Abstract
Loss of the Fragile X mental retardation protein (FMRP) is associated with presumed postsynaptic deficits in mouse models of Fragile X syndrome. However, the possible presynaptic roles of FMRP in learning-related plasticity have received little attention. As a result, the mechanisms whereby FMRP influences synaptic function remain poorly understood. To investigate the cellular locus of the effects of FMRP on synaptic plasticity, we cloned the Aplysia homolog of FMRP and find it to be highly expressed in neurons. By selectively down-regulating FMRP in individual Aplysia neurons at the sensory-to-motor neuron synapse reconstituted in co-cultures, we demonstrate that FMRP functions both pre- and postsynaptically to constrain the expression of long-term synaptic depression induced by repeated pulses of FMRF-amide. In contrast, FMRP has little to no effect on long-term synaptic facilitation induced by repeated pulses of serotonin. Since other components of signaling pathways involved in plasticity appear to be conserved between Aplysia and mammalian neurons, our findings suggest that FMRP can participate in both pre- and postsynaptic regulation of enduring synaptic plasticity that underlies the storage of certain types of long-term memory.
Fragile X syndrome is the most common genetically inherited form of mental impairment and results from the loss of a single protein, FMRP, encoded by the FMR1 gene (Penagarikano et al. 2007). FMRP is an RNA binding protein that is localized throughout the cell body and dendrites of neurons and is thought to regulate the translation of proteins required for synaptic plasticity, perhaps in an activity-dependent and local fashion (Bagni and Greenough 2005). In support of this idea, mice lacking FMRP have enhanced type 1 metabotropic glutamate receptor (mGluR)–dependent hippocampal long-term depression (LTD) (Huber et al. 2002), a form of plasticity that requires protein synthesis in the postsynaptic cell (Huber et al. 2001).
Despite increasing evidence regarding the postsynaptic dendritic function of FMRP, little is known about its role in the presynaptic neuron. Several findings suggest that the loss of FMRP also has presynaptic effects. For example, some Fragile X individuals exhibit structural changes in their brain that are indicative of abnormalities in both axon segregation and aberrant white matter connectivity (Barnea-Goraly et al. 2003; Haas et al. 2009). Similarly, in the hippocampus, axonal projections from granule cells in the dentate gyrus to CA3 pyramidal neurons are abnormal in Fmr1 knockout mice (Ivanco and Greenough 2002; Mineur et al. 2002). In addition, several studies in flies and rodents have demonstrated that FMRP can localize to axons and presynaptic specializations (Feng et al. 1997a; Antar et al. 2006; Christie et al. 2009), and that altered levels of FMRP affect growth cone dynamics (Antar et al. 2006; Li et al. 2009a) and axonal morphology (Morales et al. 2002; Bureau et al. 2008), as well as synapse and circuit formation (Zhang et al. 2001; Hanson and Madison 2007; Bureau et al. 2008; Gibson et al. 2008). Furthermore, FMRP is predicted to bind several mRNAs coding for proteins that are localized to axons and are involved in path-finding and synaptic plasticity (Brown et al. 2001; Miyashiro et al. 2003; Zalfa et al. 2003; Darnell et al. 2004).
These examples suggest an additional presynaptic role for FMRP. Although presynaptic FMRP has been implicated in synaptic plasticity (Bureau et al. 2008; Gibson et al. 2008; Zhang et al. 2009), no direct test of the role of FMRP in presynaptic function in forms of long-term synaptic plasticity has been undertaken. To address this question, we have cloned the homolog of FMRP in Aplysia californica (ApFMRP) and have studied its regulatory role in long-term synaptic plasticity using Aplysia sensory-to-motor neuron co-cultures. This reduced preparation is capable of expressing multiple forms of long-term synaptic plasticity that underlie sensitization and habituation, two simple forms of learning in Aplysia (Montarolo et al. 1986, 1988; Rayport and Schacher 1986) and allows the selective manipulation of pre- and postsynaptic neurons. We find that in Aplysia, FMRP plays a modulatory role during the expression of long-term synaptic depression induced by repeated pulses of FMRF-amide. In addition, we identify a novel presynaptic locus for FMRP function and confirm a postsynaptic role of FMRP in Aplysia previously described in rodents.
Results
Identification of an Aplysia FMR1 homolog (ApFMR1)
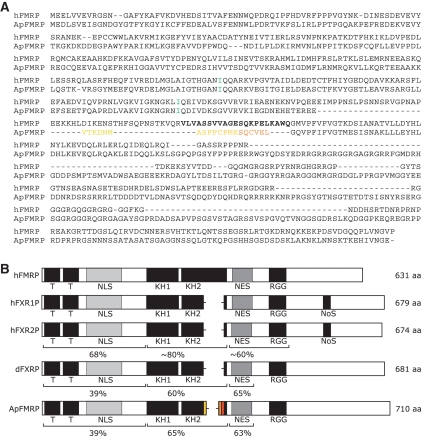
ApFMR1 was cloned using a combination of degenerate primed PCR, RACE PCR-based cloning, and isolation of two overlapping clones from a cDNA library constructed from Aplysia central nervous system mRNA. The resulting full-length clone codes for a protein approximately 710 amino acids in length (Fig. 1A). Comparison of Aplysia, mammalian, and Drosophila Fragile X-related proteins reveals both marked conservation and potentially important differences (Fig. 1B). Overall, ApFMRP is 40% identical to human FMRP, with significantly higher conservation in regions containing identified functional domains. Importantly, ApFMRP contains all of the key domains found in the family of Fragile X-related proteins including RNA binding motifs and elements involved in subcellular localization. The amino-terminal region of FMRP mediates protein–protein interactions and contains two relatively well-conserved Tudor domains that are common to RNA binding proteins (Maurer-Stroh et al. 2003; Ramos et al. 2006). Human and Aplysia FMRP share ∼39% identity in this region. The two KH domains in ApFMRP are ∼60% identical to human FMRP and retain a conserved isoleucine residue that is important for FMRP function (De Boulle et al. 1993; Feng et al. 1997b; Darnell et al. 2005b; Zang et al. 2009). ApFMRP also contains an RGG box, a poorly defined domain that is involved in RNA binding (Kiledjian and Dreyfuss 1992). Homology scores between human and Aplysia FMRP in this region are relatively low because this domain is defined only as containing a high concentration of arginines and glycines. Furthermore, it is unclear whether there are multiple distinct RGG boxes in the carboxy-terminal region of ApFMRP or if this domain is merely expanded.
Figure 1.
Conservation among the Fragile X–related family of proteins. (A) Amino acid alignment of human FMRP and the A. californica homolog. Yellow and orange amino acids represent products of alternative splicing. (Green) Conserved isoleucines in KH domains. (Boldface) Amino acids coded for by human FMR1 exon 12. (B) Alignment of human, Drosophila, and Aplysia Fragile X-related proteins reveal that ApFMRP contains all of the key structural elements found in human FMRP (hFMRP) including amino acids corresponding to regions that are absent in the Fragile X-related family members FXR1P and FXR2P. Scores are expressed as percent identity to hFMRP. (NLS) Nuclear localization signal; (KH) K homology domain; (NES) nuclear export signal; (RGG) arginine glycine box; (NoS) nucleolar signal sequence.
ApFMR1 is alternatively spliced
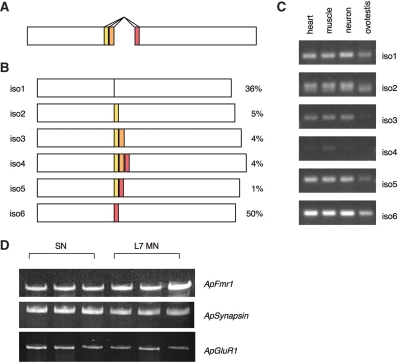
Mammalian FMR1 undergoes significant alternative splicing giving rise to at least 12 possible protein isoforms (Ashley et al. 1993; Verkerk et al. 1993). Interestingly, many of the ApFMR1 clones identified during screening contained small insertions of variable lengths in the second KH domain. Analysis of genomic sequence between coding regions flanking these splice sites revealed that these insertions arise from alternative 5′ donor and 3′ acceptor splice sites in two adjacent exons (Fig. 2A). Alternative splicing at these positions does not shift the reading frame of downstream ApFMR1 sequences and can produce six possible isoforms (Fig. 2B). These insertions give rise to amino acids that exhibit some identity to those coded for by exons 11 and 12 of mammalian FMR1, regions that are not present in other Fragile X-related genes, FXR1 and FXR2, or in the Drosophila FMR1 homolog (Siomi et al. 1995; Zhang et al. 1995; Wan et al. 2000; Kirkpatrick et al. 2001). Analysis of Drosophila genomic sequence in analogous regions revealed no conventional splice sites that could produce similar alternative splicing, nor are there genomic sequences that can potentially code for homologous insertions. This further underscores the similarity of Aplysia and mammalian FMRP.
Figure 2.
ApFMR1 is alternatively spliced. (A) Alternatively spliced isoforms of ApFMR1 result from alternate 5′ donor and 3′ acceptor splice sites of adjacent exons. (B) Schematic depiction of the resulting six possible isoforms and their relative abundance in neurons. (C) RT-PCR for specific ApFMR1 isoforms demonstrates that they are expressed in various tissues including neurons. (D) ApFMR1 is expressed equally well in Aplysia sensory and L7 motor neurons using single-cell RT-PCR. These results show the representative triplicates of the experiments performed (n = 6). The synaptic markers ApSynapsin and ApGluR2 were used as internal controls (Li et al. 2009b).
ApFMR1 is widely expressed
To determine the expression profile of ApFMRP isoforms among multiple tissues, ApFMR1 was amplified from RNA harvested from different organs from adult Aplysia using isoform-specific primers. ApFMR1 has a widespread expression pattern among various tissues, with abundant expression in neurons (Fig. 2C). This is consistent with FMR1 expression in other species including flies, zebrafish, frogs, and mammals (Hinds et al. 1993; Wan et al. 2000; Tucker et al. 2004; Lim et al. 2005). In neurons, mRNA corresponding to ApFMRP isoform six is more abundant than other alternatively spliced forms as detected by semiquantitative RT-PCR (Fig. 2C). This finding is corroborated by quantification of the frequency of different isoforms identified among clones during ApFMR1 screening; of 109 clones identified from CNS-derived cDNA, ApFMRP isoforms were represented as follows: isoform 1: 36%; isoform 2: 5%; isoform 3: 4%; isoform 4: 4%; isoform 5: 1%; and isoform 6: 50%. Isoform 6 contains an insertion in the region of alternative splicing, which based on homology comparisons suggests that it is more like FMRP than the FXRs (Kirkpatrick et al. 2001).
Since Aplysia sensory-to-motor neuron co-cultures have been widely used for studies of long-term synaptic plasticity, we verified the expression of ApFMR1 in these neurons using single-cell RT-PCR (Fig. 2D). Because each isoform varies only in a small region, it is not feasible to design nested primer pairs that are specific for each isoform. Thus, we adopted nested primer pairs that are common to all ApFMR1 isoforms. We found that ApFMR1 is expressed equally well in both Aplysia sensory and L7 motor neurons (Fig. 2D).
ApFMRP is distributed throughout neuronal cell bodies and neurites
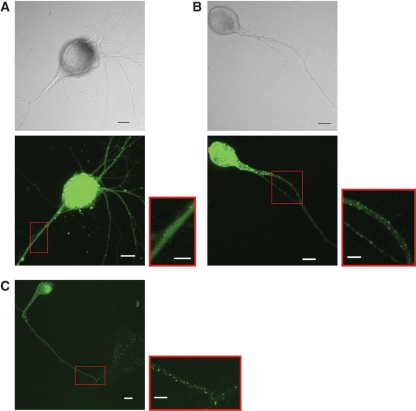
In all species studied to date, the subcellular localization of FMRP has been predominantly cytoplasmic. Because antibodies to ApFMRP do not exist, we expressed the most abundant ApFMR1 isoform in Aplysia CNS, isoform 6, as an ApFMRP-ECFP fusion protein to determine the subcellular localization of ApFMRP. Aplysia sensory neurons were injected with a DNA construct coding for either ECFP or an ApFMRPiso6–ECFP fusion protein and were imaged by confocal microscopy. Whereas ECFP labeled neurons uniformly (Fig. 3A), ApFMRPiso6–ECFP was found in the cell body and extended into neurites in a granular pattern reminiscent of FMRP localization in primary cultures of rodent neurons (Fig. 3B; Antar et al. 2005, 2006). These structures extend throughout sensory cell neurites regardless of whether cells are cultured alone (Fig. 3B) or in the presence of postsynaptic target neurons (Fig. 3C).
Figure 3.
ApFMRPiso6–ECFP localization in Aplysia neurons. When expressed in isolated sensory neurons in culture, (A) ECFP uniformly fills the extent of neurites, but (B) ApFMRPiso6–ECFP forms granules that extend throughout neurites. (C) In sensory–motor neuron co-cultures, ApFMRPiso6–ECFP forms granules that extend throughout neurites of sensory neurons. Scale bar, 20 µm. Red boxes show higher magnification views with scale bar, 10 µm.
Reduction of ApFMRP enhances long-term depression induced by FMRFamide
Although FMRP has been shown to play a role in several long-lasting forms of synaptic plasticity in mammals (Huber et al. 2002; Li et al. 2002; Koekkoek et al. 2005; Larson et al. 2005; Zhao et al. 2005; Desai et al. 2006; Nosyreva and Huber 2006; Lauterborn et al. 2007; Meredith et al. 2007; Wilson and Cox 2007; Hu et al. 2008; Shang et al. 2009; Auerbach and Bear 2010), the relative contribution of FMRP located in either the pre- or postsynaptic compartment has not been determined. Since ApFMR1 is expressed throughout the Aplysia nervous system and pre- and postsynaptic cells can be selectively manipulated in sensory–motor neuron co-cultures, we have directly examined the role of both pre- and postsynaptic ApFMRP in regulating long-term synaptic plasticity. To deplete ApFMRP, we injected individual cultured neurons with an antisense oligonucleotide designed to selectively target and degrade all isoforms of ApFMR1 mRNA (Supplemental Fig. S1).
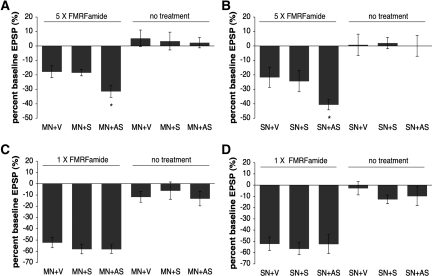
To evaluate possible postsynaptic roles of ApFMRP, we injected motor neurons in sensory–motor neuron co-cultures with either an antisense oligonucleotide targeted against ApFMR1, a sense (control) oligonucleotide, or vehicle alone. We then induced protein synthesis-dependent long-term synaptic depression by stimulation with five pulses of 1 µM FMRF-amide. In cultures with motor neurons injected with vehicle alone or with a sense oligonucleotide, the mean amplitude of the EPSP in the sensory–motor neuron synapse decreased 24 h following repeated exposure to FMRF-amide (sense oligo [S] %EPSP, −18.45% ± 2.2%, n = 11; vehicle alone [V] %EPSP, −17.8% ± 4.11%, n = 15; Fig. 4A). In contrast, in cultures with motor neurons injected with ApFMR1 antisense oligonucleotides, the decrease in mean EPSP amplitude 24 h after FMRF-amide treatment was significantly enhanced (%EPSP, −31.38% ± 4.14%, n = 16; P = 0.015 vs. V; P = 0.027 vs. S; Fig. 4A). Oligonucleotide injection did not affect EPSPs recorded 24 h post-injection in the absence of FMRF-amide treatment (V %EPSP, +5.29% ± 5.74%, n = 7; S %EPSP, +3.38% ± 6.16%, n = 8; antisense oligo [AS] %EPSP, +2.30% ± 3.62%, n = 10; P > 0.05), indicating that postsynaptic ApFMRP does not modulate basal synaptic transmission. The enhancement of long-term depression in Aplysia due to the depletion of ApFMR1 is similar to findings in Fmr1 knockout mice that identify enhanced forms of late-phase LTD in the hippocampus and cerebellum (Huber et al. 2002; Koekkoek et al. 2005) that are thought to have a predominantly postsynaptic mechanism of expression (Huber et al. 2001; Koekkoek et al. 2005).
Figure 4.
Down-regulation of ApFMRP enhances long-term depression induced by FMRF-amide. Repeated exposure to FMRF-amide (1 µM) causes a long-term depression of synaptic transmission measured 24 h after induction. Injection of an ApFMR1 antisense oligonucleotide (AS) into either postsynaptic motor neurons (A) or presynaptic sensory neurons (B) enhances FMRF-amide-induced long-term depression of synaptic transmission 24 h after induction, whereas injection of sense oligonucleotide (S) or vehicle alone (V) does not. In contrast, neither post- nor presynaptic injection of ApFMR1 AS or S oligonucleotides affected short-term depression induced with a single 5-min pulse of 1 µM FMRF-amide (C and D, respectively). Oligonucleotide injections did not affect basal transmission recorded post-injection in the absence of FMRF-amide.
To determine whether FMRP also has a presynaptic contribution to long-lasting forms of synaptic depression, we looked at the effect of injecting presynaptic sensory neurons in sensory–motor neuron co-cultures with an ApFMR1 antisense oligonucleotide on the expression of long-term synaptic depression induced by stimulation with five pulses of 1 µM FMRF-amide (Fig. 4B). Whereas the mean amplitude of the EPSP at the sensory–motor neuron synapse decreased 24 h following repeated exposure to FMRF-amide in cultures with sensory neurons injected with vehicle alone or with a sense control oligonucleotide (S %EPSP, −24.32% ± 7.35%, n = 18; V %EPSP, −21.67% ± 6.99%, n = 18), injection of sensory neurons with ApFMR1 antisense oligonucleotides significantly enhanced the decrease in mean EPSP amplitude in response to FMRF-amide (%EPSP, −40.59% ± 3.62%, n = 27; P = 0.024 vs. V; P = 0.048 vs. S; Fig. 4B). Again, oligonucleotide injection did not affect basal synaptic transmission recorded 24 h post-injection in the absence of FMRF-amide treatment (V %EPSP, +0.77% ± 7.40%, n = 13; S %EPSP, +2.06% ± 3.93%, n = 18; AS %EPSP, +0.056% ± 7.19%, n = 18; P > 0.05).
Taken together, these results suggest a general role for FMRP in processes specific to regulating the maintenance of synaptic depression. Furthermore, while experiments in Fmr1 knockout mice have not been able to distinguish pre- from postsynaptic roles of FMRP in hippocampal LTD, our data in Aplysia offer direct evidence for a presynaptic as well as a postsynaptic role for FMRP in long-term synaptic depression.
Reduction of ApFMRP does not affect FMRF-amide-induced short-term depression
We next asked whether ApFMRP has a general role in regulating processes that underlie synaptic depression or whether its role is specific to long-lasting forms of depression. To test whether FMRP plays a postsynaptic role in short-term depression, we injected motor neurons in sensory–motor neuron co-cultures with either antisense oligonucleotide targeted against ApFMR1, a sense control oligonucleotide, or vehicle alone and induced short-term synaptic depression by stimulating co-cultures with a single 5-min pulse of 1 µM FMRF-amide. The mean amplitude of the EPSP in the sensory–motor neuron synapse decreased 5 min after exposure to one pulse of FMRF-amide (V %EPSP, −52.20% ± 4.69%, n = 10) and was unaffected by injection of antisense or sense oligonucleotides (S %EPSP, −58.00% ± 4.23%, n = 8; AS %EPSP, −58.11% ± 3.74%, n = 9; P > 0.05; Fig. 4C). Injection of motor neurons with oligonucleotide did not affect basal synaptic transmission in the absence of FMRF-amide treatment (V %EPSP, −11.75% ± 4.73%, n = 4; AS %EPSP, −13.2% ± 6.45%, n = 5; S %EPSP, −6.17% ± 7.64%, n = 6; P > 0.05). Similarly, we found that injection of either antisense oligonucleotides targeted against ApFMR1 or control oligonucleotides into sensory neurons in sensory–motor neuron co-cultures had no effect on the mean amplitude of the EPSP in response to a single pulse of 1 µM FMRF-amide (V %EPSP, −52.13% ± 6.00%, n = 8; S, −56.55% ± 5.30%, n = 11; AS, −52.33% ± 8.40%, n = 9; P > 0.05; Fig. 4D). We again found that oligonucleotide injection into sensory neurons did not affect basal synaptic transmission in the absence of FMRF-amide treatment (V %EPSP, −2.71% ± 6.00%, n = 7; S, −12.57% ± 3.84%, n = 7; AS, −9.75% ± 8.35%, n = 8; P > 0.05). These results indicate that neither pre- nor postsynaptic ApFMRP contributes to short-term synaptic depression or basal synaptic transmission.
Synaptic facilitation induced by 5-HT is not changed by reduction of ApFMRP
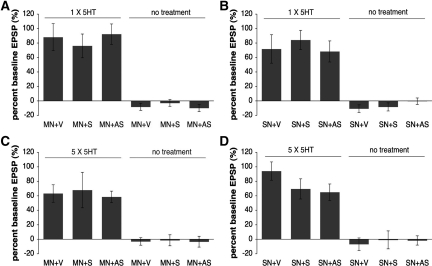
Finally, we asked whether ApFMRP regulates processes underlying long-term synaptic plasticity in general or if it is specific to synaptic depression. We first asked whether ApFMRP plays a role in the induction of short-term facilitation induced by serotonin (5-HT). Postsynaptic motor neurons in sensory–motor neuron co-cultures were injected with an antisense oligonucleotide targeting ApFMR1, a sense control oligonucleotide, or vehicle alone, and short-term facilitation was induced by stimulation with a single 5-min pulse of 10 µM 5-HT. All groups demonstrated comparable increases in EPSP amplitude 5 min following exposure to 5-HT (V %EPSP, +88.11% ± 18.61%, n = 9; S %EPSP, +76.08% ± 16.40%, n = 12; AS %EPSP, +92.27% ± 14.13%, n = 11; P > 0.05; Fig. 5A). Oligonucleotide injection also did not affect basal synaptic transmission in the absence of 5-HT treatment (V %EPSP, −8.11% ± 4.9%, n = 9; S %EPSP, −2.80% ± 4.62%, n = 10; AS %EPSP, −9.80% ± 4.82%, n = 10; P > 0.05). Similarly, oligonucleotide injection into presynaptic sensory neurons in sensory–motor neuron co-cultures also did not affect short-term potentiation induced by one pulse of 5-HT (V %EPSP, +71.80% ± 19.69%, n = 10; S %EPSP, +84.22% ± 13.30%, n = 9; AS %EPSP, +68.30% ± 14.56%, n = 10; P > 0.05) or basal synaptic transmission (V %EPSP, −10.67% ± 5.19%, n = 9; S %EPSP, −8.25% ± 5.62%, n = 8; AS %EPSP, −0.27% ± 4.46%, n = 15; P > 0.05; Fig. 5A). These results indicate that neither pre- nor postsynaptic ApFMRP modulates the induction of short-term facilitation by 5-HT.
Figure 5.
Down-regulation of ApFMRP does not affect long-term facilitation induced by 5-HT. Short-term facilitation was induced with one 5-min pulse of 10 µM 5-HT. The mean amplitude of the EPSP in the sensory–motor neuron synapse increased 5 min after exposure to 5-HT and was unaffected by injection of antisense (AS) or sense (S) ApFMR1 oligonucleotides into postsynaptic motor neurons (A) or presynaptic sensory neurons (B). Repeated exposure to 5-HT (10 µM) causes a long-term facilitation of synaptic transmission measured 24 h after induction. Injection of antisense (AS) or sense (S) ApFMR1 oligonucleotides into postsynaptic motor neurons (C) or presynaptic sensory neurons (D) did not change 5-HT-induced long-term facilitation of synaptic transmission 24 h after induction. Oligonucleotide injections did not affect basal transmission recorded post-injection in the absence of 5-HT.
We next addressed whether ApFMRP has a role in the long-term facilitation induced by repeated exposure to 5-HT. Motor neurons in sensory–motor neuron co-cultures were injected with an antisense oligonucleotide targeted to ApFMR1, a sense oligonucleotide, or vehicle alone, and long-term potentiation was induced by stimulation with five pulses of 10 µM 5-HT. All groups demonstrated comparable increases in EPSP amplitude 24 h following exposure to 5-HT (V %EPSP, +63.14% ± 12.09%, n = 7; S %EPSP, +67.90% ± 24.45%, n = 10; AS %EPSP, +58.54% ± 7.95%, n = 13; P > 0.05), and oligonucleotide injection did not affect basal synaptic transmission recorded at 24 h in the absence of 5-HT treatment (V %EPSP, −3.0% ± 5.15%, n = 8; S %EPSP, −1.43% ± 7.64%, n = 7; AS %EPSP, −3.33% ± 7.50%, n = 6; P > 0.05; Fig. 5C). Similarly, injection into the presynaptic sensory neuron did not affect basal synaptic transmission recorded at 24 h in the absence of 5-HT treatment (V %EPSP, −6.78% ± 8.61%, n = 9; S %EPSP, −0.83% ± 12.45%, n = 6; AS %EPSP, −1.67% ± 6.36%, n = 6; P > 0.05), or long-term facilitation of EPSP amplitude 24 h following repeated exposure to 5-HT (V %EPSP, +94.1% ± 12.58%, n = 20; S %EPSP, +69.5% ± 13.94%, n = 18; AS %EPSP, +65% ± 11.36%, n = 17; P > 0.05; Fig. 5D). The inability of the knockdown of ApFMRP to affect processes required for the induction or expression of LTF is similar to several findings in Fmr1 knockout mice that have largely failed to identify a role of FMRP in multiple forms of hippocampal long-term potentiation (LTP) (Godfraind et al. 1996; Paradee et al. 1999; Li et al. 2002; Larson et al. 2005; but see Lauterborn et al. 2007; Hu et al. 2008; Shang et al. 2009; Auerbach and Bear 2010).
Discussion
Aplysia express a homolog of FMR1
Comparison with mammalian FMR1 demonstrates that ApFMR1 represents the Aplysia homolog of the Fragile X gene family. Consistent with expression profiles of FMR1 orthologs in other species, ApFMR1 appears to be widely expressed, with the highest levels of expression in central neurons, and relatively low, but significant levels in all other tissues tested. This pattern of expression is compatible with findings in vertebrates and flies (Hinds et al. 1993; Schenck et al. 2001; Tucker et al. 2004; Lim et al. 2005).
The characterization of several cDNAs identified during screening for ApFMRP did not identify additional Fragile X-related transcripts in Aplysia, nor did analysis of more than 190,000 ESTs from Aplysia CNS libraries (Moroz et al. 2006). Given the degree of sequence homology in the FMRP KH domains among multiple species, it is unlikely that these cloning methods would have missed additional family members. Thus, it appears that, as with the Drosophila FMRP homolog (Wan et al. 2000), a single gene in Aplysia represents the family of vertebrate Fragile X-related proteins. The theory of gene duplication as a major genetic force permitting the evolution of vertebrates from invertebrates (Ohno 1970) is now generally accepted, and it is not uncommon for invertebrate genomes to contain single genes that are orthologous to entire vertebrate gene families (e.g., Araki et al. 1996; Stock et al. 1997). This single gene in invertebrates has most likely evolved via duplication to give rise to the Fragile X-related family of genes in mammals.
ApFMRP contains three distinct classes of highly conserved RNA binding domains that are characteristic of Fragile X-related proteins in other species: Tudor, KH, and RGG box domains. These regions share significant similarity with human FMRP in regions that confer RNA binding capacity, including the conservation of isoleucine residues important for FMRP KH domain function. ApFMRP is also homologous to the Fragile X-related proteins in regions involved in ribosome association and protein–protein interactions. In addition, the ApFMRP–ECFP fusion protein forms granular structures that are distributed throughout the neurites of cultured Aplysia neurons in a pattern similar to the distribution of mammalian FMRP in dendrites (Antar et al. 2005, 2006; Ferrari et al. 2007). The subcellular distribution and these RNA binding domains are central to the function of FMRP in processes including its regulation of RNA stability (Zalfa et al. 2007), transport and trafficking (De Diego Otero et al. 2002; Antar et al. 2005; Ferrari et al. 2007), and association with polyribosomes (Khandjian et al. 1996; Tamanini et al. 1996; Corbin et al. 1997; Feng et al. 1997b; Stefani et al. 2004; Darnell et al. 2005a). The dendritic and synaptic localization of FMRP (Feng et al. 1997b; Ferrari et al. 2007) and its association with mRNAs coding for proteins involved in synaptic plasticity (Zalfa et al. 2003; Iacoangeli et al. 2008) and synthesized in response to synaptic signals (Park et al. 2008; Waung et al. 2008) support a role for FMRP in local protein synthesis at the synapse, a cellular process thought to underlie changes in the morphology and stability of synapses associated with the expression of long-term plasticity (Martin and Zukin 2006). Indeed, studies in rodents have identified the importance of the KH2 RNA binding domain in the formation of dendritically localized FMRP granules (Darnell et al. 2005a) and elimination of dendritic spines (Pfeiffer and Huber 2007), as well as alterations in synaptic plasticity and behaviors associated with the loss of FMRP function in mice (Zang et al. 2009).
Further characterization of ApFMRP may provide valuable insight into the function of human FMRP. Human FMRP contains a stretch of amino acids encoded by exons 11 and 12 that are unique among the Fragile X-related family of proteins. While vertebrate FXRP proteins and the Drosophila Fragile X homolog do not contain these sequences, several alternatively spliced neuronal isoforms of ApFMRP contain inserts exhibiting some homology to amino acids in this unique region. Structure studies of mammalian FMRP and other proteins containing KH domains suggest that these extra amino acids are part of a variable loop region in the second KH domain of FMRP that contains charged residues in an exposed position (Valverde et al. 2007). Whereas little is known about the function of this loop, its inclusion appears to alter the affinity of FMRP for certain RNA targets (Denman and Sung 2002; Xie et al. 2009), and alternative splicing in this region may serve to increase the functional diversity of FMRP by affecting its RNA binding and target recognition properties. In addition, since the FXRPs lack these sequences and do not appear to function as repressors of protein synthesis in vitro (Laggerbauer et al. 2001), this loop may also affect FMRP-mediated regulation of translation.
FMRP function in synaptic plasticity
To begin to study the pre- and postsynaptic roles of FMRP in long-term plasticity, we used a reduced neuronal culture system that reconstitutes critical components of the circuitry underlying a simple form of behavioral learning in Aplysia. Similar to findings that long-term depression is enhanced in mice lacking FMRP (Huber et al. 2002; Koekkoek et al. 2005), we find that injection of individual cultured neurons with an antisense oligonucleotide designed to selectively target and degrade ApFMR1 mRNA leads to an enhancement of FMRF-amide-induced long-term depression. In contrast, antisense oligonucleotide injection does not affect long-term facilitation induced by repeated exposure to 5-HT at these synapses. This result is consistent with findings from several studies that suggest some forms of hippocampal long-term potentiation are unaffected in Fmr1 knockout mice (Godfraind et al. 1996; Paradee et al. 1999; Li et al. 2002; Larson et al. 2005). Together, these studies highlight the similarities between forms of long-term plasticity in Aplysia and the mammalian hippocampus and suggest that FMRP normally functions to regulate molecules critical for the expression of long-term synaptic depression.
What may underlie the context specificity of the effect of FMRP knockdown on the expression of these forms of long-term plasticity? One possibility is that FMRF-amide induction of protein synthesis-dependent LTD specifically recruits signaling pathways that engage FMRP, for example, mGluR signaling. If 5-HT induction of LTF does not require the same signaling pathways, FMRP may not be recruited. Alternatively, FMRP may regulate the synthesis of a subset of mRNAs that are specifically required for the expression of LTD, but not LTF. Indeed, a recent study suggests that local protein synthesis can be both transcript- and stimulus-specific (Wang et al. 2009).
Whereas FMRP knockdown did not affect protein synthesis-dependent LTF induced by 5-HT, it remains a possibility that FMRP is involved in other forms of long-lasting synaptic facilitation. For example, some forms of LTP are altered in the cortex (Li et al. 2002; Larson et al. 2005; Zhao et al. 2005; Meredith et al. 2007; Wilson and Cox 2007; Harlow et al. 2010) and in the hippocampus (Lauterborn et al. 2007; Hu et al. 2008; Shang et al. 2009; Auerbach and Bear 2010) of Fmr1 knockout mice. It is known that distinct forms of long-term plasticity can have different mechanisms underlying their expression. Furthermore, it is possible that the function of FMRP varies in a neuron- or synapse-specific manner, depending on the signaling pathways required for the induction or expression of distinct forms of plasticity, as well as the developmental stage of the animal. Alternatively, distinct molecular profiles of populations of cells or individual synapses may influence the regulation of FMRP or the availability of its RNA targets.
What is the locus for the influence of FMRP on synaptic plasticity? Previous investigations of synaptic depression in Fmr1 knockout mice have suggested a postsynaptic role for FMRP by regulating mGluR-stimulated protein synthesis (Huber et al. 2001; Koekkoek et al. 2005). While our findings confirm a postsynaptic role of FMRP in the expression of protein synthesis-dependent long-term depression in Aplysia, they also demonstrate an additional presynaptic role for FMRP in this persistent form of plasticity. Since other mechanisms underlying plasticity appear to be conserved between Aplysia and mammalian neurons (Pittenger and Kandel 2003), it is tempting to suggest that FMRP may also have a coordinated pre- and postsynaptic role in regulating some forms of long-term plasticity in the mammalian brain. For example, although the form of type 1 mGluR-dependent hippocampal LTD that is enhanced in Fmr1 knockout mice has been shown to require de novo protein synthesis in dendrites (Huber et al. 2001), a presynaptic locus has not been directly investigated, but does contribute to other forms of mGluR-dependent LTD in the hippocampus (Zakharenko et al. 2002; Feinmark et al. 2003). Moreover, while the major postsynaptic mechanism for this form of mGluR-LTD is thought to be the internalization of postsynaptic AMPA receptors (Snyder et al. 2001), the levels of AMPA receptor surface expression in acute hippocampal slices from Fmr1 knockouts appear to be comparable to controls in both basal conditions and following the induction and expression of mGluR-LTD (Nosyreva and Huber 2006). The discrepancy between these results could be accounted for by a presynaptic mechanism, such as the one we describe here for Aplysia, that also contributes to the enhanced expression of this form of plasticity in Fmr1 knockout mice.
What, then, are the mechanisms by which FMRP regulates FMRF-amide-induced LTD presynaptically? Putative mRNA targets of FMRP have been identified that code for proteins important for presynaptic function, including munc13 and PP2A, as well as mRNAs coding for proteins important for normal axonal morphology, including MAP1b (Darnell et al. 2001; Castets et al. 2005). As FMRP is able to interact with or regulate the production of factors involved in structural rearrangements (Brown et al. 2001; Darnell et al. 2001; Schenck et al. 2001; Zhang et al. 2001; Miyashiro et al. 2003), it is possible that one or more of those factors is needed for the structural rearrangements leading to synapse destabilization or elimination that is associated with long-term depression (Bailey and Chen 1983, 1988; Bailey et al. 1992). In addition, since FMRP appears to inhibit the translation of its target RNAs (Laggerbauer et al. 2001; Li et al. 2001; Mazroui et al. 2002), it is possible that FMRP normally represses the synthesis of one of the proteins needed for expression of LTD, perhaps via mechanisms involving recruitment of RNAi machinery (Siomi et al. 2004) or interactions with the 4E-BP-like repressor of translation CYFIP1 (Napoli et al. 2008). In this model, down-regulation of FMRP removes an inhibitory constraint on this form of plasticity by increasing the translation of specific mRNAs necessary for long-term plasticity leading to enhanced LTD.
Finally, what might be the relationship between the cognitive impairment and abnormal immature-looking dendritic spines observed in Fragile X individuals and the enhancement of LTD observed following knockdown of FMRP in animal models? It is possible that enhanced LTD leading to enhanced synapse elimination (Bailey and Chen 1983, 1988; Bailey et al. 1992) can slow down the synaptic maturation, resulting in immature-looking dendritic spines and cognitive impairment (Pfeiffer and Huber 2007).
Materials and Methods
Cloning of ApFMRP
Total RNA was extracted from Aplysia pleural and pedal ganglia with Trizol (Invitrogen), and cDNA was synthesized with the Stratascript First Strand cDNA Synthesis Kit (Stratagene). Degenerate primers for PCR were designed against two regions of homology in the Fragile X-related proteins among multiple species: IUPAC code sequences from the KH2 domain corresponding to KVIGKNG (AARGTNATHGGNAARAAYGG) and KEVEQLR (GKNARYTGYTCNACYTCYTT). Bands of predicted size were subcloned into vector PCR2.1 using TOPO-TA cloning (Invitrogen). Similarity to Fragile X-related proteins was determined using the BLAST search program (NCBI).
The 5′ end of ApFMR1 cDNA was identified using the SMART RACE cDNA amplification system (Clontech). To identify the entire ApFMRP coding region, the 5′ half of the ApFMR1 cDNA was then used to screen a phage cDNA library made from Aplysia central nervous system (a gift from Dusan Bartsch). Homology was determined using ClustalW alignments with Fragile X-related proteins from other species: hFMRP accession number NP_002015, hFXR1P accession number EAW78371, hFXR2P accession number NP_004851, and dFXRP accession number NP_731443. Scores are measures of amino acid identity and are calculated as percent identity of a length of sequence.
Subcloning
For expression of ApFMRPiso6–ECFP in Aplysia neurons, ApFmr1 full-length cDNA was amplified from Aplysia cDNA using primers HindIII–FMRstart (CCCAAGCTTCGATGGAGGATCTTTCGGTTGAAATC) and FMRstop–BamHI (CGGGATCCCTATTCCCCATTCACTATATGCTC) and cloned into the HindIII/BamHI sites of pECFP-C1 (Clontech). An NheI/BamHI fragment from pECFPC1–ApFMR1 was subcloned into XbaI/BamHI sites in Aplysia expression vector pNEX3.
RT-PCR
RNA was extracted from multiple tissues using RNAqueous-4-PCR (Ambion), and RT-PCR was performed using HF-PROSTAR RT-PCR kit (Stratagene) with 50 ng of total RNA per reaction and an ApFMRP universal reverse primer (RT-QAKLE R: TTCCAGTTTGGCTTGCCG) and isoform unique forward primers (iso1: AGGGAAGAGGGTCAGGTA; iso2: AGACAACATGGGTCAGGTA; iso3: TGTTGAACTGGGTCAGGTA; iso4: GTTGAACTGGCCTCTTTCT; iso5: AGACAACATGGCCTCTTTCT; iso6: AGGGAAGAGGCCTCT-TTC).
Single-cell RT-PCR was performed as previously described (Li et al. 2009b). The nested primer pairs are as follows: F1: CAGCGGAGACGAAGGAGGAAAAAC; F2: AGGAGGAAAAACCGTCCCCGAGGC; R1: GGCCCCTGAAGTGGCCGACGCAGT; R2: GGCCGACGCAGTCGCAGAGCTGTT.
Oligonucleotide and plasmid DNA injection
Antisense oligonucleotides were chosen from nucleotide sequence contained in all isoforms of ApFMR1 that exhibited the lowest degree of homology to entries in the nonredundant BLAST database; antisense: GTCTTGGATGTTCCGCCCAT; sense: ATGGGCGGAACATCCAAGAC. DNA constructs for injection were purified by equilibrium centrifugation in CsCl–ethidium bromide gradients (Sambrook et al. 1989). DNA and oligonucleotides were dissolved in water and diluted to a concentration of 25 ng/uL (oligo) and 1 µg/uL (DNA) in injection buffer (10 mM Tris-HCl at pH 7.6, 100 mM KCl, 0.1% fast green) and were pressure-injected into motor and sensory neurons with a picospritzer (General Valve) as previously described (Kaang 1996). Oligonucleotides were injected 3 h prior to FMRF-amide or 5-HT treatment.
Sensory and motor neuron co-cultures and electrophysiology
Co-cultures of sensory neurons and the motor neuron L7 of Aplysia californica were prepared as previously described (Montarolo et al. 1988). Electrical recordings were made from motor neurons using intracellular microelectrodes filled with 2.5 M KCl using an Axoclamp-2A amplifier. EPSPs were evoked by stimulating the sensory neurons extracellularly with depolarizing pulses as previously described (Montarolo et al. 1986).
Confocal imaging
Fluorescent images of sensory neurons expressing injected DNA constructs were acquired with Zeiss Axiovert microscopes mounted on an LSM Pascal (Zeiss) laser confocal scanning microscope. Images were taken with a 40 × , NA 0.75 (MRC1000) objective, and the gains and neutral density filters were adjusted to prevent saturation of the detection threshold.
Statistical analysis
All data are presented as mean percentage change ±SEM in the EPSP amplitude as compared to the initial amplitude. A two-factor (treatment/oligo) ANOVA and post hoc Fisher's LSD test were used for multiple comparisons.
Acknowledgments
We thank C.H. Bailey for his critical comments on the manuscript, L. Moroz, A.B. Kohn, and T.J. Ha for assistance in cloning full-length ApFMR1; and Huixiang “Vivian” Zhu and Edward Konstantinov for Aplysia culture preparation. This work is supported by grants from the FRAXA Research Foundation (to S.M.T.), the Howard Hughes Medical Institute (to E.R.K.), the Simons Foundation (to E.R.K.), and National Institutes of Health grant NS053415 (to Y.-B.C.).
Footnotes
[Supplemental material is available for this article.]
References
- Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ 2005. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav 4: 350–359 [DOI] [PubMed] [Google Scholar]
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ 2006. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci 32: 37–48 [DOI] [PubMed] [Google Scholar]
- Araki I, Terazawa K, Satoh N 1996. Duplication of an amphioxus myogenic bHLH gene is independent of vertebrate myogenic bHLH gene duplication. Gene 171: 231–236 [DOI] [PubMed] [Google Scholar]
- Ashley CT, Sutcliffe JS, Kunst CB, Leiner HA, Eichler EE, Nelson DL, Warren ST 1993. Human and murine FMR-1: Alternative splicing and translational initiation downstream of the CGG-repeat. Nat Genet 4: 244–251 [DOI] [PubMed] [Google Scholar]
- Auerbach BD, Bear MF 2010. Loss of the fragile X mental retardation protein decouples metabotropic glutamate receptor dependent priming of long-term potentiation from protein synthesis. J Neurophysiol 104: 1047–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni C, Greenough WT 2005. From mRNP trafficking to spine dysmorphogenesis: The roots of fragile X syndrome. Nat Rev Neurosci 6: 376–387 [DOI] [PubMed] [Google Scholar]
- Bailey CH, Chen M 1983. Morphological basis of long-term habituation and sensitization in Aplysia. Science 220: 91–93 [DOI] [PubMed] [Google Scholar]
- Bailey CH, Chen M 1988. Long-term memory in Aplysia modulates the total number of varicosities of single identified sensory neurons. Proc Natl Acad Sci 85: 2373–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Montarolo P, Chen M, Kandel ER, Schacher S 1992. Inhibitors of protein and RNA synthesis block structural changes that accompany long-term heterosynaptic plasticity in Aplysia. Neuron 9: 749–758 [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Hedeus M, Menon V, White CD, Moseley M, Reiss AL 2003. White matter tract alterations in fragile X syndrome: Preliminary evidence from diffusion tensor imaging. Am J Med Genet B Neuropsychiatr Genet 118: 81–88 [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, et al. 2001. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 107: 477–487 [DOI] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM, Svoboda K 2008. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J Neurosci 28: 5178–5188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castets M, Schaeffer C, Bechara E, Schenck A, Khandjian EW, Luche S, Moine H, Rabilloud T, Mandel JL, Bardoni B 2005. FMRP interferes with the Rac1 pathway and controls actin cytoskeleton dynamics in murine fibroblasts. Hum Mol Genet 14: 835–844 [DOI] [PubMed] [Google Scholar]
- Christie SB, Akins MR, Schwob JE, Fallon JR 2009. The FXG: A presynaptic fragile X granule expressed in a subset of developing brain circuits. J Neurosci 29: 1514–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin F, Bouillon M, Fortin A, Morin S, Rousseau F, Khandjian EW 1997. The fragile X mental retardation protein is associated with poly(A)+ mRNA in actively translating polyribosomes. Hum Mol Genet 6: 1465–1472 [DOI] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB 2001. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 107: 489–499 [DOI] [PubMed] [Google Scholar]
- Darnell JC, Warren ST, Darnell RB 2004. The fragile X mental retardation protein, FMRP, recognizes G-quartets. Ment Retard Dev Disabil Res Rev 10: 49–52 [DOI] [PubMed] [Google Scholar]
- Darnell JC, Mostovetsky O, Darnell RB 2005a. FMRP RNA targets: Identification and validation. Genes Brain Behav 4: 341–349 [DOI] [PubMed] [Google Scholar]
- Darnell JC, Fraser CE, Mostovetsky O, Stefani G, Jones TA, Eddy SR, Darnell RB 2005b. Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev 19: 903–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, Van den Bos F, de Graaff E, Oostra BA, Willems PJ 1993. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet 3: 31–35 [DOI] [PubMed] [Google Scholar]
- De Diego Otero Y, Severijnen LA, van Cappellen G, Schrier M, Oostra B, Willemsen R 2002. Transport of fragile X mental retardation protein via granules in neurites of PC12 cells. Mol Cell Biol 22: 8332–8341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman RB, Sung YJ 2002. Species-specific and isoform-specific RNA binding of human and mouse Fragile X mental retardation proteins. Biochem Biophys Res Commun 292: 1063–1069 [DOI] [PubMed] [Google Scholar]
- Desai NS, Casimiro TM, Gruber SM, Vanderklish PW 2006. Early postnatal plasticity in neocortex of Fmr1 knockout mice. J Neurophysiol 96: 1734–1745 [DOI] [PubMed] [Google Scholar]
- Feinmark SJ, Begum R, Tsvetkov E, Goussakov I, Funk CD, Siegelbaum SA, Bolshakov VY 2003. 12-Lipoxygenase metabolites of arachidonic acid mediate metabotropic glutamate receptor-dependent long-term depression at hippocampal CA3-CA1 synapses. J Neurosci 23: 11427–11435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM 1997a. Fragile X mental retardation protein: Nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci 17: 1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST 1997b. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol Cell 1: 109–118 [DOI] [PubMed] [Google Scholar]
- Ferrari F, Mercaldo V, Piccoli G, Sala C, Cannata S, Achsel T, Bagni C 2007. The Fragile X mental retardation protein-RNP granules show an mGluR-dependent localization in the post-synaptic spines. Mol Cell Neurosci 34: 343–354 [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM 2008. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of Fragile X syndrome. J Neurophysiol 100: 2615–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind JM, Reyniers E, De Boulle K, D'Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ 1996. Long-term potentiation in the hippocampus of fragile X knockout mice. Am J Med Genet 64: 246–251 [DOI] [PubMed] [Google Scholar]
- Haas BW, Barnea-Goraly N, Lightbody AA, Patnaik SS, Hoeft F, Hazlett H, Piven J, Reiss AL 2009. Early white-matter abnormalities of the ventral frontostriatal pathway in fragile X syndrome. Dev Med Child Neurol 51: 593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE, Madison DV 2007. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of Fragile X syndrome. J Neurosci 27: 4014–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A 2010. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron 65: 385–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, Schalling M 1993. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet 3: 36–43 [DOI] [PubMed] [Google Scholar]
- Hu H, Qin Y, Bochorishvili G, Zhu Y, van Aelst L, Zhu JJ 2008. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J Neurosci 28: 7847–7862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF 2001. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol 86: 321–325 [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF 2002. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci 99: 7746–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, Brosius J, Denman RB, Khandjian EW, Kindler S, Tiedge H 2008. On BC1 RNA and the fragile X mental retardation protein. Proc Natl Acad Sci 105: 734–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanco TL, Greenough WT 2002. Altered mossy fiber distributions in adult Fmr1 (FVB) knockout mice. Hippocampus 12: 47–54 [DOI] [PubMed] [Google Scholar]
- Kaang BK 1996. Parameters influencing ectopic gene expression in Aplysia neurons. Neurosci Lett 221: 29–32 [DOI] [PubMed] [Google Scholar]
- Khandjian EW, Corbin F, Woerly S, Rousseau F 1996. The fragile X mental retardation protein is associated with ribosomes. Nat Genet 12: 91–93 [DOI] [PubMed] [Google Scholar]
- Kiledjian M, Dreyfuss G 1992. Primary structure and binding activity of the hnRNP U protein: Binding RNA through RGG box. EMBO J 11: 2655–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick LL, McIlwain KA, Nelson DL 2001. Comparative genomic sequence analysis of the FXR gene family: FMR1, FXR1, and FXR2. Genomics 78: 169–177 [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, et al. 2005. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron 47: 339–352 [DOI] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U 2001. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet 10: 329–338 [DOI] [PubMed] [Google Scholar]
- Larson J, Jessen RE, Kim D, Fine AK, du Hoffmann J 2005. Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J Neurosci 25: 9460–9469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, Gall CM 2007. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci 27: 10685–10694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y 2001. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res 29: 2276–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Pelletier MR, Perez Velazquez JL, Carlen PL 2002. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci 19: 138–151 [DOI] [PubMed] [Google Scholar]
- Li C, Bassell GJ, Sasaki Y 2009a. Fragile X mental retardation protein is involved in protein synthesis-dependent collapse of growth cones induced by semaphorin-3A. Front Neural Circuits 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Huang BS, Vishwasrao H, Sutedja N, Chen W, Jin I, Hawkins RD, Bailey CH, Kandel ER 2009b. Dscam mediates remodeling of glutamate receptors in Aplysia during de novo and learning-related synapse formation. Neuron 61: 527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Luo T, Sargent TD, Fallon JR 2005. Developmental expression of Xenopus fragile X mental retardation-1 gene. Int J Dev Biol 49: 981–984 [DOI] [PubMed] [Google Scholar]
- Martin KC, Zukin RS 2006. RNA trafficking and local protein synthesis in dendrites: An overview. J Neurosci 26: 7131–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer-Stroh S, Dickens NJ, Hughes-Davies L, Kouzarides T, Eisenhaber F, Ponting CP 2003. The Tudor domain “Royal Family”: Tudor, plant Agenet, Chromo, PWWP and MBT domains. Trends Biochem Sci 28: 69–74 [DOI] [PubMed] [Google Scholar]
- Mazroui R, Huot ME, Tremblay S, Filion C, Labelle Y, Khandjian EW 2002. Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Hum Mol Genet 11: 3007–3017 [DOI] [PubMed] [Google Scholar]
- Meredith RM, Holmgren CD, Weidum M, Burnashev N, Mansvelder HD 2007. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signaling in mice lacking fragile X gene FMR1. Neuron 54: 627–638 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Sluyter F, de Wit S, Oostra BA, Crusio WE 2002. Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus 12: 39–46 [DOI] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J 2003. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron 37: 417–431 [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S 1986. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science 234: 1249–1254 [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Kandel ER, Schacher S 1988. Long-term heterosynaptic inhibition in Aplysia. Nature 333: 171–174 [DOI] [PubMed] [Google Scholar]
- Morales J, Hiesinger PR, Schroeder AJ, Kume K, Verstreken P, Jackson FR, Nelson DL, Hassan BA 2002. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron 34: 961–972 [DOI] [PubMed] [Google Scholar]
- Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L, et al. 2006. Neuronal transcriptome of Aplysia: Neuronal compartments and circuitry. Cell 127: 1453–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, et al. 2008. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell 134: 1042–1054 [DOI] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM 2006. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of Fragile X Syndrome. J Neurophysiol 95: 3291–3295 [DOI] [PubMed] [Google Scholar]
- Ohno S 1970. Evolution by gene duplication. Springer-Verlag, New York [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST 1999. Fragile X mouse: Strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience 94: 185–192 [DOI] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. 2008. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron 59: 70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Mulle JG, Warren ST 2007. The pathophysiology of fragile X syndrome. Annu Rev Genomics Hum Genet 8: 109–129 [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM 2007. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J Neurosci 27: 3120–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Kandel ER 2003. In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philos Trans R Soc Lond B Biol Sci 358: 757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Hollingworth D, Adinolfi S, Castets M, Kelly G, Frenkiel TA, Bardoni B, Pastore A 2006. The structure of the N-terminal domain of the fragile X mental retardation protein: A platform for protein–protein interaction. Structure 14: 21–31 [DOI] [PubMed] [Google Scholar]
- Rayport SG, Schacher S 1986. Synaptic plasticity in vitro: Cell culture of identified Aplysia neurons mediating short-term habituation and sensitization. J Neurosci 6: 759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T 1989. Molecular cloning: A laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Schenck A, Bardoni B, Moro A, Bagni C, Mandel JL 2001. A highly conserved protein family interacting with the fragile X mental retardation protein (FMRP) and displaying selective interactions with FMRP-related proteins FXR1P and FXR2P. Proc Natl Acad Sci 98: 8844–8849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Wang H, Mercaldo V, Li X, Chen T, Zhuo M 2009. Fragile X mental retardation protein is required for chemically-induced long-term potentiation of the hippocampus in adult mice. J Neurochem 111: 635–646 [DOI] [PubMed] [Google Scholar]
- Siomi MC, Siomi H, Sauer WH, Srinivasan S, Nussbaum RL, Dreyfuss G 1995. FXR1, an autosomal homolog of the fragile X mental retardation gene. EMBO J 14: 2401–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi H, Ishizuka A, Siomi MC 2004. RNA interference: A new mechanism by which FMRP acts in the normal brain? What can Drosophila teach us? Ment Retard Dev Disabil Res Rev 10: 68–74 [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF 2001. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci 4: 1079–1085 [DOI] [PubMed] [Google Scholar]
- Stefani G, Fraser CE, Darnell JC, Darnell RB 2004. Fragile X mental retardation protein is associated with translating polyribosomes in neuronal cells. J Neurosci 24: 7272–7276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock DW, Quattro JM, Whitt GS, Powers DA 1997. Lactate dehydrogenase (LDH) gene duplication during chordate evolution: The cDNA sequence of the LDH of the tunicate Styela plicata. Mol Biol Evol 14: 1273–1284 [DOI] [PubMed] [Google Scholar]
- Tamanini F, Meijer N, Verheij C, Willems PJ, Galjaard H, Oostra BA, Hoogeveen AT 1996. FMRP is associated to the ribosomes via RNA. Hum Mol Genet 5: 809–813 [DOI] [PubMed] [Google Scholar]
- Tucker B, Richards R, Lardelli M 2004. Expression of three zebrafish orthologs of human FMR1-related genes and their phylogenetic relationships. Dev Genes Evol 214: 567–574 [DOI] [PubMed] [Google Scholar]
- Valverde R, Pozdnyakova I, Kajander T, Venkatraman J, Regan L 2007. Fragile X mental retardation syndrome: Structure of the KH1–KH2 domains of fragile X mental retardation protein. Structure 15: 1090–1098 [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, de Graaff E, De Boulle K, Eichler EE, Konecki DS, Reyniers E, Manca A, Poustka A, Willems PJ, Nelson DL, et al. 1993. Alternative splicing in the fragile X gene FMR1. Hum Mol Genet 2: 399–404 [DOI] [PubMed] [Google Scholar]
- Wan L, Dockendorff TC, Jongens TA, Dreyfuss G 2000. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol 20: 8536–8547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DO, Kim SM, Zhao Y, Hwang H, Miura SK, Sossin WS, Martin KC 2009. Synapse- and stimulus-specific local translation during long-term neuronal plasticity. Science 324: 1536–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM 2008. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59: 84–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BM, Cox CL 2007. Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice. Proc Natl Acad Sci 104: 2454–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Dolzhanskaya N, LaFauci G, Dobkin C, Denman RB 2009. Tissue and developmental regulation of fragile X mental retardation 1 exon 12 and 15 isoforms. Neurobiol Dis 35: 52–62 [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Zablow L, Siegelbaum SA 2002. Altered presynaptic vesicle release and cycling during mGluR-dependent LTD. Neuron 35: 1099–1110 [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C 2003. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell 112: 317–327 [DOI] [PubMed] [Google Scholar]
- Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SG, et al. 2007. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci 10: 578–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang JB, Nosyreva ED, Spencer CM, Volk LJ, Musunuru K, Zhong R, Stone EF, Yuva-Paylor LA, Huber KM, Paylor R, et al. 2009. A mouse model of the human Fragile X syndrome I304N mutation. PLoS Genet 5: e1000758 doi: 10.1371/journal.pgen.1000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, O'Connor JP, Siomi MC, Srinivasan S, Dutra A, Nussbaum RL, Dreyfuss G 1995. The fragile X mental retardation syndrome protein interacts with novel homologs FXR1 and FXR2. EMBO J 14: 5358–5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K 2001. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 107: 591–603 [DOI] [PubMed] [Google Scholar]
- Zhang J, Hou L, Klann E, Nelson DL 2009. Altered hippocampal synaptic plasticity in the FMR1 gene family knockout mouse models. J Neurophysiol 101: 2572–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, et al. 2005. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 47: 859–872 [DOI] [PubMed] [Google Scholar]