Abstract
Background
Previous studies on blood pressure (BP) indices as predictor of coronary heart disease (CHD) have provided equivocal results and generally relied upon Cox Proportional Hazards regression methodology with age and gender accounting for most of the predictive capability of the model.
Objective
The aim of the present study was to use serially-collected BP measurements to examine age- and gender-related differences in BP indices for predicting CHD.
Methods
We investigated the predictive accuracy of time-dependent BP indices for CHD using a method of risk prediction based on posterior probabilities calculated from mixed-effects regression to utilize intra-individual differences in serial BP measurements according to age changes within gender groups. Data were collected prospectively from two community-dwelling cohort studies in the U.S. and Europe, with a total of 152,633 participants (ages 30 to 74) and 610,061 BP measurements.
Results
During mean follow-up of 7.5 years, 2,457 non-fatal and fatal CHD events were observed. In both study populations, pulse pressure (PP) and systolic blood pressure (SBP) performed best as individual predictors of CHD in females [areas under the receiver operating characteristic curves (AUC) between 0.83 and 0.85 for PP, and 0.77 and 0.81 for SBP]. Mean arterial pressure (MAP) and diastolic blood pressure (DBP) performed better for males than for females. The degree of discrimination was overall greater but more varied over all BP indices for females than males in both populations.
Conclusion
Our findings indicate differences in discrimination between men and women in the accuracy of longitudinally collected BP measurements for predicting CHD, implicating the usefulness of gender-specific BP indices to assess individual CHD risk.
Keywords: blood pressure indices, coronary heart disease, epidemiology, gender, prospective study, random effects models
Introduction
The relation of blood pressure (BP) to coronary heart disease (CHD) is well known and characterized by a continuous, strong and graded effect that is etiologically significant and independent of other CHD risk factors.1 Numerous epidemiological studies have examined the accuracy of the various BP components for predicting CHD and more generally cardiovascular disease (CVD), with the available data being equivocal.2–9 Analyses from the Framingham Heart Study4 indicate that for CVD, combining pulse pressure (PP) with the steady factors mean arterial pressure (MAP), systolic blood pressure (SBP) and diastolic blood pressure (DBP) yielded results that had better discrimination in terms of area under the receiver operating characteristic curve (AUC) than were the single BP components alone. In addition, PP was reported to be superior to either SBP or DBP alone in predicting CHD.5 By contrast, in the Chicago Heart Association Detection Project6 AUC estimates all indicated better predictive utility for SBP and DBP compared with PP for all cardiovascular outcomes, including CHD and heart failure death, with the predictive value of PP being especially modest in individuals <50 years. With the stiffening of arteries, SBP progressively increases with age while DBP remains constant or even declines with age.10,11 PP increases with age in both men and women; however, it has been reported that women have higher pulse pressures than men, particularly after midlife.11–14
Previous studies reported in the literature on the accuracy of BP indices for predicting CHD or CVD exclusively relied upon the use of Cox Proportional Hazards regression methodology, where age is usually considered as a continuous risk factor which may violate the proportionality assumption of the model. In addition, age combined with sex often accounts for the large majority of the ability of the model to classify risk as measured by the c statistic or overall concordance with little improvement in prediction when BP and other risk factors are introduced in the model.15–18 However, while CHD risk increases with age, disease and aging are not synonymous, since by contrast to aging which occurs in everyone with passage of time, disease is a selective event that occurs in only some individuals of the population. Although aging is usually accompanied by several age-related diseases, subgroups of healthy older individuals may exhibit characteristics of relatively younger individuals.19–21 Prediction methods based on the Cox model thus may fail to recognize the substantial heterogeneity in individual aging.
In the present study, we applied a method of risk prediction based on posterior probabilities calculated from a mixed-effects regression model22,23 to allow for differences between average values of covariates with regard to sex and age groups, and individual longitudinal changes within each group to estimate the predictive accuracy of serial BP measurements for CHD. We used data from two prospective, community-dwelling cohort studies from the U.S. and Europe.
Methods
Study Populations
The Baltimore Longitudinal Study of Aging (BLSA), begun in 1958 and conducted by the Intramural Research Program of the National Institute on Aging, is a prospective open cohort study of volunteer participants who are predominantly Caucasian, healthy, well-educated, middle- to upper-middle-class, and community-residing in and near the Baltimore-Washington, DC metropolitan area. The BLSA recruits men and women ages 17 to 96 years to participate in repeated medical examinations and assessments of physical and psychological performance. Examinations occur approximately every 2 years with a 2 to 3 day visit to the research center in Baltimore. Participants are given a careful health screening at baseline to ensure that they are in excellent health with no known diseases. A more detailed description of the original program methodology has been reported previously.24–26 Study participants provided informed consent at each examination visit and the study is approved by the Institutional Review Board of the MedStar Research Institute.
The Vorarlberg Health Monitoring and Promotion Program (VHM&PP)27,28 started in 1985 and is conducted by the Agency for Social and Preventive Medicine in Vorarlberg, the westernmost province of Austria. VHM&PP is one of the world’s largest ongoing population-based risk factor surveillance programs. All adults of the region are invited to participate by a combination of different measures including written invitations, television, radio, and newspaper reports. Active follow-up of study participants is performed through a recall-system of written biennial re-invitation letters. Sociodemographic data are recorded, and a voluntary physical examination is conducted regularly in a standardized manner by trained local physicians and internists. There is a representative range of participants across social classes and the vast majority is of Austrian origin with only a small minority of migrant workers participating in the study. In addition, the majority of VHM&PP participants are white-collar or self-employed workers. A more detailed description of the program methodology has been reported elsewhere.27,28 All participants signed informed consents to have personal data stored and processed. Institutional review board approval for this study was obtained by the Ethics Committee of the province of Vorarlberg.
The sample for the present investigation included all male and female BLSA (n=1,966) and VHM&PP (n=150,667) participants ages 30 to 74 years at the start of follow-up. Twelve female and 70 male BLSA participants had evidence of overt CHD at the onset of follow-up and thus were excluded from the analysis.
Blood Pressure Assessment
A total of 610,061 BP measurements were obtained. For both populations, BP measurements were performed in the morning after 5 minutes of rest while the participant was in a seated position. BP was measured three times in both arms using a mercury sphygmomanometer appropriately sized to the arm of the participant, and the arithmetic mean of the measurements was used in the analysis. MAP (in mm Hg) was determined as one third the sum of SBP and twice DBP with PP (in mm Hg) determined by the difference between SBP and DBP [4]. BP determinations were made without regard to the use of antihypertensive medication. For both study populations, smoking status was determined at each visit and individuals who were presently smoking or were smoking within the last 2 years were considered as smokers.
Coronary Endpoints
For the BLSA, overt CHD was used as the primary study end-point; for the VHM&PP, CHD mortality only was investigated, since CHD morbidity data was not routinely available. In the BLSA, overt CHD was defined as cardiac death or nonfatal myocardial infarction (MI). Cardiac death was defined by death due to acute MI, congestive heart failure, or a sudden death not due to another cause. The cause of death was determined by the consensus of three BLSA physicians who examined the individual’s death certificate, medical records, autopsy report, and other relevant information. Nonfatal MI included clinical, characterized by chest pain accompanied by serial electrocardiogram (ECG) changes or enzyme elevation, or silent, characterized by Q-wave abnormalities on resting ECG (Minnesota codes 1:1 or 1:2) with conformation by an independent BLSA cardiologist who reviewed all ECG results.
For the VHM&PP, date and cause of death information was provided by the local health authority and was linked in the database with the use of a validated procedure. All deaths were identified from death certificates that were confirmed by authorized physicians only. In cases of unclear causes of death, autopsies were performed. CHD deaths were coded according to the International Classification of Diseases (10th Revision), including deaths of acute and sub-acute forms of CHD (ICD-10 I21 to I24), death of chronic forms including occlusive CHD and its complications (ICD-10 I20, I25 (excluding I25.5)), and death of coronary artery disease related to congestive heart failure (ICD-10 I25.5, I50).28
Statistical Methods
A method of risk prediction based on posterior probabilities calculated from a mixed-effects regression model was used to utilize intra-individual differences in repeated BP measurements for predicting CHD. The method is appropriate for repeated measurements on the same individuals over an extended period of observation.22,23 Following a method for predicting preclinical disease using linear mixed-effects models, longitudinal trajectories were estimated for each of the four BP indices over all study participants. The model included fixed-effects terms for the participant’s first examination age, length of time in the study, and a categorical variable for CHD. Quadratic terms for time and interaction terms involving CHD with first age, time and time2 were tested for inclusion as fixed-effects terms in the model. Additional tests for adjustment of smoking status were performed. Random-effects terms in the model included intercept to account for between-person variability as well as possibly for time, and time2 as tested for inclusion by a likelihood-ratio χ2 test. 29 The marginal distributions from the mixed-effects model for being a CHD event and non-event for each individual are then computed and using Bayes theorem posterior probabilities of having CHD are obtained at each serial BP measurement for each individual.22,23 Since it was not possible to perform a mixed-effects regression analysis on the entire VHM&PP population because of the enormous amount of required computer memory, a random sample was chosen representing approximately 5% of the VHM&PP study population to obtain the mixed-effects estimates for the subsequent CHD predictions. Additional analyses were performed for the combinations of the BP variables SBP and DBP as well as MAP and PP, using a multivariate mixed-effects regression model approach.23
The ability of the multiple serial BP measurements to discriminate between participants who did and did not develop CHD during the study period was assessed using the area under the receiver operating characteristic curve (AUC) or c statistic.30 The calibration of the models were assessed using a modified Hosmer-Lemeshow χ2 statistic where a small or non-significant χ2 value suggests that the prediction model is more accurate in terms of calibration and has adequate fit to the data.31 All statistical analyses were performed separately for females and males using SAS/STAT software v 9.1 (SAS Institute, Inc.).
Results
Characteristics of study populations
Characteristics of the study populations are shown in Table 1. 1,966 participants from the BLSA and 150,667 participants from the VHM&PP with a total of 610,061 serial BP measurements were available for analyses for the current investigation. Mean age at baseline was 49.5 and 48.9 years, respectively for female and male BLSA participants, and 44.1 and 44.3 years for female and male VHM&PP participants. Average length of follow-up in the BLSA was 9.1 years for females and 13.0 years for males; the corresponding numbers for the VHM&PP were 7.8 years and 7.1 years, respectively. More than 80% of BLSA and 70% of VHM&PP participants had 2 or more study visits during follow-up. CHD mortality rates were similar in BLSA and VHM&PP females with a higher CHD mortality rate in BLSA males compared to VHM&PP males, likely due to longer follow-up for BLSA males (Table 1) and a higher percentage of males 60 years or older in the BLSA.
Table 1.
Characteristics of Study Populations
| Population | N (%) | Blood Pressure Measurements | Average Length Of Follow-up |
% CHD | |||
|---|---|---|---|---|---|---|---|
| Median | % ≥ 2 | Maximum | Morbidity | Mortality | |||
| BLSA | |||||||
| Females | 911 (46.3) | 3 | 81 | 18 | 9.1 ± 8.1 | 5.0 | 1.0 |
| Males | 1,055 (53.7) | 5 | 88 | 26 | 13.0 ± 10.6 | 18.4 | 5.8 |
| VHM&PP | |||||||
| Females | 80,152 (53.2) | 3 | 73 | 21 | 7.8 ± 6.8 | n.a.* | 1.0 |
| Males | 70,515 (46.8) | 3 | 69 | 21 | 7.1 ± 6.7 | n.a.* | 1.9 |
n.a. not available.
Tables 2 and 3 give baseline values of the BP indices and smoking status for the BLSA and VHM&PP for participants 30–59 and 60–74 years of age at baseline, stratified by sex and CHD outcome. BP indices were higher for both females and males in the VHM&PP compared to their respective BLSA counterparts; similarly, higher rates of smoking were reported in the VHM&PP, with rates being negligible in the BLSA. In both populations SBP was higher in males than in females under age 60 while the reverse is true for those 60 years and older with females having generally higher values of SBP. PP values were considerably higher among females compared to males for those 60 and older in both populations.
Table 2.
Mean Levels (Standard Deviations) of Blood Pressure Indices and Smoking Status (%) for BLSA Study Population*
| Variable | 30 – 59 years | 60 – 74 years | ||||||
|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | |||||
| Non-CHD n = 681 |
CHD N = 9 |
Non-CHD n = 648 |
CHD n = 120 |
Non-CHD N = 184 |
CHD n = 37 |
Non-CHD n = 213 |
CHD n = 74 |
|
| SBP | 118 (16.5) | 117 (10.6) | 122 (15.4) | 128 (16.0) | 135 (20.4) | 139 (28.4) | 134 (19.7) | 143 (21.4) |
| DBP | 76.0 (10.5) | 73.9 (7.01) | 80.1 (10.1) | 84.6 (11.3) | 79.3 (11.1) | 78.3 (11.8) | 81.8 (11.2) | 85.5 (12.7) |
| MAP | 90.0 (11.6) | 88.2 (7.9) | 94.0 (10.9) | 99.0 (11.9) | 97.9 (12.8) | 98.5 (16.2) | 99.1 (12.9) | 105 (14.8) |
| PP | 42.0 (11.5) | 43.0 (6.1) | 41.7 (11.4) | 43.1 (11.5) | 55.6 (16.1) | 60.6 (20.9) | 51.8 (14.2) | 57.0 (13.9) |
| Smoking (%) | 3 | 11 | 7 | 3 | 3 | 3 | 4 | 3 |
CHD represents overt CHD.
Table 3.
Mean Levels (Standard Deviations) of Blood Pressure Indices and Smoking Status (%) for VHM&PP*
| Variable | 30 – 59 years | 60 – 74 years | ||||||
|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | |||||
| Non-CHD n = 68904 |
CHD n = 165 |
Non-CHD n = 61574 |
CHD n = 611 |
Non-CHD n = 10399 |
CHD n = 684 |
Non-CHD n = 7573 |
CHD n = 757 |
|
| SBP | 125 (19.3) | 146 (24.0) | 131 (17.4) | 145 (22.5) | 148 (21.6) | 156 (21.7) | 145 (21.0) | 149 (20.6) |
| DBP | 78.7 (11.0) | 86.5 (14.6) | 82.0 (10.7) | 87.7 (12.2) | 86.0 (10.7) | 87.2 (10.7) | 85.0 (10.8) | 86.1 (10.9) |
| MAP | 94.1 (12.8) | 106 (16.5) | 98.2 (11.8) | 107 (14.4) | 106 (12.9) | 110 (12.7) | 105 (12.8) | 107 (12.3) |
| PP | 46.0 (13.6) | 59.4 (16.8) | 48.6 (12.8) | 57.2 (16.4) | 62.8 (17.4) | 68.4 (18.0) | 60.3 (16.5) | 63.8 (17.3) |
| Smoking (%) | 24 | 30 | 32 | 41 | 7 | 7 | 17 | 21 |
CHD represents CHD mortality.
Predicting Coronary Heart Disease by Time-dependent Blood Pressure Indices
Linear mixed-effects models were fit to the longitudinal BP indices of SBP, DBP, PP and MAP with a bivariate model used for SBP+DBP along with MAP+PP. The estimates of the fixed effects are shown in Table 4 for the six different models applied separately to BLSA females and males. The values in the table represent estimates of initial population averages or average rates of change in blood pressure with regard to the initial age in the study (Fage), time in the study (Time), disease status (CHD) and necessary interaction terms corresponding to these variables. For example, in the SBP models, the intercept values of 85.9 for females and 101.8 for males represent the predicted average initial SBP levels independent of the other variables or terms in the models. Thus, a 40 year-old female at baseline (Time = 0) with no occurrence of CHD during follow-up would have a predicted average SBP value of 114.8 mmHg compared to a similar aged male with no CHD having a predicted SBP value of 120.8 mmHg. Note that smoking was not a significant variable in any of the BLSA models (all p>0.25) due to the small overall percentage of smokers in the BLSA.
Table 4.
Blood Pressure Indices Models for BLSA Population
| Parameters | Females | Males | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP + DBP |
MAP | PP | MAP+ PP |
SBP | DBP | SBP + DBP |
MAP | PP | MAP+ PP |
|
| Intercept | 85.9 | 84.4 | 72.6 | 72.4 | 101.8 | 99.8 | 81.3 | 81.5 | ||||
| Fage* | 0.722 | 0.751 | 0.379 | 0.381 | 0.476 | 0.505 | 0.274 | 0.268 | ||||
| Time | 0.374 | 0.962 | 1.061 | 1.342 | 0.554 | 0.542 | 1.541 | 1.521 | ||||
| Time2 | -- | −0.011 | −0.014 | −0.028 | -- | 0.008 | −0.034 | −0.031 | ||||
| Fage×Time | -- | −0.011 | −0.019 | −0.024 | −0.003 | −0.0007 | −0.027 | −0.026 | ||||
| Fage×Time2 | -- | -- | -- | 0.0003 | -- | −0.0003 | 0.0005 | 0.0004 | ||||
| CHD | −16.7 | −21.0 | −1.154 | 1.55 | −13.4 | 5.36 | 3.42 | 4.34 | ||||
| CHD×Fage | 0.293 | 0.360 | -- | -- | 0.309 | -- | -- | -- | ||||
| CHD×Time | 0.436 | 0.500 | 0.517 | -- | 0.253 | -- | 0.162 | -- | ||||
| Intercept | 65.8 | 65.7 | 18.7 | 18.2 | 72.4 | 72.3 | 29.6 | 27.5 | ||||
| Fage | 0.214 | 0.216 | 0.533 | 0.542 | 0.146 | 0.149 | 0.313 | 0.357 | ||||
| Time | 1.86 | 1.90 | −1.067 | −1.052 | 1.99 | 2.011 | −1.485 | −1.470 | ||||
| Time2 | −0.051 | −0.051 | 0.043 | 0.047 | −0.051 | −0.0505 | 0.0549 | 0.0589 | ||||
| Fage×Time | −0.039 | −0.340 | 0.031 | 0.031 | −0.038 | −0.0389 | 0.0385 | 0.0382 | ||||
| Fage×Time2 | 0.0007 | 0.0007 | −0.006 | −0008 | 0.0008 | 0.0008 | −0.00098 | −0.0011 | ||||
| CHD | −2.603 | −2.517 | −17.2 | 4.88 | 4.023 | 3.85 | −17.3 | 1.487 | ||||
| CHD×Fage | -- | -- | 0.332 | -- | -- | -- | 0.336 | -- | ||||
| CHD×Time | 0.533 | 0.523 | -- | -- | -- | -- | −0.1314 | -- | ||||
| CHD×Time2 | -- | -- | -- | -- | -- | -- | 0.0122 | -- | ||||
| χ2† | 15.9 | 21.0 | 5.06 | 15.8 | 14.2 | 9.77 | 4.96 | 17.9 | 10.3 | 6.76 | 14.6 | 8.13 |
Fage denotes initial age or age at first examination.
Chi-square statistic for Hosmer-Lemeshow goodness of fit test using deciles of risk.
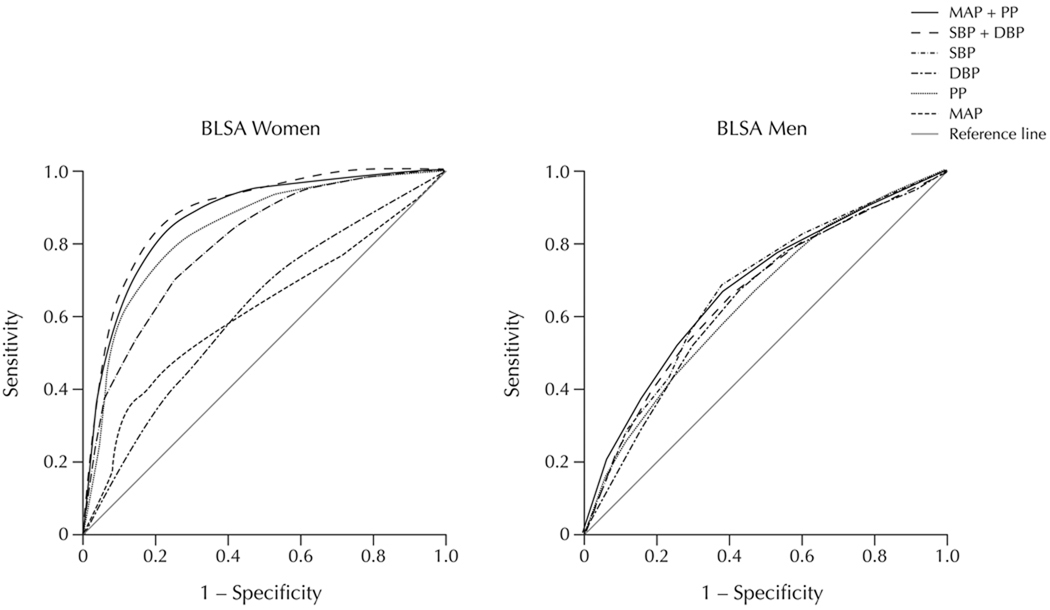
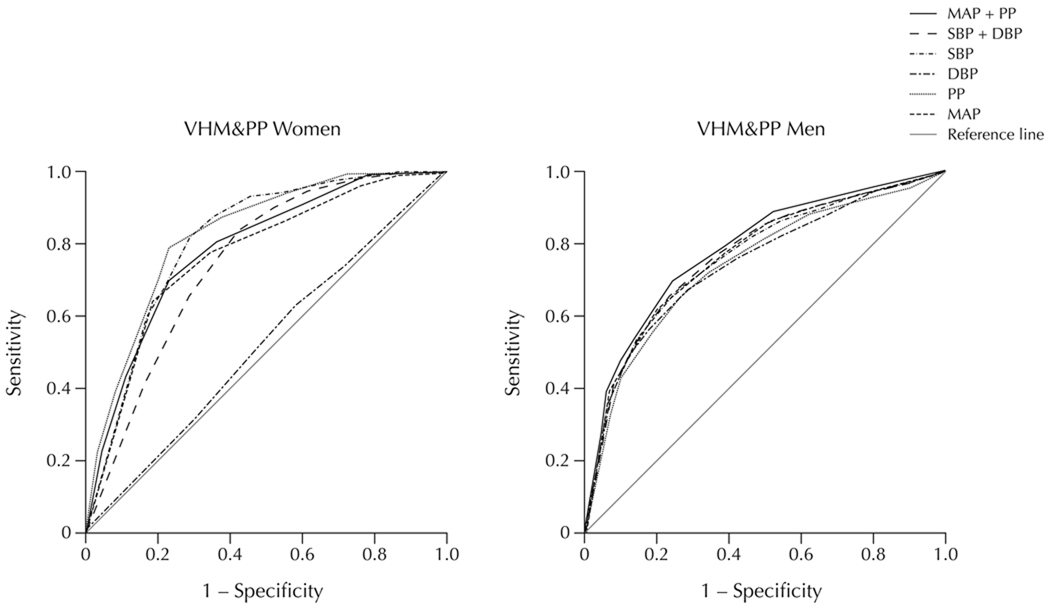
Figure 1 shows receiver operator characteristic (ROC) curves for the CHD classifications in the BLSA obtained from the posterior probabilities from the corresponding marginal distributions of the different BP indices models. For females SBP+DBP performed slightly better than PP alone with corresponding AUC values of 0.88 compared to 0.83 (Table 5). Also, SBP alone (AUC = 0.79) had considerably better accuracy in predicting CHD than either MAP or DBP (AUC = 0.60) alone. For BLSA males, however, all the BP models had a similar predictability for CHD. Figure 2 shows ROC curves for the different BP indices for the VHM&PP. For females, PP and SBP gave the best accuracy in predicting CHD mortality with AUC values of 0.83 and 0.81, respectively. Models of MAP+PP (AUC = 0.79) and SBP+DBP (AUC = 0.78) also performed well followed by MAP alone (AUC = 0.75). As with BLSA females, DBP alone performed the worst in VHM&PP females with a value little better than by chance (AUC = 0.52, Table 5).
Figure 1.
Receiver operating characteristic curves for prediction of overt CHD using longitudinally measured BP indices in the Baltimore Longitudinal Study of Aging (BLSA) in females (n=911) and males (n=1,055).
Table 5.
Areas Under Receiver Operator Characteristic Curves for Both Sexes in BLSA and VHM&PP Study Populations
| Variable | BLSA* | VHM&PP† | ||||
|---|---|---|---|---|---|---|
| Females | Males | Females | Females‡ | Males | Males‡ | |
| SBP | 0.79 | 0.66 | 0.81 | 0.77 | 0.76 | 0.71 |
| DBP | 0.60 | 0.65 | 0.52§ | 0.69 | 0.75§ | 0.65 |
| SBP+DBP | 0.88 | 0.68 | 0.78§ | 0.85 | 0.77§ | 0.70 |
| MAP | 0.60 | 0.67 | 0.75§ | 0.76 | 0.77§ | 0.65 |
| PP | 0.83 | 0.63 | 0.83 | 0.85 | 0.75 | 0.70 |
| MAP+PP | 0.83 | 0.67 | 0.79§ | 0.83 | 0.78§ | 0.71 |
Outcome measure is overt CHD.
Outcome measure is CHD death.
BLSA estimates employed in prediction model.
Linear mixed-effects prediction model included a fixed effect for smoking.
Figure 2.
Receiver operating characteristic curves for prediction of CHD mortality using longitudinally measured BP indices in the Vorarlberg Health Monitoring and Prevention Program (VHM&PP) in females (n=80,152) and males (n=70,515).
A comparison of the results from the BLSA and VHM&PP with regard to sex suggests that the prediction accuracy for both SBP and PP alone were better for females in both populations, while both DBP and MAP alone performed better for males in both studies. In addition, the bivariate predictions of SBP+DBP and MAP+PP performed much better for females in the BLSA and only slightly better for females in the VHM&PP (Table 5).
When applying the mixed-model estimates obtained from the BLSA (Table 4) to perform CHD mortality predictions for the VHM&PP, AUC values were very similar indicating good generalizability of our model to other populations. In VHM&PP females, AUC estimates derived from BLSA model estimation vs. VHM&PP model estimation were 0.85 vs. 0.83 for PP, 0.83 vs. 0.79 for MAP+PP, 0.76 vs. 0.75 for MAP, 0.85 vs. 0.78 for SBP+DBP, and 0.69 vs. 0.52 for DBP. In VHM&PP males, BLSA estimates gave slightly lower AUC values than using the VHM&PP model estimates for all the BP variables with the smallest difference in AUC values for SBP (0.71 vs. 0.76) and PP (0.70 vs. 0.75, Table 5).
Model Calibration
Calibration for the different models was assessed by comparing the observed risk to that estimated from the model using a modified Hosmer-Lemeshow goodness of fit test. For the BLSA models, the accuracy of the calibration in females was best for the SBP+DBP (χ2 = 5.06, P = 0.75) and MAP+PP (χ2 = 9.77, P = 0.28). Other BLSA female prediction models exhibited adequate fit, while for BLSA males, prediction fit was best for SBP (χ2 = 4.96, P = 0.76) and MAP (χ2 = 6.76, P = 0.56) with only DBP (χ2 = 17.9, P = 0.02) showing a lack of good calibration (Table 4). For the VHM&PP, all of the models for both females and males showed a good calibration with the exception of DBP for females (χ2 = 21.0, P = 0.01).
Discussion
The aim of the present investigation was to use serially-collected BP measurements to examine age-and gender-related differences in BP indices for predicting CHD in two prospective community-dwelling cohorts in the U.S. and Europe. Different from previous investigations that traditionally relied upon Cox Proportional Hazards regression methodology,2–9 we here employed a method of risk prediction based on mixed-effects regression utilizing estimates of individual differences and trajectories in BP levels.
As overall discriminators, BP indices performed better in predicting CHD in females than in males with the exception of DBP and MAP. In men, all BP indices performed about equally in terms of AUC values with the prediction models demonstrating good calibration for all BP indices with the exception of DBP whose model had a borderline calibration. For women, results were more varied with PP performing better than in men in both the BLSA and VHM&PP. These gender-related differences may be partly explained by BP changes that occur with advancing age. Data from the Third National Health and Nutrition Examination Study (NHANES III) suggested a greater PP and greater prevalence of isolated systolic hypertension among older females.32 In addition, it has been reported that increases in risk factor levels including blood pressure are associated with increasing rates of CHD with advancing age, especially in women.33
Methods of risk prediction carried out utilizing the Cox Proportional Hazards model have found that the effects of BP and other risk factors, including cholesterol (total, HDL and LDL), diabetes and smoking, are relatively small when compared to the effects of age alone.8,18 For example, in the risk score developed by Wilson and colleagues,16,18 age alone yielded an AUC of 0.65 for predicting CHD, compared to the highest AUC of 0.74 for men and 0.77 for women. More recently, Wang and colleagues17 reported c statistics for predicting all-cause mortality of 0.75 with age and sex as predictors, 0.80 with age, sex and the conventional risk factors for CVD, and 0.82 with all the previous predictors plus multiple biomarkers including predictors such as B-type natriuretic peptide level and urinary albumin-to-creatinine ratio. These traditional methods of individual risk assessment are based on the summation of risk or risk score from identifiable risk factors which includes age. Although age contributes greatly to the resulting risk score, all of the major risk factors with the exception of age are thought to be direct causes of CHD.34 While it has been recognized that including age in the Framingham risk score accounts somewhat for the accumulation of atherosclerotic plaque, doing so has the limitation of providing a relatively poor predictor in making an individual CHD assessment.
Our study had some strengths and potential limitations that should be considered. Major strengths are the statistical-analytical approach utilizing repeated BP measurements from the prospective study design, the large sample size, length of follow-up, and the standardized protocol performed by experienced physicians. Potential limitations of our investigation are the lack of information on antihypertensive medications and hormonal status (including eventual hormone replacement therapy) in females. In addition, other risk factors related to hypertension, including unfavorable lifestyle factors such as stress, lack of physical activity, unhealthy diet, overweight body, alcohol consumption and genetic factors potentially might have confounded gender differences in the predictive ability of our models. A final limitation of our investigation is that there is an under representation of minority populations in the BLSA. While the BLSA study population is not representative of the U.S. population, the study population consists of participants chosen from a large group of waiting-list volunteers so that the age distribution of the BLSA sample is comparable to the overall age distribution in the U.S. In addition, many of the important confounding risk factor variables are controlled for through baseline selection and thus, longitudinal changes in blood pressure indices are believed to be characterized by the physiological and etiological changes related to the occurrence of CHD. There is little smoking in the BLSA and so smoking is not a predictor variable in that population.
In the VHM&PP, smoking was a significant variable for many BP indices and was used in their prediction algorithms. However, when the BLSA algorithm without smoking was applied to the Vorarlberg data, prediction results often were better than using VHM&PP estimates for VHM&PP females. This possibly might suggest that physiological changes in BP measures are the most important thing to consider since smoking would only overall raise BP measures and eventually have minimal effect on the rates of change.
Conclusions
In summary, we aimed to investigate the accuracy of different BP indices in predicting CHD using longitudinal, repeated measurements data from two community-dwelling cohorts in the U.S. and Europe. Different from previous investigations, we calculated age- and sex-specific trajectories, employing a method of discrimination based on estimates of individual differences in BP and rates of change. We found that in both study populations PP and SBP as individual predictors performed quite well for predicting CHD in females, and that MAP and DBP alone performed better in males. Given differences in discrimination between men and women in the accuracy of BP for predicting CHD, gender-specific BP indices using age-generated trends may be useful to assess individual CHD risk.
Acknowledgments
Funding sources: National Institute on Aging Intramural Research Program (LJ Brant)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: All authors: None
References
- 1.Safar ME, Boudier HS. Vascular development, pulse pressure, and the mechanisms of hypertension. Hypertension. 2005;46:205–209. doi: 10.1161/01.HYP.0000167992.80876.26. [DOI] [PubMed] [Google Scholar]
- 2.Vaccarino V, Holford TR, Krumholz HM. Pulse pressure and risk for myocardial infarction and heart failure in the elderly. J Am Coll Cardiol. 2000;36:130–138. doi: 10.1016/s0735-1097(00)00687-2. [DOI] [PubMed] [Google Scholar]
- 3.Darne B, Girerd X, Safar M, Cambien F, Guize L. Pulsatile versus steady component of blood pressure: A cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension. 1989;13:392–400. doi: 10.1161/01.hyp.13.4.392. [DOI] [PubMed] [Google Scholar]
- 4.Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, Vasan RS, Levy D. Single versus combined blood pressure components and risk for cardiovascular disease. The Framingham Heart Study. Circulation. 2009;119:243–250. doi: 10.1161/CIRCULATIONAHA.108.797936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting coronary heart disease? The Framingham Heart Study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 6.Mosley WJ, Greenland P, Garside DB, Lloyd-Jones DM. Predictive utility of pulse pressure and other blood pressure measures for cardiovascular outcomes. Hypertension. 2007;49:1256–1264. doi: 10.1161/HYPERTENSIONAHA.106.083592. [DOI] [PubMed] [Google Scholar]
- 7.Hardoon SL, Whincup PH, Lennon LT, Wannamethee SG, Capewell S, Morris RW. How much of the recent decline in the incidence of myocardial infarction in British men can be explained by changes in cardiovascular risk factors? Evidence from a prospective population-based study. Circulation. 2008;117:598–604. doi: 10.1161/CIRCULATIONAHA.107.705947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asia Pacific Cohort Studies Collaboration. The impact of cardiovascular risk factors on the age-related excess risk of coronary heart disease. Int J Epidemiol. 2006;35:1025–1033. doi: 10.1093/ije/dyl058. [DOI] [PubMed] [Google Scholar]
- 9.Somes GW, Pahor M, Shorr RI, Cushman WC, Applegate WB. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med. 1999;159:2004–2009. doi: 10.1001/archinte.159.17.2004. [DOI] [PubMed] [Google Scholar]
- 10.Pearson JD, Morrell CH, Brant LJ, Landis PK, Fleg JL. Age-associated changes in blood pressure in a longitudinal study of healthy men and women. J Gerontol Med Sci. 1997;52A:M177–M183. doi: 10.1093/gerona/52a.3.m177. [DOI] [PubMed] [Google Scholar]
- 11.Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 12.Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol. 2001;37:1374–1380. doi: 10.1016/s0735-1097(01)01166-4. [DOI] [PubMed] [Google Scholar]
- 13.Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, et al. Noninvase (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007;49:1248–1255. doi: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension. 2008;51:1123–1128. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, D’Agostino RB, Mosca L, Burke GL, Wilson PWF, Rader DL, et al. Cardiovascular risk assessment based on US cohort studies. Findings from a National Heart, Lung, and Blood Institute workshop. Circulation. 2001;104:491–496. doi: 10.1161/01.cir.104.4.491. [DOI] [PubMed] [Google Scholar]
- 19.Rodeheffer DJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects. Circulation. 1984;69:203–213. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- 20.Lighart GJ, Schult HRE, Hijmans W. Natural killer cell function is not diminished in the healthy aged and is proportional to the number of NK cells in the peripheral block. Immunology. 1989;68:396–402. [PMC free article] [PubMed] [Google Scholar]
- 21.Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. J Gerontol Biol Sci Med Sci. 2009;64:215–222. doi: 10.1093/gerona/gln024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brant LJ, Sheng SL, Morrell CH, Verbeke GN, Carter HB, Lesaffre E. Screening for prostate cancer using random effects models. J Roy Stat Soc A. 2003;166:51–62. [Google Scholar]
- 23.Sheng SL, Brant LJ. Predicting preclinical disease using the mixed-effects regression model. In: Balakrishnan N, editor. Handbook of Methods and Applications of Statistics: Life and Health Sciences. New York, NY: Wiley; 2009. pp. 613–633. [Google Scholar]
- 24.Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin JP. Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, DC: U.S. Govt. Printing Office; 1984. [Google Scholar]
- 25.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 27.Ulmer H, Kelleher C, Diem G, Concin H. Long-term tracking of cardiovascular risk factors among men and women in a large population-based health system: The Vorarlberg Health Monitoring & Promotion Programme. Eur Heart J. 2003;24:1004–1013. doi: 10.1016/s0195-668x(03)00170-2. [DOI] [PubMed] [Google Scholar]
- 28.Strasak AM, Kelleher CC, Klenk J, Brant LJ, Ruttmann E, Rapp K, et al. Longitudinal change in serum gamma-glutamyltransferase and cardiovascular disease mortality: a prospective population-based study in 76,113 Austrian adults. Arterioscler Thromb Vasc Biol. 2008;28:1857–1865. doi: 10.1161/ATVBAHA.108.170597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrell CH, Pearson JD, Brant LJ. Linear transformations of linear mixed-effects models. Am Statistician. 1997;51:338–343. [Google Scholar]
- 30.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 31.Lemeshow S, Hosmer DW. A review of goodness of fit tests for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 32.Martins D, Nelson K, Pan D, Tareen N, Norris K. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: Data from NHANES III. J Gend Specif Med. 2001;4:10–13. [PubMed] [Google Scholar]
- 33.Jousilahiti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease. A prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99:1165–1172. doi: 10.1161/01.cir.99.9.1165. [DOI] [PubMed] [Google Scholar]
- 34.Grundy SM. Age as a risk factor: You are as old as your arteries. Am J Cardiol. 1999;83:1455–1457. doi: 10.1016/s0002-9149(99)00125-3. [DOI] [PubMed] [Google Scholar]