Abstract
Genetically engineered mice cancer models are among the most useful tools for testing the in vivo effectiveness of the various chemopreventive approaches. The p53-null mouse model of mammary carcinogenesis was previously characterized by us at the cellular, molecular, and pathological levels. In a companion article, Medina et al. (2009) analyzed the efficacy of bexarotene, gefitinib, and celecoxib as chemopreventive agents in the same model. Here we report the global gene expression effects on mammary epithelium of such compounds, analyzing the data in light of their effectiveness as chemopreventive agents. SAGE was used to profile the transcriptome of p53 null mammary epithelium obtained from mice treated with each compound Vs controls. This information was also compared with SAGE data from p53-null mouse mammary tumors. Gene expression changes induced by the chemopreventive treatments revealed a common core of 87 affected genes across treatments (p<0.05). The effective compounds, bexarotene and gefitinib may at least in part exert their chemopreventive activity by affecting a set of 34 genes related to specific cellular pathways. The gene expression signature revealed various genes previously described to be associated with breast cancer, such as, the AP-1 complex member Fos like antigen 2, Early growth response1, Gelsolin and Tumor protein translationally-controlled 1, among others. The concerted modulation of many of these transcripts prior to malignant transformation appears conducive to predominantly decrease cell proliferation. This study has revealed candidate key pathways that can be experimentally tested in the same model system and may constitute novel targets for future translational research.
Keywords: Chemoprevention, gene expression profile, SAGE, Bexarotene, Gefitinib, Celecoxib
INTRODUCTION
Human breast cancer therapeutic and preventive agents are primarily grouped based on their mechanism of action regarding the tumor's estrogen receptor alpha (ERα) status. Human clinical trials have shown that the selective ER modulators (SERMs) such as: tamoxifen, raloxifene, and aromatase inhibitors are ineffective for the most part in the treatment of ERα negative breast cancers [1–2]. Agents such as retinoids, cycloxygenase-2 (COX2) inhibitors, and EGFR-TK inhibitors, are being tested for the prevention and treatment of hormonally unresponsive ERα (−) breast cancers. Thus, there is much interest in testing these chemopreventive agents in pre-clinical models of breast cancer. Transgenic and other genetically engineered mice cancer models are among the most useful tools for testing the in vivo effectiveness of the various chemopreventive approaches [3].
The p53-null mice model of mammary carcinogenesis is a unique in vivo model of pre-neoplastic and neoplastic progression that reproduces many of the critical features of human breast cancer [4–5]. In this model, BALB/c p53 null mammary epithelial cells are transplanted into cleared mammary fat pads of p53 wild type syngeneic hosts. Over 60% of these isogenic orthotopic transplants develop invasive mammary adenocarcinomas without hormonal stimulation [4]. Most of these tumors are intraductal in origin and pre-malignant lesions can be observed closely mimicking human breast cancer [5]. Importantly, the deregulation of transcripts related to the control of cell proliferation, differentiation and apoptosis in tumors arising from p53-null mice and human mammary gland have been reported to be strikingly similar [6].
The effects of chemopreventive agents at the gene transcriptional level is poorly understood [7]. In order to identify biomarkers of effectiveness and to elucidate molecular mechanisms of action, we performed a comparative transcriptome profiling from p53 null mammary epithelium obtained from mice treated with three chemopreventive agents: a retinoid × receptor agonist (bexarotene, LGD1069), an EGFR-TK inhibitor (gefitinib, ZD1839), and a Cox-2 inhibitor (celecoxib, SC58635). In a companion article, we assessed the anti-tumorigenic effectiveness of the same compounds in the same p53-null mammary epithelial cancer model [8]. That study demonstrated a significant decrease in mammary tumorigenicity when p53 null mammary epithelium recipient virgin mice were treated with bexarotene (75% reduction) or gefitinib (50% reduction) (p<0.05); but no effect was observed when animals were treated with celecoxib.
In this manuscript we report gene expression changes detected in p53 null mammary epithelium as a result of treating mice with the aforementioned chemopreventive agents. The results are presented and analyzed in light of the anti-tumorigenic effectiveness of two of the three compounds studied.
MATERIAL AND METHODS
Chemopreventive agents
The RXR-selective retinoid used in this study LGD1069 (bexarotene, Targretin) was obtained from Ligand Pharmaceutical, Inc (San Diego, CA), ZD1839 (gefitinib, Iressa) was obtained from AstraZeneca (Macclesfield, U.K.) and SC58635 (celecoxib, Celebrex) was purchased from SIGMA (St. Louis, MO).
p53-null mice mammary model and treatments
Housing of mice and all experiments performed with mice were done in accordance with NIH guidelines and regulations in AALAC accredited facilities. Balb/c p53-null mammary epithelium was transplanted into the cleared mammary fat pads of three-week old wild type Balb/c mice [4]. Transplanted mice were separated at random in two groups for each reagent (experimental vs. control). Thus, each group included age-matched vehicle treated controls and bexarotene-treated, gefitinib-treated or celecoxib-treated mice respectively. All mice were treated 6 days/week for 2 months starting at 11 weeks of age. The rexinoid bexarotene (100 mg/kg) was administered by gastric gavage using a 20-gauge gavage needle in a volume of 0.1-ml sesame seed oil. Mice were treated with gefitinib (100 mg/kg) suspended in distilled water containing 1% Tween 80. ZD1839 was administered in 0.1 ml by gastric gavage with a 20-gauge gavage needle. Celecoxib treatment was provided with the diet of mice supplemented with 500 ppm SC58635.
SAGE methodology
To decrease the chances of potential artifacts due to sample heterogeneity, RNA for SAGE was extracted from a pool of mammary epithelial samples (8–10 fat pads per pool: three separate pools from each treatment group) collected at 2 months after initiation of treatment with the chemopreventive agent. Mammary epithelium enriched samples (>90% epithelial cells) were used for the analyses [9]. All SAGE libraries were generated following standard procedures as described previously [10]. Briefly total RNA was extracted from frozen samples using TRIzol (Invitrogen, Carlsbad, CA, USA). SAGE library construction was performed with the I-SAGE kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol and introducing only minor modifications. The anchoring enzyme was NlaIII and the tagging enzyme used was BsmFI. Concatemerized ditags were cloned into pZERO-1 and sequenced with an ABI 3700 DNA Analyzer (Applied Biosystems, Foster City, CA, USA). SAGE libraries were generated at an approximate resolution of 60,000 tags per library [6, 9].
SAGE data processing and statistical analyses
SAGE tag extraction from sequencing files was performed by using the SAGE2000 software version 4.0 (a kind gift of Dr. Kenneth Kinzler, John Hopkins University, Baltimore, MD). SAGE data management, tag to gene matching, as well as additional gene annotations and links to publicly available resources such as Gene Ontology (GO), UniGene, and Entrez gene ID, were performed using a suite of web-based SAGE library tools developed by us. In our analyses we only considered tags with single tag-to gene reliable matches.
In order to obtain a more complete picture to identify transcripts of potential relevance as biomarkers and to identify transcripts of relevance in chemoprevention we performed two types of analyses. 1) The gene expression signature for each chemopreventive agent in normal p53 null mammary epithelum was obtained. To this end SAGE profiles of each chemopreventive agent was compared to its corresponding control and 2) in order to identify transcripts whose modulation could be of relevance in prevention of carcinogenesis, SAGE profiles obtained from each chemopreventive agent were also compared with transcripts deregulated in p53-null mammary tumors.
The mouse mammary tumors used developed spontaneously from intra-mammary fat pad transplanted p53-null mammary epithelium [4]. As normal control for the SAGE analysis of tumors, p53-null enriched mammary epithelium derived from Balb/c female mice unexposed to hormonal stimulation was used as described previously [9]. To decrease the chances of potential artifacts due to sample heterogeneity, the normal sample (MN2) represents a pool of mammary epithelial samples from five age-matched separate mice. In addition, two p53-null mammary tumor SAGE libraries (T2532 and T2539) derived from p53 null Balb/c female mice unexposed to hormonal stimulation were selected for the comparative analysis [6]. These SAGE mammary tumor libraries were pooled, averaged and normalized to 60,000 tags.
To compare the control (vehicle) vs. treatment SAGE libraries for each chemopreventive agent (e.g.: untreated p53-null mammary epithelium vs. Celecoxib treatment SAGE libraries) and the p53-null normal mammary epithelium (MN2 SAGE library) vs. p53-null mammary tumors (T2532 and T2539 pooled SAGE libraries); we utilized the Audic and Claverie's significance test [11]. Tags with total counts of less than four in compared libraries were filtered out before the analysis. First, we compared the differences in gene expression profiles between p53-null normal mammary epithelium (SAGE library, MN2) and two pooled p53-null mammary tumors, (SAGE librariesT2532 and T2539) previously generated by us [6, 9]. Second, we compared the differentially expressed transcripts from each chemopreventive treatment (treated vs untreated epithelium) with the transcripts detected as differentially expressed between normal and tumor.
Statistical analysis and scatter plot visualization of SAGE libraries were done with the Discovery Space 4 software (Genome Science Centre, BC Cancer Agency, Canada, Vancouver) [http://www.bcgsc.ca/platform/bioinfo/software/ds]. For automated functional annotation and classification of genes of interest based on Gene Ontology terms, we used the EASE [12] available at the Database for Annotation, Visualization and Integrated Discovery (DAVID) [13]. All of the raw SAGE data reported as supplementary files in this article are publicly available.
Identification of commonly deregulated genes among chemopreventive agents
Differentially expressed genes were compiled into an Excel spreadsheet pivot-Table for comparison of overlapping data between rexinoid LGD1069, Gefitinib and Celecoxib chemopreventive agents. Any combination of two lists was compared for matching gene-identity. The number and identity of genes commonly affected in two chemopreventive agents (e.g. LGD1069 vs. Celecoxib) was determined. We used the normal approximation to the binomial distribution as previously described [14] to calculate whether the number of matching genes derived from each cross-platform comparison was of statistical significance (p< 0.05). To enable illustration of the co-ocurring deregulated genes between transgenic mice models, we used the TIGR MultiExperiment Viewer (MeV 3.0) software. This tool was used for average clustering of SAGE based on the fold change of tag counts for each transcript comparing treatment to control (vehicle) in each chemopreventive agent.
RESULT AND DISCUSSION
p53-null mice SAGE libraries
We generated six mouse SAGE libraries from mammary epithelium obtained from the described p53-null mice model from virgin mice treated with bexarotene, gefitinib or celecoxib and their corresponding controls. In addition, we compared these data with SAGE profiles obtained from p53-null normal mammary epithelium (MN2) and from two p53-null mammary tumors (T2532 and T2539) [6, 9]. This resulted in a dataset of almost 540,000 tags representing over 25,000 transcripts from a total of 9 SAGE libraries. The study approach, underwent three phases: i) identification of differentially expressed genes in mammary epithelium as a result of each chemopreventive agent treatment; ii) identification of commonly deregulated transcripts among treatments; followed by iii) assessment of modulation of the identified transcripts in p53-null mammary tumors.
Bexarotene (Rexinoid agonist) treatment
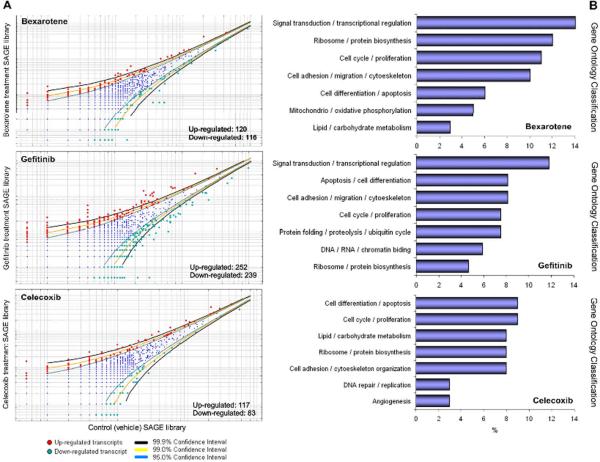
Retinoids are biologically active derivatives of vitamin A that play essential roles modulating cell proliferation, differentiation and apoptosis. Signal-transduction is mediated by two classes of nuclear retinoid-dependent transcriptional activators: the retinoic acid receptors (RAR α, β, γ) and the retinoid × receptor (RXR α, β, γ). A highly selective RXR agonist, the rexinoid bexarotene (Targretin) can inhibit the growth of normal and malignant breast cells, and was shown to suppress the development of breast cancer transgenic mice models without apparent side effects [15–16]. The chemopreventive effects of bexarotene have been attributed to transcriptional modulation of cell proliferation, cell death / apoptosis and cell differentiation related genes [17]. Our statistical analysis revealed 236 genes differentially expressed (p< 0.05) between vehicle treated p53-null mammary epithelium and bexarotene treatment (Figure 1A). Among these transcripts, 120 were up-modulated and 116 were down-modulated by bexarotene treatment (see Supplementary table 1). GO annotation of the 236 differentially expressed genes showed that approximately 14% of the transcripts are involved in signal transduction / transcriptional regulation, 12% are related to ribosome / protein biosynthesis, 11% are related to cell cycle / proliferation (Figure 1B). Table 1 shows the most highly deregulated transcripts by bexarotene treatment in p53-null mammary epithelium (Fold change ≥ 7; p< 0.01).
FIGURE 1.
Deregulated transcripts by treatment with chemoprentive agents in the p53-null mammary model. (A) Scatter-plot representation of differentially expressed genes between bexarotene, gefitinib and celecoxib treatments and control SAGE libraries (p< 0.05). (B) Gene ontology (GO) classification of differentially expressed transcripts as a result of each chemopreventive agent treatment. Relative representation of the deregulated transcripts with specific GO term annotations related to Biological processes or molecular function.
Table 1.
Most highly deregulated transcripts in mammary epithelium from p53-null transgenic mice for each chemopreventive treatment assessed (Fold change ≥ 7; p < 0.01).
| Tag | Gene | Description | Entrez Gene | Fold Change |
|---|---|---|---|---|
| Bexarotene treatment | ||||
| GTTTGCTGTA | Serpinb6a | Serine (or cysteine) peptidase inhibitor | 20719 | 17.0 |
| AGTCTCGAGG | Slc1a5 | Solute carrier family 1 | 20514 | 12.0 |
| GGTTTGGGGG | Jup | Junction plakoglobin | 16480 | 11.0 |
| TGCGTGCTGG | Timp2 | Tissue inhibitor of metalloproteinase 2 | 21858 | 11.0 |
| TTGAAATTAC | BC061494 | CDNA sequence | 381832 | 11.0 |
| GATTTCTTTG | Gpc3 | Glypican 3 | 14734 | 10.0 |
| TAACCAAAAA | Itgb4 | Integrin beta 4 | 192897 | 10.0 |
| CCCAGTCCCT | Ltbp4 | Latent transforming growth factor bindin. prot. 4 | 108075 | 8.0 |
|
| ||||
| GACTCTATAT | Csn2 | Casein beta | 12991 | −15.0 |
| CAATAAAACA | Sar1b | SAR1 gene homolog B (S. Cerevisiae) | 66397 | −11.0 |
| GCAGCGATTC | Nme2 | Expressed in non-metastatic cells 2 | 18103 | −10.0 |
| TGTTCTATGG | Laptm5 | Lysosomal-associated protein transmembrane 5 | 16792 | −9.0 |
| GTGTTTTGCT | AI451557 | Expressed sequence | 102084 | −9.0 |
| CTAGGTGGTG | Glycam1 | Glycosylation dependent cell adhesion molecule 1 | 14663 | −8.8 |
| TAAAGTCAAT | Muc15 | Mucin15 | 269328 | −8.0 |
| TCAGAGTGAG | Igh-6 | Immunoglobulin heavy chain 6 | 16019 | −7.5 |
|
| ||||
| Gefitinib treatment | ||||
| TGGATCCTGA | Hbb-b1 | Hemoglobin beta adult major chain | 15129 | 25.0 |
| ACTACTGAGG | Stno | Strawberry notch homolog (Drosophila) | 216161 | 18.0 |
| CAAGAGGTTG | Fxyd3 | FXYD domain-containing ion transport regulator 3 | 17178 | 18.0 |
| CTTATCTGTT | Vil2 | Villin 2 | 22350 | 15.0 |
| GAAATGATGA | Pfdn5 | Prefoldin 5 | 56612 | 13.8 |
| CTTTGGGGAC | Dscr1 | Down syndrome critical region homolog 1 (human) | 54720 | 13.0 |
| ATTCTCTGGA | Atp2a2 | ATPase, Ca++ transporting | 11938 | 13.0 |
| CTTCCCTGTT | Ctnna1 | Catenin alpha 1 (cadherin associated protein) | 12385 | 13.0 |
|
| ||||
| TCCTAAAAAA | Myh9 | Myosin, heavy polypeptide 9, non-muscle | 17886 | −33.0 |
| ACACCAAAAA | Aebp1 | AE binding protein 1 | 11568 | −22.0 |
| ATACAAATTA | Jak2 | Janus kinase 2 | 16452 | −14.0 |
| CACTGATTTA | Ywhab | Tyrosine 3-monooxygenase/tryptophan 5-monoox. | 54401 | −13.0 |
| GTGTGAAATA | Ranbp2 | RAN binding protein 2 | 19386 | −13.0 |
| CTTCCCTAAT | 6720456B07 | RIKEN cDNA 6720456B07 gene | 101314 | −13.0 |
| ACACCCCTTC | Rhoj | Ras homolog gene family, member J | 80837 | −12.0 |
| TAATGATATT | Ncoa7 | Nuclear receptor coactivator 7 | 211329 | −12.0 |
|
| ||||
| Celecoxib treatment | ||||
| CCCAAGTGTA | Igl-V1 | Immunoglobulin lambda chain, variable 1 | 16142 | 15.0 |
| AAATTTGTTC | AW555464 | Expressed sequence | 217882 | 11.0 |
| TGAATGGCCT | Klhdc2 | Kelch domain containing 2 | 69554 | 11.0 |
| CAACTGTATT | Aco2 | Aconitase 2, mitochondrial | 11429 | 10.0 |
| CCTGCTCTGT | Prpf19 | PRP19/PSO4 pre-mRNA procc. factor 19 homolog. | 28000 | 10.0 |
| GATGGTACAT | Stc2 | Stanniocalcin 2 | 20856 | 10.0 |
| TGAAAATCTA | Abp1 | Amiloride binding protein 1 | 76507 | 8.5 |
| AACAATCTGA | Pck2 | Phosphoenolpyruvate carbokinase 2 | 74551 | 7.0 |
|
| ||||
| TGTATAAATA | Map2k1ip1 | Mitogen-activated protein kinase 1 interacting pro.1 | 56692 | −11.0 |
| AATACACTTG | Fam18b | Family with sequence similarity 18, member B | 67510 | −10.0 |
| TCGTTTTTTA | Akt1 | Thymona viral proto-oncogene 1 | 11651 | −9.0 |
| GGGTTCAGCT | Rbck1 | RanBP-type and C3HC4-type zinc finger | 24105 | −9.0 |
| CAGGGAAACC | Polr2e | Polymerase (RNA) II polypeptide E | 66420 | −9.0 |
| TTGAAAATAA | Anapc1 | Anaphase promoting complex subunit 1 | 17222 | −9.0 |
| CAGGCCATCC | Dkkl1 | Dickkopf-like 1 | 50722 | −8.0 |
| GGGATATAAA | Dnaja1 | DnaJ (Hsp40) homolog, subfamily A, member 1 | 15502 | −7.0 |
Up-regulated transcripts for each treatment are represented by positive fold changes and down-regulated transcripts are represented by negative fold changes.
Gefitinib (EGFR-TK inhibitor) treatment
The epidermal growth factor receptor (EGFR) family members (HER1–4) are commonly over-expressed in ERα (−) human breast carcinomas, providing a new target for anticancer drug development. The EGFR signaling network activates several pathways involved in the G1-S transition as well as disables pro-apoptotic molecules thus leading to deregulated proliferation and enhanced tumor cell survival [18]. Gefitinib (Iressa) is a synthetic anilinoquinazoline tyrosine kinase inhibitor selective for EGFR that can effectively block the tumorigenic potential that arises from the EGF signaling pathway. Recent studies have demonstrated that gefitinib prevents ERα (−) tumor formation in MMTV-ErbB-2 mice [19]. Our statistical analysis revealed 491 genes differentially expressed (p< 0.05) between untreated p53-null mammary epithelium and gefitinib treatment (Figure 1A). Among these transcripts, 252 were up-modulated and 239 were down-modulated by gefitinib treatment (see Supplementary table 1). GO annotation of the 491 differentially expressed genes showed that approximately 16% of the transcripts are involved in cell cycle / proliferation and apoptosis / cell differentiation, 12% are related to signal transduction / transcriptional regulation, 8% are related to cell adhesion / migration and cytoskeleton organization (Figure 1B). Table 1 shows the most highly deregulated transcripts by gefitinib treatment in p53-null mammary epithelium (Fold change ≥ 7; p< 0.01).
Celecoxib (COX-2 inhibitor) treatment
Cox-2 is one of the rate-limiting enzymes in converting free arachidonic acid to PGG2. Cox-2 is upregulated in response to tumour promoters, growth factors, cytokines and it is responsive to various oncogenes such as v-src, v-Ha-ras, Wnt1 and HER-2/neu [20]. Cox-2 is over-expressed in approximately 40% of breast cancers including in situ lesions. Celecoxib, a selective Cox-2 inhibitor has been tested for its ability as chemopreventive agent, showed to significantly reduce the incidence of mammary tumors formation in some transgenic mice models [20]. Our statistical analysis revealed 200 genes differentially expressed (p< 0.05) between p53-null mammary epithelium from vehicle treated Vs. Celecoxib treated mice (Figure 1A). Among these transcripts, 117 were up-modulated and 83 were down-modulated by Celecoxib treatment (see Supplementary table 1). GO annotation of the 200 differentially expressed genes showed that approximately 18% of the transcripts are involved in apoptosis / cell differentiation, and cell cycle / proliferation (Figure 1B). Table 1 shows the most highly deregulated transcripts by Celecoxib treatment in p53-null mammary epithelium (Fold change ≥ 7; p< 0.01).
Three-way comparison of genes deregulated by the tested chemopreventive agents
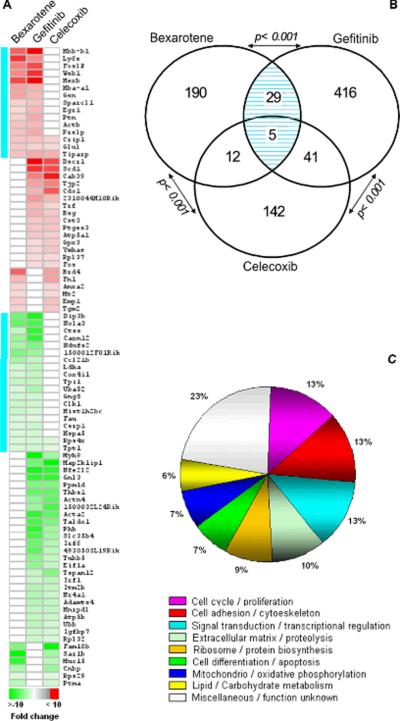
In order to identify a common core of effectors genes among the three chemopreventive agents, we performed a three-way comparison of the above-described SAGE datasets. Among the three treatments, a total of 835 genes were identified as deregulated in p53-null mammary epithelium obtained from treated mice. Eighty-seven genes were identified as commonly deregulated by more than one of the chemopreventive agents (Figure 2A) (see Supplementary table 2). Thirty-four genes were identified as co-deregulated in bexarotene and gefitinib treatments, representing a non-random significant number of overlapping genes based on normal approximation to the binomial distribution (p< 0.001) (Figure 2B). Forty-five genes were co-deregulated in gefitinib and celecoxib treatments (p< 0.001), and seventeen genes were identified between bexarotene and celecoxib treatments (p< 0.001) (Figure 2B). Only five genes were identified as co-deregulated by all three treatments, these are: the common up-regulation of TCDD-inducible poly (ADP-ribose) polymerase (Tiparp), Cysteine-rich protein 1 (Crip1), Glutamate-ammonia ligase (Glul); and down-regulation of Tumor protein translationally-controlled 1 (Tpt1) and Ribosomal protein S4 (Rps4x). Gene Ontology annotation of the 87 commonly deregulated genes showed that 13% of the transcripts are involved in cell cycle / proliferation, 13% are related to signal transduction / transcriptional regulation, 13% are related to cell adhesion / cytoskeleton organization and 10% are related to extracellular matrix / proteolysis (Figure 2C).
FIGURE 2.
Co-occurring differentially expressed genes among bexarotene, gefitinib and celecoxib treatments in p53-null `normal' mammary epithelium. Eighty-seven genes were identified modulated by more than one treatment. (A) Heat map of the 87 deregulated transcripts. Color scale at the bottom depicts the approximate fold change in expression for each transcript and library relative to control mammary gland. Negative fold change (e.g.: transcripts with decreased expression in bexarotene treatment) is represented in green, and positive fold change (e.g.: transcripts with over-expression in bexarotene treatment) is represented in red. Aquamarine lines on left: co-ocurring transcripts modulated both by bexarotene and gefitinib treatments. (B) Venn diagram showing the overlap between transcripts modulated by bexarotene, gefitinib and celecoxib treatments. Statistical analysis showed a significant number of overlapping genes between treatments (p< 0.001). Hatched area with blue lines: number of genes commonly modulated by both bexarotene and gefitinib treatments. (C) Gene ontology classification of the 87 transcripts deregulated by the chemopreventive treatments.
Transcriptomic changes relevant to p53-null mammary mice tumor development
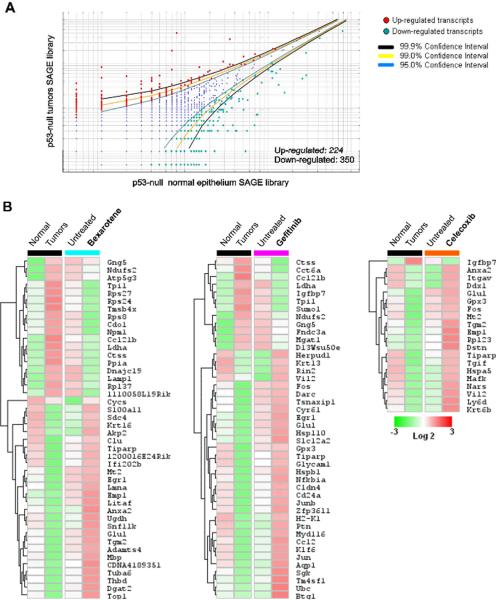
To identify the deregulated genes of relevance to tumorigenesis, we compared the chemopreventive agents SAGE profiles with genes identified as differentially expressed in p53-null mice mammary tumors. We identified 574 differentially expressed genes (p<0.05) when comparing SAGE data from the p53-null mammary tumors vs. p53-null normal mammary epithelium (Figure 3A). Among the 574 transcripts, 224 were up-modulated and 350 were down-modulated transcripts in p53-null mammary tumors (see Supplementary table 3).
FIGURE 3.
Transcripts identified as deregulated in p53-null mammary tumors that were observed to be modulated in the opposite direction as the result of treatment with chemopreventive agents in normal mammary epithelium (i.e. up in tumors, down in the treated epithelium or viceversa). (A) Scatter-plot representation of differentially expressed genes between p53-null `normal' epithelium and p53-null tumors SAGE libraries (p< 0.05). (B) Heat maps of the transcripts modulated in the opposite direction in tumors vs treated normal epithelium: p53-null tumors (Black cluster) and bexarotene treated normal p53 null mice epithelium (aquamarine cluster), gefitinib treated (fuchsia cluster) and celecoxib treated (orange cluster). Color scale at the bottom depicts the approximate fold change in expression for each transcript and library relative to control mammary gland. Negative fold change is represented in green, and positive fold change is represented in red.
Bexarotene treatment of p53-null `normal' mammary epithelium affects the expression of 44 transcripts commonly deregulated in p53-null mammary tumors. Among these transcripts 26 were up-modulated and 18 were down-modulated in p53-null mammary epithelium in opposite way to how the same transcripts are affected in p53-null mammary tumors (Figure 3B). Gefitinib and celecoxib treatment of p53-null mammary epithelium affects the expression of 44 and 20 transcripts respectively that are also deregulated in p53-null mammary tumors (Figure 3B). Among these transcripts, 32 genes were up-modulated by gefitinib treatment (12 down-modulated) and 19 genes were up-modulated by celecoxib treatment (1 down-modulated transcripts) in opposite way to how the same transcripts are affected in p53-null mammary tumors (Figure 3B).
Although transcripts modulated by the three chemopreventive agents share significant overlap, bexarotene and gefitinib treatments affect the expression of more transcripts (44 genes each one) deregulated in p53-null mammary tumors than celecoxib treatment (20 genes). Interestingly, both bexarotene and gefitinib, at a 100mg/kg dose, were effective anti-tumorigenic agents in the p53 null mammary model, reducing tumor incidence by 75% and 50% respectively in virgin mice [8]. On the other hand, celecoxib treatment did not affect tumorigenicity in either the virgin or hormone stimulated mice.
The heat map shown in Figure 2A and table 2 display 34 transcripts commonly deregulated (in the same direction) by the bexarotene and gefitinib treatments. Within this list we find some genes on which little is known but we also find genes previously described to be associated with human breast cancer, such as, Fos like antigen 2 (Fosl2), Early growth response 1 (Egr1), Gelsolin (Gsn) and Tumor protein translationally-controlled 1 (Tpt1), among others.
Table 2.
Common core of transcripts significantly deregulated by bexarotene and gefitinib treatments in mammary epithelium from p53-null mice.
| Tag | Gene | Description | Fold change* | B | G | T |
|---|---|---|---|---|---|---|
| Transcriptional regulation /signal transduction | ||||||
| CATCTGTATT | Fosl2 | Fos-like antigen 2 | 6.0 | ↑ | ↑ | - |
| GGTTTTGTTT | Wsb1 | WD repeat and SOCS box-containing 1 | 6.2 | ↑ | ↑ | - |
| GGATATGTGG | Egr1 | Early growth response 1 | 2.1 | ↑ | ↑ | ↓ |
| GCGCCCTTCC | Ccl21b | Chemokine (C-C motif) ligand 21b | −2.8 | ↓ | ↓ | ↑ |
| GATTTCTGTC | Gng5 | Guanine nucleotide binding protein gamma 5 | −1.7 | ↓ | ↓ | ↑ |
| Cell cycle /proliferation | ||||||
| AAATCCTTTC | Ptn | Pleiotrophin | 2.6 | ↑ | ↑ | - |
| TATAGTATGT | Glul | Glutamate-ammonia ligase | 1.9 | ↑ | ↑ | ↓ |
| CAGCATAAAT | Dip3b | Dip3 beta | −7.2 | ↓ | ↓ | - |
| GCCAAACCAA | Clk1 | CDC-like kinase 1 | −1.9 | ↓ | ↓ | - |
| Cytoskeleton organization / extracellular matrix remodeling | ||||||
| CTCCTGGACA | Gsn | Gelsolin | 3.1 | ↑ | ↑ | - |
| TTAACTCTGA | Prelp | Proline arginine-rich end leucine-rich repeat | 2.8 | ↑ | ↑ | - |
| CCCTGAGTCC | Actb | Actin, beta, cytoplasmic | 2.5 | ↑ | ↑ | - |
| CTGAGGAAGT | Sparcl1 | SPARC-like 1 | 2.1 | ↑ | ↑ | - |
| ATAGCCCCAA | Ctss | Cathepsin S | −5.1 | ↓ | ↓ | ↑ |
| TTTTATTCTC | Capn12 | Calpain 12 | −6.0 | ↓ | ↓ | - |
| GGCTGTTGAA | Csrp1 | Cysteine and glycine-rich protein 1 | −1.6 | ↓ | ↓ | - |
| Protein metabolism | ||||||
| TTACCATTGC | Tiparp | TCDD-inducible poly(ADP-ribose) polymerase | 2.8 | ↑ | ↑ | ↓ |
| CCAGGTTATT | Nola3 | Nucleolar protein family A, member 3 | −6.5 | ↓ | ↓ | - |
| TGACCCCGGG | Uba52 | Ubiquitin A-52 residue ribosomal protein | −2.3 | ↓ | ↓ | - |
| TGGTGTAGGA | Hspa5 | Heat shock 70kD protein 5 | −1.5 | ↓ | ↓ | - |
| TGGGTTGTCT | Tpt1 | Tumor protein translationally-controlled 1 | −1.5 | ↓ | ↓ | - |
| GTGAAACTAA | Rps4x | Ribosomal protein S4, X-linked | −1.5 | ↓ | ↓ | - |
| CTAATAAAGC | Fau | Finkel-Biskis-Reilly murine sarcoma virus | −1.3 | ↓ | ↓ | - |
| Miscellaneous | ||||||
| TGGATCCTGA | Hbb-b1 | Hemoglobin, beta adult major chain | 15.5 | ↑ | ↑ | - |
| TAAATTAAGA | Hexb | Hexosaminidase B | 8.5 | ↑ | ↑ | - |
| GAGGACTGCC | Ly6e | Lymphocyte antigen 6 complex, locus E | 6.2 | ↑ | ↑ | - |
| CCCTTCTTCT | Hba-a1 | Hemoglobin alpha, adult chain 1 | 3.3 | ↑ | ↑ | - |
| CCAGGCCTTA | Crip1 | Cysteine-rich protein 1 | 2.2 | ↑ | ↑ | - |
| TGCACTATTG | 1500012F01 | RIKEN cDNA 1500012F01 gene | −4.4 | ↓ | ↓ | - |
| AACTAGAAAA | Ndufs2 | NADH dehydrogenase Fe-S protein 2 | −4.2 | ↓ | ↓ | ↑ |
| TAAGGGAAAT | Tpi1 | Triosephosphate isomerase 1 | −2.3 | ↓ | ↓ | ↑ |
| CCAAATAAAA | Ldha | Lactase dehydrogenase A | −2.2 | ↓ | ↓ | ↑ |
| CTAATAAAAG | Cox4i1 | Cytochrome c oxidase subunit IV isoform 1 | −2.1 | ↓ | ↓ | - |
| TAAAGCAAAA | Hist1h2bc | Histone 1, H2bc | −1.9 | ↓ | ↓ | - |
Up-regulated transcripts are represented by positive average fold changes and down-regulated transcripts are represented by negative average fold changes among bexarotene and gefitinib treatments. B: Bexarotene treatment, G: Gefitinib treatment, T: p53-null mammary tumors.
Within the functional group of Transcriptional regulation, among the most prominently upregulated by both chemopreventive compounds we find the transcription factor Fosl2 (also known as Fra2) a Fos familiy member. Interestingly, an antitumor promoter, the phenolic antioxidant tertbutylhydroquinone (BHQ), was reported to induce expression of Fra2 (Fosl2) as well as Fra1. Furthermore, the authors concluded that inhibitory AP-1 complexes composed of Jun-Fra heterodimers, induced by BHQ, antagonize the transcriptional effects of the tumor promoter TPA, which are mediated by Jun-Fos heterodimers [21]. Similarly, inhibition of IL6 stimulated cell growth of human myeloma and mouse hybridoma cells was shown to be associated with increased expression of Fra2 protein [22]. Fra2 has also been associated with differentiation in epidermis and exogenous expression of Fra-2 (Fosl2) repressed AP-1 transcriptional activity in TPA-treated keratinocytes and play an opposing role to that of Fos [23]. In ovary, expression of Fra2 and JunD is induced and maintained by luteinizing hormone with the transition of proliferating granulosa cells to terminally differentiated, non-dividing luteal cells [24]. Perhaps the observed upregulation of Fosl2 in mammary gland epithelium of animals exposed to the effective chemopreventive agents is conducive to tilting the balance for the formation of AP1 complexes with growth inhibitory properties.
Also within the group of Transcriptional regulators (Table 2) we find Egr1, a member of the immediate early gene group of transcription factors in a family that includes the tumor suppressor WT1. Egr1 is rapidly and transiently expressed after stimulation of cells with serum, growth factors, phorbol ester tumor promoters, ionizing or nonionizing irradiation [25]. Human EGR1 plays an important role in cell growth, differentiation and development. Huang et al. (1997) demonstrated that the suppressive activity of Egr1 is applicable to several different types of human tumor cell lines including breast carcinoma, glioblastoma, and osteogenic sarcoma and fibrosarcoma [26]. It was shown previously that EGR1 acts like a tumor-suppressor gene, with its expression repressed in breast carcinomas. Recently, was reported that the EGR1 gene is deleted in ER-negative human breast carcinomas [27]. Interestingly, we detected significant up-modulation of Egr1 gene expression in p53-null mammary epithelium of mice treated with bexarotene and gefitinib.
Among other transcripts of interest in cancer detected in our study and within the functional group associated with the cytoskeleton, we observed that both bexarotene and gefitinib treatment significantly up-modulate Gelsolin expression in p53-null `normal' mammary epithelium. Gelsolin encodes a calcium-dependent protein that regulates actin filament length. This protein was suggested to play critical roles in actin cytoskeleton organization, cell motility and apoptosis. Interestingly, and in agreement with our findings, loss of gelsolin expression is one of the most frequently alterations in mammary cancer across at species [9, 28]. Approximately seventy percent of human invasive breast carcinomas and 56% of ductal carcinomas in situ were reported to be deficient in the gelsolin protein expression [29–30]. It is very intriguing to observe that mammary epithelia from mice treated with the two most effective chemopreventive agents in our study, displayed increase in Gelsolin expression.
Cysteine cathepsins are a family of lysosomal proteases that have recently emerged as important players in cancer, and have been involved in apoptosis, angiogenesis, cell proliferation, and invasion [31]. The expression and activity levels of some cysteine cathepsins are commonly upregulated in human and mouse cancers. Increased levels of cathepsins D, B, and L have been reported to be indicators of aggressive tumor behavior in human breast tumors [32]. Recently was demonstrated an important role for Cathepsin S (Ctss) in regulating angiogenesis and tumor growth in genetically engineered mouse model of pancreatic cancer [33]. We observed significant up-modulation of Ctss expression in p53-null mammary tumors compared with normal mammary epithelium (Fold change= 3.6). More important, Ctss gene expression was significantly down-modulated in p53-null mammary epithelium of mice treated with bexarotene and gefitinib (average fold change= −5.1) (Table 2). Intriguingly, we also observed another cysteine protease, Calpain 12 (Capn12) very significantly down-regulated (average fold change= −6) (Table 2). In general calpains are cysteine proteases involved in a variety of cellular processes including apoptosis, cell division, modulation of integrin-cytoskeletal interactions, and synaptic plasticity (Dear et al., 2000), however no information is available on the specific role of Capn12.
Among genes related to protein modifications, TIPARP (also known as PARP1/PARP7) encodes a poly (ADP-ribose) polymerase (PARP) catalyzes the transfer of the ADP-ribose moiety from its substrate NAD+, to a limited number of proteins involved in chromatin architecture, DNA repair and DNA metabolism. Poly (ADP-ribosylation) is a post-translational modification of nuclear proteins in response to DNA damage that activates the base excision repair machinery [34]. The generation of PARP-deficient mice demonstrated the importance of PARP in the maintenance of genomic integrity due to its function in base excision repair [35, 36]. In our study we detected that treatment with all three chemopreventive agents, bexarotene, celecoxib and gefitinib, up-modulates Tiparp gene expression in p53-null mammary epithelium (Table 2). On the other hand, we observed significant down modulation of Tiparp expression when comparing p53-null mammary tumors compared with normal mammary epithelium. Perhaps, treatment with chemopreventive agents such as those here studied, increases DNA repair activity in mammary epithelium at pre-neoplastic stages and a biomarker of this increased activity is the observed Tiparp overexpression.
In summary, our analyses of differentially expressed genes in mammary epithelium of mice exposed to each chemopreventive agent revealed significant similarities across treatments. These results are particularly relevant in light of the findings of Medina et al. 2009, in which bexarotene and gefitinib were observed to be effective as chemopreventive agents in the p53 null mammary epithelium cancer model, while celecoxib did not show any preventive effect. Most importantly, the comprehensive comparison of gene expression profiles allowed us to identify a substantial set of transcripts that behave almost identically in mammary epithelia from mice exposed exclusively to the effective anti-tumorigenic agents (bexarotene and gefitinib), thus, generating a gene expression signature that could be a biomarker of chemopreventive effectiveness in this model. Furthermore, our data provides insight into the molecular bases at play distinguishing the effective from the ineffective chemopreventive interventions and of relevance in mammary tumor development. Not surprisingly, bexarotene and gefitinib appear to exert their chemopreventive activity by affecting multiple cellular pathways, such as modulating the expression of genes related to cell proliferation, cytoskeleton and extracellular matrix remodeling. A somewhat surprising but important observation is that these agents commonly modulate cell adhesion and protein biosynthesis pathways in addition to the more expected cell proliferation and apoptosis pathways.
Further studies will be required focusing on the functional characterization and mechanistic aspects of key cellular pathways identified by our gene expression analysis. The pathways of interest can be first experimentally tested in the described mouse model and in the future may become targets of interest for translational research.
Supplementary Material
Supplementary data file 1. SAGE dataset of differentially expressed genes on normal mammary epithelium from p53 null mice treated with vehicle control vs. bexarotene, gefitinib or celecoxib treatments (p< 0.05).
Supplementary data file 2. Co-occurring deregulated transcripts in normal mammary epithelium from p53 null mice exposed to the various chemopreventive treatments (p< 0.05).
Supplementary data file 3. Differentially expressed genes between p53-null normal epithelium and p53-null mammary tumors (p< 0.05).
ACKNOWLEDGMENTS
This work was supported by NIH-NCI R01 CA101211 (PB), U19 CA84978 (CMA), U01 CA84243 and NIEHS center grant ES-07784. We thank AstraZeneca for kindly providing the compound gefitinib to perform the studies here described.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
REFERENCES
- 1.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC. The effect of raloxifene on risk of breast cancer in postmenospausal women: results form the MORE randomized trial. Multiple Outcomes of Raloxifene evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 3.Shen Q, Brown PH. Transgenic mouse models for the prevention of breast cancer. Mutat Res. 2005;576:93–110. doi: 10.1016/j.mrfmmm.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Jerry DJ, Kittrell FS, Kuperwasser C, Laucirica R, Dickinson ES, Bonilla PJ, Butel JS, Medina D. A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene. 2000;19:1052–8. doi: 10.1038/sj.onc.1203270. [DOI] [PubMed] [Google Scholar]
- 5.Medina D, Kittrell FS, Shepard A, Stephens LC, Jiang C, Lu J, Allred DC, Mccarthy M, Ullrich RL. Biological and genetic properties of the p53 null preneoplastic mammary epithelium. FASEB J. 2002;16:881–3. doi: 10.1096/fj.01-0885fje. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Sun H, Drake J, Kittrell F, Abba MC, Deng L, Gaddis S, Sahin A, Baggerly K, Medina D, Aldaz CM. From mice to humans: identification of commonly deregulated genes in mammary cancer via comparative SAGE studies. Cancer Res. 2004;64:7748–55. doi: 10.1158/0008-5472.CAN-04-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayanan BA. Chemopreventive agents alters global gene expression pattern: predicting their mode of action and targets. Current Cancer Drug Targets. 2006;6:711–727. doi: 10.2174/156800906779010218. [DOI] [PubMed] [Google Scholar]
- 8.Medina D, Kittrell F, Hill J, Hilsenbeck S, Brown PH. Prevention of tumorigenesis in p53 null mammary epithelium by rexinoid baxarotene, tyrosine kinase inhibitor gefitinib and celecoxib. Cancer Prevent Res. 2009 doi: 10.1158/1940-6207.CAPR-08-0107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldaz CM, Hu Y, Daniel R, Gaddis S, Kittrell F, Medina D. Serial analysis of gene expression in normal p53 null mammary epithelium. Oncogene. 2002;21:6366–76. doi: 10.1038/sj.onc.1205816. [DOI] [PubMed] [Google Scholar]
- 10.Charpentier AH, Bednarek AK, Daniel RL, Hawkins KA, Laflin KJ, Gaddis S, MacLeod MC, Aldaz CM. Effects of estrogen on global gene expression: identification of novel targets of estrogen action. Cancer Res. 2000;60:5977–83. [PubMed] [Google Scholar]
- 11.Audic S, Claverie J. The significance of digital gene expression profiles. Genome Res. 1997;7:986–995. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- 12.Hosack DA, Dennis G, Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:R60. [PubMed] [Google Scholar]
- 14.Smid M, Dorssers LCJ, Jenster G. Venn Mapping: clustering of heterologous microarray data based on the number of co-occurring differentially expressed genes. Bioinformatic. 2003;19:2065–2071. doi: 10.1093/bioinformatics/btg282. [DOI] [PubMed] [Google Scholar]
- 15.Wu K, Kim H, Rodríguez JL, Hilsenbeck SG, Mohsin SK, Xu X, Lamph WW, Kuhn JG, Green JE, Brown PH. Suppression of mammary tumorigenesis in transgenic mice by the RXR-selective retinoid, LGD1069. Cancer Epidemiol Biomarkers Prev. 2002;11:467–474. [PubMed] [Google Scholar]
- 16.Wu K, Zhang Y, Xu X, Hill J, Celestino J, Kim H, Mohsin SK, Hilsenbeck SG, Lamph WW, Bissonette R, Brown PH. The retinoid X receptor-selective retinoid, LGD1069, prevent the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002;62:6376–80. [PubMed] [Google Scholar]
- 17.Kim HT, Kong G, Denardo D, Li Y, Uray I, Pal S, Mohsin S, Hilsenbeck SG, Bissonnette R, Lamph WW, Johnson K, Brown PH. Identification of biomarkers modulated by the rexinoid LGD1069 (bexarotene) in human breast cells using oligonucleotide arrays. Cancer Res. 2006;66:12009–18. doi: 10.1158/0008-5472.CAN-05-2515. [DOI] [PubMed] [Google Scholar]
- 18.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887–8895. [PubMed] [Google Scholar]
- 19.Lu C, Speers C, Zhang Y, Xu X, Hill J, Steinbis E, Celestino J, Shen Q, Kim H, Hilsenbeck S, Mohsin SK, Wakeling A, Osborne CK, Brown PH. Effect of epidermal growth factor receptor inhibitor on development of estrogen receptor-negative mammary tumors. J Natl Cancer Inst. 2003;95:1825–33. doi: 10.1093/jnci/djg117. [DOI] [PubMed] [Google Scholar]
- 20.Howe LR, Dannenberg AJ. COX-2 inhibitors for the prevention of breast cancer. J Mamm Gland Biol Neoplas. 2003;8:31–43. doi: 10.1023/a:1025731204719. [DOI] [PubMed] [Google Scholar]
- 21.Yoshioka K, Deng T, Cavigelli M, Karin M. Antitumor promotion by phenolic antioxidants: inhibition of AP-1 activity through induction of Fra expression. Proc Natl Acad Sci USA. 1995;92:4972–6. doi: 10.1073/pnas.92.11.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezzonico R, Loubat A, Lallemand D, Pfarr CM, Proudfoot A, Rossi B, Ponzio G. Cyclic AMP stimulates a JunD/Fra-2 AP-1 complex and inhibits the proliferation of interleukin-6-dependent cell lines. Oncogene. 1995;11:1069–78. [PubMed] [Google Scholar]
- 23.Rutberg SE, Saez E, Lo S, Jang SI, Markova N, Spiegelman BM, Yuspa SH. Opposing activities of c-Fos and Fra-2 on AP-1 regulated transcriptional activity in mouse keratinocytes induced to differentiate by calcium and phorbol esters. Oncogene. 1997;15:1337–46. doi: 10.1038/sj.onc.1201293. [DOI] [PubMed] [Google Scholar]
- 24.Sharma SC, Richards JS. Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulose cells. Relation of JunD and Fra2 to terminal differentiation. J Biol Chem. 2000;275:33718–28. doi: 10.1074/jbc.M003555200. [DOI] [PubMed] [Google Scholar]
- 25.Huang RP, Liu C, Fan Y, Mercola D, Adamson ED. Egr-1 negatively regulates human tumor cell growth via the DNA-binding domain. Cancer Res. 1995;55:5054–5062. [PubMed] [Google Scholar]
- 26.Huang RP, Fan Y, de Belle I, Niemeyer C, Gottardis MM, Mercola D, Adamson ED. Decreased Egr-1 expression in human, mouse and rat mammary cells and tissues correlate with tumor formation. Int J Cancer. 1997;72:102–109. doi: 10.1002/(sici)1097-0215(19970703)72:1<102::aid-ijc15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Ronski K, Sanders M, Burleson JA, Moyo V, Benn P, Fange M. Early growth response gene 1 (EGR1) is deleted in estrogen receptor-negative human breast carcinoma. Cancer. 2005;104:925–930. doi: 10.1002/cncr.21262. [DOI] [PubMed] [Google Scholar]
- 28.Dong Y, Asch HL, Ying A, Asch BB. Molecular mechanism of trasncriptonal repression of gelsolin in human breast cancer cells. Exp Cell Res. 2002;276:328–336. doi: 10.1006/excr.2002.5534. [DOI] [PubMed] [Google Scholar]
- 29.Asch HL, Winston JS, Edge SB, Stomper PC, Asch BB. Down-regulation of gelsolin expression in human breast ductal carcinoma in situ with and without invasion. Breast Cancer Res Treat. 1999;55:179–188. doi: 10.1023/a:1006203632228. [DOI] [PubMed] [Google Scholar]
- 30.Winston JS, Asch HL, Zhang PJ, Edge SB, Hyland A, Asch BB. Downregulation of gelsolin correlates with the progression to breast carcinoma. Breast Cancer Res Treat. 2001;65:11–21. doi: 10.1023/a:1006446108411. [DOI] [PubMed] [Google Scholar]
- 31.Joyce JA, Hanahan D. Multiple roles for cysteine cathepsins in cancer. Cell Cycle. 2004;3:1516–1619. doi: 10.4161/cc.3.12.1289. [DOI] [PubMed] [Google Scholar]
- 32.Nomura T, Katunuma N. Involvement of cathepsins in the invasion, metastasis and proliferation of cancer cells. J Med Invest. 2005;52:1–9. doi: 10.2152/jmi.52.1. [DOI] [PubMed] [Google Scholar]
- 33.Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, Kalluri R, Shi G. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem. 2006;281:6020–6029. doi: 10.1074/jbc.M509134200. [DOI] [PubMed] [Google Scholar]
- 34.Amé J, Rolli V, Schreiber V, Niedergang C, Apiou F, Decker P, Muller S, Höger T, Nurcia JM, de Murcia G. PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J Biol Chem. 1999;274:17860–17868. doi: 10.1074/jbc.274.25.17860. [DOI] [PubMed] [Google Scholar]
- 35.De Murcia JM, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, Oliver FJ, Masson M, Dierich A, LeMeur M, Walztinger C, Chambon P, de Murcia G. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci USA. 1997;94:7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masson M, Niedergang C, Schreiber V, Muller S, Murcia JM, de Murcia G. XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol Cell Biol. 1998;18:3563–3571. doi: 10.1128/mcb.18.6.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data file 1. SAGE dataset of differentially expressed genes on normal mammary epithelium from p53 null mice treated with vehicle control vs. bexarotene, gefitinib or celecoxib treatments (p< 0.05).
Supplementary data file 2. Co-occurring deregulated transcripts in normal mammary epithelium from p53 null mice exposed to the various chemopreventive treatments (p< 0.05).
Supplementary data file 3. Differentially expressed genes between p53-null normal epithelium and p53-null mammary tumors (p< 0.05).