Abstract
A number of mechanisms participate in the hepatic injury that occurs during and following liver transplantation. A normal allograft regenerative response is probably essential for a successful transplant outcome. In this study, the effect of cyclosporine, a potent immunosuppressant used routinely after liver transplantation, on the regenerative response of the liver after partial hepatectomy was investigated. Male Wistar rats were pretreated for one week with either cyclosporine or the olive oil vehicle and were subjected to either a two-thirds partial hepatectomy or a sham operation. Animals were sacrificed at various times postoperatively and the remnant livers were weighed to determine the liver weight to body weight ratio, two biochemical measures of a regenerative response (cytosolic ornithine decarboxylase activity and thymidine kinase activity), and the hepatic content of estrogen and androgen receptors, as the content of these receptors has been shown to modulate, at least in part, the subsequent hepatic regenerative response. The preoperative hepatic cytosol content of ornithine decarboxylase, thymidine kinase, and estrogen receptor was significantly greater (P < 0.05) in rats pretreated with cyclosporine than in those treated with the vehicle alone. A significant increase in ornithine decarboxylase and thymidine kinase activities occurred after partial hepatectomy in both the cyclosporine-pretreated and vehicle-pretreated animals. The absolute levels for each parameter were also greater in the cyclosporine-treated animals than in the vehicle-treated controls at 24 hr after partial hepatectomy (P < 0.05). The pattern of change in the hepatic cytosolic content of estrogen and androgen receptors in both groups of animals was comparable with those described previously for regenerating liver. These data suggest that cyclosporine may predispose the liver to respond to either a regenerative signal or perceived need and thereby fortuitously enhance liver graft performance after successful surgical implantation.
Keywords: cyclosporine, liver transplantation, hepatic regenerative response, rat, estrogen, androgen, cytosol
The capacity of the liver to regenerate in response to an injury has been well documented (1, 2). In recent years, transplantation of the liver has become an established treatment for individuals with end-stage liver disease (3, 4). During transplantation, the liver graft is subjected to a variety of potential hepatic injuries including ischemic injury associated with organ harvesting and the obligate storage prior to revascularization, reperfusion injury following revascularization, immunological attack mounted by the immune system of the recipient, and, not infrequently, by postoperative bacterial, fungal, and viral infection. Furthermore, several of the drugs used following transplantation, particularly cyclosporine, are known to be hepatotoxic (5, 6).
It is conceivable that the hepatic toxicity of cyclosporine might either enhance or impair hepatic regeneration following liver transplantation and thereby affect transplant results. To investigate this important clinical question, the following studies were performed.
MATERIALS AND METHODS
Animals and Chemicals
Adult male inbred Wistar rats weighing 250–300 g were purchased from Harlan Sprague Dawley (Indianapolis, Indiana). New England Nuclear (Boston, Massachusetts) was the source for the [14C]ornithine (57.6 mCi/mmol), [3H]estradiol (99 Ci/mmol), [3H]R1881 (87 Ci/mmol), and unlabeled R1881. Tritiated thymidine (5 Ci/mmol) and ACS scintillation fluid were obtained from Amersham (Arlington Heights, Illinois). Absolute ethanol and DEAE-cellulose paper were purchased from U.S. Industrial Chemicals Company (Tuscola, Illinois) and BioRad (Richmond, California), respectively. Sigma Chemical Company (St. Louis, Missouri) was the source for the unlabeled ornithine, pyridoxal phosphate, Tris base, adenosine triphosphate, diethylstilbestrol, sodium molybdate, nicotinamide adenine dinucleotide, calf thymus DNA, and bovine serum albumin. All other chemicals were obtained from Fisher Chemical Company (Pittsburgh, Pennsylvania).
Surgical Procedures
The rats were assigned randomly to three groups and treated as follows: the rats in group I received cyclosporine in olive oil 25 mg/kg orally for seven days and on the eighth day, immediately after an intravenous bolus injection of cyclosporine in saline (5 mg/kg), were subjected to a standard two-thirds partial hepatectomy (7); The rats in group II were given the olive oil vehicle orally for seven days and on the eighth day, immediately after an intravenous injection of saline, were subjected to a standard two-thirds partial hepatectomy. The rats in group III received cyclosporine in the same treatment schedule as group I but were subjected to a sham operation on the eighth day rather than a two-thirds hepatectomy.
All surgical procedures were performed between 9:00 and 11:00 AM, using light ether anesthesia to eliminate the effects of any diurnal rhythms on the results (8).
At various times up to 72 hr after partial hepatectomy and sham operation, animals were anesthetized with ether and weighed. Blood was drawn from the abdominal aorta and the remnant liver was removed, weighed, and homogenized in four volumes of ice-cold buffer consisting of 0.25 M sucrose, 1.5 mM EDTA, 10 mM mercaptoethanol, and 10 mM Tris HCl (pH 7.4) using a Brinkman Polytron homogenizer. Hepatic cytosol was prepared by centrifugation at 103,000g for 1 hr at 4° C. All cytosolic enzyme assays were performed immediately after preparation of the cytosol.
Ornithine Decarboxylase Activity
The amount of 14CO2 released from labeled ornithine incubated with rat liver cytosol was used to determine the ornithine decarboxylase activity (9). In brief, 0.4 ml cytosol was preincubated for 5 min at 37° C with a mixture containing 0.2 mM pyridoxal phosphate, 5 mM dithiothreitol, 1.5 mM l-ornithine in 10 mM Tris HCl (pH 8.0). Thereafter 0.5 μCi, [1-14C]-dl-ornithine was added to the mixture and 250 μl of ethanolamine–ethylene glycol (2:1) was placed in a center well to act as a CO2 trap. The mixture was allowed to incubate for 1 hr at 37° C, and then the reaction was terminated by the addition of 0.1 ml saturated trichloracetic acid solution. After maintaining the reaction mixture at 37° C for an additional hour, the CO2 trapping solution was removed and placed into a glass scintillation vial containing 10 ml ACS scintillation fluid. A Packard Tri-Carb 460 CD liquid scintillation system (Downers Grove, Illinois) was used to measure the radioactivity present in the vial.
Thymidine Kinase Activity
The in vitro conversion of thymidine to thymidine phosphate by rat liver cytosol was used to determine the level of thymidine kinase activity (10): The reaction mixture was made up by 0.1 ml cytosol, 850 μl of incubation buffer consisting of 5 mM adenosine triphosphate and 3.6 mM MgCl2 in 50 mM Tris HCl (pH 8.0), and 50 μl 1μM [3H]thymidine. The reaction was allowed to proceed for 10 min at 37° C and terminated by immersion in boiling water for 2 min. Denatured protein was removed by centrifugation at 1500g for 5 min at 4° C. A 0.l-ml aliquot of the supernatant was spotted on a square of DEAE-cellulose paper. The paper squares were washed in 1 mM ammonium formate for 5 min, followed by distilled water for 3 min and placed in glass scintillation vials. A 0.1 M HCl–0.2 M KCl mixture was added to elute the radioactivity into solution. Fifteen minutes later, 10 ml ACS scintillation fluid was added to each vial and the radioactivity present in the vial was counted in a Packard Tri-Carb 460 CD liquid scintillation system (Downers Grove, Illinois).
Estrogen and Androgen Receptor Content of Hepatic Cytosol
Cytosolic estrogen receptor content was determined by measuring the specific binding of a saturating concentration of [3H]estradiol (11). The estrogen receptors in hepatic cytosol were stabilized by diluting the hepatic cytosol 1:1 with buffer consisting of 4 mM sodium molybdate, 1.5 mM EDTA, and 10 mM Tris HCl (pH 7.4). Total binding of the [3H]estradiol was measured by adding 24 μl 30 mM [3H]estradiol and 25 μl ethanol to 200 μl of the diluted cytosol. Parallel assays in which the ethanol was replaced with 25 μl 3μM unlabeled DES dissolved in ethanol were used to determine the nonspecific binding. After an incubation period of 2 hr at 4° C, the reaction was terminated by the addition of 0.4 ml 1% dextrancoated charcoal to remove unbound estradiol. The mixture was centrifuged at 1500g for 5 min at 4° C, and the supernatant carefully transferred to a scintillation vial containing 8 ml ACS scintillation fluid. Again, the radio-activity was counted in a Packard Tri-Carb 460 CD liquid scintillation system (Downers Grove, Illinois).
The cytosolic androgen receptor assay was similar to the one described above with minor exceptions (12). Tritiated R1881, a synthetic androgen, was used as the labeled steroid to determine the total binding and unlabeled R1881 was used to determine nonspecific binding. Binding of R1881 to glucocorticoid receptors was blocked by adding 5 μM triamcinolone acetonide overnight at 4° C. The reaction was terminated by the addition of 0.4 ml 1% dextran-coated charcoal. The mixture was centrifuged at 1500g for 5 min at 4° C. Thereafter the supernatant was placed in a scintillation vial with 8 ml ACS scintillation fluid and the radioactivity was counted as described above.
Miscellaneous Methods
The cytosolic protein concentration was determined using the method of Lowry et al (13), with bovine serum albumin as the standard. Serum measures of liver injury were determined using a Random Access Chemistry Analyzer (Technicon RA 500).
All results are presented as mean values ± SEM. Statistical analysis of the data was performed using the Student’s t test. A P value of < 0.05 was considered to represent a significant difference.
RESULTS
The liver injury parameters (Figure 1) assessed in the animals studied were similar regardless of their preoperative therapy. A significant increase in serum AST levels occurred with the hepatic injury associated with partial hepatectomy in both operated groups. A small, but nonetheless significant, increase in serum AST levels also occurred after the sham operation. Interestingly, the hepatic injury experienced, as determined by AST levels after partial hepatectomy, was less in the rats pretreated with cyclosporine than it was in the vehicle-treated controls.
Fig 1.
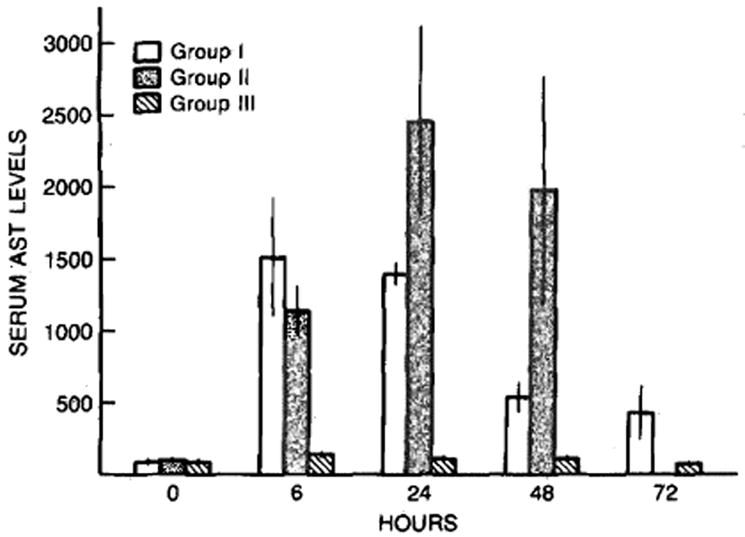
Serum AST levels after sham operation (group III) and after partial hepatectomy in cyclosporine- (group I) and vehicle- (group II) treated rats. Mean ± SEM (N = 4–8 animals per group).
The rate of hepatic growth after partial hepatectomy in cyclosporine- and vehicle-treated animals and after a sham operation is shown in Figure 2. The liver weight to body weight (LW/BW) ratio remained unchanged after the sham operation despite cyclosporine treatment. There was a steady increase in the LW/BW ratio in the animals receiving a two-thirds hepatectomy between 6 and 72 hr after the surgical procedure. At 72 hr after partial hepatectomy, the LW/BW ratios in the cyclosporine-treated rats were similar to those of the unoperated controls, whereas the LW/BW ratios in the vehicle-treated animals were still reduced significantly (P < 0.05) as compared to the unoperated control animals. Thus the LW/BW ratios at 72 hr after partial hepatectomy in cyclosporine-treated rats were greater than those of the vehicle-treated animals.
Fig 2.
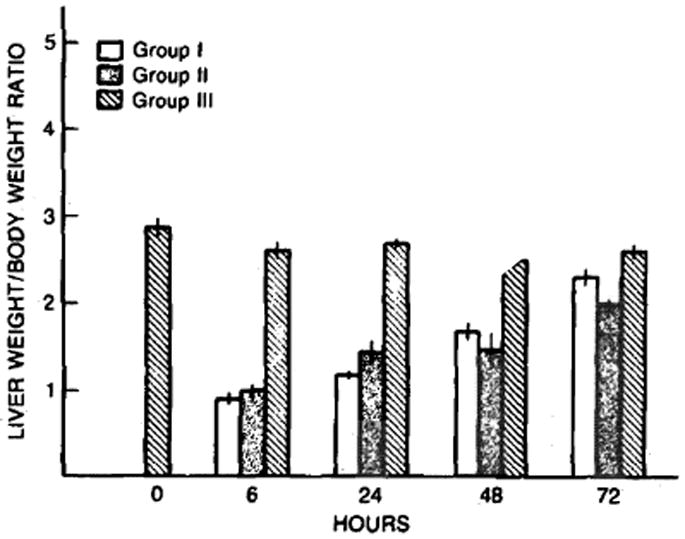
The changes in liver weight to body weight ratio after sham operation (group III) and after partial hepatectomy in cyclosporine- (group I) and vehicle- (group II) treated rats. Mean ± SEM (N = 4–8 animals per group).
As shown in Figure 3, treatment with cyclosporine resulted in a significantly higher preoperative level of ornithine decarboxylase activity in the liver (P < 0.01) compared to that present in the vehicle treated animals. Moreover, at 6 hr following a two-thirds hepatectomy, hepatic levels of ornithine decarboxylase activity were elevated significantly in all three groups compared to their respective preoperative levels (P < 0.01). Importantly, the activity level was greatest in the rats treated with cyclosporine that were subjected to a partial hepatectomy. The hepatic ornithine decarboxylase activity returned to basal preoperative levels at 24 hr in the rats subjected to a sham hepatectomy but remained elevated (P < 0.05) in both groups experiencing a two-thirds partial hepatectomy; again, the ODC activity was greater in the rats treated with cyclosporine than in the animals receiving the vehicle, although both groups experienced an identical two-thirds partial hepatectomy.
Fig 3.
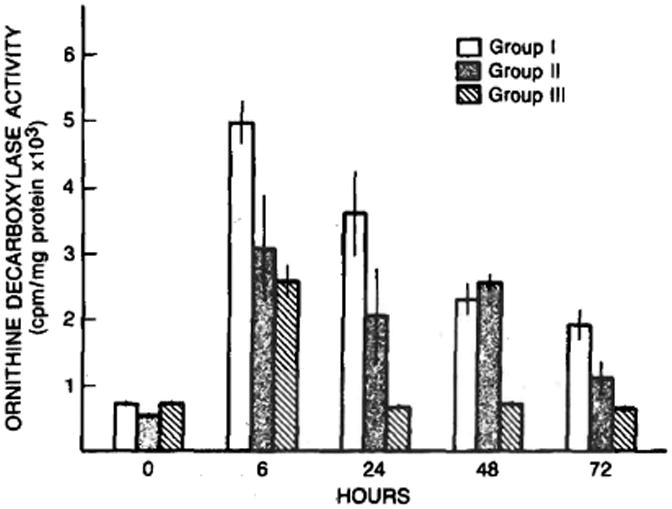
Ornithine decarboxylase (cpm/mg protein) after sham operation (group III) and after partial hepatectomy in cyclosporine- (group I) and vehicle- (group II) treated rats. Mean ± SEM (N = 4–8 animals per group).
The levels of thymidine kinase activity within the hepatic cytosol of the groups of animals across time are shown in Figure 4. Preoperative levels of hepatic thymidine kinase activity were significantly greater in the rats pretreated with cyclosporine (P < 0.01) than in vehicle-treated controls. Hepatic thymidine kinase activity was lower in the sham-operated animals at 24 hr (P < 0.05) than it was in the same animals preoperatively. A significant increase in thymidine kinase activity occurred at 24 hr in both groups of animals receiving a two-thirds partial hepatectomy, with the levels being greater (P < 0.05) in the rats pretreated with cyclosporine than in the vehicle-treated controls. An increase in hepatic thymidine kinase activity was seen at 48 and 72 hr after the sham operation with the levels at 72 hr being significantly greater than the preoperative levels in the vehicle-treated animals (both P < 0.05). In both groups of animals subjected to a two-thirds partial hepatectomy, hepatic thymidine kinase activity remained elevated at 48 and 72 hr with levels being greater (P < 0.05) in the rats pretreated with cyclosporine (group I).
Fig 4.
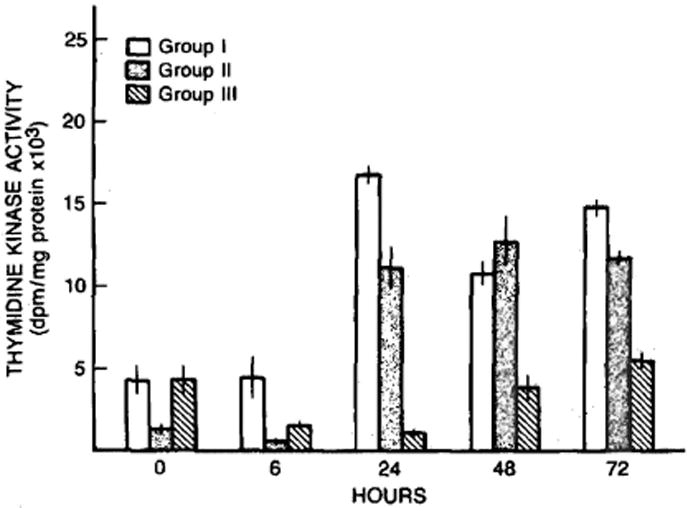
Thymidine kinase activity (dpm/mg protein) after sham operation (group III) and after partial hepatectomy in cyclosporine- (group I) and vehicle- (group II) treated rats. Mean ± SEM (N = 4–8 animals per group).
As presented in Figure 5, the preoperative basal hepatic estrogen receptor content was greater in the two groups of rats pretreated with cyclosporine (P < 0.01) as compared to the vehicle-treated animals. A significant reduction in hepatic cytosolic estrogen receptor content occurred at 6 hr in all three groups of animals with the reduction being in the range of 20–50%. Thereafter, a steady decline in hepatic cytosolic estrogen receptor content occurred in the sham-operated cyclosporine-treated animals with the levels at 72 hr being approximately half the preoperative levels in the same group. The hepatic estrogen receptor content of the cytosol in this group, however, was significantly greater (P < 0.05) than that present in the vehicle-treated animals receiving a two-thirds partial hepatectomy. In contrast, the cytosolic content of estrogen receptor after partial hepatectomy in the cyclosporine-treated rats decreased to approximately one quarter of the preoperative levels at 24 hr, then rapidly increased and returned almost to the preoperative levels by 72 hr. A similar initial reduction in the cytosolic estrogen receptor content at 6 hr after partial hepatectomy was seen in the vehicle-treated rats. Moreover the cytosolic estrogen receptor content increased rapidly thereafter and was back to preoperative levels by 72 hr after the partial hepatectomy.
Fig 5.
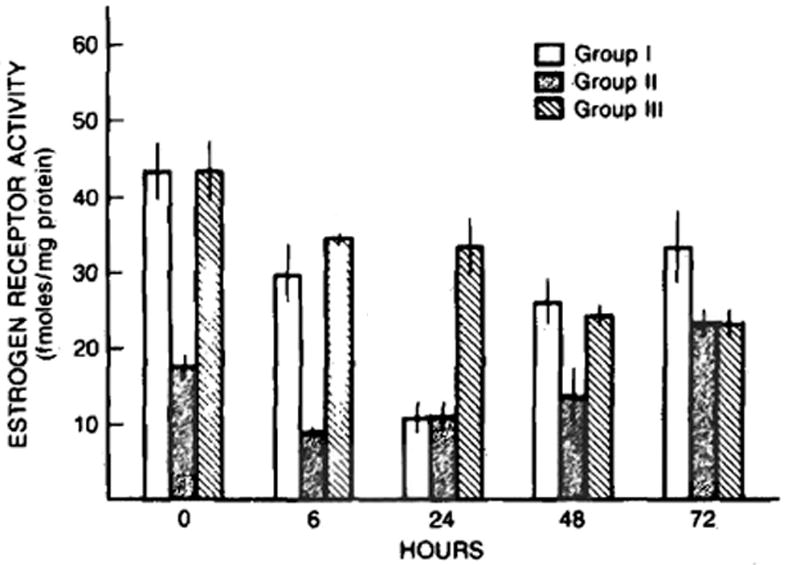
Hepatic cytosolic estrogen receptor content (fmols/mg protein) in rats subjected to sham operation (group III) and partial hepatectomy after pretreatment with cyclosporine (group I) and vehicle (group II). Mean ± SEM (N = 4–8 animals per group).
The preoperative hepatic cytosolic androgen receptor content in the animals studied is shown in Figure 6. Basal androgen receptor content was not affected by pretreatment with either cyclosporine or the olive oil vehicle. A decline in the androgen receptor content was observed 6 hr postoperatively in all three groups of animals studied. Thereafter, however, the androgen receptor content remained low after the sham operation and only returned to basal preoperative levels at 72 hr, whereas the levels in the groups undergoing a two-thirds partial hepatectomy returned to preoperative levels between 24 and 48 hr. The androgen receptor content was greatest at 24 and 48 hr in the rats treated with cyclosporine and experiencing a partial hepatectomy (Figure 6).
Fig 6.
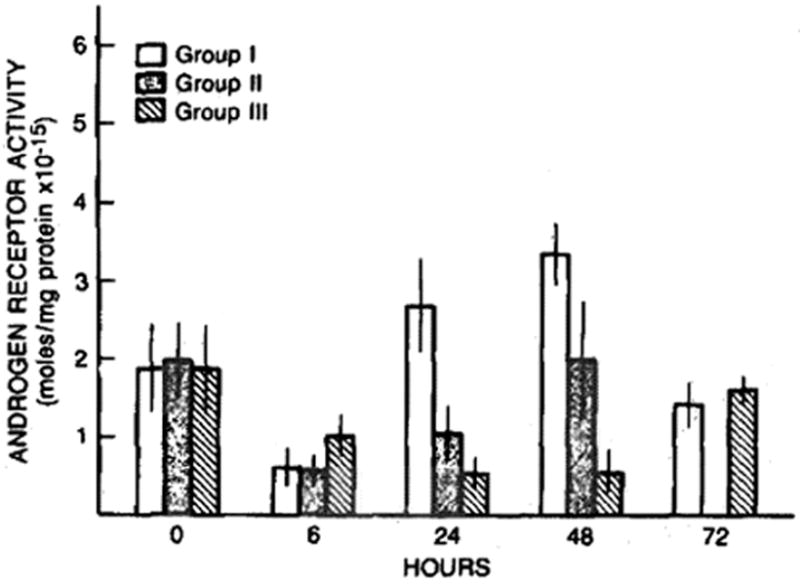
Hepatic cytosolic androgen receptor content (fmols/mg protein) after sham operation (group III) and after partial hepatectomy in cyclosporine- (group I) and vehicle- (group II) treatment rats. Mean ± SEM (N = 4–8 animals per group).
DISCUSSION
In this study the effect of cyclosporine on the hepatic regenerative response that occurs after partial hepatectomy was investigated. Rats were treated with either cyclosporine or the olive oil vehicle for one week and then subjected to either a two-thirds partial hepatectomy or a sham operation. The endpoints utilized were LW/BW ratio and the cytosolic ornithine decarboxylase, thymidine kinase, and estrogen and androgen receptor content.
Ornithine decarboxylase activity was assessed because it is known to be the rate-limiting enzyme in polyamine synthesis, and an increase in polyamine synthesis is a prerequisite for cell proliferation (8, 14, 15). Moreover, polyamine synthesis is thought to be critical for the initiation of hepatic regeneration following a wide variety of hepatic injuries (10). Thymidine kinase activity was assessed because it is required for the phosphorylation of thymidine before it is incorporated into DNA. Thus it is essential for DNA synthesis, and an increase in its activity has been shown to occur before the initiation of hepatic regeneration in all currently available models of hepatic regeneration (10, 16-18). Both the thymidine kinase and ornithine decarboxylase activities were significantly greater prior to partial hepatectomy in the livers of the rats pretreated with cyclosporine. This suggests that these animals were primed for a regenerative response. An additional significant increase in both ornithine decarboxylase and thymidine kinase activity occurred following partial hepatectomy in the cyclosporine-treated rats and also occurred in the vehicle-treated control rats subjected to the same operative procedure. In contrast to what was seen in the cyclosporine-treated animals, thymidine kinase activity in liver cytosol obtained from vehicle-treated control animals was reduced prior to partial hepatectomy, but at 6 hr and at 24 hr after partial hepatectomy increased significantly. After partial hepatectomy, the increase in thymidine kinase activity in hepatic cytosol occurred faster and to a greater extent in the animals pretreated with cyclosporine.
The liver weight to body weight ratio at 72 hr after partial hepatectomy, the ultimate measure of hepatic regeneration, was greater in the rats pretreated with cyclosporine than in those receiving the vehicle (Figure 2). Thus, using several different indices of hepatic regeneration, a greater regenerative response was seen after a two-thirds partial hepatectomy in rats pretreated with cyclosporine than in vehicle-treated controls.
Interestingly, a significant increase in hepatic ornithine decarboxylase activity was noted at 6 hr in the cyclosporine-treated rats after the sham operation. Similarly, the hepatic thymidine kinase activity in rats pretreated with cyclosporine and subjected a sham operation was increased significantly above preoperative levels in the same animals. These data suggest that cyclosporine treatment primes the liver for a regenerative response, but, in the absence of an injury, no regeneration occurs. Thus, the liver weight to body weight ratios remained unchanged after the sham operation despite cyclosporine pretreatment.
Taken together, the data presented are consistent with an increased hepatic regenerative response after partial hepatectomy in rats treated with cyclosporine. Cyclosporine is well known to have hepatotoxic potential (5, 6), and this untoward action of the drug may account for the enhanced regenerative response seen after partial hepatectomy in cyclosporine-treated animals. The regenerative priming seen in cyclosporine-treated and sham-operated animals probably reflects the low-level hepatic injury associated with cyclosporine treatment per se. Since the preoperative liver injury measures assessed in the rats studied that were pretreated with cyclosporine were similar to those of vehicle-treated animals, the hepatic injury produced by cyclosporine treatment alone was probably inadequate to initiate an overt regenerative response, despite adequate regenerative priming. In this regard, it should be noted that the hepatic injury induced by cyclosporine, at the doses used in these experiments and clinically, may be subclinical and therefore not sufficient to be detected by routine liver injury parameters and inadequate to initiate a “primed” regenerative response.
Previous studies have shown that hepatic regeneration in male rats is associated with a loss of certain male-specific hepatic characteristics (12). Moreover, serum estradiol and total hepatic estrogen receptor levels increase while serum testosterone and hepatic androgen receptor levels decrease following a partial hepatectomy (19). During regeneration, the total hepatic estrogen receptor level increases, whereas the cytosolic estrogen receptor content actually decreases as the receptors shift from the cytosol to the nucleus. This movement of the estrogen receptor to the nucleus is thought to play a critical role in the initiation of the hepatic regenerative response (19). The cytosolic estrogen receptor content in rats treated with cyclosporine was greater than that of the vehicle-treated controls. Nonetheless, the pattern of the estrogen receptor changes seen after partial hepatectomy in both groups (cyclosporine-treated and vehicle-treated animals undergoing partial hepatectomy) was compatible with those described previously in untreated animals (19). The shift of one estrogen receptor from the hepatic cytosol to the nucleus in the cyclosporine-treated animals might account, at least in part, for the greater regenerative response seen after partial hepatectomy as evidenced by the increased levels of ornithine decarboxylase and thymidine kinase activity present in the cytosol of the cyclosporine-treated animals.
The preoperative levels of androgen receptor content in the livers of the cyclosporine- and vehicle-treated rats were similar. Moreover, the cytosolic content of the androgen receptor measured after partial hepatectomy and sham operation were compatible with those described previously (19). This observation suggests that the hepatic control of estrogen receptor, but not the androgen receptor, is important for the regenerative process.
Various mechanisms for hepatic injury occur with liver transplantation and include ischemia, rejection, infection, and drug toxicity. In the majority of patients, the sum of these hepatic injuries is minor. However, it can be severe in some cases. The capacity of the liver to regenerate after such injuries may be crucial for a successful transplant outcome. With this in mind, it is important to know how cyclosporine, a potent immunosuppressive agent used routinely following liver transplantation, might affect the regenerative response of the liver. The present studies address this important issue and suggest that cyclosporine, probably acting as an intrinsic hepatotoxin, primes the regenerative response and thereby enhances posttransplantation hepatic regenerative capacity.
Because of the critical shortage of suitable pediatric liver donors, some transplant centers have resorted to using partially hepatectomized adult liver grafts in children (20). A normal or enhanced regenerative response following transplantation of a reduced-size adult liver graft placed into a child is particularly critical for a successful outcome in such cases. The present observations appear applicable to these cases (21).
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant AM 29961 and from the NIDDK 32556 and from the NIAAA AA06601.
Dr. D. Kahn was supported by a grant from the Medical Research Council of South Africa.
References
- 1.Bucher NLR, Malt RA. Regeneration of Liver and Kidney. Boston: Little Brown and Company; 1971. [Google Scholar]
- 2.Francavilla A, Porter KA, Benichou J, Jones AF, Starzl TE. Liver regeneration in dogs: Morphological and chemical changes. J Surg Res. 1979;25:409–419. doi: 10.1016/s0022-4804(78)80005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Iwatsuki S, Van Thiel DH, Gartner JC, Zitelli BJ, Malartack JJ, Schnock RR, Shaw BW, Jr, Hakala TR, Rosenthal JR, Porter KA. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwatsuki S, Starzl TE, Todo S, Gordon RD, Esquivel CO, Izakis AG, Makowka L, Marsh JW, Koneru B, Streber A. Experience in 1,000 liver transplants under cyclosporine-steroid therapy. A survival report. Transplant Proc. in press. [PMC free article] [PubMed] [Google Scholar]
- 5.Calne RY, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC, Craddock GN, Pentlon BD, Rolles K. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978;2:1323–1327. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Weil R, III, Iwatsuki S, Klintmalm G, Schröter GPJ, Kaep LJ, Waki Y, Terasaki PI, Porter K. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151:17–26. [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins GM, Anderson RM. Experimental pathology of the liver. Restoration of the liver of the white rat following surgical removal. Arch Pathol. 1931;12:186–201. [Google Scholar]
- 8.Bucher NLR. Regeneration of mammalian liver. Int Rev Cytol. 1963;15:245–300. doi: 10.1016/s0074-7696(08)61119-5. [DOI] [PubMed] [Google Scholar]
- 9.McGowan JA, Fausto A. Ornithine decarboxylase activity and the onset of deoxyribonucleic acid synthesis in regenerating liver. Biochem J. 1978;170:123–127. doi: 10.1042/bj1700123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn D, Stadler J, Terblanche J, Van Hoorn-Hickman R. Thymidine kinase: An inexpensive index of liver regeneration in a large animal model. Gastroenterology. 1980;79:907–911. [PubMed] [Google Scholar]
- 11.Eagon PK, Fisher SE, Imhoff AF, Porter LE, Stewart RR, Van Thiel DH, Lester R. Estrogen-binding proteins of male rat liver: Influences of hormonal changes. Arch Biochem Biophys. 1980;201:486–499. doi: 10.1016/0003-9861(80)90537-8. [DOI] [PubMed] [Google Scholar]
- 12.Francavilla A, Eagon PK, DiLeo A, Polimeno L, Panella C, Aquilino AM, Ingrosso M, Van Thiel DH, Starzl TE. Sex hormone-related functions in regenerating male rat liver. Gastroenterology. 1986;91:1263–1270. doi: 10.1016/s0016-5085(86)80026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AC, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Russell DH, Snyder SH. Amino synthesis in rapidly growing tissues: Ornithine decarboxylase activity in regenerating rat liver, chick embryo and various tumors. Proc Natl Acad Sci USA. 1968;60:1420–1426. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandt JT, Pierce DA, Fausto N. Ornithine decarboxylase activity and polyamine synthesis during kidney hypertrophy. Biochim Biophys Acta. 1972;279:184–189. doi: 10.1016/0304-4165(72)90253-x. [DOI] [PubMed] [Google Scholar]
- 16.Bollum FJ, Potter VR. Incorporation of thymidine into deoxyribonucleic acid by enzymes from rat tissues. J Biol Chem. 1958;233:478–482. [PubMed] [Google Scholar]
- 17.Kizer DE, Holman L. Purification and properties of thymidine kinase from regenerating rat liver. Biochim Biophys Acta. 1974;350:193–200. doi: 10.1016/0005-2744(74)90217-4. [DOI] [PubMed] [Google Scholar]
- 18.Kahn D, Svanas GW, Eagon PK, Porter LE, Makowka L, Podesta L, Chopchap P, Starzl TE, Van Thiel DH. Liver regeneration in rats treated with the antiandrogen flutamide. J Invest Surg. 1988 doi: 10.3109/08941938809141085. in press. [DOI] [PubMed] [Google Scholar]
- 19.Eagon PK, Porter LE, Francavilla A, DiLeo A, Van Thiel DH. Estrogen and androgen receptors in liver: Their role in liver disease and regeneration. Semin Liver Dis. 1985;5:59–69. doi: 10.1055/s-2008-1041758. [DOI] [PubMed] [Google Scholar]
- 20.Salizzori M, Yandza T, Kestens PJ, et al. Indication, technique and results of liver graft volume reduction before orthotopic liver transplantation in children. Transplant Proc 1988. 1988;20:704–710. [PubMed] [Google Scholar]
- 21.Van Thiel DH, Gavaler JS, Kam I, Francovilla A, Polimeno L, Schade RR, Smith J, Diven W, Penkrot RJ, Starzl TE. Rapid growth of an intact human liver transplanted into a recipient larger than the donor. Gastroenterology. 1987;93:1414–1419. doi: 10.1016/0016-5085(87)90274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


