Abstract
Myopia is the result of a mismatch between the optical power and the length of the eye, with the latter being too long. Driving the research in this field is the need to develop myopia treatments that can limit axial elongation. When axial elongation is excessive, as in high myopia, there is an increased risk of visual impairment and blindness due to ensuing pathologies such as retinal detachments. This article covers both clinical studies involving myopic children, and studies involving animal models for myopia. Atropine, a nonselective muscarinic antagonist, has been studied most extensively in both contexts. Because it remains the only drug used in a clinical setting, it is a major focus of the first part of this article, which also covers the many shortcomings of topical ophthalmic atropine. The second part of this article focuses on in vitro and animal-based drug studies, which encompass a range of drug targets including the retina, retinal pigment epithelium and sclera. While the latter studies have contributed to a better understanding of how eye growth is regulated, no new antimyopia drug treatments have reached the clinical setting. Less conservative approaches in research, and in particular, the exploration of new bioengineering approaches for drug delivery, are needed to advance this field.
Keywords: animal models, atropine, clinical trials, dopamine, eye growth, growth factors, myopia
Myopia describes the refractive error in which light entering the eye from distant objects is focused in front of the retina, leading to blurred vision. The condition is most commonly the result of excessive elongation of the posterior vitreous chamber of the eye, increasing the risk of retinal detachment and some degenerative retinal conditions, and rendering high myopia a major cause of visual impairment and blindness [1–6]. Myopia has become a major public health concern owing to rapid rises in the prevalence of myopia, first noted in East Asian populations. For example, as of the year 2000, the prevalence of myopia had reached 84% for Taiwanese adolescents between 16 and 18 years of age. Of the 18-year-olds, 21% were highly myopic, putting them at high risk for pathologic conditions in the future [7]. Similar trends are evident in recently published myopia prevalence figures for the USA, although they are not as high as East Asian figures [8–11].
The management of myopia has been mostly directed at correcting the mismatch between the eye’s optical power and its length using either optical means, such as single-vision spectacles and contact lenses, or refractive surgeries, such as photorefractive keratectomy (PRK) and laser-assisted in situ keratomileusis (LASIK), which both involve reshaping and thus modifying the optical power of the cornea. While these options restore sharp distance vision in myopes, they do nothing to control myopia progression. On the other hand, the possibility that myopia progression can be controlled optically has seen a recent upsurge in interest, driven in part by the demonstration in animal models that positive lenses can slow eye growth. While comprehensive analysis of this literature is beyond the scope of this article, it is appropriate to acknowledge the promising results from some recent contact lens studies. Specifically, dramatic slowing of eye elongation has been reported in early, albeit small-scale myopia studies involving two different types of contact lenses, one being a concentric bifocal soft contact lens [12–16], and the other being a rigid lens worn overnight to flatten and thereby reduce the power of the central cornea (a procedure known as orthokeratology or corneal refractive therapy) [17,18]. CooperVision (CA, USA) recently released the MiSight® multifocal soft contact lens intended for myopia control [19]. The reported treatment effects are substantially greater than those of progressive addition spectacles (PALs), for which a large-scale clinical trial, the Correction of Myopia Evaluation Trial (COMET), found their benefit to be relatively small and limited to a subgroup of myopes exhibiting binocular vision-related or accommodative (near focusing) abnormalities [20,21].
There are currently no pharmaceutical agents approved by the US FDA for use as myopia treatments, although three drugs, namely atropine, pirenzepine and 7-methylxanthine (7-MX), have been targeted in recent clinical trials [22–26]. Topical atropine is also widely used off-label in East Asian countries where myopia-related public health concerns are highest. In this article, a summary of early clinical investigations of drugs for controlling myopia provides an historical perspective and backdrop to more recent clinical and drug studies using in vitro and animal models for myopia, which have sought to understand the complex signaling cascade that underlies myopic eye growth and so identify new potential drug treatments.
Clinical myopia control studies
Atropine
Early history
Atropine, a nonselective muscarinic receptor antagonist, remains the only drug in current clinical use for myopia control and is also the most extensively studied drug in the context of myopia control. Its topical ophthalmic applications date back to the Renaissance period, when Italian women used atropine to enlarge their pupils to make themselves appear interested in any suitor or subject [27]. The name of the plant from which it is extracted, Atropa belladonna, is attributed to atropine’s former use as a cosmetic. In the late 1700s, atropine gained notice for its inhibitory effect on accommodation (cycloplegia). Both pupil dilation and cycloplegia represent problematic side-effects without therapeutic benefit for myopia control.
Starting with early studies by Bedrossian in the 1960s and 1970s [28–30], there have been numerous studies of atropine’s topical effect on myopia progression. This application of atropine was a logical extension of arguments linking myopia with excessive near work and presumed excessive accommodation. However, serious deficiencies in the designs of these early studies lead to questioning of the general conclusion that atropine slows myopia progression. For example, in the Bedrossian studies, refractive errors are the only outcome measure, yet alone, such data do not distinguish between reductions in myopia due to long-term cycloplegia and those due to reduced axial elongation, the desired treatment outcome for limiting the pathological complications of myopia. The apparent lack of follow-up of study drop-outs and inconsistencies in the vehicle used to deliver the atropine represent other weaknesses in these studies.
Other early studies making the case for the effectiveness of atropine as a myopia-control treatment include one by Kelly et al. [31] and another by Gimbel [32]. Kelly retrospectively analyzed patient records that were organized into five groups, including a control and two atropine groups. However, treatment regimens varied significantly within the groups, and treatments were not randomized. Gimbel’s study is the first to suggest that the antimyopia effect of atropine may be limited in duration – in this case, to the first of 3 years of treatment. The utility of the data is limited by deficiencies in the study’s design, including the lack of a control group and the use of spectacles, along with treatment regimens that vary the number of drops applied and their frequency of administration [32].
Challenges associated with clinical trials of atropine
Despite the design flaws of early atropine studies, encouraging results from these and related animal studies, along with the increased clinical motivation to control myopia progression, have spawned other clinical investigations. Nonetheless, a truly definitive study has not been offered, largely owing to ocular effects particular to atropine. For example, even in well-controlled, randomized studies, the tell-tale pupil dilation caused by atropine makes truly double-blind studies impossible. Furthermore, the cycloplegic action of atropine requires that atropine-treated patients use bifocal or multifocal lenses to compensate for their loss of accommodation, and as already noted, these lenses may have their own growth-limiting effects [12–16,20,33–37], which must be decoupled from any drug treatment. A study intended to address this problem included placebo single-vision and multifocal spectacles control groups [38], and although no difference in myopia progression was found between these groups, this result is at odds with other relevant reports [20,21,39]. In addition, because the atropine groups also wore multifocal lenses, the potential additive or interactive effects of atropine and multifocal lens treatments cannot be ruled out as explanations for their slowed myopia progression. Finally, as alluded to earlier, difficulties in achieving a level of cycloplegia in untreated eyes comparable with that produced by chronic treatment with atropine, the most potent of clinical cycloplegic agents, will lead to a relative underestimation of myopia in atropine-treated eyes.
Another challenge faced in atropine studies is ensuring compliance, on which treatment efficacy is dependent [40]. This point is demonstrated well by the results of a study by Chiang et al., in which the fully compliant group, which comprised 70% of 706 subjects, recorded a minimal (0.08 D) change in refractive error, compared with 0.23 D for the partially compliant group [41]. The many potential causes of noncompliance in topical atropine studies include ocular discomfort, most commonly due to photophobia, as well as stinging, red eyes, headaches and allergic reactions with longer term use. A variety of strategies have been adopted in more recent studies to obtain reliable data on compliance, including weighing returned solution bottles to determine usage [42,43], written or oral questionnaires about usage [44], calendar records of usage [43], and the use of electronic bottle caps that track their removal [45–47]. While these methods are helpful in tracking compliance in the forgetful, none are foolproof and they do not deal with subject drop-outs, which in one study reached 9% with a 1% atropine treatment [46]. The same study reported lower drop-out rates for 0.25 and 0.1% solutions [46], presumably reflecting their less severe and more short-lived ocular side-effects.
Atropine & myopia control
Despite the aforementioned challenges of clinical trials involving atropine, published studies leave little doubt that atropine treatment combined with multifocal lenses can control myopia progression. From the long list of relevant studies shown in Table 1, two of the best designed studies, one by Shih et al. [38] and another by Chua et al. [43], are selected for illustrative purposes. Although each study has weaknesses, the weaknesses are outweighed by the merits.
Table 1.
Summary of clinical trials of antimuscarinic drugs as myopia control treatments.
| Authors (year) |
Age of subjects (years); treatment period |
Initial refractive error (D) |
Key study features |
Treatment q.h.s. unless otherwise specified (subjects; n) |
Control (subjects; n) |
Cycloplegic refractions (Y/N; agent) |
Mean refractive error change in treatment and control groups, respectively, in D/year (SD) |
Mean axial length increase in treatment and control groups, respectively, in mm/year (SD) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Bedrossian et al. (1966) | 7–13; 1 year | −0.5 to −5 | M, NR | 1% Atr or Scop drops (35) | Untreated fellow eye (35) | Y, 1% Tp | +0.18*; −0.91 | NA | [28] |
| Bedrossian (1971) | 7–13; 2 years | 0 to −5 | M, NR | 1% Atr or Scop drops (75) | Untreated fellow eye (75) | Y, 1% Tp | +0.20; −0.85 | NA | [29] |
| Gimbel (1973) | 5–15; 1 year | <0.25 | NM, NR | 1% Atr drops; frequency varied (279) | Untreated myopes (572) | Y, 1% Tp | +0.59; −0.61 | NA | [32] |
| Bedrossian (1979) | 8–13; 2 years | −2.3 (average) | M, R | 1% Atr drops (90) | Untreated fellow eye (90) | Y, Tp | +0.17; −0.99 | NA | [30] |
| Brodstein et al. (1984) | 0–18+; 1–107 months | <−0.5 | NM, NR | 1% Atr drops + BF (222) | Untreated myopes (146) | Inconsistent | −0.01 (0.03); −0.03 (0.02) | NA | [49] |
| Gruber (1985) | 2–21; 1–2 years | ? | NM, NR | 1% Atr drops (100) | Untreated myopes (100) | ? | −0.11; −0.28 | NA | [227] |
| Yen et al. (1989) | 6–14; 1 year | −0.5 to −4.0 | M, R | 1% Atr drops; q.o.d. (32) or 1% Cp drops (32) | Saline drops (32) | Y, 1% Cp + 1% Tp | −0.22 (0.54)*; −0.58 (0.49)*; −0.91 (0.58) | NA | [228] |
| Chou et al. (1997) | 10.6 (average); 26–48 or 28–48 months | −6.25 to −12.0 | M, NR | 0.5% Atr drops + BF (12) or 0.5% Atr drops + BF (8) | Pretreatment Tp (12) or pretreatment untreated (8) | Y, 0.5% Tp | −0.01 (0.04)*; −0.12 (0.09); −0.04 (0.06)*; −0.14 (0.07) (D/month) | NA | [50] |
| Shih et al. (1999) | 6–13; up to 2 years | ? | M?, R | 0.5% Atr, or 0.25% Atr, or 0.1% Atr all drops | Placebo drops | ? | −0.04 (0.63)*; −0.45 (0.55)*; −0.47 (0.91)*; −1.06 (.61) | NA | [46] |
| Romano et al. (2000) | 5–15; 2–24 months | ? | NM, NR | 1% Atr drops + BF always compliant (19) | Partially compliant (12) or never compliant to treatment (4) | Y, 1% Cp | +0.07; −0.18; −0.17 | NA | [40] |
| Chiang et al. (2001) | 6–16; >10 years | <−0.5 | NM, NR | 1% Atr drops + BF (496) | Partially compliant to treatment (210) | ? | 0.08*; 0.23 | NA | [41] |
| Syniuta et al. (2001) | 4–13; 3–96 months | <−6.0 | R, M | 1% Atr (drops?) + BF (15) | Untreated myopes (15) | Y, 1% Cp | 0.05 (0.67); 0.84 (0.26) | NA | [229] |
| Shih et al. (2001) | 6–13; 18+ months | −3.3 (average) | R, NM | 0.5% Atr drops + MF (66) | Placebo drops + MF (61) or + SV (61) | Y, 1% Tp | −0.42 (0.07)**; −1.19 (0.07); −1.40 (0.09) (progression after 18 months) | 0.22 (0.03)**; 0.49 (0.03) | [38] |
| Kennedy et al. (2000) | 6–15; 18 weeks–11.5 years | 0 to −9.2 | R, M | Atr (frequency and dose unknown) (176) | Untreated myopes (retrospective data) | Inconsistent | 0.16*; 0.35 (noncyclopleged) | NA | [230] |
| Siatkowski et al. (2004) | 8–12; 1 year | −0.75 to −4.0 | R, M | 2% Pir gel; b.i.d (174) | Placebo gel (57) | Y, 0.5% Prop + 1% Cp + 1% Tp | −0.26 (0.36)**; −0.53 (0.50) | 0.19 (0.24); 0.23 (0.35) | [53] |
| Tan et al. (2005) | 6–12; 1 year | −0.75 to −4.0 | R, M | 2% Pir gel; b.i.d or q.d | Placebo gel | Y, 0.5% Prop + 1% Cp + 1% Tp | −0.47**; −0.70; −0.84 | 0.33*; 0.30*; 0.20 | [52] |
| Lee et al. (2006) | 6–12; 1 year | −0.5 to −5.5 | NM, NR | 0.05% Atr drops (21) | Untreated myopes (36) | Y, 1% Cp + 1% Tp | −0.28 (0.26)**; −0.75 (0.35) | NA | [47] |
| Chua et al. (2006) | 6–12; 2 years | −1.0 to −6.0 | M, R | 1% Atr drops (166) | Vehicle drops, fellow eye (190) | Y, Prop + 1% Cp | −0.28 (0.92)**; −1.20 (0.69) (progression after 2 years) | 0.02 (0.35); 0.38 (0.38) (>2 years) | [43] |
| Fan et al. (2007) | 5–10; 1 year | <−3.0 | M, NR | 1% Atr ointment (23) | Untreated myopes (23) | Y, 1% Cp | +0.06 (0.79)**; −1.19 (2.48) | 0.09 (0.19)**; 0.70 (0.63) | [22] |
| Liang et al. (2008) | 6–15; ≥6 months | <−0.5 | M, R | 0.5% Atr (23) or 0.25% Atr (26) + AA; all drops | 0.25% Atr drops | Y, 1% Cp | −0.15 (0.15)*; −0.21 (0.23)*; −0.38 (0.32) | 0.12 (0.12); 0.14 (0.11); 0.16 (0.09) | [24] |
| Fang et al. (2010) | 6–12; ≥1 year | (+1 to −1 D) | M, NR | 0.025% Atr (24) | Untreated premyopes (+1 to −1 D) (26) | Y, 1% Cp + 1% Tp | −0.14 (0.24)**; −0.58 (0.34) | NA | [231] |
Frequency of administration is daily unless otherwise noted. Studies are only listed if annual refractive error changes were included in the study.
p ≤ 0.05;
p ≤ 0.01.
AA: Stimulation with auricular acupoints; Atr: Atropine; BF: Bifocal glasses; b.i.d: Twice daily; Cp: Cyclopentalate; Drops: Eye drops; M: Matched groups; MF: Multifocal glasses; N: No; NA: Not available; NM: Groups not matched; NR: Not randomized; Pir: Pirenzepine; Prop: Proparicaine; q.h.s.: Every night; q.o.d.: Every other day; R: Randomization; Scop: Scopolamine; SD: Standard deviation; SV: Single-vision glasses; Tp: Tropicamide; Y: Yes.
The study by Shih et al. compared changes in both the refractive errors and axial lengths of a group wearing multifocal spectacles treated daily with 0.5% atropine with those of two placebo control groups [38]. Over an 18-month treatment period, the atropine-treated group recorded a mean increase in myopia of 0.42 D, which was significantly lower than the changes of 1.2 and 1.4 D for the multifocal and single-vision spectacle-wearing control groups, respectively. A correspondingly smaller increase in axial length was recorded for the atropine-treated group (0.22 vs 0.49 and 0.59 mm), implying that the intergroup differences in myopia progression were at least partly a consequence of inhibited eye elongation. The rate of lens thickening in atropine-treated eyes was also slower than that of control eyes.
The Atropine in the Treatment of Myopia (ATOM) study led by Chua et al. [43] differs in design from that of Shih et al. in two important ways. First, the treatment was monocular, removing the need for multifocal spectacles. Second, a higher (1%) concentration of atropine was used. The study found decreased myopia progression with negligible increase in axial length for atropine-treated eyes over the 2-year treatment period. By contrast, the untreated fellow eyes grew an average of 0.38 mm over the same period. Curiously, a small yet statistically significant decrease in axial length was recorded in treated eyes over the first 12 months of the study. This effect of atropine was attenuated in the second year. In the 12 months following cessation of atropine treatment (in year 3), the rate of myopia progression in eyes previously treated with atropine increased to be more than double that of the fellow eyes, although the mean progression rate was still lower in atropine-treated compared with control eyes over the 3-year period [48]. The post-treatment increase in growth rate raises the question of whether eyes have internal refractive error set points that guide eye growth. Drug-induced altered receptor sensitivity, a well-documented effect of chronic drug treatments, offers an alternative explanation for this rebound phenomenon. The monoocular nature of the study design also introduces interocular interactions as a confounding factor, with potential to influence both treatment and recovery effects. There has been no rigorous study of lower doses of atropine; although the refractive error data from a study by Lee et al. involving a 0.05% atropine are provocative, no axial length data are available to verify an inhibitory effect on myopic eye growth [47].
Age, myopia magnitude & progression, & control with atropine
Published studies provide only limited insight into the clinically relevant question of whether there is an optimum age for atropine treatment. Specifically, is the efficacy of atropine dependent on age, given that children tend to exhibit higher rates of myopia progression than adolescents and young adults? In the only study of relevance, Brodstein et al. grouped his subjects by age, and found that atropine was most effective in the 8- to 12-year-old group and the 18 years or over group [49]. However, these results are difficult to interpret as the older group had been treated for the longest period of time and the younger of the two groups recorded the fastest myopia progression rate. More studies are needed to determine if atropine is most effective in young children.
The possible dependence of atropine’s efficacy on the extent of myopia at the initiation of treatment has also not been adequately investigated. In published studies, initial refractive errors typically range from −1.0 to −6.0 D. One exception is a study by Chou et al. that included children with refractive errors ranging from −6.25 to −12.0 D [50]. Although 0.5% atropine was reported to be effective in slowing myopia in the latter group, the control group used in the study were the same patients tracked before atropine treatment, adding age-related slowed progression as a confounding factor. In another study in which children with low levels of myopia above −3.0 D were excluded, Fan et al. reported 1% atropine drops to be effective in slowing myopia [42]. However, treatments were not randomized, and the control group was left untreated instead of receiving a placebo treatment.
Pirenzepine
An early animal-based study involving pirenzepine, a relatively selective M1 /M4 muscarinic receptor antagonist, held promise that it could supplant atropine as an antimyopia treatment, but with fewer ocular side-effects [51]. For children engaged in outdoor activities, the mydriatic effect of atropine raises long-term safety concerns associated with the increased light reaching the lens and retina. Pirenzepine is reported to have reduced mydriatic and cycloplegic effects in humans [45,52], and is already approved in oral form for the treatment of dyspepsia [45]. After establishment of the safety and tolerability of a 0.5% ophthalmic gel formulation in a US-based study [45], a follow-up Asia-based double-masked, placebo-controlled randomized study in which 2% pirenzepine gel was administered twice-daily found myopia progression to be decreased by approximately 50% over 12 months [53]. Results of a more recently published US-based clinical trial of 2% pirenzepine were similar and the drug treatment was also found to have a clinically acceptable safety profile [25,53]. However, clinical trials of this drug have been suspended, presumably owing to its reduced efficacy as an antimyopia treatment compared with topical atropine, and the requirement of twice-daily rather than once-daily dosing.
Other antimuscarinic drugs
Two other ophthalmic antimuscarinic drugs, tropicamide and scopolamine, have also been reported to slow myopia progression in early studies [54,55]. Nightly tropicamide administration was reported to reduce myopia progression by approximately 50% in an early study involving 136 treated and 164 control eyes [55]. However, treatments were not randomized and there have been no follow-up studies, even though both the short duration of action of this drug and the night-time treatment regimen used should minimize day-time pupillary and accommodative side-effects. By contrast, the pharmacokinetic profile of scopolamine is similar to that of atropine [56], and thus it offers little advantage as an antimyopia drug over atropine with respect to ocular side-effects.
Other miscellaneous drugs
Intraocular pressure-lowering drugs
Clinical testing of other drugs for control of myopia progression has been mostly limited to intraocular pressure (IOP)-lowering drugs, motivated by data, albeit equivocal, linking myopia with increased IOP [57–63]. β-blockers have been studied more than other IOP-lowering drugs. However, in the best-designed study in this category, which used timolol maleate in a randomized study of 159 myopic school children, Jensen found no evidence of slowed progression of myopia after 2 years, despite an average decrease in IOP of approximately 3 mm Hg [64]. Epinephrine also lowers IOP [65], although the primary motivation for its use in a private practice-based study in 1931 by Wiener was to biochemically mimic exercise in his myopic patients, all of whom he felt were lacking in physical activity [66]. Wiener reported curtailment of myopia progression in 79 out of his 99 patients, with myopia control being defined as progression of less than 0.25 D per year. However, his study design had many weaknesses; no controls were used, his patients included a wide range of ages and amounts of myopia, and no axial length data were collected [66]. Nonetheless, Weiner was seemingly ahead of his time in prescribing daily outdoor exercise to his patients, a strategy supported by recently published studies reporting a protective effect against myopia of outdoor activity [67]. While a slightly later, unrelated clinically based study also found epinephrine to be effective in limiting myopia progression [68], it was also not well controlled, and there have been no recent clinical studies of either epinephrine or other ocular hypotensive drugs.
Methylxanthine
A 36-month placebo-controlled pilot study of oral 7-MX in children [26] was motivated by promising effects on scleral ultra-structure and eye growth rates from studies in rabbits [69] and guinea pigs [70], reviewed later. While oral therapy has practical advantages for children, and oral 7-MX was found to be safe, its effect on myopia progression was only very small. For example, the mean overall progression rates for the 7-MX and placebo groups over the first 12 months of the study differed by less than 0.1 D (0.67 and 0.76 D, respectively). Furthermore, myopes showing high progression rates seemed to benefit least from 7-MX treatment, another limitation of this treatment option.
Animal model studies of myopia
History
Progress in therapeutic drug development is contingent on there being suitable animal models for childhood myopia. Following the serendipitous discovery that animals can be made myopic through visual manipulations, there are now a number of alternative models, most involving optical intervention and each with their own strengths and weaknesses. Of historical significance is a model developed by Young, who, in attempting to mimic the effect of reading, kept monkeys for up to 6 months in restraining chairs under hoods to restrict their visual space. Using this experimental paradigm in a study of eight monkeys, Young carried out the first animal-based test of atropine as an antimyopia drug [71]. This study failed to uncover an antimyopia effect of atropine, but did not deter other researchers from pursuing the possibility that myopia progression could be controlled through drug intervention. Such research has been aided by the development of two additional experimental models of myopia featuring increased eye elongation (i.e., ‘myopic eye growth’), as in human myopia. These models are introduced here.
Experimental models of myopia
An incidental discovery that lid-suturing induces myopia led to more refined methods of covering the eye to stimulate eye growth, an approach that is generally referred to as form-deprivation myopia (FDM). Additional optical defocus methods for inducing myopia experimentally were subsequently developed. The first report of FDM appears in a 1977 publication on cat striate cortex, in which Wilson and Sherman noted as an aside to a neurological study that “interestingly, the deprived eye was usually 1–2 D more myopic than the nondeprived eye” [72]. Months later, Wiesel and Raviola reported myopic shifts in refractive errors in the eyes of macaque monkeys closed surgically for 19 days to 26 months [73]. Refractive errors ranged from 0 to −13.5 D; lower refractive errors correlated with shorter treatment periods or older ages. Importantly, these myopic eyes were found to share many anatomical features with myopic human eyes. For example, they were enlarged, more so in the axial direction (21% increase) than in the equatorial dimension (7% increase). The posterior sclera was also thinner than normal, as in myopic human eyes [74], while corneal curvature and thickness, as well as choroidal and retinal thicknesses, were found to be unchanged [73], although later studies report choroidal thinning in some animal myopia models [75–77]. Equally important was the finding that lid-fused eyes did not enlarge in monkeys reared in the dark [78], implying that visual stimulation through the translucent eye lid was necessary for the axial elongation.
The development of small animal myopia models has been critical to progress in this field. Lid suture myopia models were initially developed, with relevant publications covering tree shrews [79], rabbits [80] and chicks [81]. Today, lid suture is rarely used, with preference being given to translucent plastic diffuser devices, which have two major advantages. One is that they avoid the potential compromise to corneal physiology of lid closure, and the other is that they may be shaped to obscure either the total visual field or a section of it. These form-deprivation models have analogies in congenital cataracts and other ocular pathologies leading to significant retinal image degradation in young children. Young chickens show robust responses to form-deprivation treatments and much larger refractive error changes than their mammalian counterparts, with myopia levels as high as −20 D reported in one early study [81]. The latter features, as well as their rapid response to myopia-inducing stimuli, relatively small size and comparatively low cost underlie the widespread use of chickens in myopia research today [82–84]. For pharmacological studies, the main drawback of chickens is a bilayered scleral structure; in addition to a fibrous layer that is similar in structure to the mammalian sclera, the chick sclera includes an inner cartilaginous layer, which thickens in experimental myopia instead of thinning [85].
The demonstration, first in chicks, that eyes can adjust their growth to compensate for imposed optical defocus by increasing their growth in response to hyperopic defocus (imposed with negative lenses) and slowing their growth in response to myopic defocus [86] is now accepted to be an expression of active emmetropization. This developmental phenomenon has since also been demonstrated in rhesus monkeys [87], marmosets [88], tree shrews [89], guinea pigs [90,91] and mice [92]. The principal differences between these various animal models lie in the maximum achievable compensation and the time required for full compensation. In all cases, the defocus-induced increased growth is coupled to myopia, which is referred to as lens-induced myopia (LIM) elsewhere in this article. The defocus imposed by LIM has clinical analogies in the increased accommodative lags and peripheral (off-axis) hyperopia that have been linked to juvenile-onset myopia, although causality is yet to be established (see [93] for review). Nonetheless, this lens model is generally considered a more plausible model for juvenile-onset myopia than the form-deprivation alternative.
Local control of eye growth
Another early discovery that has shaped the direction of more recent myopia animal studies is that eye growth regulation is largely confined to local ocular circuits (see [93] for review). The first indication that myopia development was not dependent on the brain came in 1985 from data collected from rhesus monkeys showing that lesioning of the visual pathways did not prevent lid suture-induced myopia, although this result was not confirmed in stumptail monkeys [94]. While the small number of animals in this study left the role of local eye growth regulation unresolved, results from subsequent studies involving optic nerve section in chicks [95–97] and, more recently, in guinea pigs [98] all corroborate the aforementioned finding in the rhesus monkey: that myopic eye enlargement does not require input from the brain. The same conclusion of local eye growth regulation is reached from results of two other types of studies. The first involves the use of intravitreal tetradotoxin to silence retinal ganglion cells, and hence prevent retinal communication with the brain; in both tree shrews and chicks, myopic eye growth is still observed in response to appropriate experimental manipulation [99,100]. The second involves visual manipulations that are restricted to part of the retina through the use of half diffusers or half lenses, first studied in chicks [79,83,96,101] and, more recently, in monkeys [102] and guinea pigs [103,104]. With such treatments, induced vitreous chamber dimensional changes are limited to the visually manipulated segment, providing further argument for regulation at a local level, as there are no known central (nonretinal) circuits suited to such localized growth modulation.
Despite the provocative evidence linking excessive near work with myopia in humans, manipulations used to prevent accommodation in chicks, including Edinger–Westphal nucleus lesions [105], ciliary nerve section [106] or cycloplegia [107], all have no effect on experimental myopia. These results are consistent with the observation that experimental myopia can be induced in the Eastern grey squirrel, despite it having no measurable accommodation [108]. The implication of these studies and the aforementioned model of local eye growth regulation is that the central accommodation control circuit is not involved in eye growth regulation. This also represents an argument against targeting accommodation, specifically the ciliary muscle, in antimyopia drug studies.
A significant amount of the animal-based research exploring drug interventions for myopia control has been directed at verifying and understanding the antimyopia action of atropine. Other pharmacological studies in animals have been directed at the more general question of how the dimensions of the vitreous chamber and, thus, eye size, are regulated. Studies of the retinal signaling pathways underlying myopia, the role of the retinal pigment epithelium (RPE) in relaying derived growth-modulatory signals to the choroid and sclera, and the mechanisms underlying the myopic growth changes in the choroid and sclera have contributed to a broader understanding of the myopic growth process. As our understanding of these visually triggered events has grown, so too has the list of potential therapeutic options. The following sections summarize key findings from studies reporting pharmacological effects on eye growth, starting with studies involving atropine and related antimuscarinic drugs. Other drugs are organized by presumed site of action, starting at the retina. The same drugs are also listed by pharmacological category in Tables 2–5, and summarized in terms of their sites of action in Figure 1.
Table 2.
Muscarinic drugs reported to influence eye growth or related manifestations in animal models.
| Drug treatments† |
Description | Observations | Ref. |
|---|---|---|---|
| Carbachol | Nonselective M-receptor agonist | Increases scleral fibroblast proliferation; increases TGF-β release by RPE cells | [131,132] |
| Atropine | Nonselective M-receptor antagonist | Inhibits FDM and LIM; inhibits scleral GAG synthesis and fibroblast proliferation | [51,110,114,119,120,132,139,232] |
| Scopolamine | Nonselective M-receptor antagonist (M1 > M2) | Partially inhibits FDM | [120] |
| Tropicamide | Nonselective M-receptor antagonist (some selectivity for M4) | Partially inhibits FDM | [120] |
| Dexetimide | Nonselective M-receptor antagonist (M1, M4 > M2, M3) | Partially inhibits FDM | [120] |
| MT7 | Selective M1-receptor antagonist | Partially inhibits LIM; inhibits scleral fibroblast proliferation | [121,132] |
| Pirenzepine | Selective M1/M4-receptor antagonist | Inhibits FDM and LIM; inhibits scleral GAG synthesis and fibroblast proliferation | [51,116,117,119,120,126–128,132,139] |
| Telenzepine | Preferential M1-receptor antagonist | Inhibits LIM; inhibits scleral GAG synthesis | [121,139] |
| Oxyphenonium | Preferential M1-receptor antagonist | Inhibits FDM | [120] |
| Propantheline | M1-receptor antagonist | Partially inhibits FDM; toxic to retina | [120] |
| Benztropine | Selective M1/M2-receptor antagonist (M1 > M2) | Partially inhibits FDM | [120] |
| Dicyclomine | Preferential M1-receptor antagonist; least selective for M2 | No effect on FDM | [120] |
| Mepenzolate | Selective M1, M4, M2-receptor antagonist | No effect on FDM | [120] |
| Methoctramine | Selective M2-receptor antagonist | No effect on FDM | [51,120] |
| McN-A-343 | Selective M2-receptor antagonist | No effect on scleral GAG synthesis | [139] |
| Gallamine | Preferential M2-receptor antagonist | No effect on FDM; toxic to retina; no effect on scleral GAG synthesis | [120,139] |
| Himbacine | Selective M2/M4-receptor antagonist | Inhibits scleral fibroblast proliferation | [132] |
| 4-DAMP | Preferential M1/M3-receptor antagonist | Does not limit FDM [51] except with associated retinal damage [120]; inhibits scleral GAG synthesis and cell proliferation | [51,120,132,139] |
| Hexahydro-siladifenidol | Preferential M3, M4, M2-receptor antagonist (M3, M4 > M2) | Partially inhibits FDM | [120] |
| p-fluorohexahydrosila-difenidol | Preferential M3-receptor antagonist | Partially inhibits FDM | [120] |
| AFDX-116 | Preferential M2-receptor antagonist | Partially inhibits FDM | [120] |
| Quinuclidinyl benzilate | Preferential M2-receptor antagonist | Partially inhibits FDM; toxic to retina | [120] |
| MT3 | M4-specific receptor antagonist | Partially inhibits FDM and LIM; no effect on scleral GAG synthesis | [115,121] |
Drugs delivered by intravitreal injection unless otherwise indicated.
4-DAMP: 4-diphenylacetoxy-N-methylpiperidine; FDM: Form-deprivation myopia; GAG: Glycosaminoglycan; LIM: Lens-induced myopia; M1 Muscarinic receptor type 1; M2 Muscarinic receptor type 2; M3 Muscarinic receptor type 3; M4 Muscarinic receptor type 4; M5 Muscarinic receptor type 5; McN-A-343: 4-(m-chlorophenyl-carbamoyloxy)-2-butynyltrimethylammonium chloride; MT3: Muscarinic toxin 3; RPE: Retinal pigment epithelium.
Table 5.
Miscellaneous drugs reported to influence eye growth or related manifestations of myopia in animal models.
| Drug treatments† | Description | Observations | Ref. |
|---|---|---|---|
| VIP | Retinal neurotransmitter (amacrine cells) | Low doses associated with FDM | [170,172] |
| l-NAME (affects NO, retinal neurotransmitter and blood flow modulator) | NO synthase inhibitor | Inhibits FDM and LIM | [189,190] |
| Retinoic acid | Growth modulator | Enhances normal eye growth, inhibits scleral fibroblast proliferation | [137,158,200,203] |
| Disulfiram | Inhibitor of retinoic acid synthesis | Inhibits FDM but not LIM | [202,244] |
| PGF2α (modulates aqueous outflow pathways) | Prostaglandin | Inhibits FDM | [219] |
| Latanoprost | Prodrug for PGF2α | No effect on FDM | [219] |
| Basic FGF | Growth factor | Inhibits myopic eye growth (FDM), stimulates proliferation of scleral fibroblasts and chondrocytes | [136,138,208,210,245] |
| Acidic FGF | Growth factor | Inhibits myopic eye growth (FDM) | [208] |
| TGF-β | Growth factor | Antagonizes inhibitory effect of basic FGF (FDM), increases in vitro scleral collagen synthesis, modulates cell contraction, stimulates proliferation of fibroblasts, as well as that of scleral chondrocytes in cell culture but not organ culture | [133–136,138,210] |
| 7-methylxanthine | Adenosine antagonist | Inhibits myopic growth (FDM), increases collagen and decreases GAG content in normal rabbit sclera, increases collagen fibril diameter in normal rabbit sclera and in sclera of normal and form-deprived guinea pig eyes | [69,70] |
| β-xyloside | Inhibits proteoglycan synthesis | Inhibits myopic eye growth (FDM) | [246] |
Drugs delivered by intravitreal injection unless otherwise indicated.
FDM: Form-deprivation myopia; GAG: Glycosaminoglycan; LIM: Lens-induced myopia; l-NAME: N-nitro-l-arginine methyl ester; NO: Nitric oxide; PGF2α Prostaglandin F2α; VIP: Vasoactive intestinal peptide.
Figure 1. Potential sites of action of drug classes reported to limit myopic eye growth.
RA: Retinoic acid.
Drug studies involving animal models for myopia
Antimuscarinic drugs
Atropine
Early studies using chicks to investigate the antimyopia effects of atropine, a nonselective muscarinic antagonist, provided important insight into the mechanism of its action, as well as further evidence against a role for accommodation in the development of myopia. The first of these studies in 1991 involved subconjunctival injections of atropine to monocularly lid-sutured chicks [51]. Reduced axial elongation of atropine-treated deprived eyes was observed, although the in vitro method of measuring axial lengths with vernier calipers on enucleated eyes was relatively inaccurate compared with the in vivo high-frequency A-scan ultrasonography technique now widely used in such studies. In contrast to the mammalian ciliary muscle, which is comprised of smooth muscle with muscarinic receptors, the post-hatch chick ciliary muscle has striated muscle with no muscarinic receptors [109]. Consequently, accommodation in the chick was not inhibited by atropine, and thus the aforementioned result indirectly rules out inhibition of accommodation as the mechanism for its antimyopia effect.
Atropine’s ability to limit myopia progression in the chick by a nonaccommodative mechanism was further confirmed in another study, in which diffusers were used to induce myopia and atropine was delivered by intravitreal injection [110]. Ultrasonography measurement of the ocular components revealed decreases in vitreous chamber depth to be the origin of axial length reductions. Equatorial ocular dimensions were also found to be significantly reduced, although to a lesser extent than the axial dimensions, and contrasting with the minimal effects on equatorial dimensions reported in the 1991 study. Ruling out an accommodative mechanism for atropine’s antimyopia effect, atropine was shown to have no effect on carbachol-induced accommodation. This study raised the possibility of a retinal site of action, since muscarinic receptors were known to exist in the neural retina. Puzzlingly, higher doses of atropine were needed to inhibit eye growth in this and later atropine studies involving intravitreal injections than in the 1991 study, even though the drug was delivered closer to the retina.
Pirenzepine
Pirenzepine, an M1/M4 selective antagonist, was included in the 1991 chick study referred to earlier [51], and was also found to be effective in limiting FDM over the dose range tested. Ongoing interest in this drug as an antimyopia drug treatment stems from reports in other studies that mammalian ciliary muscle does not have Ml receptors [111–113]. Thus, as a potential antimyopia therapy, pirenzepine held promise of reduced cycloplegia, an undesired side-effect of atropine. An inhibitory effect on myopic eye growth was also recorded for pirenzepine in later studies in chicks, although there are significant interstudy differences in reported effective doses, as also noted previously for atropine studies. For example, in one such study, near toxic dose levels were required to completely suppress the development of FDM, and the effect of pirenzepine on LIM was also weak [114]. In this context, pirenzepine is apparently also less potent than atropine. Thus, in one recent study, a tenfold higher dose of pirenzepine over atropine was needed for equivalent inhibition of FDM (Table 6) [115].
Table 6.
Comparison of designs and outcomes of three studies examining the effect of pirenzepine injected either subconjunctivally, or intravitreally on experimentally induced myopia in chicks.
| Study design parameter |
Study | ||||
|---|---|---|---|---|---|
| Stone et al. [51] | Rickers et al. [126] | Leech et al. [127] | |||
| Injection mode | Subconjunctival (daily) | Intravitreal (2 doses over 7 days) | Subconjunctival | Intravitreal (daily) | Subconjunctival (daily) |
| Ocular treatment | Unilateral lid suture | Binocular goggle occlusion | Binocular goggle occlusion | Monocular goggle occlusion | Monocular goggle occlusion |
| Age at onset (years), duration of treatment | 1, 14 days | 11, 7 days | 11, 7 days | 7, 5 days | 7, 5 days |
| Fellow eye treatment | Saline injection | Goggle occlusion + saline injection | Goggle occlusion + saline injection | None | None |
| Ocular dimension used as index of effect | Axial length | Axial length | Axial length | Vitreous chamber depth | Vitreous chamber depth |
| Refractive error limiting dose | Unknown | 1 mg | None found up to 2 mg | 200 µg | 5 mg |
| Threshold growth-inhibiting dose (dose for total inhibition in brackets) | 3.5 µg (unknown) | 500 µg (2 mg, toxic) | None found up to 2 mg | 500 µg (500 µg) | None found up to 7.5 mg – only anterior chamber growth limited |
The efficacy of pirenzepine as an antimyopia treatment has also been investigated in tree shrews and monkeys. In tree shrews, daily subconjunctival injections of pirenzepine over a 12-day period were able to limit vitreous chamber elongation for both LIM and FDM, and also limited equatorial expansion. No toxic effects in the retina or sclera were seen by histology [116–118]. A trend toward myopia inhibition was also observed in the pirenzepine-treated eyes of rhesus monkeys wearing black contact lenses. However, refractive error and axial length differences between drug- and vehicle-treated eyes were not statistically significant, perhaps owing to the limited sample size and interanimal variability in induced myopia [119]. Pirenzepine also caused small but significant reductions in vitreous chamber depth in otherwise untreated eyes of tree shrews [116,117], and similar trends were found with rhesus monkeys [119]. These observations call into question the specificity of pirenzepine’s action for myopic eye growth.
Other antimuscarinic drugs
While many other muscarinic antagonists have been tested for their ability to inhibit FDM in chicks (summarized in Table 2), only intravitreal injection of oxyphenonium [120], a nonselective antimuscarinic drug, and telenzepine [121], an M1-selective antagonist, were able to completely inhibit FDM and LIM, respectively. However, oxyphenonium induced an inflammatory response at high (100-mM) doses, but a 10-mM dose was sufficient to inhibit eye growth. In terms of inhibiting myopic eye growth, the efficacies of many other muscarinic antagonists do not vary in any systematic way with muscarinic receptor selectivity (Table 2), suggesting that the few antagonists that do strongly inhibit FDM may be acting through noncholinergic mechanisms, although differences in bioavailability of these drugs could also contribute to differences in efficacy. A further complication is introduced: should any of these drugs have access to, and interaction with, presynaptic M2 receptors, activation of which typically increases acetylcholine (Ach) release [122], the net result would be a decrease in any inhibitory effect mediated through postsynaptic receptors.
Site of action of antimuscarinic drugs
Currently, the site of action for the antimyopia effects of antimuscarinic drugs remains unresolved, introducing a significant impediment to research aimed at optimizing drug treatments and delivery methods. The distribution of muscarinic receptors in ocular tissue is not by itself informative, as these receptors appear to be widely distributed. Data for relevant species are summarized in Table 7. Neither the cm1 (analogous to mammalian M1) or cm5 receptors have been conclusively shown to be present in the chick eye [114,123], perhaps explaining why high doses of pirenzepine were required to inhibit experimentally induced myopia in the chick. By contrast, all five receptor subtypes have been identified in the retina, choroid and sclera of the tree shrew [124] and guinea pig [125], rendering all of these tissues plausible sites of action for the antimyopia effects of antimuscarinic drugs (Table 7).
Table 7.
Summary of the ocular localization of M1 to M5 muscarinic receptors, from studies of chick, guinea pig, tree shrew and humans.
| Author (year) |
Model | Technique | Muscarinic receptor subtype | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | ||||
| Liu et al. (2007) | Human limbal and conjunctival epithelial and conjunctival fibroblast cultures | RT-PCR, immuno-staining, Western blot | Limbus, conjunctiva | Limbus, conjunctiva | Limbus, conjunctiva | Limbus, conjunctiva | Limbus, conjunctiva | [233] |
| Collison et al. (2000) | Human lens epithelial culture; human eyes | RT-PCR | Lens, iris, retina, sclera | Lens, retina, sclera | Lens, retina, sclera | Lens, iris, retina, sclera | Lens, iris, retina, sclera | [234] |
| Fischer et al. (1998) | Chick eyes† | Immunostaining | Lens, retina, sclera | Ciliary body, retina (AC, GC, RPE), choroid | Ciliary body, retina (AC, BC, RPE), choroid | Ciliary body, retina (AC, GC, RPE), choroid | [235] | |
| Ishizaka et al. (1998) | Human sphincter and dilator muscle cell culture | Immunostaining and dialator | Iris | Iris | Iris | Iris | Iris | [236] |
| Liu et al. (2007) | Guinea pig eyes | QT-PCR, Western blot | Iris/ciliary body, retina, choroid, sclera | Iris/ciliary body, retina, choroid, sclera | Iris/ciliary body, retina, choroid, sclera | Iris/ciliary body, retina, choroid, sclera | Iris/ciliary body, retina, choroid, sclera | [125] |
| Vessey et al. (2002) | Chick eyes† | Radioligand binding | Retina, choroid (assay not specific to muscarinic receptor subtypes) | Retina, choroid (assay not specific to muscarinic receptor subtypes) | Retina, choroid (assay not specific to muscarinic receptor subtypes) | Retina, choroid (assay not specific to muscarinic receptor subtypes) | Retina, choroid (assay not specific to muscarinic receptor subtypes) | [237] |
| Ali et al. (1983) | Chick eyes† | Binding assay | Retina (assay not specific to muscarinic receptor subtypes) | Retina (assay not specific to muscarinic receptor subtypes) | Retina (assay not specific to muscarinic receptor subtypes) | Retina (assay not specific to muscarinic receptor subtypes) | Retina (assay not specific to muscarinic receptor subtypes) | [238] |
| Schliebs et al. (1982) | Rat eyes | Binding assay | Retina, brain (assay not specific to muscarinic receptor subtypes) | Retina, brain (assay not specific to muscarinic receptor subtypes) | Retina, brain (assay not specific to muscarinic receptor subtypes) | Retina, brain (assay not specific to muscarinic receptor subtypes) | Retina, brain (assay not specific to muscarinic receptor subtypes) | [239] |
| Qu et al. (2006) | Human scleral fibroblast culture Human sclera culture |
RT-PCR, Western blot, immunocytochemistry Immunostaining |
Scleral fibroblasts Sclera |
Scleral fibroblasts Sclera |
Scleral fibroblasts Sclera |
Scleral fibroblasts Sclera |
Scleral fibroblasts Sclera |
[240] |
| Cha et al. (2002) | Chick scleral fibroblast culture† | Immunostaining, Western blot | Sclera | Sclera | Sclera | Sclera | [114] | |
| McBrien et al. (2009) | Tree shrew eyes | RT-PCR Immunohistochemistry |
Cornea, iris/ciliary body, retina, choroid, sclera Retina, choroid, sclera |
Iris/ciliary body, retina, choroid, sclera Retina, choroid, sclera |
Cornea, iris/ciliary body, retina, choroid, sclera Retina, choroid, sclera |
Cornea, iris/ciliary body, retina, choroid, sclera Retina, choroid, sclera |
Cornea, iris/ciliary body, retina, choroid, sclera Retina, choroid, sclera |
[124] |
The analogous muscarinic receptors in the chick are cm1 to cm5 [237].
AC: Amacrine cell; BC: Bipolar cell; GC: Ganglion cell; RPE: Retinal pigment epithelium.
Results from pharmacokinetic studies are also inconclusive about the sites of action of atropine and pirenzepine. In terms of the abilities of these two drugs to inhibit experimental myopia, intravitreal delivery has proven to be more effective than subconjunctival delivery in both chicks and tree shrews, perhaps reflecting differences in intraocular drug distributions [126–128]. For example, in tree shrews peak retinal levels of pirenzepine are 2.5-times higher than scleral levels when pirenzepine is injected intravitreally, but four- to five-times lower than scleral levels when injected subconjunctivally [128]. This evidence might be interpreted as evidence for a retinal site of action, if pirenzepine levels were not also higher in all ocular tissues – including the sclera – with intravitreal compared with subconjunctival injections. Thus, these study results do not exclude an extraretinal site of action.
The notion that the RPE serves as a relay for growth-modulating signals generated in the retina, and acts on the choroid and sclera [129,130], opens the possibility that the RPE could also be the site of action of antimuscarinic drugs. A study using cultured human RPE cells in which atropine blocked carbachol-induced mRNA expression and secretion of TGF-β2 [131] lends support for this idea, given evidence from other studies linking this growth factor with scleral growth modulation [132–138], although the requirement that secretion is from the basal (choroid-facing) surface of RPE was not established in the in vitro study. The role of growth factors as modulators of eye growth is discussed in a later section.
The strongest support for a scleral site of action for antimuscarinic drugs comes from in vitro studies assessing their effects on scleral cells or tissue. The first of these studies reported an inhibitory effect of atropine on cell proliferation in cultured whole-chick sclera [139]. Similar results were obtained with atropine in subsequent studies of cultured scleral fibroblasts from chicks [114], mice and humans [132]. Carbachol, a nonselective cholinergic agonist, had the opposite effect. Four other muscarinic antagonists – pirenzepine, himbacine, 4-diphenylacetoxy-N-methylpiperidine (4-DAMP) and MT7 – covering a broad range of selectivities – M1/M4, M2/M4, M3 and M1, respectively – all showed inhibitory influences on cell proliferation with mouse and human scleral fibroblast cultures [132]. These data do not offer insight into the receptor mechanism involved, although, in further testing, atropine, pirenzepine and MT7 were shown to block the enhanced proliferation seen with the addition of MT1, an M1-specific agonist, to the same cultures. The same study implicated three growth factors as possible downstream mediators of the actions of carbachol and atropine. In mouse scleral fibroblast cultures, levels of FGF-2 were increased by atropine and decreased by carbachol, while the levels of both TGF-β1 and EGF-2 changed in the opposite direction.
The glycosaminoglycan (GAG) content of the sclera, which is altered in myopic eyes (decreased in mammalian eyes and increased in the cartilaginous sclera of chick eyes; see [140,141] for review), is also reported to be affected by muscarinic receptor antagonists, although studies have been limited to chicks and inconsistencies in results appear across studies. In one such study, atropine inhibited GAG synthesis in cultured cartilaginous sclera from normal and form-deprived eyes [139], and pirenzepine and telenzepine had similar effects but were less potent [139]. By contrast, in other studies, no effect on scleral GAG synthesis was observed with either pirenzepine or M4-selective antagonist MT3, although inhibitory effects on FDM were observed in vivo with both drugs [115]. The timing of measurements may provide a partial explanation for differences in study outcomes, as another in vivo study reports the effect of pirenzepine to be transient; GAG synthesis in the cartilaginous sclera of form-deprived chicks was decreased 2 h after treatment but not after 6 h [118]. The M1/M3 selective antagonist, 4-DAMP, also inhibited scleral GAG synthesis in vitro, although in this case the effect was greater for tissue from normal compared with form-deprived eyes. Neither gallamine, an M2 antagonist, MT3 or 4-(m-chlorophenyl-carbamoyloxy)-2-butynyltrimethylammonium chloride (McN-A-343), an M1 agonist, altered GAG synthesis by chondrocytes [115,139]. Comparison of cartilagenous and fibrous sclera indicated that drug-induced changes in GAG synthesis were restricted to the cartilaginous layer of chick sclera, with the fibrous layer being unaffected [139]. This observation is consistent with an independent report of differential changes in GAG synthesis in the cartilaginous and fibrous layers of sclera from myopic chick eyes [142]. It also calls into question the relevance of these results for human myopia.
Nicotinic cholinergic analogs
In the only relevant study involving FMD in chicks, inhibitory effects were reported for a range of nicotinic analogs [143], with mecamylamine and chlorisondamine, both nonselective antagonists, having the greatest inhibitory effects. However, the multiphasic dose–response relationship of mecamylamine included enhancement of myopia with low doses, and the two highest doses of chlorisondamine appeared toxic to the RPE. It is thus difficult to see how this line of research might be productively extended to develop an antimyopia treatment. Nonetheless, it is interesting to note that two survey-based studies, one based in the USA and the other in Singapore, both report an apparent protective effect against myopia of passive, smoking-related exposure to nicotine in children [144,145], although differences in education – a possible influence on parental smoking habits – complicate the interpretation of these data.
Acetylcholine esterase inhibitors
There are two studies of relevance to Ach esterase inhibitors, both involving form-deprived chicks. One investigated systemically administered chlorpyrifos to simulate environmental exposure to this organophosphate insecticide [146], and the other tested diisopropylfluorophosphate [147] given by intravitreal injection. As inhibitors of Ach esterase, both drugs are expected to promote Ach accumulation. Yet, both drugs also inhibited FDM without effects on normal development, even though both antimuscarinic and antinicotinic drugs, which block Ach transmission, also show antimyopia activities. These apparently paradoxical results presumably reflect the complexity of signaling pathways underlying FDM. The added complexity of using indirectly acting drugs opens up as possible sites of action multiple subtypes of cholinergic receptors with diffuse distributions throughout the eye. While this line of research is clearly not productive in terms of developing antimyopia treatments, it has potential clinical implications of relevance to the unexplained reports of organophosphate-induced myopia in Japan [148,149].
Dopamine agonists
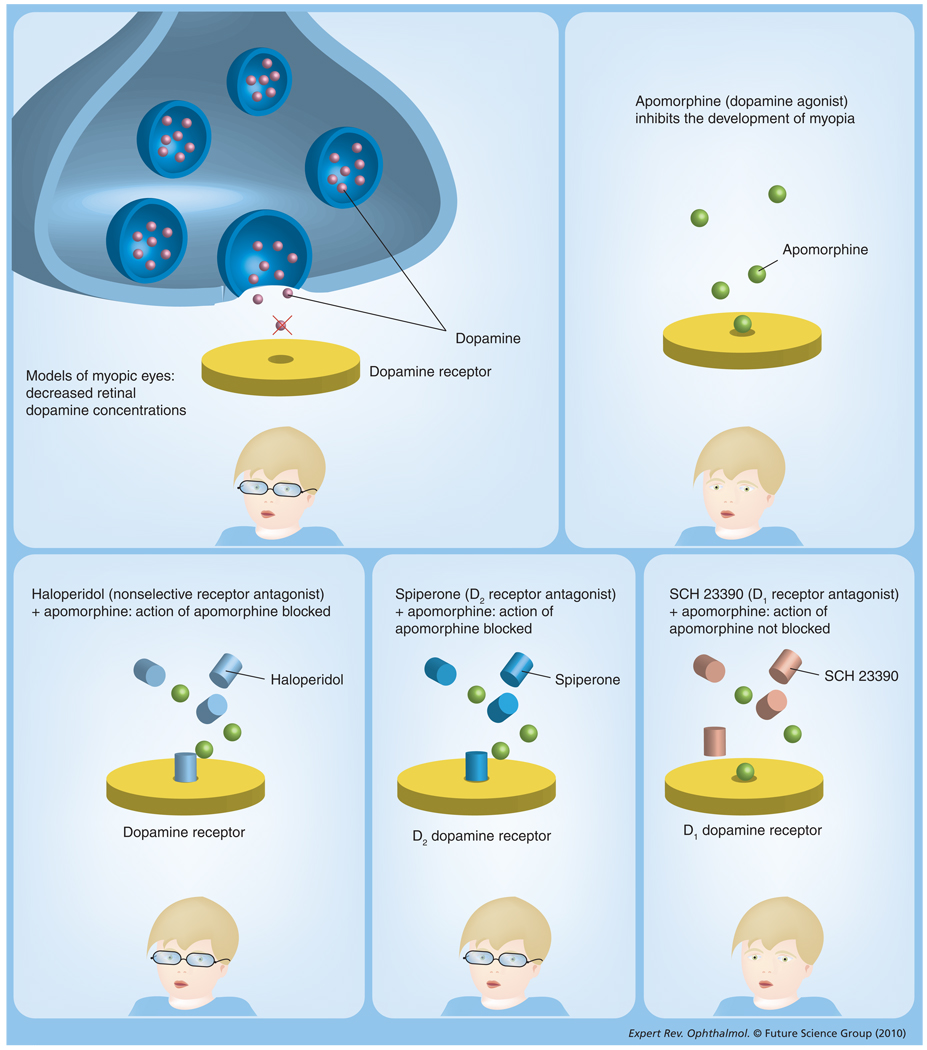
Interest in dopamine agonists as antimyopia drugs stems from early observations of altered retinal dopamine activity in form-deprived myopic eyes. In monkeys form deprived with either opaque contact lenses or by lid suture, retinal levels of both dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC), its primary metabolite, were decreased, as was the activity of tyrosine hydroxylase, a rate-limiting enzyme involved in dopamine biosynthesis [150]. Similar changes were found in a study of form-deprived myopic chicks [151]. Furthermore, when select areas of the retina are deprived by hemispherical diffusers, changes in retinal dopamine metabolism were restricted to the deprived retinal area [152]; changes did not extend to choroid or sclera [152]. Directly linking reduced retinal dopamine with FDM in chicks is the observation that subconjunctival injection of apomorphine, a nonselective dopamine receptor agonist, exerts a dose-dependent antimyopia effect in chicks, with an appropriately high dose of apomorphine completely inhibiting excessive axial elongation [151]. Other studies using intravitreal injections of apomorphine in chicks have confirmed its inhibitory effect on FDM [153,154] and describe a similar inhibitory effect on LIM [153,154]. In another recent study, form deprivation-induced downregulation of retinal ZENK, avian acronym for the orthologous mammalian genes zif-268, egr-1, ngfi-a and krox-24 [155], was prevented by dopamine agonist 2-amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene hydrobromide [156]. Dopamine also inhibits FDM rabbits [157] and rhesus monkeys [158], albeit with less consistency in the monkeys – perhaps a reflection of the greater variability in their responses to myopia-inducing stimuli [159,160]. Other related studies in chicks have implicated D2 receptors in the antimyopia effect of apomorphine. Specifically, the D2 receptor antagonists haloperidol and spiperone both eliminate the antimyopia effect of apomorphine [151,153], while SCH 23390, a D1 receptor antagonist, does not. These drug interactions are summarized in Figure 2. A plausible signal pathway for local eye growth regulation that has support from RPE cell culture studies [129,130] is one in which the dopamine released during retinal image processing interacts with D2 receptors on the apical (retinal-facing) surface of RPE, thereby triggering a signaling cascade directed at the choroid and sclera. While the aforementioned studies (summarized in Table 3) strongly implicate retinal dopamine in FDM, there are many unresolved questions, including whether the same or different retinal (signaling) pathways underlie LIM and FDM, what retinal cells are involved, and what role retinal dopamine plays in normal growth. For example, depletion of retinal dopaminergic amacrine cells with the neurotoxin 6-hydroxydopamine does not have any effect on LIM but suppresses deprivation-induced myopia [161–163]. This suggests that different pathways underlie these two forms of experimental myopia, although the effect on FDM is also paradoxical, given that dopamine agonists also show antimyopia activity. On the other hand, reserpine inhibits both FDM and LIM [152], although its actions are more complex, depleting retinal serotonin as well as dopamine stores. In a recent study, exposure of chicks to very high illuminance was found to slow the development of LIM and inhibit FDM by approximately 60%. Spiperone negated the influence of light on FDM, implicating retinal dopamine in this light effect [164]. Interestingly, dopamine receptor antagonists do not exert any growth-enhancing action in when injected into normal eyes [153]. Together, these observations open the possibility of more than one dopaminergic signaling pathway controlling eye growth, at least at the input end, with feedback circuits providing a plausible explanation for the paradoxical actions of 6-hydroxydopamine and apparently robust nature of normal eye growth. Two other studies address the question of whether the antimyopia effects of dopaminergic and antimuscarinic drugs involve the same or different pathways. In one, the antimyopia effects of apomorphine and atropine were found not to be additive when injected intravitreally into the eyes of lens-wearing chicks, implying interactions between the dopaminergic and muscarinic cholinergic pathways [154]. Results from the other, more recent study point to a retinal site for this interaction, at least for FDM [156].
Figure 2. Predicted effects of interactions between dopamine analogs on myopia based on evidence from animal model studies.
Table 3.
Dopamine analogs reported to influence eye growth or related manifestations of myopia in animal models.
| Drug treatments† | Description | Observations | Ref. |
|---|---|---|---|
| Dopamine | Nonselective D-receptor agonist | Low levels associated with myopic eye growth; inhibits FDM | [150,151,156] |
| Apomorphine | D1/D2-receptor agonist | Inhibits FDM and LIM | [151,153,154,157] |
| Haloperidol | Nonselective D-receptor antagonist | Antagonizes antimyopia effect of apomorphine in FDM | [151] |
| SCH 23390 | D1-receptor antagonist | Does not antagonize antimyopia effect of apomorphine of di-isopropylfluorophosphate in FDM; inhibits FDM | [147,153,242] |
| Spiperone | D2-receptor antagonist | Antagonizes antimyopia effect of apomorphine, DFP and MT3 in FDM | [147,153,241] |
| 6-OHDA | Selectively neurotoxic to dopaminergic cells | Inhibits myopic eye growth (selectively affects FDM, no effect on LIM) | [160–162,242] |
| Reserpine | Depletes dopamine and 5HT (retinal stores with intravitreal injection) | Inhibits FDM and LIM | [152,242,243] |
Drugs delivered by intravitreal injection unless otherwise indicated.
5HT: Serotonin; 6-OHDA: 6-Hydroxydopamine; DFP: Di-isopropyl fluorophosphate; FDM: Form-deprivation myopia; LIM: Lens-induced myopia.
γ-aminobutyric acid
γ-aminobutyric acid (GABA), a neurotransmitter targeted by the pharmaceutical industry for its central inhibitory influences, is also present in retinal neurons, including calretinin-immunoreactive horizontal cells [165] and star-burst amacrine cells for which GABA plays an important role in their directional selectivity [166] (also see review [167]). Two studies in chicks, one involving FDM [168] and the other LIM [169], both report antimyopia effects for a range of GABA antagonists, with GABAC-selective antagonists proving to be more potent than either GABAA- or GABAB -selective antagonists. Interestingly, retinal GABAC receptors appear to be involved in the modulation by GABA of a retinoic acid (RA) pathway [170], a link made more interesting by other studies implicating RA in eye growth regulation. The relevant RA literature is reviewed in a later section. Indirect evidence for a role of retinal GABA in eye growth regulation in monkeys is provided by evidence of increased immunoreactivity in GAD65-immunoreactive amacrine cells in eyes exposed to myopic defocus; cells in eyes exposed to diffuser blur showed less reactivity. However, there has been no follow-up to this study and no relevant studies of other mammalian models.
Retinal neuropeptides: vasoactive intestinal peptide, substance P & enkephalins
The first neuropeptides investigated in the context of eye growth modulation were vasoactive intestinal peptide (VIP) and substance P [155,171]. In the retinas of many species, both have been localized to subsets of amacrine cells [155,172]. Suggestive evidence for a role of VIP in eye growth regulation comes from an immunohistochemical study of retinal tissue from six monkey eyes closed for durations varying from 0.5 to 120 days by lid fusion. Deprived eyes, which also exhibited myopia in five out of the six cases, showed consistent increases in immunoreactivity for VIP, but not for substance P relative to their fellow open eyes, despite also having undergone denervation procedures. More direct evidence of a role of VIP as a growth modulator comes from a study in chickens, in which daily intravitreal doses of either of two VIP antagonists completely abolished FDM. Inhibition of FDM was also observed with intravitreal injection of porcine VIP but complete inhibition was not achieved [172]. The antagonist fragments of VIP, rather than VIP itself, underlie its growth inhibitory action. This conclusion is supported by the finding that another analog of VIP, thought to contain fewer antagonist fragments than the more labile parent peptide, was without effect.
While these findings suggest a role for VIP in eye growth modulation, results from other studies are equivocal at best. For example, although intravitreal quisqualic acid substantially depletes VIP-immunoreactive cells [173], it does not prevent FDM [174]. However, a definitive conclusion based on the latter observation is not possible as quisqualic acid also destroys enkephalin- and Ach-immunoreactive amacrine cells [173]. Likewise, colchicine, which causes eye enlargement [175], depletes VIP-immunoreactive cells but also inactivates ganglion cells [175]. Note that VIP was recently localized in intrinsic choroidal neurons in chicks [176], although its potential role as a modulator of choroidal thickness remains to be investigated.
In another study in chicks, daily intravitreal injections of naloxone, a nonselective opioid antagonist, as well as a κ-receptor selective antagonist, were found to inhibit FDM. However, retinal proenkephalin levels were unaffected by FDM, leaving the authors to interpret their results as inconclusive [177]. While this and the other peptide studies cited earlier offer insight into the signaling mechanisms underlying the development of myopia – specifically, they point to involvement of the inner retina in signal processing – there have been no recent follow-up studies of those covered in the aforementioned discussion.
Glucagon, IGF-1 & insulin
Retinal glucagon-containing amacrine cells show increased immunoreactivity to the immediate-early response gene product ZENK [178] in response to imposed myopic defocus or cessation of form deprivation in chicks and, conversely, decreased immunoreactivity in response to hyperopic defocus or form deprivation [179,180]. This observation brought attention to glucagon as a potential modulator of eye growth and antimyopia treatment. The finding of increased retinal proglucagon mRNA induced by imposed myopic defocus in chicks also supports this notion [181], as do results from pharmacological studies using intravitreally injected glucagon analogs, although some qualification is required. The finding of choroidal thickening after injection of glucagon into form-deprived and negative lens-wearing eyes of chicks [182] is consistent with inhibition of both FDM [183] and LIM [184] with the glucagon agonist, Lys17,18,Glu21-glucagon-amide. However, in one of the FDM studies, ultrastructural changes in rod photoreceptors and RPE were observed with doses in the 10−6–10−5 M range, below which little inhibition of FDM was seen [183]. No signs of retinal damage were seen, with doses in the 0.05–2.5 nmol range found to be inhibitory in the LIM study [184]. The relative selectivity of these toxic effects may reflect the greater density of glucagonergic receptors on RPE compared with other cells in the retina and choroid [185]. Interestingly, intravitreal colchicine, which eliminates glucagonergic amacrine cells, leads to excessive eye growth in normal eyes [175], while it suppresses the ability to respond to negative lenses but leaves compensation to positive lenses relatively unchanged [186]. In normal eyes glucagon inhibits equatorial eye growth and glucagon antagonists enhance it [187]. These regional, equatorial biases of the growth effects of colchicine and glucagon analogs are consistent with the bias in the distribution of glucagon-containing bullwhip and mini-bullwhip cells in the chick retina. Glucagon receptors have also been identified on chick scleral cells in the same region [187]. However, it is currently unknown whether there are parallels in mammalian and primate eyes.
Results from studies involving glucagon (summarized in Table 4) have triggered interest in the possible roles in eye growth modulation of IGF-1 and insulin, the latter having opposite effects on blood glucose to glucagon. In two recently published studies in chicks, intravitreal insulin was found to increase the growth-enhancing effect of negative lenses [188], and both insulin and IGF-1, injected intravitreally, increased eye elongation in otherwise untreated eyes [182]. Consistent with the myopia-inducing action of insulin, its intravitreal injection also reduced the number of ZENK-immunoreactive cells, although retinal ZENK mRNA levels were increased [188]. These paradoxical findings along with the atypical nature of the induced axial myopia, which largely reflects anterior, rather than vitreous chamber growth, raise more questions than they answer, including whether drug interventions that reduce intraocular insulin would be effective in controlling myopia.
Table 4.
Glucagon analogs reported to influence eye growth or related manifestations of myopia in animal models.
| Drug treatments† | Description | Observations | Ref. |
|---|---|---|---|
| Glucagon | Nonselective glucagon receptor agonist | Inhibits FDM and LIM | [179–182,183,184,187] |
| Lys17,18,Glu21–glucagon | Glucagon receptor agonist | Inhibits FDM and LIM | [183,184] |
Drugs delivered by intravitreal injection unless otherwise indicated.
FDM: Form-deprivation myopia; LIM: Lens-induced myopia.
Nitric oxide modulation via retinal &/or choroidal mechanisms
Reports in chicks that recovery from either FDM or LIM can be inhibited by a single intravitreal injection of the nitric oxide synthase (NOS) inhibitor N-nitro-l-arginine methyl ester (l-NAME) add to accumulating evidence implicating nitric oxide (NO) in myopia [189,190]. Inhibiting the neuronal isoform of NOS (nNOS) appears to be as effective in inhibiting compensation to positive lenses as the nonselective antagonist, l-NAME [191,192], with retinal amacrine cells being a major source of NO [193] and thus a possible drug target. Electrophysiological studies reporting l-NAME-induced short-term reductions in the amplitude of the second oscillatory potential (OP2) wave in form-deprived eyes [189], suppression of ON responses, and enhanced OFF response in lens-treated eyes [190], are also consistent with an inner retinal site of action. However, an alternative choroidal site of action for the aforementioned ocular growth effects of NOS inhibitors has also been conjectured based on two lines of evidence. One is the inhibitory effect of l-NAME on the choroidal thickness increases seen in eyes recovering from form deprivation or fitted with positive lenses [192]. The other is the presence in the choroid of nNOS-positive intrinsic neurons and nerve terminals [176,194,195]. Note that the ubiquitous distribution of NOS and the ready diffusion and short life span of NO make it unlikely that this avenue could be effectively exploited for control of myopia.
RA as an eye (scleral) growth modulator
Retinoic acid, an oxidized form of vitamin A, is well known as a developmental regulator, with a diverse range of actions including the regulation of cell-lineage decisions and differentiation in embryonic tissues, including the eye [196–199]. It is thus perhaps not surprising that it has also been implicated in early eye growth regulation, in a variety of studies that encompass chick, guinea pig and marmoset models, as well as in vitro studies.
The earliest of these studies, which were in chicks, raised the possibility that RA was involved in the bidirectional regulation of eye growth with direct involvement in scleral remodeling. Specifically, expression of RA receptor β was found to be increased in the sclera of form-deprived eyes [137], and RA was found to inhibit the proliferation of both scleral chondrocytes and fibroblasts in a concentration-dependent manner in vitro while also strongly inducing RA receptor β expression in both cell types. RA is synthesized in a variety of ocular tissues with potential ties to eye growth regulation. For example, in a co-culture study, the sclera was shown to accumulate RA synthesized from applied retinol by the adjacent choroid [200]. In related in vivo studies, the rate of choroidal synthesis of RA was found to decrease in response to form deprivation and negative lenses, and increase – by four- to fivefold – in eyes recovering from FDM or wearing positive lenses. The retina/RPE also synthesized RA in the same direction but at a much slower rate. In more direct tests implicating RA’s role in eye growth regulation, RA and 13-cis RA administered orally to young chickens both increased the rate of ocular elongation while citral, an inhibitor of RA synthesis, inhibited ocular elongation [201]. RA was ascribed a role in the coordinated, nonvisual regulation of eye growth, on the basis of near normal emmetropization in RA-treated eyes in the latter study, although in another related study inhibition of RA synthesis with systemic disulfiram inhibited FDM but not LIM [202]. The reason for the apparent selectivity of disulfiram for FDM is unresolved but could reflect other non-RA actions of this drug.
Studies in guinea pigs and marmorsets have confirmed some of the results of studies in chicks, but have also revealed important species-related differences that may reflect structural differences between avian and mammalian choroid and sclera (birds have well-developed choroidal lymphatic lacunae and an additional inner cartilage layer in their sclera). Thus, contrasting with the results from chicks, RA levels significantly increased, rather than decreased, in both retina and combined choroid–sclera samples from guinea pig eyes made myopic by either form deprivation or with negative lenses, with the opposite trend found in eyes recovering from FDM or wearing positive lenses [203]. The results from a study in marmosets provided a more direct link between RA synthesis and ocular elongation: in response to form deprivation, increased ocular elongation was associated with significantly increased RA synthesis in both retina and choroid/RPE, while eyes becoming shorter than normal in response to the same treatment showed unaltered RA synthesis [158]. The species-related differences in study outcomes serve to highlight the importance of verifying in mammals results of ocular growth studies in chicks.
The findings summarized previously demonstrating the importance of RA as an ocular growth modulator also raise the question of whether RA analogs could be used as antimyopia treatments. Reports of RA-induced reduced scleral GAG synthesis, demonstrated in vitro in primate [158] and chick [200] tissue, make the sclera a plausible site of action. However, the required reduction in RA activity may be associated with significant side-effects, given the critical role of RA in development.
Other growth factors
It is logical that FGF and TGF-β would be targets in experimental myopia studies, as members of both families have been linked to growth modulation in nonocular connective tissues [204–207]. In one study involving form-deprived chicks, both basic FGF (known as both bFGF and FGF-2) and acidic FGF (aFGF) inhibited vitreous chamber elongation and thus FDM. The potency of bFGF was 160-times greater than that of aFGF, which also resulted in retinal complications when injected intravitreally [208]. bFGF also inhibited anterior chamber growth when injected intravitreally, while subconjunctival injections of bFGF affected only vitreous chamber growth. The ubiquitous distribution of receptors for FGF in the eye renders these data uninformative in terms of possible mechanisms for their inhibitory growth effects; they are found in nearly all cells in the retina, choroid and scleral chondrocytes, including dopamine- and VIP-containing amacrine cells [209]. However, intravitreal injection of bFGF was without effect on three retinal changes previously linked to form deprivation in chicks: decreased tyrosine hydroxylase, decreased dopamine synthesis and decreased expression of c-fos, a putative transcriptional regulator of the tyrosine hydroxylase gene [210]. This result suggests that bFGF is acting either on an alternate nondopaminergic retinal pathway or on an extraretinal site such as the choroid or sclera to achieve its antimyopia effect.
In support of a scleral site of action for bFGF, is has been shown to be a potent stimulator of chick scleral chondrocyte and fibroblast proliferation in culture [138], although this may not reflect its in vivo activity, as the bFGF content of the sclera appears unaffected by FDM [136]. With LIM in tree shrews, as with FDM in chicks, scleral levels of bFGF were unaltered [211]. Tree shrew sclera from myopic eyes also showed no change in bFGF mRNA expression, although there was a significant upregulation of scleral FGF receptor-1 mRNA and expression levels returned to normal during recovery from induced myopia.
There are data from both chicks and tree shrews implicating TGF-β in eye growth regulation, with the sclera being the most plausible site of action. The mRNA levels of all three isoforms of TGF-β were found to be significantly downregulated in the sclera of form-deprived tree shrew eyes [135], opposite to the trend reported for sclera from form-deprived chick eyes, which also showed increased TGF-β in the combined retina–RPE–choroid tissues. The decreases in tree shrew correlated with the reduction in collagen synthesis by cultured scleral fibroblasts when treated with a cocktail of TGF-β isoforms that simulated levels recorded in vitro [135], as well as a decrease in α smooth muscle actin expression when cells were also placed under stress [212]. The observation that cultured human embryonic retinal pigment cells also increase secretion of TGF-β1 and TGF-β2 directed towards the sclera in response to apomorphine, a dopamine agonist, is also consistent with a scleral growth-inhibitory role for this family of growth factors [213]. While the sclera is also a plausible site of action for bFGF in both chicks and mammals, based on the aforementioned data, the picture may be more complex, with bFGF and TGF-β acting on multiple sites [136].
Ocular hypotensive & other miscellaneous drugs
Adrenergic analogs
In agreement with clinical myopia studies [64], topical timolol, a β-adrenergic receptor antagonist (β-blocker), lowered IOP but did not inhibit experimental myopia in chicks [214]. By contrast, the normal eyes of cynomolgus monkeys treated with topical timolol became more myopic than control eyes and those treated with topical epinephrine, another ocular hypotensive agent [215]. Apart from its IOP-lowering effect, epinephrine is known to decrease cytosolic calcium in chick RPE, thereby regulating fluid transport from retina to choroid [216], and providing a plausible mechanism for epinephrine’s effect on myopia progression. There have been no recent follow-up studies for drugs in this group.
Prostaglandins
The ability of prostaglandins to affect eye growth has received only limited attention, despite prostaglandin receptors being distributed widely throughout the eye, including in the sclera, and prostaglandin analogs now being the most widely prescribed ocular hypotensive drugs for glaucoma. Their hypotensive effects have been linked to increased extracellular matrix remodeling, leading to increased uveoscleral outflow [217,218]. In the only experimental myopia study, intravitreal injection of prostaglandin F2α (PGF2α), but not prostaglandin E2 (PGE2) or latanoprost, significantly reduced the axial growth response to form deprivation compared with that recorded with saline (control) injections in chicks. Neither PGF2α, PGE2 or latanoprost had any antimyopia effect when delivered as topical eyedrops or injected subconjunctivally [219]. In the same study, indomethacin, a nonsteroidal drug known to inhibit prostaglandin synthesis, was without effect on myopia development, irrespective of whether delivered by subconjunctival, intravitreal or intramuscular routes. The mechanism for the antimyopia effect of PGF2α remains to be elucidated, although a retinal site of action is consistent with the improved efficacy with intravitreal injection. In vitro observations of prostaglandin-induced increases in human scleral permeability and levels of matrix metalloproteinases 2 and 3 [220] argue for the judicious use of prostaglandins in the treatment of glaucoma in myopic patients, until the effects of their chronic use on scleral remodeling and compliance are established.
7-methylxanthine
In the first study of relevance to myopia involving 7-MX, an adenosine antagonist and metabolite of caffeine, favorable biochemical and ultrastructural changes were observed in normal rabbit sclera [69]. Specifically, 7-MX increased posterior sclera collagen content and fibril size diameter, opposite to trends normally seen in myopic eyes. These observations are also consistent with the results of an independent study involving form-deprived guinea pigs; animals receiving a 300 mg/kg daily oral dose of 7-MX showed approximately 50% less ocular elongation and myopia than the vehicle control animals, and these effects were coupled to increases in both collagen fibril diameters and overall thickness of the posterior sclera, presumably contributing to the antimyopia effect [70]. As indicated earlier, this drug has entered human clinical trials [26], although initial results seem less promising than the results of the aforementioned guinea pig study. Confirmation of its efficacy with LIM in the same animal model may provide insight into these apparently different outcomes. Further animal testing to better understand the mechanism for the antimyopia effect of 7-MX seem warranted. Nonetheless, a promising outcome of the clinical trial was the apparent absence of any rebound effect on ocular growth when the treatment was terminated.
Expert commentary
As the field stands today, pharmacological options for treating myopia, either by slowing its progression or preventing its onset, are limited to just one drug, atropine. However, its use as a topical ophthalmic formulation remains limited by the significant ocular side-effects of currently used doses and, more recently, by questions about tolerance with long-term use and rebound effects on myopia progression after termination of treatment. The only other drugs to reach clinical trial, pirenzepine and 7-MX, both appear substantially less effective than atropine in controlling myopia.
One is led to ask the question of why progress in this field has been so slow. A contributing factor is the relatively recent nature of the myopia epidemic, first reported in Asian countries in the 1990s and only very recently acknowledged to be affecting the US population [11]. A second factor is the only slow acceptance of the notion that myopia is a treatable condition. The common misconception that myopia is simply determined by genetics has been coupled to a further misconception that it is also untreatable. A third factor is the conservative approaches used to investigate myopia treatment options. For example, all human clinical trials of atropine have used topical ocular delivery, which logically is not the most effective way to treat a condition that primarily affects the posterior vitreous chamber, and specifically, its outer scleral coat. The need to limit myopia progression is now ever more urgent as the age of onset becomes progressively younger [221,222], leading to an increasing prevalence of pathological myopia.
Five-year view
The wealth of recent animal-based research summarized in this article has provided many new insights into the local neurochemical pathways driving the accelerated eye elongation underlying myopia. For drugs targeting the retina, the presumed origin of myopia-generating signals, the protective barriers provided by retinal vascular endothelium and RPE pose obstacles to drug delivery, even for systemically delivered drugs. Local intravitreal injections and implants are accompanied by significant safety risks, including cataracts and retinal detachment. The wisdom of targeting the retina is further called into question by the potential for unintended effects on vision, given the young age of the target population, and the long duration of required treatment. Treatment duration could be up to 20 years, when myopia susceptibility and progression rates can be safely assumed to decline.
As an alternative target for myopia-controlling drugs, the sclera, which is isolated from the sensitive retina by the vascular choroid, would seem a suitably safe choice, warranting more attention from researchers. However, the greatest changes in myopia involve the posterior sclera, which is relatively inaccessible to drugs delivered in traditional topical ophthalmic formulations owing to the multiple hydrophobic and hydrophilic barriers imposed by the intervening ocular structures, the long diffusion distances, and the rapid drug clearance caused by blinking and tear drainage. Nonetheless, these challenges also offer the opportunity to exploit advances in nanotechnologies and biomaterials.
Modern polymer-based formulations for drug delivery are within reach and could be employed to deliver atropine directly to the sclera, should it prove to be the site of its antimyopia action. Extended drug-release profiles suitable for targeted (localized) delivery of low doses to the external sclera would reduce side-effects (see [223–226] for recent reviews of ocular drug delivery). The application of these emerging drug delivery technologies, together with further advances in our understanding of myopia signal pathways and thus identification of new drug options, may provide the much needed and overdue breakthrough in antimyopia therapies.
Key issues
High myopia is a leading cause of visual impairment and blindness and thus a major public health concern, yet there are no US FDA-approved pharmaceutical treatments for myopia. Decreases in age of onset of myopia, now quite common among preschool children in some populations, represent a particular challenge to drug development owing to associated safety issues.
Off-label use of topical ophthalmic atropine, a nonselective muscarinic antagonist, is currently the only treatment used for slowing myopia progression. Atropine use is largely limited to East-Asian countries with the highest prevalence figures owing to its many ocular side-effects, including photophobia and problems with near focusing.
Elucidation of the mechanism underlying the antimyopia action of atropine could lead to new drugs with better selectivity profiles and reduced side-effects, as well as solutions to the problems of loss of efficacy with long-term use and rebound accelerated myopia progression when treatment is terminated. To date, clinical trial results for topical ophthalmic pirenzepine, an M1/M4 selective antimuscarinic drug, showed slowed myopia progression, but its reduced efficacy compared with atropine and twice-daily dosing routine likely explain the apparent loss of interest in this drug.
Studies using animal models have confirmed the role of vision in myopia development, with the retina being the likely origin of myopia-generating signals that are plausible targets for new antimyopia treatments. Interest in dopamine analogs as antimyopia treatment originates from findings in animal studies that retinal dopamine and its metabolites are reduced in eyes induced to become myopic, although the merits of such an approach may be outweighed by the risk of unwanted visual side-effects, given the young age of the target population.
Scleral growth changes, including increased remodeling in mammalian and primate eyes, underlie the increased rate of vitreous chamber elongation associated with myopia. The sclera is a relatively safe target for drug therapy, being separated from the sensitive neural retina by the intervening vascular choroid and retinal pigment epithelium, which serve as a selective barrier and likely also as a signal relay for myopia-generating signals. Understanding the nature of the latter signals may open up other new drug treatment options.
The application of modern biotechnological tools to develop novel drug delivery systems may allow direct targeting of the posterior sclera, where the changes are greatest. Polymer- and nanoparticle-based drug delivery systems, used alone or in combination, have potential in this context as extended local low-dose drug delivery devices with fewer side effects than topical ophthalmic formulations.
The use of an oral route of delivery, as in the clinical trial of 7-methylxanthine, has a practical advantage for young subjects, but increases the potential for side-effects. This drug is an adenosine antagonist and represents the first exploration in humans of more novel drugs. However, while scleral collagen fiber and overall thickening were observed in animal-based testing of this drug, clinical trial results showed only limited slowing of ocular elongation, although the lack of a rebound effect after the termination of treatment is promising.
Acknowledgments
Financial & competing interests disclosure
The authors would like to acknowledge the funding support from the National Institutes of Health grant from National Eye Institute (NEI) (R01-EY012392). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Ghafour IM, Allan D, Foulds WS. Common causes of blindness and visual handicap in the west of Scotland. Br. J. Ophthalmol. 1983;67(4):209–213. doi: 10.1136/bjo.67.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology. 2006;113(8):1354–1362. doi: 10.1016/j.ophtha.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Buch H, Vinding T, Nielsen NV. Prevalence and causes of visual impairment according to World Health Organization and United States criteria in an aged, urban Scandinavian population: the Copenhagen City Eye Study. Ophthalmology. 2001;108(12):2347–2357. doi: 10.1016/s0161-6420(01)00823-5. [DOI] [PubMed]
- 4.Xu L, Wang Y, Li Y, et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology. 2006;113(7):1134.e1–1134.e11. doi: 10.1016/j.ophtha.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Cedrone C, Culasso F, Cesareo M, et al. Incidence of blindness and low vision in a sample population: the Priverno Eye Study, Italy. Ophthalmology. 2003;110(3):584–588. doi: 10.1016/S0161-6420(02)01898-5. [DOI] [PubMed] [Google Scholar]
- 6.Hsu WM, Cheng CY, Liu JH, Tsai SY, Chou P. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2004;111(1):62–69. doi: 10.1016/j.ophtha.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann. Acad. Med. Singapore. 2004;33(1):27–33. [PubMed] [Google Scholar]
- 8.Fan DS, Lam DS, Lam RF, et al. Prevalence, incidence, and progression of myopia of school children in Hong Kong. Invest. Ophthalmol. Vis. Sci. 2004;45(4):1071–1075. doi: 10.1167/iovs.03-1151. [DOI] [PubMed] [Google Scholar]
- 9.Shelton L, Rada JS. Effects of cyclic mechanical stretch on extracellular matrix synthesis by human scleral fibroblasts. Exp. Eye Res. 2007;84(2):314–322. doi: 10.1016/j.exer.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saw SM, Tong L, Chua WH, et al. Incidence and progression of myopia in Singaporean school children. Invest. Ophthalmol. Vis. Sci. 2005;46(1):51–57. doi: 10.1167/iovs.04-0565. [DOI] [PubMed] [Google Scholar]
- 11. Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch. Ophthalmol. 2009;127(12):1632–1639. doi: 10.1001/archophthalmol.2009.303. •• Myopia is often considered to be a problem of East Asian countries where increases in its prevalence were first reported; this study dispels this myth, reporting on significant increases in the prevalence of myopia in the same population in the USA over a 30-year period.
- 12.Aller T. Myopia progression with bifocal soft contact lenses – a twin study. Optom. Vis. Sci. 2000;77(S79) [Google Scholar]
- 13.Aller T, Grisham D. Myopia progression control using bifocal contact lenses. Optom. Vis. Sci. 2000;77(S187) [Google Scholar]
- 14. Aller TA, Wildsoet C. Bifocal soft contact lenses as a possible myopia control treatment: a case report involving identical twins. Clin. Exp. Optom. 2008;91(4):394–399. doi: 10.1111/j.1444-0938.2007.00230.x. • This study is an example of optical treatments for myopia under clinical investigation; shows that currently available contact lenses, intended for the correction of presbyopia, have the potential to slow eye growth, at least in some subjects.
- 15.Aller TA, Wildsoet C. Results of a one-year prospective clinical trial (CONTROL) of the use of bifocal soft contact lenses to control myopia progression. Ophthalmic Physiol. Opt. 2006;26(S1):8–9. [Google Scholar]
- 16.Howell E. Comparison of multifocal contact lenses with multifocal spectacle lenses for myopia control. Presented at: 12th International Myopia Conference; 8–12 July 2008; Queensland, Australia. [Google Scholar]
- 17. Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr. Eye Res. 2005;30(1):71–80. doi: 10.1080/02713680590907256. • Describes the myopia control effect of corneal reshaping therapy; its intended clinical application was to provide sharp uncorrected vision to myopes as an alternative to refractive surgery.
- 18.Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br. J. Ophthalmol. 2009;93(9):1181–1185. doi: 10.1136/bjo.2008.151365. [DOI] [PubMed] [Google Scholar]
- 19.Myopia control moves forward. Optician. 2010 April 9; [Google Scholar]
- 20.Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest. Ophthalmol. Vis. Sci. 2003;44(4):1492–1500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- 21.Gwiazda JE, Hyman L, Norton TT, et al. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest. Ophthalmol. Vis. Sci. 2004;45(7):2143–2151. doi: 10.1167/iovs.03-1306. [DOI] [PubMed] [Google Scholar]
- 22.Fan DS, Lam DS, Chan CK, Fan AH, Cheung EY, Rao SK. Topical atropine in retarding myopic progression and axial length growth in children with moderate to severe myopia: a pilot study. Jpn. J. Ophthalmol. 2007;51(1):27–33. doi: 10.1007/s10384-006-0380-7. [DOI] [PubMed] [Google Scholar]
- 23. Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116(3):572–579. doi: 10.1016/j.ophtha.2008.10.020. • One of only a small number of studies to address the important question of whether the benefit of atropine is retained after the cessation of treatment or whether there is a rebound effect on eye growth.
- 24.Liang CK, Ho TY, Li TC, et al. A combined therapy using stimulating auricular acupoints enhances lower-level atropine eyedrops when used for myopia control in school-aged children evaluated by a pilot randomized controlled clinical trial. Complement. Ther. Med. 2008;16(6):305–310. doi: 10.1016/j.ctim.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25. Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K. Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J. AAPOS. 2008;12(4):332–339. doi: 10.1016/j.jaapos.2007.10.014. • Describes one of two clinical studies to address the safety and efficacy of pirenzepine as an antimyopia treatment, and is the only US-based trial. While the results were promising, the pharmaceutical company involved is apparently not pursuing further testing of this drug.
- 26. Trier K, Munk Ribel-Madsen S, Cui D, Brogger Christensen S. Systemic 7-methylxanthine in retarding axial eye growth and myopia progression: a 36-month pilot study. J. Ocul. Biol. Dis. Infor. 2008;1(2–4):85–93. doi: 10.1007/s12177-008-9013-3. • Describes a European clinical trial of 7-methylxanthine, a novel oral antimyopia treatment. There are ongoing investigations of this drug, although compared with topical atropine, its effect on myopia progression is not very encouraging.
- 27.Beasley R, Danesi M. Persuasive Signs: The Semiotics of Advertising. Berlin, Germany: Mouton de Gruyer; 2002. [Google Scholar]
- 28.Bedrossian RH. Treatment of progressive myopia with atropine. Presented at: The XX International Congress of Ophthalmology; 14–19 August 1966; Munich, Germany. [Google Scholar]
- 29.Bedrossian RH. The effect of atropine on myopia. Ann. Ophthalmol. 1971;3(8):891–897. [PubMed] [Google Scholar]
- 30.Bedrossian RH. The effect of atropine on myopia. Ophthalmology. 1979;86(5):713–719. doi: 10.1016/s0161-6420(79)35455-0. [DOI] [PubMed] [Google Scholar]
- 31.Kelly TS, Chatfield C, Tustin G. Clinical assessment of the arrest of myopia. Br. J. Ophthalmol. 1975;59(10):529–538. doi: 10.1136/bjo.59.10.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gimbel HV. The control of myopia with atropine. Can. J. Ophthal. 1973;8:527–532. [PubMed] [Google Scholar]
- 33.Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Invest. Ophthalmol. Vis. Sci. 2002;43(9):2852–2858. [PubMed] [Google Scholar]
- 34.Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom. Vis. Sci. 2000;77(8):395–401. doi: 10.1097/00006324-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Fulk GW, Cyert LA, Parker DE. A randomized clinical trial of bifocal glasses for myopic children with esophoria: results after 54 months. Optometry. 2002;73(8):470–476. [PubMed] [Google Scholar]
- 36.Goss DA, Grosvenor T. Rates of childhood myopia progression with bifocals as a function of nearpoint phoria: consistency of three studies. Optom. Vis. Sci. 1990;67(8):637–640. doi: 10.1097/00006324-199008000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Cheng D, Schmid KL, Woo GC, Drobe B. Randomized trial of effect of bifocal and prismatic bifocal spectacles on myopic progression: two-year results. Arch. Ophthalmol. 2010;128(1):12–19. doi: 10.1001/archophthalmol.2009.332. [DOI] [PubMed] [Google Scholar]
- 38. Shih Y-F, Hsiao CK, Chen C-J, Chang C-W, Hung PT, Lin LLK. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmol. Scand. 2001;79(3):233–236. doi: 10.1034/j.1600-0420.2001.790304.x. •• Describes one of the best controlled early clinical trials of atropine for myopia control; the inclusion of drug-free single vision and multifocal spectacle control groups allowed the potentially confounding effect of the latter optical intervention to be quantified and thus the effect of atropine to be isolated.
- 39.Brown B, Edwards MH, Leung JT. Is esophoria a factor in slowing of myopia by progressive lenses? Optom. Vis. Sci. 2002;79(10):638–642. doi: 10.1097/00006324-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Romano PE, Donovan JP. Management of progressive school myopia with topical atropine eyedrops and photochromic bifocal spectacles. Binocul. Vis. Strabismus. Q. 2000;15(3):257–260. [PubMed] [Google Scholar]
- 41.Chiang MF, Kouzis A, Pointer RW, Repka MX. Treatment of childhood myopia with atropine eyedrops and bifocal spectacles. Binocul. Vis. Strabismus. Q. 2001;16(3):209–215. [PubMed] [Google Scholar]
- 42.Chua W, Balakrishnan V, Chan Y ATOM Study Group. ARVO Analysis of Safety Data for Atropine in the Treatment of Myopia (ATOM) study. Invest. Ophthalmol. Vis. Sci. 2002;43 (E-Abstract 3329) [Google Scholar]
- 43. Chua W-H, Balakrishnan V, Chan Y-H, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285–2291. doi: 10.1016/j.ophtha.2006.05.062. •• Describes a study involving monocular atropine treatment of subjects, allowing the contralateral (fellow) eyes to serve as a control. Although this design offers a more sensitive approach for quantifying atropine’s action, the treatment-induced anisometropia was a source of controversy in the clinical community.
- 44.Chua W, Balakrishnan V, Tan D, Chan Y ATOM Study Group. Analysis of Safety Data for Atropine in the Treatment of Myopia (ATOM) study. Invest. Ophthalmol. Vis. Sci. 2002;43 Suppl. (E-Abstract 3329) [Google Scholar]
- 45.Bartlett JD, Niemann K, Houde B, Allred T, Edmondson MJ, Crockett RS. A tolerability study of pirenzepine ophthalmic gel in myopic children. J. Ocul. Pharmacol. Ther. 2003;19(3):271–279. doi: 10.1089/108076803321908392. [DOI] [PubMed] [Google Scholar]
- 46. Shih YE, Chen CH, Chou AC, Ho TC, Lin LLK, Hung PT. Effects of different concentrations of atropine on controlling myopia in myopic children. J. Ocul. Pharmacol. Ther. 1999;15(1):85–90. doi: 10.1089/jop.1999.15.85. • The significant ocular side-effects of topical atropine have been a major obstacle to its wider use for myopia control. This study provides important insight into the dose-dependency of both the antimyopia effects and side effects of atropine.
- 47.Lee JJ, Fang PC, Yang IH, et al. Prevention of myopia progression with 0.05% atropine solution. J. Ocul. Pharmacol. Ther. 2006;22(1):41–46. doi: 10.1089/jop.2006.22.41. [DOI] [PubMed] [Google Scholar]
- 48. Chua W-H, Tan D, Balakrishnan V, Chan Y-H ATOM Study Group. Progression of childhood myopia following cessation of atropine treatment. Invest. Ophthalmol. Vis. Sci. 2005;46 Suppl. (E-Abstract 4625) •• Describes the first of a small number of studies to address the important question of whether the benefit of atropine is retained after the cessation of treatment or whether there is a rebound effect on eye growth. The paper more fully describing this study is also referenced in [23].
- 49.Brodstein RS, Brodstein DE, Olson RJ, Hunt SC, Williams RR. The treatment of myopia with atropine and bifocals. A long-term prospective study. Ophthalmology. 1984;91(11):1373–1379. doi: 10.1016/s0161-6420(84)34138-0. [DOI] [PubMed] [Google Scholar]
- 50.Chou AC, Shih YF, Ho TC, Lin LLK. The effectiveness of 0.5% atropine in controlling high myopia in children. J. Ocul. Pharmacol. Ther. 1997;13(1):61–68. doi: 10.1089/jop.1997.13.61. [DOI] [PubMed] [Google Scholar]
- 51. Stone RA, Lin T, Laties AM. Muscarinic antagonist effects on experimental chick myopia. Exp. Eye Res. 1991;52(6):755–758. doi: 10.1016/0014-4835(91)90027-c. •• Describes the first study to demonstrate the antimyopia effect of atropine in an animal model of myopia; also reports on the effectiveness of several other more selective muscarinic antagonists in limiting myopia progression.
- 52. Tan DT, Lam DS, Chua WH, Shu-Ping DF, Crockett RS. One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology. 2005;112(1):84–91. doi: 10.1016/j.ophtha.2004.06.038. • Clinical study that addresses both the safety and efficacy of pirenzepine. Describes a Singapore-based clinical investigation of the safety and efficacy of pirenzepine as an antimyopia treatment; the second related study was undertaken in the US-based trial. The results from this and a closely related US-based study were promising, but are not currently being followed up.
- 53.Siatkowski RM, Cotter S, Miller JM, Scher CA, Crockett RS, Novack GD. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Arch. Ophthalmol. 2004;122(11):1667–1674. doi: 10.1001/archopht.122.11.1667. [DOI] [PubMed] [Google Scholar]
- 54.Gostin SB. Prophylatic management of progressive myopia. South Med. J. 1962;55:916–920. doi: 10.1097/00007611-196209000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Abraham S. Control of myopia with tropicamide. A progess report. J. Pediatr. Ophthalmol. 1966;3:10–22. [Google Scholar]
- 56.Bartlett JD, Jaanus SD. Clinical Ocular Pharmacology. MO, USA: Butterworth-Heinemann/Elsevier; 2008. [Google Scholar]
- 57.Bengtsson B. Some factors affecting the distribution of intraocular pressures in a population. Acta Ophthalmol. (Copenh.) 1972;50(1):33–46. doi: 10.1111/j.1755-3768.1972.tb05639.x. [DOI] [PubMed] [Google Scholar]
- 58.Bonomi L, Mecca E, Massa F. Intraocular pressure in myopic anisometropia. Int. Ophthalmol. 1982;5(3):145–148. doi: 10.1007/BF00149143. [DOI] [PubMed] [Google Scholar]
- 59.David R, Zangwill LM, Tessler Z, Yassur Y. The correlation between intraocular pressure and refractive status. Arch. Ophthalmol. 1985;103(12):1812–1815. doi: 10.1001/archopht.1985.01050120046017. [DOI] [PubMed] [Google Scholar]
- 60.Edwards MH, Brown B. IOP in myopic children: the relationship between increases in IOP and the development of myopia. Ophthalmic Physiol. Opt. 1996;16(3):243–246. [PubMed] [Google Scholar]
- 61.Puell-Marin MC, Romero-Martin M, Dominguez-Carmona M. Intraocular pressure in 528 university students: effect of refractive error. J. Am. Optom. Assoc. 1997;68(10):657–662. [PubMed] [Google Scholar]
- 62.Quinn GE, Berlin JA, Young TL, Ziylan S, Stone RA. Association of intraocular pressure and myopia in children. Ophthalmology. 1995;102(2):180–185. doi: 10.1016/s0161-6420(95)31038-x. [DOI] [PubMed] [Google Scholar]
- 63.Tomlinson A, Phillips CI. Unequal axial length of eyeball and ocular tension. Acta Ophthalmol. (Copenh.) 1972;50(6):872–876. doi: 10.1111/j.1755-3768.1972.tb06625.x. [DOI] [PubMed] [Google Scholar]
- 64. Jensen H. Myopia progression in young school children. A prospective study of myopia progression and the effect of a trial with bifocal lenses and β blocker eye drops. Acta Ophthalmol. Suppl. 1991;200:1–79. • Described the only well-controlled clinical study to evaluate the effects of β-blockers on myopia progression. It was undertaken in Europe. The results were disappointing.
- 65.Vaughan D, Shaffer R, Riegelman S. A new stabilized form of epinephrine for the treatment of open-angle glaucoma. Arch. Ophthalmol. 1961;66:232–235. doi: 10.1001/archopht.1961.00960010234014. [DOI] [PubMed] [Google Scholar]
- 66.Wiener M. The use of epinephrine in progessive myopia. Am. J. Ophthalmol. 1931;14:520–522. [Google Scholar]
- 67.Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115(8):1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 68.Macdiarmid DC. The treatment of myopia. Trans. Ophthalmol. Soc.N. Z. 1964;16:66–72. [PubMed] [Google Scholar]
- 69. Trier K, Olsen EB, Kobayashi T, Ribel-Madsen SM. Biochemical and ultrastructural changes in rabbit sclera after treatment with 7-methylxanthine, theobromine, acetazolamide, or l-ornithine. Br. J. Ophthalmol. 1999;83(12):1370–1375. doi: 10.1136/bjo.83.12.1370. • The scleral changes reported for 7-methylxanthine in this animal study support an antimyopia effect, although early results from a human clinical trial of this drug suggest reduced efficacy compared with atropine as an antimyopia treatment.
- 70.Cui D, Trier K, Zeng J, et al. Effects of 7-methylxanthine on the sclera in form deprivation myopia in guinea pigs. Acta Ophthalmol. 2009 doi: 10.1111/j.1755-3768.2009.01688.x. DOI: 10.1111/j.1755-3768.2009.01688.x (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 71.Young FA. The effect of atropine on the development of myopia in monkeys. Am. J. Optom. Arch. Am. Acad. Optom. 1965;42:439–449. doi: 10.1097/00006324-196508000-00001. [DOI] [PubMed] [Google Scholar]
- 72.Wilson JR, Sherman SM. Differential effects of early monocular deprivation on binocular and monocular segments of cat striate cortex. J. Neurophysiol. 1977;40(4):891–903. doi: 10.1152/jn.1977.40.4.891. [DOI] [PubMed] [Google Scholar]
- 73.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266(5597):66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 74.Curtin BJ, Teng CC. Scleral changes in pathological myopia. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1958;62(6):777–788. discussion 788–790. [PubMed] [Google Scholar]
- 75.Wallman J, Wildsoet CF, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35(1):37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 76.Troilo D, Nickla DL, Wildsoet CF. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest. Ophthalmol. Vis. Sci. 2000;41(6):1249–1258. [PubMed] [Google Scholar]
- 77.Hung L-F, Wallman J, Smith E. Vision-dependent changes in the choroidal thickness of Macaque monkeys. Invest. Ophthalmol. Vis. Sci. 2000;41(6):1259–1269. [PubMed] [Google Scholar]
- 78.Raviola E, Wiesel TN. Effect of dark-rearing on experimental myopia in monkeys. Invest. Ophthalmol. Vis. Sci. 1978;17(6):485–488. [PubMed] [Google Scholar]
- 79.Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124(1):154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- 80.Mohan M, Rao VA, Dada VK. Experimental myopia in the rabbit. Exp. Eye Res. 1977;25(1):33–38. doi: 10.1016/0014-4835(77)90243-3. [DOI] [PubMed] [Google Scholar]
- 81.Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201(4362):1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- 82.Hayes BP, Fitzke FW, Hodos W, Holden AL. A morphological analysis of experimental myopia in young chickens. Invest. Ophthalmol. Vis. Sci. 1986;27(6):981–991. [PubMed] [Google Scholar]
- 83.Hodos W, Kuenzel WJ. Retinal-image degradation produces ocular enlargement in chicks. Invest. Ophthalmol. Vis. Sci. 1984;25(6):652–659. [PubMed] [Google Scholar]
- 84.Yinon U, Koslowe KC, Lobel D, Landshman N, Barishak YR. Lid suture myopia in developing chicks: optical and structural considerations. Curr. Eye Res. 1982;2(12):877–882. doi: 10.3109/02713688209020025. [DOI] [PubMed] [Google Scholar]
- 85.Gottlieb MD, Joshi HB, Nickla DL. Scleral changes in chicks with form-deprivation myopia. Curr. Eye Res. 1990;9(12):1157–1165. doi: 10.3109/02713689009003472. [DOI] [PubMed] [Google Scholar]
- 86. Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28(5):639–657. doi: 10.1016/0042-6989(88)90113-7. • The technique of using a negative ‘spectacle’ lens to induce myopia in chickens, pioneered by these authors and described for the first time in this study, is now the most widely used experimental model of myopia.
- 87. Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat. Med. 1995;1(8):761–765. doi: 10.1038/nm0895-761. • The study described in this paper confirmed that negative lenses, which had been shown to induce myopia in chickens, are also effective in inducing myopia in monkeys.
- 88. Whatham AR, Judge SJ. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001;41(3):267–273. doi: 10.1016/s0042-6989(00)00250-9. • The study described in this paper demonstrated that defocusing contact lenses are a suitable substitute for spectacle lenses for altering eye growth in the marmoset.
- 89. Siegwart JT, Norton TT. Refractive and ocular changes in tree shrews raised with plus or minus lenses. Invest. Ophthalmol. Vis. Sci. 1993;34(4):1208. • The important finding that the tree shrew, a small mammal, sometimes considered a primitive primate, shows compensatory eye growth changes in response to defocusing lenses, as in chickens, is described in this paper. The tree shrew model for myopia has been widely used for studies of scleral changes in myopia.
- 90. Howlett MHC, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49(2):219–227. doi: 10.1016/j.visres.2008.10.008. • The demonstration that guinea pigs, another small mammal, show similar responses to defocusing lenses as tree shrews, as reported in this paper, has allowed their use as an alternative to the tree shrew in myopia research. Their greater accessibility is reflected in the appearance of new investigators in the field of experimental myopia.
- 91.McFadden S, Wallman J. Guinea pig eye growth compensates for spectacle lenses. Invest. Ophthalmol. Vis. Sci. 1995;36 Suppl.:758. [Google Scholar]
- 92.Beuerman RW, Barathi VA, Weon SR, Tan D. Two models of experimental myopia in the mouse. Invest. Ophthalmol. Vis. Sci. 2003;44 Suppl.:4338. [Google Scholar]
- 93. Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–468. doi: 10.1016/j.neuron.2004.08.008. • Provides a comprehensive overview of potential biological mechanisms underlying eye growth regulation and myopia, including how biochemical and image-derived signals influence eye growth.
- 94.Raviola E, Wiesel TN. An animal model of myopia. N. Engl. J. Med. 1985;312(25):1609–1615. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- 95.Wildsoet CF, Pettigrew JD. Experimental myopia and anomalous eye growth patterns unaffected by optic nerve section in chickens: evidence for local control of eye growth. Clin. Vision Sci. 1988;3(2):99–107. [Google Scholar]
- 96.Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr. Eye Res. 1987;6(8):993–999. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- 97. Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks - insights from selective lesions of the optic nerve and ciliary nerve. Curr. Eye Res. 2003;27(6):371–385. doi: 10.1076/ceyr.27.6.371.18188. • The results reported in this paper, by ruling out the need for intact optic and ciliary nerves for experimental myopia, help to limit the scope of plausible antimyopia treatments. They specifically lend support for targeting the retina, choroid and sclera.
- 98.Wildsoet CF, McFadden SA. Optic nerve section does not prevent form deprivation-induced myopia or recovery from it in the mammalian eye. Presented at: Association for Research in Vision and Opthalmology, Annual Meeting; 2–6 May 2010; FL, USA. [Google Scholar]
- 99.Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis. Neurosci. 1994;11(1):143–153. doi: 10.1017/s0952523800011184. [DOI] [PubMed] [Google Scholar]
- 100. Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35(9):1175–1194. doi: 10.1016/0042-6989(94)00233-c. •• Describes the first evidence for choroidal thickness modulation as a component of emmetropization, and thus a plausible target for anti-myopia drugs. Choroidal thinning was linked to myopia development.
- 101.Gottlieb MD, Fugate-Wentzek LA, Wallman J. Different visual deprivations produce different ametropias and different eye shapes. Invest. Ophthalmol. Vis. Sci. 1987;28(8):1225–1235. [PubMed] [Google Scholar]
- 102.Smith EL, 3rd, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest. Ophthalmol. Vis. Sci. 2009;50(11):5057–5069. doi: 10.1167/iovs.08-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McFadden S. Partial occlusion produces local form deprivation myopia in the guinea pig eye. Invest. Ophthalmol. Vis. Sci. 2002;41(4):189. [Google Scholar]
- 104.Zeng G, McFadden S. Regional variation in susceptibility to myopia from partial form deprivation in the guinea pig. Invest. Ophthalmol. Vis. Sci. 2010;51 (E-Abstract 10-A-4887-ARVO) [Google Scholar]
- 105.Schaeffel F, Troilo D, Wallman J, Howland HC. Developing eyes that lack accommodation grow to compensate for imposed defocus. Vis. Neurosci. 1990;4(2):177–183. doi: 10.1017/s0952523800002327. [DOI] [PubMed] [Google Scholar]
- 106.Wildsoet CF, Howland HC, Falconer S, Dick K. Chromatic aberration and accommodation: their role in emmetropization in the chick. Vision Res. 1993;33(12):1593–1603. doi: 10.1016/0042-6989(93)90026-s. [DOI] [PubMed] [Google Scholar]
- 107.Schwahn HN, Schaeffel F. Chick eyes under cycloplegia compensate for spectacle lenses despite six-hydroxy dopamine treatment. Invest. Ophthalmol. Vis. Sci. 1994;35(9):3516–3524. [PubMed] [Google Scholar]
- 108.McBrien NA, Moghaddam HO, New R, Williams LR. Experimental myopia in a diurnal mammal (Sciurus carolinensis) with no accommodative ability. J. Physiol. 1993;469:427–441. doi: 10.1113/jphysiol.1993.sp019821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pilar G, Nunez R, McLennan IS, Meriney SD. Muscarinic and nicotinic synaptic activation of the developing chicken iris. J. Neurosci. 1987;7(12):3813–3826. doi: 10.1523/JNEUROSCI.07-12-03813.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest. Ophthalmol. Vis. Sci. 1993;34(1):205–215. • The finding reported in this paper represented an important step forward in understanding the antimyopia effect of atropine, overturning a commonly held belief among clinicians that atropine’s cycloplegic action was responsible for this effect.
- 111.Honkanen RE, Abdel-Latif AA. Characterization of cholinergic muscarinic receptors in the rabbit iris. Biochem. Pharmacol. 1988;37(13):2575–2583. doi: 10.1016/0006-2952(88)90249-3. [DOI] [PubMed] [Google Scholar]
- 112.Honkanen RE, Howard EF, Abdel-Latif AA. M3-muscarinic receptor subtype predominates in the bovine iris sphincter smooth muscle and ciliary processes. Invest. Ophthalmol. Vis. Sci. 1990;31(3):590–593. [PubMed] [Google Scholar]
- 113.Konno F, Takayanagi I. Comparison of the muscarinic cholinoceptors in the rabbit ciliary body and the guinea-pig ileum. Eur. J. Pharmacol. 1986;132(2–3):171–178. doi: 10.1016/0014-2999(86)90602-3. [DOI] [PubMed] [Google Scholar]
- 114.Cha YL, Weon SR, Beuerman RW. Effect of muscarinic agents and the role of sclera fibroblasts in experimental myopia. Invest. Ophthalmol. Vis. Sci. 2002;43 Suppl.:2411. [Google Scholar]
- 115.Morgan I, van Rijswijk P, Megaw P. M4 muscarinic acetylcholine receptors mediate muscarinic block of axial elongation in the chicken. Invest. Ophthalmol. Vis. Sci. 2005;46 Suppl. (E-Abstract 3342) [Google Scholar]
- 116. Cottriall CL, McBrien NA. The M1 muscarinic antagonist pirenzepine reduces myopia and eye enlargement in the tree shrew. Invest. Ophthalmol. Vis. Sci. 1996;37(7):1368–1379. • Represents the first demonstration in a mammalian model that pirenzepine limits myopia progession, confirming earlier results in chickens, and bringing clinical testing in humans one step closer.
- 117.McBrien NA, Cottriall CL. Pirenzepine reduces axial elongation and myopia in monocularly derived tree shrews. Invest. Ophthalmol. Vis. Sci. 1993;34 Suppl.:2493. [PubMed] [Google Scholar]
- 118.Truong HT, Cottriall CL, Gentle A, McBrien NA. Pirenzepine affects scleral metabolic changes in myopia through a non-toxic mechanism. Exp. Eye Res. 2002;74(1):103–111. doi: 10.1006/exer.2001.1107. [DOI] [PubMed] [Google Scholar]
- 119. Tigges M, Iuvone PM, Fernandes A, et al. Effects of muscarinic cholinergic receptor antagonists on postnatal eye growth of rhesus monkeys. Optom. Vis. Sci. 1999;76(6):397–407. doi: 10.1097/00006324-199906000-00020. • Describes a primate investigation of muscarinic antagonists, including pirenzepine, as antimyopia drugs.
- 120. Luft WA, Ming Y, Stell WK. Variable effects of previously untested muscarinic receptor antagonists on experimental myopia. Invest. Ophthalmol. Vis. Sci. 2003;44(3):1330–1338. doi: 10.1167/iovs.02-0796. • Describes the most comprehensive investigation of muscarinic antagonists as potential antimyopia drugs; the inclusion of drugs with selectively for different muscarinic receptor subtypes provide some limited new insights into the mechanism of action of atropine, a nonselective muscarinic antagonist.
- 121.Diether S, Schaeffel F, Fritsch C, Trendelenburg AU, Payor R, Lambrou GN. Role of M1 and M4 receptors in development of myopia: study with muscarinic receptor antagonists and muscarinic toxins. Invest. Ophthalmol. Vis. Sci. 2005;46 Suppl. (E-Abstract 1986) [Google Scholar]
- 122.North RA, Slack BE, Surprenant A. Muscarinic M1 and M2 receptors mediate depolarization and presynaptic inhibition in guinea-pig enteric nervous system. J. Physiol. 1985;368:435–452. doi: 10.1113/jphysiol.1985.sp015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yin GC, Gentle A, McBrien NA. Muscarinic antagonist control of myopia: a molecular search for the M1 receptor in chick. Mol. Vis. 2004;10(93):787–793. [PubMed] [Google Scholar]
- 124. McBrien NA, Jobling AI, Truong HT, Cottriall CL, Gentle A. Expression of muscarinic receptor subtypes in tree shrew ocular tissues and their regulation during the development of myopia. Mol. Vis. 2009;15:464–475. • Provides a useful catalog of the muscarinic receptor subtypes present in mammalian ocular tissues and their respective locations, with potential application in drug development research.
- 125.Liu Q, Wu J, Wang X, Zeng J. Changes in muscarinic acetylcholine receptor expression in form deprivation myopia in guinea pigs. Mol. Vis. 2007;13:1234–1244. [PubMed] [Google Scholar]
- 126.Rickers M, Schaeffel F. Dose-dependent effects of intravitreal pirenzepine on deprivation myopia and lens-induced refractive errors in chickens. Exp. Eye Res. 1995;61(4):509–516. doi: 10.1016/s0014-4835(05)80147-2. [DOI] [PubMed] [Google Scholar]
- 127.Leech EM, Cottriall CL, McBrien NA. Pirenzepine prevents form deprivation myopia in a dose dependent manner. Ophthalmic Physiol. Opt. 1995;15(5):351–356. [PubMed] [Google Scholar]
- 128.Cottriall CL, McBrien NA, Annies R, Leech EM. Prevention of form-deprivation myopia with pirenzepine: a study of drug delivery and distribution. Ophthalmic Physiol. Opt. 1999;19(4):327–335. [PubMed] [Google Scholar]
- 129. Rymer J, Wildsoet CF. The role of the retinal pigment epithelium in eye growth regulation and myopia: a review. Vis. Neurosci. 2005;22(3):251–261. doi: 10.1017/S0952523805223015. • In reviewing the evidence supporting the important role of the retinal pigment epithelium in relaying retinal growth modulatory signals to the choroid and sclera, this paper also provides information on target locations and drug groups for pharmacological interventions for myopia.
- 130.Zhang Y, Maminishkis A, Zhi C, et al. Apomorphine regulates TGF-β1 and TGF-β2 expression in human fetal retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2009;50 Suppl.:3845. [Google Scholar]
- 131. Tan J, Deng ZH, Liu SZ, Wang JT, Huang C. TGF-β2 in human retinal pigment epithelial cells: expression and secretion regulated by cholinergic signals in vitro. Curr. Eye Res. 2010;35(1):37–44. doi: 10.3109/02713680903374190. • The finding described in this paper, that muscarinic receptors on the retinal pigment epithelium regulate the secretion of TGF-β, brings together seemingly disparate observations reported in other papers implicating the growth factor in scleral remodeling and atropine, a muscarinic antagonist, in myopia inhibition.
- 132. Barathi VA, Weon SR, Beuerman RW. Expression of muscarinic receptors in human and mouse sclera and their role in the regulation of scleral fibroblasts proliferation. Mol. Vis. 2009;15:1277–1293. •• The results reported in this paper offer a scleral mechanism for the antimyopia effect of atropine, involving muscarinic receptors on scleral fibroblasts.
- 133.Honda S, Fujii S, Sekiya Y, Yamamoto M. Retinal control on the axial length mediated by transforming growth factor-β in chick eye. Invest. Ophthalmol. Vis. Sci. 1996;37(12):2519–2526. [PubMed] [Google Scholar]
- 134.Jobling AI, Gentle A, Metlapally R, McGowan BJ, McBrien NA. Regulation of scleral cell contraction by transforming growth factor-β and stress: competing roles in myopic eye growth. J. Biol. Chem. 2009;284(4):2072–2079. doi: 10.1074/jbc.M807521200. [DOI] [PubMed] [Google Scholar]
- 135. Jobling AI, Nguyen M, Gentle A, McBrien NA. Isoform-specific changes in scleral transforming growth factor-β expression and the regulation of collagen synthesis during myopia progression. J. Biol. Chem. 2004;279(18):18121–18126. doi: 10.1074/jbc.M400381200. • The results reported in this paper implicate TGF-β in the regulation of scleral collagen synthesis in the tree shrew. That it might be a suitable target for myopia therapy is supported by the additional observation of altered expression of TGF-β isoforms during myopia progression.
- 136.Seko Y, Shimokawa H, Tokoro T. Expression of bFGF and TGF-β 2 in experimental myopia in chicks. Invest. Ophthalmol. Vis. Sci. 1995;36(6):1183–1187. [PubMed] [Google Scholar]
- 137. Seko Y, Shimokawa H, Tokoro T. In vivo and in vitro association of retinoic acid with form-deprivation myopia in the chick. Exp. Eye Res. 1996;63(4):443–452. doi: 10.1006/exer.1996.0134. • The changes in retinoic acid (RA) receptor expression in a form deprivation myopia model of chick, coupled with the ability of RA to regulate cell proliferation reported in this paper, represent the first of a number of studies pointing to an important role for RA in eye growth regulation. The therapeutic implications of these results are the subject of ongoing investigations.
- 138.Seko Y, Tanaka Y, Tokoro T. Influence of bFGF as a potent growth stimulator and TGF-β as a growth regulator on scleral chondrocytes and scleral fibroblasts in vitro. Ophthalmic Res. 1995;27(3):144–152. doi: 10.1159/000267651. [DOI] [PubMed] [Google Scholar]
- 139. Lind GJ, Chew SJ, Marzani D, Wallman J. Muscarinic acetylcholine receptor antagonists inhibit chick scleral chondrocytes. Invest. Ophthalmol. Vis. Sci. 1998;39(12):2217–2231. • Reports the first investigation into the effects of muscarinic antagonists on isolated chicken scleral cells, in pursuit of the hypothesis that atropine-like drugs may exercise their antimyopia effect directly on the sclera. Although interpretation of the results reported therein is the subject of ongoing debate, interest in the sclera as a tissue target for antimyopia drugs remains.
- 140.Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp. Eye Res. 2006;82(2):185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 141.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog. Ret. Eye Res. 2003;22(3):307–338. doi: 10.1016/s1350-9462(02)00063-0. [DOI] [PubMed] [Google Scholar]
- 142.Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest. Ophthalmol. Vis. Sci. 1997;38(9):1726–1739. [PubMed] [Google Scholar]
- 143. Stone RA, Sugimoto R, Gill AS, Liu J, Capehart C, Lindstrom JM. Effects of nicotinic antagonists on ocular growth and experimental myopia. Invest. Ophthalmol. Vis. Sci. 2001;42(3):557–565. • Investigated the ability of a range of nicotinic analogs to limit form-deprivation myopia in chicks. Nicotinic analogs are of interest, given provocative data suggesting that smoking might protect against myopia. The results of the study are complicated, with some drugs showing multiphasic and others incomplete inhibitory responses.
- 144.Saw SM, Chia KS, Lindstrom JM, Tan DT, Stone RA. Childhood myopia and parental smoking. Br. J. Ophthalmol. 2004;88(7):934–937. doi: 10.1136/bjo.2003.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stone RA, Wilson LB, Ying GS, et al. Associations between childhood refraction and parental smoking. Invest. Ophthalmol. Vis. Sci. 2006;47(10):4277–4287. doi: 10.1167/iovs.05-1625. [DOI] [PubMed] [Google Scholar]
- 146.Geller AM, Abdel-Rahman AA, Peiffer RL, Abou-Donia MB, Boyes WK. The organophosphate pesticide chlorpyrifos affects form deprivation myopia. Invest. Ophthalmol. Vis. Sci. 1998;39(7):1290–1294. [PubMed] [Google Scholar]
- 147.Cottriall CL, Brew J, Vessey KA, McBrien NA. Diisopropylfluorophosphate alters retinal neurotransmitter levels and reduces experimentally-induced myopia. Naunyn Schmiedebergs Arch. Pharmacol. 2001;364(4):372–382. doi: 10.1007/s002100100460. [DOI] [PubMed] [Google Scholar]
- 148.Ishikawa S, Miyata M. Development of myopia following chronic organophosphate pesticide intoxication: an epidemiological and experimental study. In: Merrigan WH, Weiss B, editors. Neurotoxicity of the Visual System. NY, USA: Raven Press; 1980. pp. 233–254. [Google Scholar]
- 149.Dementi B. Ocular effects of organophosphates: a historical perspective of Saku disease. J. Appl. Toxicol. 1994;14(2):119–129. doi: 10.1002/jat.2550140214. [DOI] [PubMed] [Google Scholar]
- 150. Iuvone PM, Tigges M, Fernandes A, Tigges J. Dopamine synthesis and metabolism in rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Vis. Neurosci. 1989;2(5):465–471. doi: 10.1017/s0952523800012360. • Evaluated the effect of form deprivation on dopamine synthesis and metabolism changes in monkeys, paralleling a similar study around the same time in chickens.
- 151. Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc. Natl Acad. Sci. USA. 1989;86(2):704–706. doi: 10.1073/pnas.86.2.704. •• Linked reduced retinal dopamine with eye growth in the chicken. The reported results have served as the stimulus for subsequent investigation of the role of dopamine in eye growth regulation and exploration of the application of dopamine agonists in myopia therapy.
- 152.Ohngemach S, Hagel G, Schaeffel F. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Vis. Neurosci. 1997;14:493–505. doi: 10.1017/s0952523800012153. [DOI] [PubMed] [Google Scholar]
- 153. Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis. Neurosci. 1993;10(3):447–453. doi: 10.1017/s0952523800004673. • A specific subtype of dopamine receptor through which dopamine may be acting to exert its antimyopia effect is identified in this paper.
- 154. Schmid KL, Wildsoet CF. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom. Vis. Sci. 2004;81(2):137–147. doi: 10.1097/00006324-200402000-00012. • The results reported in this paper support roles for both dopaminergic and cholinergic pathways in eye growth regulation and provide insight into how drugs targeting each of these pathways may interact.
- 155.Stone RA, Laties AM, Raviola E, Wiesel TN. Increase in retinal vasoactive intestinal polypeptide after eyelid fusion in primates. Proc. Natl Acad. Sci. USA. 1988;85(1):257–260. doi: 10.1073/pnas.85.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ashby R, McCarthy CS, Maleszka R, Megaw P, Morgan IG. A muscarinic cholinergic antagonist and a dopamine agonist rapidly increase ZENK mRNA expression in the form-deprived chicken retina. Exp. Eye Res. 2007;85(1):15–22. doi: 10.1016/j.exer.2007.02.019. • The study reported in this paper revisits the site of action of known antimyopia antimuscarinic and dopamine agonist drugs; observed drug-induced changes in retinal gene expression are offered as evidence of retinal sites of action.
- 157.Gao Q, Liu Q, Ma P, Zhong X, Wu J, Ge J. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch. Clin. Exp. Ophthalmol. 2006;244(10):1329–1335. doi: 10.1007/s00417-006-0254-1. [DOI] [PubMed] [Google Scholar]
- 158. Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest. Ophthalmol. Vis. Sci. 1991;32(5):1674–1677. • Describes the first primate study of the antimyopia action of a dopamine receptor agonist. The trends in the data were consistent with already published results for chickens.
- 159. Troilo D, Nickla DL, Mertz JR, Summers Rada JA. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest. Ophthalmol. Vis. Sci. 2006;47(5):1768–1777. doi: 10.1167/iovs.05-0298. • The results reported in this paper provide a link between changes in retinoic acid levels and scleral glycosaminoglycan synthesis in myopic eyes.
- 160.Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., 3rd Recovery from form-deprivation myopia in rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 2004;45(10):3361–3372. doi: 10.1167/iovs.04-0080. [DOI] [PubMed] [Google Scholar]
- 161.Wildsoet CF, Clark IQ. Interacting effects of kainic acid and 6-hydroxydopamine on eye growth and refraction in the chick. Invest. Ophthalmol. Vis. Sci. 1993 Suppl.:2491. [Google Scholar]
- 162.Li XX, Schaeffel F, Kohler K, Zrenner E. Dose-dependent effects of 6-hydroxy dopamine on deprivation myopia, electroretinograms, and dopaminergic amacrine cells in chickens. Vis. Neurosci. 1992;9(5):483–492. doi: 10.1017/s0952523800011287. [DOI] [PubMed] [Google Scholar]
- 163.Schaeffel F, Hagel G, Bartmann M, Kohler K, Zrenner E. 6-hydroxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Res. 1993;34:143–149. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- 164.Ashby RS, Schaeffel F. The effect of bright light on lens-compensation in chicks. Invest. Ophthalmol. Vis. Sci. 2010;51(10):5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- 165.Fischer AJ, Stanke JJ, Aloisio G, Hoy H, Stell WK. Heterogeneity of horizontal cells in the chicken retina. J. Comp. Neurol. 2007;500(6):1154–1171. doi: 10.1002/cne.21236. [DOI] [PubMed] [Google Scholar]
- 166.Araki CM, Hamassaki-Britto DE. Motion-sensitive neurons in the chick retina: a study using Fos immunohistochemistry. Brain Res. 1998;794(2):333–337. doi: 10.1016/s0006-8993(98)00307-2. [DOI] [PubMed] [Google Scholar]
- 167.Zhou ZJ, Lee S. Synaptic physiology of direction selectivity in the retina. J. Physiol. 2008;586 Pt 18:4371–4376. doi: 10.1113/jphysiol.2008.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Stone RA, Liu J, Sugimoto R, Capehart C, Zhu X, Pendrak K. GABA, experimental myopia, and ocular growth in chick. Invest. Ophthalmol. Vis. Sci. 2003;44(9):3933–3946. doi: 10.1167/iovs.02-0774. • Describes the first study to implicate retinal γ-aminobutyric acid in refractive error development. Its role as an inhibitory neurotransmitter is well established, and γ-aminobutyric acid analogs are already in clinical use. Their applicability for myopia control awaits further investigation.
- 169.Chebib M, Gavande N, Wong KY, et al. Guanidino acids act as rho1 GABA(C) receptor antagonists. Neurochem. Res. 2009;34(10):1704–1711. doi: 10.1007/s11064-009-9968-x. [DOI] [PubMed] [Google Scholar]
- 170.Song XQ, Meng F, Ramsey DJ, Ripps H, Qian H. The GABA rho1 subunit interacts with a cellular retinoic acid binding protein in mammalian retina. Neuroscience. 2005;136(2):467–475. doi: 10.1016/j.neuroscience.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 171.Stone RA, Kuwayama Y, Laties AM. Regulatory peptides in the eye. Experientia. 1987;43(7):791–800. doi: 10.1007/BF01945357. [DOI] [PubMed] [Google Scholar]
- 172.Seltner RL, Stell WK. The effect of vasoactive intestinal peptide on development of form deprivation myopia in the chick: a pharmacological and immunocytochemical study. Vision Res. 1995;35(9):1265–1270. doi: 10.1016/0042-6989(94)00244-g. [DOI] [PubMed] [Google Scholar]
- 173.Fischer AJ, Seltner RL, Poon J, Stell WK. Immunocytochemical characterization of quisqualic acid- and N-methyl-d-aspartate-induced excitotoxicity in the retina of chicks. J. Comp. Neurol. 1998;393(1):1–15. [PubMed] [Google Scholar]
- 174.Ehrlich D, Sattayasai J, Zappia J, Barrington M. Effects of selective neurotoxins on eye growth in the young chick. Ciba Found. Symp. 1990;155:63–84. doi: 10.1002/9780470514023.ch5. discussion 84–88. [DOI] [PubMed] [Google Scholar]
- 175.Fischer AJ, Morgan IG, Stell WK. Colchicine causes excessive ocular growth and myopia in chicks. Vision Res. 1999;39(4):685–697. doi: 10.1016/s0042-6989(98)00178-3. [DOI] [PubMed] [Google Scholar]
- 176.Stubinger K, Brehmer A, Neuhuber WL, Reitsamer H, Nickla D, Schrodl F. Intrinsic choroidal neurons in the chicken eye: chemical coding and synaptic input. Histochem. Cell Biol. 2010;134(2):145–157. doi: 10.1007/s00418-010-0723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Seltner RL, Rohrer B, Grant V, Stell WK. Endogenous opiates in the chick retina and their role in form-deprivation myopia. Vis. Neurosci. 1997;14(5):801–809. doi: 10.1017/s0952523800011548. [DOI] [PubMed] [Google Scholar]
- 178.Thode C, Bock J, Braun K, Darlison MG. The chicken immediate-early gene ZENK is expressed in the medio-rostral neostriatum/hyperstriatum ventrale, a brain region involved in acoustic imprinting, and is up-regulated after exposure to an auditory stimulus. Neuroscience. 2005;130(3):611–617. doi: 10.1016/j.neuroscience.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 179.Bitzer M, Schaeffel F. Defocus-induced changes in ZENK expression in the chicken retina. Invest. Ophthalmol. Vis. Sci. 2002;43(1):246–252. [PubMed] [Google Scholar]
- 180. Fischer AJ, McGuire JJ, Schaeffel F, Stell WK. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat. Neurosci. 1999;2(8):706–712. doi: 10.1038/11167. • The study reported in this paper was the first to report bidirectional regulation of retinal genes in response to different directions of imposed optical defocus. Glucagon-containing amacrine cells were among the cells showing gene-expression changes, stimulating studies into the influence of glucagon analogs on eye growth.
- 181.Feldkaemper MP, Wang HY, Schaeffel F. Changes in retinal and choroidal gene expression during development of refractive errors in chicks. Invest. Ophthalmol. Vis. Sci. 2000;41(7):1623–1628. [PubMed] [Google Scholar]
- 182. Zhu X, Wallman J. Opposite effects of glucagon and insulin on compensation for spectacle lenses in chicks. Invest. Ophthalmol. Vis. Sci. 2009;50(1):24–36. doi: 10.1167/iovs.08-1708. •• The study reported in this paper is among the first to describe effects of insulin on eye growth, which has attracted interest because of the worldwide epidemic of childhood obesity and climbing diabetes prevalence figures. The opposing effect of glucagon on eye growth is consistent with the opposing physiological roles of these hormones. These results await confirmation in a mammalian model.
- 183.Vessey KA, Lencses KA, Rushforth DA, Hruby VJ, Stell WK. Glucagon receptor agonists and antagonists affect the growth of the chick eye: a role for glucagonergic regulation of emmetropization? Invest. Ophthalmol. Vis. Sci. 2005;46(11):3922–3931. doi: 10.1167/iovs.04-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis. Neurosci. 2002;19(6):755–766. doi: 10.1017/s0952523802196064. •Describes the first study to investigate the role of glucagon in eye growth regulation.
- 185.Buck C, Schaeffel F, Simon P, Feldkaemper M. Effects of positive and negative lens treatment on retinal and choroidal glucagon and glucagon receptor mRNA levels in the chicken. Invest. Ophthalmol. Vis. Sci. 2004;45(2):402–409. doi: 10.1167/iovs.03-0789. [DOI] [PubMed] [Google Scholar]
- 186.Choh V, Padmanabhan V, Li WS, et al. Colchicine attenuates compensation to negative but not to positive lenses in young chicks. Exp. Eye Res. 2008;86(2):260–270. doi: 10.1016/j.exer.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fischer AJ, Ritchey ER, Scott MA, Wynne A. Bullwhip neurons in the retina regulate the size and shape of the eye. Dev. Biol. 2008;317(1):196–212. doi: 10.1016/j.ydbio.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 188. Feldkaemper MP, Neacsu I, Schaeffel F. Insulin acts as a powerful stimulator of axial myopia in chicks. Invest. Ophthalmol. Vis. Sci. 2009;50(1):13–23. doi: 10.1167/iovs.08-1702. • Represents one of a small number of studies to evaluate the effects of insulin on eye growth.
- 189.Fujikado T, Kawasaki Y, Fujii J, et al. The effect of nitric oxide synthase inhibitor on form-deprivation myopia. Curr. Eye Res. 1997;16(10):992–996. doi: 10.1076/ceyr.16.10.992.9021. [DOI] [PubMed] [Google Scholar]
- 190.Fujikado T, Tsujikawa K, Tamura M, Hosohata J, Kawasaki Y, Tano Y. Effect of a nitric oxide synthase inhibitor on lens-induced myopia. Ophthalmic Res. 2001;33(2):75–79. doi: 10.1159/000055647. [DOI] [PubMed] [Google Scholar]
- 191. Nickla DL, Damyanova P, Lytle G. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor. Exp. Eye Res. 2009;88(6):1092–1099. doi: 10.1016/j.exer.2009.01.012. • The finding reported in this paper that selective inhibition of neural nitric oxide synthetase (nNOS) had similar effects on eye growth to nonselective inhibitors of NOS suggests that nNOS is the target in both cases, and furthermore, that endothelial cells are not the site of action for this drug effect.
- 192. Nickla DL, Wildsoet CF. The effect of the nonspecific nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester on the choroidal compensatory response to myopic defocus in chickens. Optom. Vis. Sci. 2004;81(2):111–118. doi: 10.1097/00006324-200402000-00009. • Reports the first study to examine the role of nitric oxide in eye growth regulation; in chickens, it appears to modulate choroidal thickness and therefore refractive error.
- 193.Koistinaho J, Swanson RA, de Vente J, Sagar SM. NADPH-diaphorase (nitric oxide synthase)-reactive amacrine cells of rabbit retina: putative target cells and stimulation by light. Neuroscience. 1993;57(3):587–597. doi: 10.1016/0306-4522(93)90008-4. [DOI] [PubMed] [Google Scholar]
- 194.Poukens V, Glasgow BJ, Demer JL. Nonvascular contractile cells in sclera and choroid of humans and monkeys. Invest. Ophthalmol. Vis. Sci. 1998;39(10):1765–1774. [PubMed] [Google Scholar]
- 195.Schrodl F, De Laet A, Tassignon MJ, et al. Intrinsic choroidal neurons in the human eye: projections, targets, and basic electrophysiological data. Invest. Ophthalmol. Vis. Sci. 2003;44(9):3705–3712. doi: 10.1167/iovs.03-0232. [DOI] [PubMed] [Google Scholar]
- 196.Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 2008;9(7):541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 197.Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl. Recept. Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Luo T, Sakai Y, Wagner E, Drager UC. Retinoids, eye development, and maturation of visual function. J. Neurobiol. 2006;66(7):677–686. doi: 10.1002/neu.20239. [DOI] [PubMed] [Google Scholar]
- 199.Mori M, Ghyselinck NB, Chambon P, Mark M. Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Invest. Ophthalmol. Vis. Sci. 2001;42(6):1312–1318. [PubMed] [Google Scholar]
- 200. Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp. Eye Res. 2000;70(4):519–527. doi: 10.1006/exer.1999.0813. •• This report of altered choroidal RA synthesis linked to altered eye growth in chickens represents one of the earliest studies linking RA with eye growth regulation. Importantly, this connection has since been confirmed in other animal models, although there are species-related differences in direction of the change in RA synthesis during altered eye growth.
- 201. McFadden SA, Howlett MH, Mertz JR, Wallman J. Acute effects of dietary retinoic acid on ocular components in the growing chick. Exp. Eye Res. 2006;83(4):949–961. doi: 10.1016/j.exer.2006.05.002. • The novel finding reported in this paper is that oral retinoic acid increased eye growth did not affect the refractive error of chicks; it suggests there may be both defocus-dependent and defocus-independent growth-regulatory mechanisms. A better understanding of these mechanisms will be critical to the development of RA analogs as antimyopia treatments.
- 202.Bitzer M, Feldkaemper M, Schaeffel F. Visually induced changes in components of the retinoic acid system in fundal layers of the chick. Exp. Eye Res. 2000;70(1):97–106. doi: 10.1006/exer.1999.0762. [DOI] [PubMed] [Google Scholar]
- 203.McFadden SA, Howlett MH, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44(7):643–653. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 204.Pohlers D, Brenmoehl J, Loffler I, et al. TGF-β and fibrosis in different organs – molecular pathway imprints. Biochim. Biophys. Acta. 2009;1792(8):746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 205.Kjaer M, Langberg H, Heinemeier K, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand. J. Med. Sci. Sports. 2009;19(4):500–510. doi: 10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 206.Lincoln J, Lange AW, Yutzey KE. Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev. Biol. 2006;294(2):292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 207.Katoh M. FGFR2 abnormalities underlie a spectrum of bone, skin, and cancer pathologies. J. Invest. Dermatol. 2009;129(8):1861–1867. doi: 10.1038/jid.2009.97. [DOI] [PubMed] [Google Scholar]
- 208. Rohrer B, Stell WK. Basic fibroblast growth factor (bFGF) and transforming growth factor β (TGF-β) act as stop and go signals to modulate postnatal ocular growth in the chick. Exp. Eye Res. 1994;58(5):553–561. doi: 10.1006/exer.1994.1049. •• Administration of basic FGF and TGF-β are reported to modulate eye growth in the chick. These findings represent the first published evidence of a role for these endogenous growth factors in ocular growth regulation. These and other related findings are yet to be exploited in pursuit of pharmaceutical interventions for myopia control.
- 209.Rohrer B, Tao J, Stell WK. Basic fibroblast growth factor, its high- and low-affinity receptors, and their relationship to form-deprivation myopia in the chick. Neuroscience. 1997;79(3):775–787. doi: 10.1016/s0306-4522(97)00042-0. [DOI] [PubMed] [Google Scholar]
- 210.Rohrer B, Iuvone PM, Stell WK. Stimulation of dopaminergic amacrine cells by stroboscopic illumination or fibroblast growth factor (bFGF, FGF-2) injections: possible roles in prevention of form-deprivation myopia in the chick. Brain Res. 1995;686(2):169–181. doi: 10.1016/0006-8993(95)00370-6. [DOI] [PubMed] [Google Scholar]
- 211.Gentle A, McBrien NA. Retinoscleral control of scleral remodelling in refractive development: a role for endogenous FGF-2? Cytokine. 2002;18(6):344–348. doi: 10.1006/cyto.2002.1046. [DOI] [PubMed] [Google Scholar]
- 212.Jobling A, Gentle A, Metlapally R, McGowan B, McBrien N. Regulation of scleral cell contraction by transforming growth factor-β and stress: competing roles in myopic eye growth. J. Biol. Chem. 2009;284(4):2072–2079. doi: 10.1074/jbc.M807521200. [DOI] [PubMed] [Google Scholar]
- 213. Zhang Y, Maminishkis A, Zhi C, et al. Apomorphine regulates TGF-β1 and TGF-β2 expression in human fetal retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2009;50 Suppl.:3845. • Describes the regulation by apomorphine of TGF-β expression in retinal pigment epithelial (RPE) cells, and promotion of TGF-β secretion from the choroid/sclera-facing side of RPE cells, offering a potential mechanism through which dopamine analogs may influence myopic eye growth.
- 214. Schmid KL, Abbott M, Humphries M, Pyne K, Wildsoet CF. Timolol lowers intraocular pressure but does not inhibit the development of experimental myopia in chick. Exp. Eye Res. 2000;70(5):659–666. doi: 10.1006/exer.2000.0834. • The finding of this paper that lowering IOP does not inhibit experimental myopia supports the notion that myopic eye enlargement is not the result of passive stretch, but instead of active scleral remodeling. This result for timolol in chickens is also consistent with those of a related clinical study.
- 215.Lueitjen-Drecoll E, Kaufman PL. Long-term timolol and epinephrine in monkeys 2. Functional morphology of the ciliary processes. Trans. Ophthalmol. Soc. 1985;105:180–195. [PubMed] [Google Scholar]
- 216.Rymer JM, Wildsoet CF, Miller SS. Epinephrine decreases cytosolic calcium in chick retinal pigment epithelium (RPE) Invest. Ophthalmol. Vis. Sci. 2002;43 Suppl. (E-Abstract 719) [Google Scholar]
- 217.Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv. Ophthalmol. 2008;53 Suppl. 1:S107–S120. doi: 10.1016/j.survophthal.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Alm A, Nilsson SF. Uveoscleral outflow – a review. Exp. Eye Res. 2009;88(4):760–768. doi: 10.1016/j.exer.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 219.Jin N, Stjernschantz J. Effects of prostaglandins on form deprivation myopia in the chick. Acta Ophthalmol. Scand. 2000;78(5):495–500. doi: 10.1034/j.1600-0420.2000.078005495.x. [DOI] [PubMed] [Google Scholar]
- 220. Kim JW, Lindsey JD, Wang N, Weinreb RN. Increased human scleral permeability with prostaglandin exposure. Invest. Ophthalmol. Vis. Sci. 2001;42(7):1514–1521. • The increased scleral permeability with prostaglandin exposure reported in this paper calls for further study on the potentially deleterious effects on myopia progression of this family of drugs, which is widely used to treat glaucoma, given that myopes are over-represented among glaucoma patients.
- 221.Seet B, Wong TY, Tan DT, et al. Myopia in Singapore: taking a public health approach. Br. J. Ophthalmol. 2001;85(5):521–526. doi: 10.1136/bjo.85.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dirani M, Chan Y-H, Gazzard G, et al. Prevalence of refractive error in Singaporean Chinese children: the Strabismus, Amblyopia, and Refractive Error in Young Singaporean Children (STARS) study. Invest. Ophthalmol. Vis. Sci. 2010;51:1348–1355. doi: 10.1167/iovs.09-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov. Today. 2008;13(3–4):135–143. doi: 10.1016/j.drudis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 224.Kim SH, Lutz RJ, Wang NS, Robinson MR. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007;39(5):244–254. doi: 10.1159/000108117. [DOI] [PubMed] [Google Scholar]
- 225.Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov. Today. 2008;13(3–4):144–151. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 226.Short BG. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol. Pathol. 2008;36(1):49–62. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- 227.Gruber E. Treatment of myopia with atropine and bifocals. Ophthalmology. 1985;92(7):985. [PubMed] [Google Scholar]
- 228.Yen MY, Liu JH, Kao SC, Shiao CH. Comparison of the effect of atropine and cyclopentolate on myopia. Ann. Ophthalmol. 1989;21(5):180–182. [PubMed] [Google Scholar]
- 229.Syniuta LA, Isenberg SJ. Atropine and bifocals can slow the progression of myopia in children. Binocul. Vis. Strabismus. Q. 2001;16(3):203–208. [PubMed] [Google Scholar]
- 230.Kennedy RH, Dyer JA, Kennedy MA, et al. Reducing the progression of myopia with atropine: a long term cohort study of Olmsted County students. Binocul. Vis. Strabismus. Q. 2000;15(3):281–304. [PubMed] [Google Scholar]
- 231. Fang PC, Chung MY, Yu HJ, Wu PC. Prevention of myopia onset with 0.025% atropine in premyopic children. J. Ocul. Pharmacol. Ther. 2010;26(4):341–345. doi: 10.1089/jop.2009.0135. • Of interest owing to the exceptionally low dose of atropine used, and because it targeted premyopic instead of myopic children, with a therapeutic goal of prevention rather than slowed progression of myopia.
- 232.Diether S, Schaeffel F, Lambrou GN, Fritsch C, Trendelenburg AU. Effects of intravitreally and intraperitoneally injected atropine on two types of experimental myopia in chicken. Exp. Eye Res. 2007;84(2):266–274. doi: 10.1016/j.exer.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 233.Liu S, Li J, Tan DT, Beuerman RW. Expression and function of muscarinic receptor subtypes on human cornea and conjunctiva. Invest. Ophthalmol. Vis. Sci. 2007;48(7):2987–2996. doi: 10.1167/iovs.06-0880. [DOI] [PubMed] [Google Scholar]
- 234.Collison DJ, Coleman RA, James RS, Carey J, Duncan G. Characterization of muscarinic receptors in human lens cells by pharmacologic and molecular techniques. Invest. Ophthalmol. Vis. Sci. 2000;41(9):2633–2641. [PubMed] [Google Scholar]
- 235. Fischer AJ, McKinnon LA, Nathanson NM, Stell WK. Identification and localization of muscarinic acetylcholine receptors in the ocular tissues of the chick. J. Comp. Neurol. 1998;392(3):273–284. • Reports the most comprehensive characterization study of muscarinic receptors in chick ocular tissues, informing later pharmacological studies testing muscarinic antagonists using chick models of myopia.
- 236.Ishizaka N, Noda M, Yokoyama S, Kawasaki K, Yamamoto M, Higashida H. Muscarinic acetylcholine receptor subtypes in the human iris. Brain Res. 1998;787(2):344–347. doi: 10.1016/s0006-8993(97)01554-0. [DOI] [PubMed] [Google Scholar]
- 237.Vessey KA, Cottriall CL, McBrien NA. Muscarinic receptor protein expression in the ocular tissues of the chick during normal and myopic eye development. Brain Res. Dev. Brain Res. 2002;135(1–2):79–86. doi: 10.1016/s0165-3806(02)00309-7. [DOI] [PubMed] [Google Scholar]
- 238.Ali SF, Hong JS, Bondy SC. Alterations in retinal neurotransmitter receptors and neuropeptides of the chick by kainic acid and acrylamide. Brain Res. 1983;274(1):115–118. doi: 10.1016/0006-8993(83)90525-5. [DOI] [PubMed] [Google Scholar]
- 239.Schliebs R, Bigl V, Biesold D. Development of muscarinic cholinergic receptor binding in the visual system of monocularly deprived and dark reared rats. Neurochem. Res. 1982;7(9):1181–1198. doi: 10.1007/BF00964894. [DOI] [PubMed] [Google Scholar]
- 240. Qu J, Zhou X, Xie R, et al. The presence of m1 to m5 receptors in human sclera: evidence of the sclera as a potential site of action for muscarinic receptor antagonists. Curr. Eye Res. 2006;31(7–8):587–597. doi: 10.1080/02713680600770609. • The finding of all five subtypes of muscarinic receptors on human sclera, as reported in this paper, opens the possibility that the sclera is the site of action for the antimyopia effects of antimuscarinic drugs such as atropine.
- 241.Arumugam B, McBrien N. The D2 antagonist spiperone prevents muscarinic antagonist control of experimentally-induced myopia in chick. Presnted at: Association for Research in Vision and Ophthalmology Annual Meeting; 2–6 May 2010; FL, USA. [Google Scholar]
- 242.Schaeffel F, Bartmann M, Hagel G, Zrenner E. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res. 1995;35(9):1247–1264. doi: 10.1016/0042-6989(94)00221-7. [DOI] [PubMed] [Google Scholar]
- 243.Diether S, Schaeffel F. Long-term changes in retinal contrast sensitivity in chicks from frosted occluders and drugs: relations to myopia? Vision Res. 1999;39(15):2499–2510. doi: 10.1016/s0042-6989(99)00005-x. [DOI] [PubMed] [Google Scholar]
- 244.Xie F, Chen YG. [Influence of disulfiram on the expression of transforming growth factor-β2 in form-deprived eyes in chicks] Beijing Da Xue Xue Bao. 2008;40(6):610–615. [PubMed] [Google Scholar]
- 245.Mao J, Liu S, Wen D, Tan X, Fu C. Basic fibroblast growth factor suppresses retinal neuronal apoptosis in form-deprivation myopia in chicks. Curr. Eye Res. 2006;31(11):983–987. doi: 10.1080/02713680600910510. [DOI] [PubMed] [Google Scholar]
- 246. Rada JA, Johnson JM, Achen VR, Rada KG. Inhibition of scleral proteoglycan synthesis blocks deprivation-induced axial elongation in chicks. Exp. Eye Res. 2002;74(2):205–215. doi: 10.1006/exer.2001.1113. • The results of this study are the first to provide evidence that interference with scleral remodeling can influence eye growth.