. 2000;20:1417–1420. doi: 10.1161/01.atv.20.6.1417. Important discussion linking chronic infection to cardiovascular disease. [DOI] [PubMed] [Google Scholar]
- 9.Prasad A, Zhu J, Halcox JPJ, Waclawiw MAA, Epstein SE, Quyyumi AA. Predisposition to atherosclerosis by infections. Role of endothelial dysfunction. Circulation. 2002;106:184–190. doi: 10.1161/01.cir.0000021125.83697.21. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Nieto FJ, Horne BD, Anderson JL, Muhlestein JB, Epstein SE. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation. 2001;103:45–51. doi: 10.1161/01.cir.103.1.45. [DOI] [PubMed] [Google Scholar]
- 11.Espinola-Klein C, Rupprecht HJ, Blankenberg S, et al. Impact of infectious burden on progression of carotid atherosclerosis. Stroke. 2002;33(11):2581–2586. doi: 10.1161/01.str.0000034789.82859.a4. [DOI] [PubMed] [Google Scholar]
- 12.Frank MW, Mehlman DJ, Tsai F, Lomasney JW, Joob AW. Syphilitic aortitis. Circulation. 1999;100:1582–1583. doi: 10.1161/01.cir.100.14.1582. [DOI] [PubMed] [Google Scholar]
- 13.Vammen S, Lindholt JS, Ostergaard L, Fasting H, Henneberg EW. Randomized double-blind controlled trial of roxithromycin for prevention of abdominal aortic aneurysm expansion. Br J Surg. 2001;88:1066–1072. doi: 10.1046/j.0007-1323.2001.01845.x. [DOI] [PubMed] [Google Scholar]
- 14.Meier CR, Derby LR, Jick SS, Vasilakis C, Jick H. Antibiotics and risk of subsequent first-time acute myocardial infarction. JAMA. 1999;281:427–431. doi: 10.1001/jama.281.5.427. [DOI] [PubMed] [Google Scholar]
- 15.Stone AFM, Mendall MA, Kaski JC, et al. Effect of treatment for Chlamydia pneumoniae and Helicobacter pylori on markers of inflammation and cardiac events in patients with acute coronary syndromes. South Thames trial of antibiotics in myocardial infarction and unstable angina (stamina) Circulation. 2002;106:1219–1223. doi: 10.1161/01.cir.0000027820.66786.cf. [DOI] [PubMed] [Google Scholar]
- 16.Pilote L, Green L, Joseph L, Richard H, Eisenberg MJ. Antibiotics against Chlamydia pneumoniae and prognosis after acute myocardial infarction. Am Heart J. 2002;143:294–300. doi: 10.1067/mhj.2002.120296. [DOI] [PubMed] [Google Scholar]
- 17.Arnestad G, Scheel O, Hungnes O. Chronic infections and coronary heart disease (correspondence) Lancet. 1997;350:1028. doi: 10.1016/S0140-6736(97)26040-X. [DOI] [PubMed] [Google Scholar]
- 18.Luchsinger JA, Pablos-Mendez A, Knirsch C, Rabinowitz D, Shea S. Relation of antibiotic use to risk of myocardial infarction in the general population. Am J Cardiol. 2002;89:18–21. doi: 10.1016/s0002-9149(01)02156-7. [DOI] [PubMed] [Google Scholar]
- 19.Sedivy R, Battistutti WB. Nanobacteria promote crystallization of psammoma bodies in ovarian cancer. APMIS. 2003;111:951–954. doi: 10.1034/j.1600-0463.2003.1111006.x. [DOI] [PubMed] [Google Scholar]
- 20■.Ciftcioglu N, Mckay DS, Kajander EO. Association between nanobacteria and periodontal diseases. Circulation. 2003;108:e58–e59. doi: 10.1161/01.CIR.0000086781.16968.2D. Links infection to formation of nanoparticles. [DOI] [PubMed] [Google Scholar]
- 21.Khullar M, Sharma SK, Singh SK, et al. Morphological and immunological characteristics of nanobacteria from human renal stones of a North Indian population. Urol Res. 2004;32(3):190–195. doi: 10.1007/s00240-004-0400-3. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Shen W, Wen J, An X, Cao L, Wang B. An animal model of black pigment gallstones caused by nanobacteria. Dig Dis Sci. 2006;51:1126–1132. doi: 10.1007/s10620-006-8019-6. [DOI] [PubMed] [Google Scholar]
- 23.Bratos-Perez MA, Sanchez PL, Garcia De Cruz S, et al. Association between self-replicating calcifying nanoparticles and aortic stenosis: a possible link to valve calcification. Eur Heart J. 2008;29:371–376. doi: 10.1093/eurheartj/ehm592. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Z, Hong L, Shen X, et al. Detection of nanobacteria infection in type III prostatitis. Urology. 2008;71(6):1091–1095. doi: 10.1016/j.urology.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 25.Agababov RM, Abashina TN, Suzina NE, Vainshtein MB, Schwartsburd PM. Link between the early calcium deposition in placenta and nanobacterial-like infection. J Biosci. 2007;32(6):1163–1168. doi: 10.1007/s12038-007-0118-9. [DOI] [PubMed] [Google Scholar]
- 26■.Vali H, Mckee MD, Cifticioglu N, et al. Nanoforms: a new type of protein-associated mineralization. Geochim Cosmochim Acta. 2001;65:63–74. Good discussion of the interface between organic and inorganic mineralization. [Google Scholar]
- 27.Kumar V, Farell G, Yu S, et al. Cell biology of pathologic renal calcification: contribution of crystal transcytosis, cell-mediated calcification, and nanoparticles. J Investig Med. 2006;54(7):412–424. doi: 10.2310/6650.2006.06021. [DOI] [PubMed] [Google Scholar]
- 28.Miller VM, Rodgers G, Charlesworth JA, et al. Evidence of nanobacterial-like structures in human calcified arteries and cardiac valves. Am J Physiol Heart Circ Physiol. 2004;287:H1115–H1124. doi: 10.1152/ajpheart.00075.2004. [DOI] [PubMed] [Google Scholar]
- 29■.Young JD, Martel J, Young L, Wu CY, Young A, Young D. Putative nanobacteria represent physiological remnants and culture by-products of normal calcium homeostasis. PLoS ONE. 2009;4(2):e4417. doi: 10.1371/journal.pone.0004417. Describes link between cell-derived enzymatic processes and formation of nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young JD, Martel J, Young D, et al. Characterization of granulations of calcium and apatite in serum as pleomorphic mineralo–protein complexes and as precursors of putative nanobacteria. PLoS ONE. 2009;4(5):e5421. doi: 10.1371/journal.pone.0005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raoult D, Drancourt M, Azza S, et al. Nanobacteria are mineralo fetuin complexes. PLoS Pathog. 2008;4(2):e41. doi: 10.1371/journal.ppat.0040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kajander EO, Ciftcioglu N. Nanobacteria: an alternative mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc Natl Acad Sci USA. 1998;95:8274–8279. doi: 10.1073/pnas.95.14.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33■.Cisar JO, Xu D-Q, Thompson J, Swaim W, Hu L, Kopecko DJ. An alternative interpretation of nanobacteria-induced biomineralization. Proc Natl Acad Sci USA. 2000;97:11511–11515. doi: 10.1073/pnas.97.21.11511. Expanded concept of bacterial remnants and formation of nanoparticle biofilm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akerman KK, Juronen I, Kajander EO. Scanning electron microscopy of nanobacteria – novel biofilm producing organisms in blood. Scan Electron Microsc. 1993;15(sIII):90–91. [Google Scholar]
- 35.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295(5559):1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 36.Shiekh FA, Miller VM, Lieske JC. Do calcifying nanoparticles promote nephrolithiasis? Clin Nephrol. 2009;71:1–8. doi: 10.5414/cnp71001. [DOI] [PubMed] [Google Scholar]
- 37.Martel J, Ding E, Young J. Purported nanobacteria in human blood as calcium carbonate nanoparticles. Proc Natl Acad Sci USA. 2008;105(14):5549–5554. doi: 10.1073/pnas.0711744105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciftcioglu N, Miller-Hjelle MA, Hjelle JT, Kajander EO. Inhibition of nanobacteria by antimicrobial drugs as measured by a modified microdilution method. Antimicrob Agents Chemother. 2002;46:2077–2086. doi: 10.1128/AAC.46.7.2077-2086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathew G, McKay DS, Ciftcioglu N. Do blood-borne calcifying nanoparticles self-propagate? Int J Nanomedicine. 2008;3(2):265–275. [PMC free article] [PubMed] [Google Scholar]
- 40.Spiewak R, Dutkiewicz J. In vitro study of pro-inflammatory and anti-tumour properties of microvesicles from bacterial cell wall of pantoea agglomerans. Ann Agric Environ Med. 2008;15(1):153–161. [PubMed] [Google Scholar]
- 41.Howell RE, Boyan-Salyers B. Comparison of calcification between bacterionema matruchotii and actinomyces naeslundii. J Dent Res. 1980;59(11):1999–2005. doi: 10.1177/00220345800590111801. [DOI] [PubMed] [Google Scholar]
- 42.Macdiarmid JA, Mugridge NB, Weiss JC, et al. Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics. Cancer Cell. 2007;11(5):431–445. doi: 10.1016/j.ccr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Miller VM, Hunter LW, Chu K, et al. Biologic nanoparticles and platelet reactivity. Nanomedicine. 2009;4(7):725–733. doi: 10.2217/nnm.09.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz MA-K, Lieske JC, Kumar V, Farell-Baril G, Miller VM. Human-derived nanoparticles and vascular responses to injury in rabbit carotid arteries: proof of principle. Int J Nanomed. 2008;3:243–248. doi: 10.2147/ijn.s2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45■.Schwartz MK, Lieske JC, Hunter LW, Miller VM. Systemic injection of planktonic forms of mammalian-derived nanoparticles alters arterial response to injury in rabbits. Am J Physiol Heart Circ Physiol. 2009;296:1434–1441. doi: 10.1152/ajpheart.00993.2008. Demonstration of systemic pathogenic potential of biologic nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bjorklund M, Ciftcioglu N, Kajander EO. Extraordinary survival of nanobacteria under extreme conditions. Proc Soc Photo Opt Instrum Eng. 1998;3441:123–129. [Google Scholar]
- 47.Yonath A. Antibiotics targeting ribosomes: resistance, selectivity, synergism, and cellular regulation. Annu Rev Biochem. 2005;74:649–679. doi: 10.1146/annurev.biochem.74.082803.133130. [DOI] [PubMed] [Google Scholar]
- 48.Kohlhepp SJ, Plant SB, Mccarron DA, Gilbert DN. Gentamicin does not chelate calcium. Antimicrob Agents Chemother. 1982;21:668–669. doi: 10.1128/aac.21.4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bracamonte MP, Rud KS, Owen WG, Miller VM. Ovariectomy increases mitogens and platelet-induced proliferation of arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2002;283:H853–H860. doi: 10.1152/ajpheart.00201.2002. [DOI] [PubMed] [Google Scholar]
- 50.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 51.BacLight™ Green, package insert. Molecular Probes; OR, USA: [Google Scholar]
- 52.Kajander EO, Kuronen I, Akerman KK, Pelttari A, Cifticioglu N. Nanobacteria from blood, the smallest culturable autonomously replicating agent on earth. Proc Soc Photo Opt Instrum Eng. 1997;3111:420–428. [Google Scholar]
- 53.Hjelle JT, Miller-Hjelle MA, Nowak DM, Dombrink-Kurtzman MA, Peterson SW. Polycystic kidney disease, fungi, and bacterial endotoxin: shifting paradigms involving infection and diet. Rev Med Microbiol. 2000;11:23–35. [Google Scholar]
- 54■.Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11(3):156–164. doi: 10.1097/01.moh.0000131441.10020.87. Developed concept of cell-derived microvesicles as carriers of biological information. [DOI] [PubMed] [Google Scholar]
- 55.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Jayachandran M, Litwiller RD, Owen WG, et al. Characterization of blood borne microparticles as markers of premature coronary calcification in recently menopausal women. Am J Physiol Heart Circ Physiol. 2008;295:931–938. doi: 10.1152/ajpheart.00193.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amabile N, Guerin AP, Leroyer A, et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16(11):3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 58.Domingue GJ, Sr, Woody HB. Bacterial persistence and expression of disease. Clin Microbiol Rev. 1997;10:320–344. doi: 10.1128/cmr.10.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59■.Domingue GJ, Schlegel JU. Novel bacterial structures in human blood: cultural isolation. Infect Immun. 1977;15:621–627. doi: 10.1128/iai.15.2.621-627.1977. Classic description of forms of microbes that contribute to chronic subclinical infection. [DOI] [PMC free article] [PubMed] [Google Scholar]