Abstract
A liquid chromatography–tandem mass spectrometry (LC–MS–MS) method was developed for the analysis of marijuana cannabinoids in mouse brain tissue using an Applied Biosystems 3200 Q trap with a turbo V source for TurbolonSpray attached to a Shimadzu SCL HPLC system. The method included cannabichromene (CBC), cannabidiol (CBD), D9-tetrahydrocannabinol (THC), 11-hydroxytetrahydrocannabinol (11-OH-THC), and 11-nor-D9-tetrahydrocannabinol-9-carboxylic acid (THC-COOH). These compounds were isolated by liquid-liquid extraction using cold acetonitrile. The following transition ions were monitored by multiple reaction monitoring (MRM): m/z 315>193, 315>259 for THC/CBD/CBC; m/z 331>193, 331>105 for 11-OH-THC; m/z 345>299, 345>193 for THC-COOH;c m/z 318>196 for THC-d3; m/z 334>196 for 11-OH-THC-d3, and m/z 348>302 for THCCOOH-d3. Linearity for THC, 1-OH-THC, and THC-COOH was 1-200 ng/g; for CBC and CBD, it was 0.5–20 ng/g. Within-run and between-run precisions for all the analytes yielded coefficients of variation of < 20%. Four C57BL6 mice were sacrificed 20 min after nose-only exposure to the smoke of 200 mg of marijuana containing 0.44 mg CBC, 0.93 mg CBD, and 8.81 mg THC. The mean brain concentrations were 3.9 ± 1.5 ng/g CBC, 21 ± 3.9 ng/g CBD, 364 ± 74 ng/g THC, and 28 ± 5.9 ng/g 11-OH-THC. THCCOOH was not detected. The relative mean brain cannabinoid concentrations correlated to the amounts of the cannabinoids in the inhaled marijuana.
Introduction
Marijuana is the most commonly used illicit drug in the United States (1) and remains as one of the most widespread drugs of abuse worldwide. There are at least 95 identified cannabinoids in marijuana (2-5) with Δ9-tetrahydrocannabinol (THC) being recognized as the primary psychoactive constituent (6). Other relatively abundant cannabinoids include cannabinol (CBN), cannabidiol (CBD), and cannabichromene (CBC). The degree to which the other cannabinoids may contribute to marijuana's overall pharmacological effects, have pharmacological properties of their own, or modulate the effects of THC remains in question. To investigate the pharmacokinetics and pharmacodynamics of these other cannabinoids, it is necessary to develop reliable analytical methods to measure not only THC, but these other cannabinoids in biological specimens.
Numerous methods have been developed to detect and quantify THC and/or its metabolites, 11-nor-Δ9-tetrahydro-cannabinol-9-carboxylic acid (THC-COOH) and 11-hydroxy-Δ9-tetrahydrocannabinol (11-HO-THC), in blood, plasma, serum, or urine. Only three prior studies utilized mass spec-trometry (MS) to measure THC in brain or other solid tissues (7-9). One study applied gas chromatography (GC)–MS to measured THC disposition in tissues and fluids of the Large White Pig following intrajugular injection of varying doses of THC (7). The method used plasma calibrators for THC quantification in all specimens. The other two studies utilized liquid chromatography (HPLC)–MS to determine THC mice brain or brain structure (8,9). In one of these studies, CBD and THC were measured following marijuana inhalation (8), and the other study measured THC after intravenous injection (9). However, no validation or assay parameters are presented in either report.
We present an HPLC–MS–MS method for the identification and quantification of THC and its metabolites, and other common cannabinoids CBN, CBD, and CBC (Figure 1) in mouse brain tissue. Brain was the chosen tissue to study as it is the site of action for many of the pharmacological effects of cannabinoids. An LC–MS–MS method has been previously reported for the determination of cannabinoids other than THC and its metabolites in urine and plasma (10). The limit of detection (LOD), lower limit of quantification (LOQ), linearity, bias, precision, percent recovery, and the matrix effect in presented method were determined in accordance with the SOFT/AAFS Forensic Laboratory Guidelines (11).
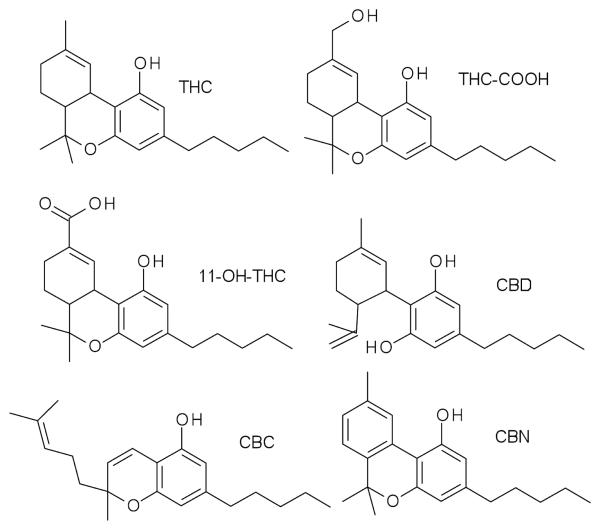
Figure 1.
Structures of THC, 11-OH-THC, THC-COOH, CBD, CBC, and CBN.
Experimental
Five male C57BL6 mice (Jackson Laboratories, Bar Harbor, ME) housed in the animal care quarters and maintained at 22 ± 2°C on a 12-h light/dark cycle with food and water available ad libitum were brought to the test environment and allowed 24 h to acclimate. The animal study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
The mice were exposed to the smoke of 200 mg of burning marijuana. The marijuana was obtained from the National Institute on Drug Abuse (Rockville, MD). An aliquot of marijuana was extracted in methanol and analyzed using the instrument method described herein but using unextracted calibrators. It was found to contain 4.4% THC, 0.22% CBC, 0.46% CBD, and 0.19% CBN. One mouse died during the exposure to the smoke of the marijuana. Twenty minutes after the exposure to the marijuana smoke, the brains of the five mice were harvested and frozen at −80°C until analysis.
Apparatus
The exposure system used was a modification of that described by Lichtman et al (12). A 15-cm corncob pipe was used to burn the plant material. The smoke from the pipe was drawn through a 27.5-cm length of tygon tubing to the manifold at a flow rate of 400 mL/min using a vacuum pump and a flow regulator. A solenoid puffing device was used to alternate the flow of smoke and fresh air to the animals every 8 s. Tygon tubing, containing 0.5 g of glass-wool fiber to sequester the smoke, was connected to the exhaust of the manifold. The mice were placed into holding tubes that fit snugly into the manifold, consisting of six ports for a nose-only exposure. They were exposed to the smoke until the plant material was completely consumed, which occurred within a 5 min time period. If the material ceased to burn at any time, it was lit again until completely consumed. The 0.5 g glass-wool fiber connected to the exhaust manifold was saved and analyzed by HPLC–MS–MS with the method described herein. The glass wool contained 159 μg THC, 10 μg CBC, 7.5 μg CBD, and 14 μg CBN.
Methods
Reagents and supplies
The THC, 11-OH-THC, THC-COOH, THC-d3, 11-OH-THC-d3, and THC-COOH-d3 were purchased from Cerilliant (Round Rock, TX). CBC and CBD were obtained from National Institute for Drug Abuse (Rockville, MD). The methanol, acetonitrile, water, and ammonium formate were purchased from Fisher Scientific (Fair Lawn, NJ) and were HPLC grade or better. Working standard solution was prepared in methanol at 1 mg/L for THC, 11-OH-THC, and THC-COOH and for 0.1 mg/L CBC and CBD. A 0.5 mg/L THCd3, 11-OH-THC-d3, and THC-COOH-d3 working internal standard solution was also prepared in methanol. A negative control and six-point calibration curves at concentrations of 0, 5, 10, 20, 50, and 100 ng/g for THC, 11-OH-THC, and THC-COOH and 0, 0.5, 1, 2, 5, and 10 ng/g for CBC and CBD in 0.5 g of drug-free mouse brain tissue were prepared with each analytical run.
Sample preparation
The drug-free and the marijuana-exposed mouse brain tissue samples were frozen at −80°C after collection. The brain tissue samples weighed between 0.35 and 0.47 g. Each sample, calibrator, or drug-free control was diluted with 1.5 g of deionized water and homogenized with a hand-held glass homogenizer.
The extractions were preformed using a modification of the procedure of Foltz et al. (13). Twenty microliters of the working internal standard was added to each homogenized brain tissue. These tissues were mixed and allowed to equilibrate overnight. The following day, 2 mL of ice-cold acetonitrile was added drop by drop to each sample while vortex mixing. The samples were then centrifuged at 3500 rpm for 10 min. After centrifuging the samples were placed in −40°C freezer for at least 2 h. The top layer containing the acetonitrile was removed via a disposable glass pipette and placed in a clean test tube. Samples were dried using a Savant AES1000. The samples were reconstituted with 100 μL of acetonitrile and placed in autosampler vial for LC–MS–MS analysis.
Instrumental analysis
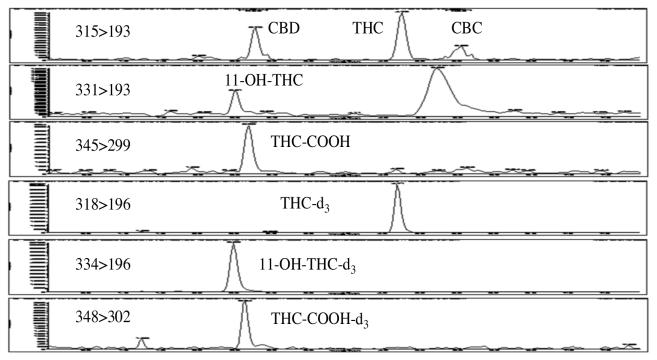
The LC-MS-MS system used was an Applied Bio systems 3200 Q trap with a turbo V source for TurbolonSpray attached to a Shimadzu SCL HPLC system controlled by Analyst 1.4.2 software. The chromatographic separation (Figure 2) was performed using a Zorbaz eclipse XDBC18 column (4.6 × 75 mm, 3.5 micron, Agilent Technologies). The mobile phase contained water/methanol (10:90, v/v) with 0.1 mM ammonium formate and was delivered at a flow rate of 0.5 mL/min. The source temperature was set at 650°C, and curtain gas had a flow rate of 30 mL/min. The ionspray voltage was 5000 V, with the ion source gases 1 and 2 having flow rates of 60 mL/min. The acquisition mode used was multiple reaction monitoring (MRM). Table I lists the transition ions monitored and the corresponding deprotonation (DP) and collision energies (CE) used for each compound. The chromatographic method resolved THC, CBD, and CBC, which all have the same transition ions, 315>193. The total run time for the analytical method was 8 min.
Figure 2.
The chromatographic separation of 2.5 ng/g THC, 11-OH-THC, and THC-COOH and 0.25 ng/g (LOD) CBC and CBD in mouse brain tissue.
Table I.
Transition Ions and Their Corresponding Deprotonation and Collision Energies
| Compound | Retention Time (min) |
Transition Ions (m/z) |
Deprotonation Energy (V) |
Collision Energy (eV) |
|---|---|---|---|---|
| THC | 4.7 | 315>193 | 46 | 27 |
| 315>259 | 46 | 27 | ||
| 11-OH-THC | 2.5 | 331>193 | 31 | 33 |
| 331>105 | 31 | 51 | ||
| THC-COOH | 2.6 | 345>299 | 46 | 23 |
| 345>193 | 46 | 23 | ||
| CBC | 5.6 | 315>193 | 31 | 23 |
| 315>293 | 31 | 23 | ||
| CBD | 2.8 | 315>193 | 46 | 29 |
| 315>259 | 41 | 29 | ||
| THC-d3 | 4.7 | 318>196 | 41 | 45 |
| 11-OH-THC-d3 | 2.6 | 334>196 | 41 | 33 |
| THC-COOH-d3 | 2.5 | 348>302 | 41 | 23 |
Bias and intra- and interassay precision
The bias was determined at 10 ng/g (n = 6) THC, THC-COOH, and 11-OH-THC and at 1 ng/g for CBC and CBD. The bias for all compounds was 80–120% of the expected value. The intrarun precision of the assay was determined by replicate (n = 3) analysis of samples with concentrations of 5, 10, 25, 50, and 100 ng/g THC, THCCOOH, and 11-OH-THC and CBC and CBD at one-tenth the concentration. The interrun precision of the assay was completed at the same concentrations (n = 3) prepared on three different days. The intrarun precision for THC, THC-COOH, 11-OH-THC, CBC, and CBD produced coefficient of variation (% CV) of less than ± 15%. The interrun precision for all of the cannabinoids tested produced % CV of less than ± 20%.
Recovery and matrix effects
Table II presents the recoveries and matrix effects for each cannabinoid. Recoveries were determined by comparing the response of drug-added specimens, which were then extracted, to the response of drug-free extracts to which the drugs were added after extraction. Thus, cannabinoid recoveries were estimated by comparing the response of 0.5 g of drug-free mouse brain tissue (n = 3) to which 10 ng/g THC, THC-COOH, and 11-OH-THC and 1 ng/g CBC and CBD were added before extraction, to the response obtained from samples 0.5 g of drug-free mouse brain tissue extracts to which the cannabinoids were added at 10 ng/g THC, THC-COOH, and 11-OH-THC and 1 ng/g CBC and CBD after extraction (n = 3). The matrix effect was determined by comparing the response obtained from unextracted samples (n = 3) at 10 ng/g of THC, THCCOOH, and 11-OH-THC and 1 ng/g for CBC and CBD and with the response obtained of samples where the analytes were add at 10 ng/g of THC, THC-COOH, 11-OH-THC and 1 ng/g for CBC and CBD to extracts (n = 3) of 0.5 g of drug-free mouse brain tissue. As presented in Table II, the brain extracts produced significant ion suppression in the electrospray system. Less ion suppression would be likely with an atmospheric pressure chemical ionization interface; however, the system would also likely be less sensitive. Our electrospray interface demonstrated sufficient sensitivity to detect CBC and CBD at relatively low concentrations.
Table II.
Recoveries and Matrix Effect for Each Cannabinoid
| Compound | % Recovery (%CV) |
% Matrix Effect (%CV) |
|---|---|---|
| THC | 77.3 (1.8) | 44.8 (2.9) |
| 11-OH-THC | 72.0 (5.6) | 73.4 (0.7) |
| THC-COOH | 34.5 (3.2) | 57.9 (1.5) |
| CBC | 83.0 (10.8) | 65.6 (9.0) |
| CBD | 88.8 (2.9) | 62.3 (10.0) |
Linear range, LOQ, and LOD
The linearity was determined by the analysis of calibration curves of 5, 10, 25, 50, and 100 ng/g THC, THC-COOH, and 11-OH-THC and CBC and CBD at one-tenth the concentrations and prepared brain tissue samples with concentrations range from 1 ng/g THC, THC-COOH, and 11-OH-THC and 0.25 ng/g CBC and CBD to 200 ng/g THC, THC-COOH, and 11-OH-THC and 20 ng/g CBC and CBD on three different days. The r2 values of the calibration curves (n = 3) for all analytes were 0.995 or better. All observed calibration values were within ± 20% of their expected values. The quantification range was administratively set to an LOQ and LOD of 1 ng/g with the upper limit of quantification of 200 ng/g for THC, THC-COOH, and 11-OH-THC. Likewise, an administrative LOD of 0.25 ng/g and LOQ of 0.5 ng/g with an upper limit of quantification of 20 ng/g were set for CBC and CBD. The % CV for all analytes at the LOQ was less than ± 20%, the signal-to-noise ratio was greater than 1:10 and LOD signal-to-noise ratio was greater than 1:3 for all analytes. The % CV for the upper limit of quantification was less than ± 20%.
Specificity
Drug-free brain extract did not yield an observable signal at the transition ions of each analyte and the internal standard at their respective retention times. The specificity of the assay was determined by analyzing a 0.5-mL aliquot of a Bio-Rad Liquichek Therapeutic Drug Monitoring Control (TDM), Level 2 control (Bio-Rad Laboratories Hercules, CA). Table III contains a list of drugs contained in the Level 2 control. None of these drugs were found to interfere with the assay; however, the drugs listed in Table III were not added to brain tissues. It is highly improbable that these drugs, which are readily extracted from serum, would yield significant interferences if extracted from brain. Additionally, the unnecessary sacrifice of a large number of additional animals to prepare matrix match samples for each of the interference tested drugs was deemed imprudent.
Table III.
Compounds Tested That Showed No Interference with the Assay
| Compound | Compound | Compound |
|---|---|---|
| Acetaminophen | Estriol | Phenytoin |
| Amikacin | Ethosuximide | Primidone |
| Amitriptyline | Flecainide | Procainamide |
| Caffeine | Gentamicin | Propranolol |
| Carbamazepine | Haloperidol | Quinine |
| Chloramphenical | Ibuprofen | Salicylate |
| Clonazepam | Imipramine | T3 |
| Cortisol | Lidocaine | T4 |
| Cyclosporine | Lithium | Theophylline |
| Desipramine | Methotrexate | TSH |
| Diazepam | N-Acetyl Procainamide | Valproic Acid |
| Digoxin | Nortriptyline | Vancomycin |
| Disopyramide | Phenobarbital |
Results and Discussion
The marijuana and the glass wool filter from the exposure system were analyzed for THC, THC-COOH, 11-OH-THC, CBC, CBD, and CBN. Neither THC-COOH nor 11-OH-THC was detected in these samples. The marijuana contained 44 μg/g of THC, 2.2 μg/g of CBC, 1.9 μg/g of CBD, and 4.7 μg/g of CBN. The glass wool filter contained 159 μg of THC, 10 μg of CBC, 7.5 μg of CBD, and 14.2 μg of CBN. The cannabinoids found in the glass wool filter confirmed that THC, CBC, and CBD were aerosolized in the marijuana smoke.
Table IV presents the brain tissue concentrations of THC, THC-COOH, 11-OHTHC, CBC, and CBD for the five mice exposed to the marijuana smoke. Mouse number five died during exposure to marijuana smoke. Therefore, the data from mouse five were not used to calculate the mean brain cannabinoid concentrations. The mean brain concentrations for the four mice that survived the exposure to the marijuana smoke and were sacrificed 20 min after exposure were 3.9 ± 1.5 ng/g CBC, 21 ± 3.9 ng/g CBD, 364 ± 74 ng/g THC, and 28 ± 5.9 ng/g 11-OH-THC. The THC concentrations observed in the presented study are consistent with those observed by Varvel et al. (8). Using the same experimental conditions of marijuana exposure, they reported a mean brain THC concentration of approximately 400 ng/g at 20 min post-exposure. THC-COOH was not detected in any of the five mice brain specimens at the LOD of 0.5 ng/g. THC-COOH is relatively more polar/nonlipophilic than the other cannabinoids; therefore, it would not likely accumulate in high concentrations in brain tissue.
Table IV.
Transition Ions and Their Corresponding Deprotonation and Collision Energies
| Sample | THC (ng/g) |
THC-COOH (ng/g) |
11-OH-THC (ng/g) |
CBC (ng/g) |
CBD (ng/g) |
|---|---|---|---|---|---|
| Mouse 1 | 386 | none detected | 21 | 6.3 | 31 |
| Mouse 2 | 215 | none detected | 14 | 2.4 | 17 |
| Mouse 3 | 485 | none detected | 22 | 4.5 | 36 |
| Mouse 4 | 374 | none detected | 29 | 4.4 | 26 |
Table V compares the relative percentages of THC, CBC, and CBD to their total amount in the brain tissue samples, the marijuana, and the glass wool filter. These results indicate that the relative percentage of THC, CBC, and CBD found in the brain tissues is closely related to their relative percentages found in the marijuana to which the mice were exposed.
Table V.
Percentages of THC, CBC, and CBD in the Five Mouse Brain Tissue Samples, the Marijuana, and the Glass Wool Fiber
| Sample | THC | CBC | CBD |
|---|---|---|---|
| Mouse 1 | 91 | 1.4 | 7.4 |
| Mouse 2 | 92 | 1.0 | 7.3 |
| Mouse 3 | 92 | 0.9 | 6.8 |
| Mouse 4 | 93 | 0.6 | 6.4 |
| Marijuana | 91 | 4.5 | 3.9 |
| Glass wool | 90 | 5.6 | 4.2 |
Conclusions
The presented HPLC–MS–MS method for the determination of THC, 11-OH-THC, THC-COOH, CBC, and CBD in mouse brain tissue was sensitive and robust for quantification. The method uses a well-established, reliable liquid-liquid extraction procedure with a simple isocratic HPLC that resolved the major marijuana cannabinoids. The observed mouse brain concentrations of THC, 11-OH-THC, CBC, and CBD were proportional to the concentrations found in the inhaled marijuana.
Acknowledgments
This project was supported by the National Institute on Drug Abuse (NIDA) Center for Drug Abuse grant R01DA02396, R01DA03672, and P50DA005274.
References
- 1.Grotenhermen F. Pharmacokinetics and pharmacodynamic of cannabinoids. Pharmacokinet. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- 2.ElSohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed SA, Ross SA, Slade D, Radwan MM, Zulfigar F, ElSohly MA. Cannabinoid ester constituents from high-potency Cannabis sativa. J. Nat. Prod. 2008;71:536–542. doi: 10.1021/np070454a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radwan MM, Ross SA, Ahmed SA, Slade D, Zulfigar F, ElSohly MA. Isolation and characterization of new cannabis constituents from a high potency variety. Planta Med. 2008;74:267–272. doi: 10.1055/s-2008-1034311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radwan MM, ElSohly MA, Slade D, Ahmed SA, Khan IA, Ross SA. Biologically active cannabinoids from high-potency Cannabis sativa. J. Nat. Prod. 2009;72:906–911. doi: 10.1021/np900067k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huestis MA. Cannabis (Marijuana)–effects on human behavior and performance. Forensic Sci. Rev. 2002;14:15–60. [PubMed] [Google Scholar]
- 7.Brunet B, Doucet C, Venisse N, Hauet T, Hébrard W, Papet Y, Mauco G, Mura P. Validation of Large White Pig as an animal model for study of cannabinoid metabolism: application to the study of THC distribution in tissues. Forensic Sci. Int. 2006;161:169–174. doi: 10.1016/j.forsciint.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Varvel S, Wiley J, Yang R, Bridgen D, Long K, Lichtman A, Martin B. Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology (Berl) 2006;186:226–234. doi: 10.1007/s00213-006-0356-9. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR. Opposing actions of D9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn. Mem. 2007;14:63–74. doi: 10.1101/lm.439007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauwiler SB, Scholer A, Drewe J. Development of a LC/MS/MS method for the analysis of cannabinoids in human EDTA-Plasma and urine after small dose of Cannabis sativa extracts. J. Chromatogr. B. 2007;850:515–522. doi: 10.1016/j.jchromb.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 11.SOFT/AAFS Forensic Laboratory Guidelines, Version 2006, section 8.3 Method calibration and validation. :12–14. [Google Scholar]
- 12.Lichtman AH, Poklis JL, Wilson DM, Poklis A, Martin BR. The pharmacological activity of inhalation exposure to marijuana in mice. Drug Alcohol Depend. 2001;63:107–116. doi: 10.1016/s0376-8716(00)00205-2. [DOI] [PubMed] [Google Scholar]
- 13.Foltz RL, McGinnis KM, Chinn DM. Quantitative measurement of delta-9-tetrahydrocannabinol and two major metabolites in physiological specimens using capillary column gas chromatography negative ion chemical ionization mass spectrometry. Biomed. Mass Spectrom. 1983;10:316–323. doi: 10.1002/bms.1200100503. [DOI] [PubMed] [Google Scholar]