Abstract
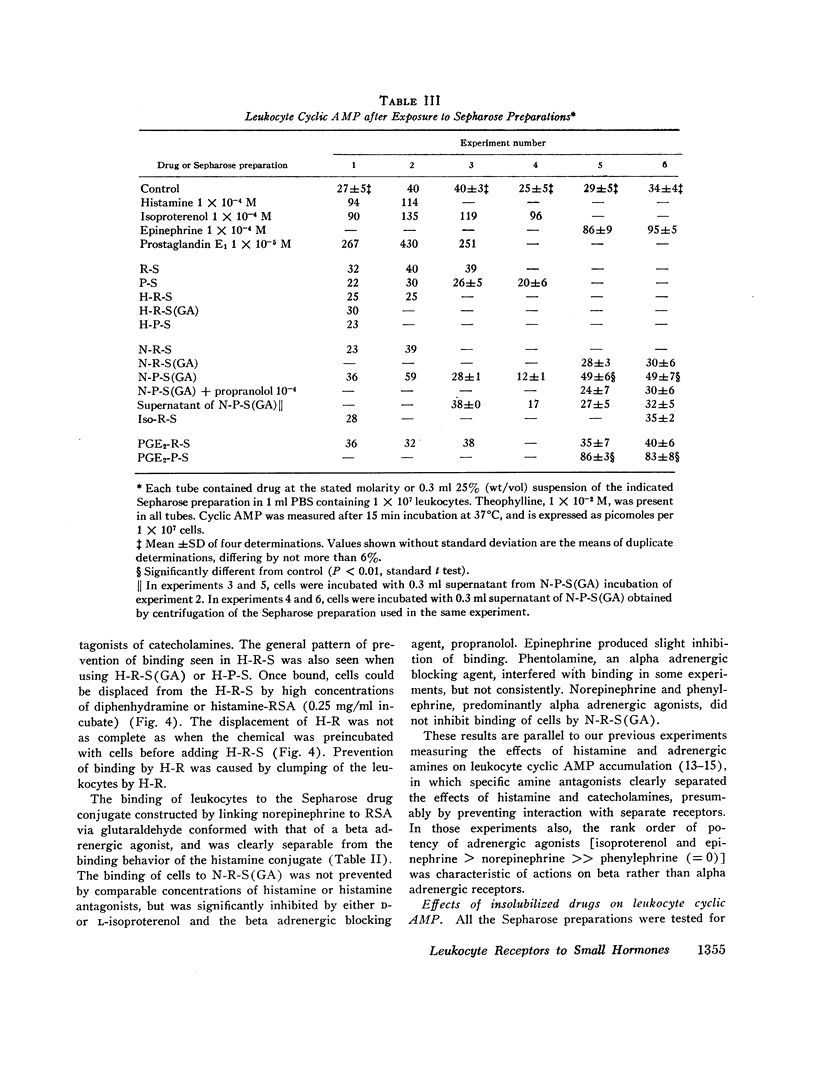
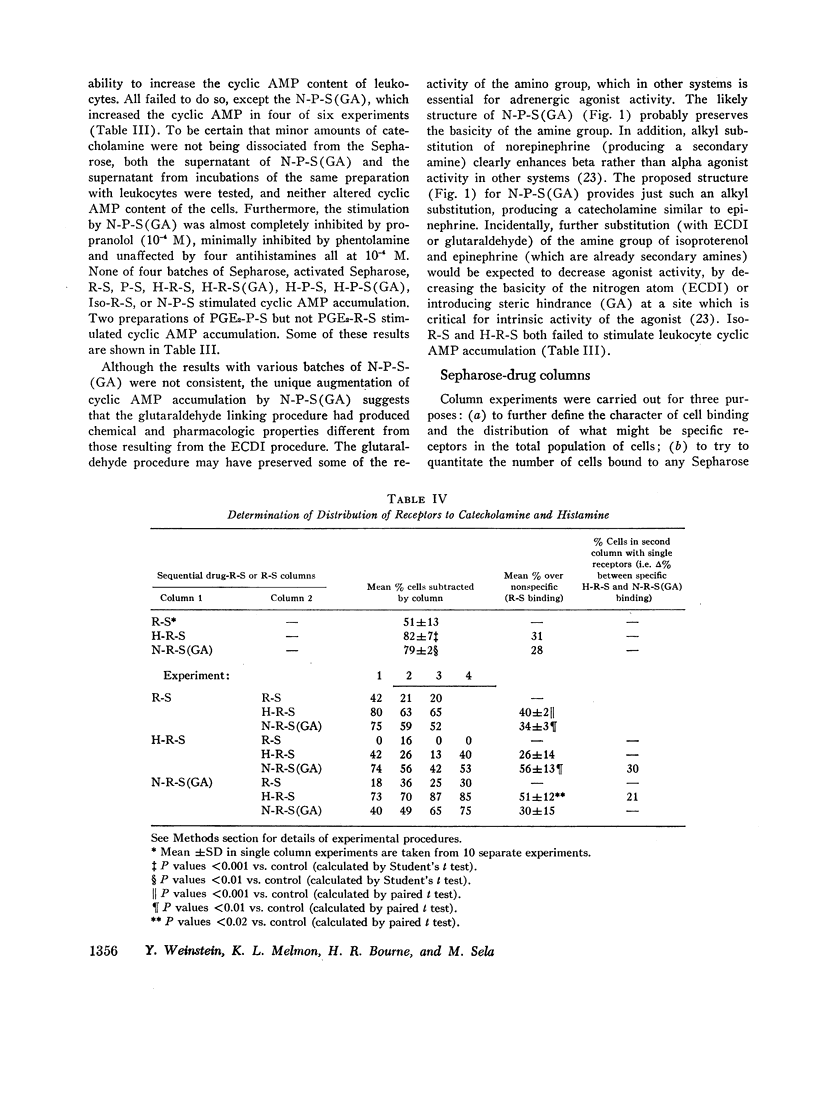
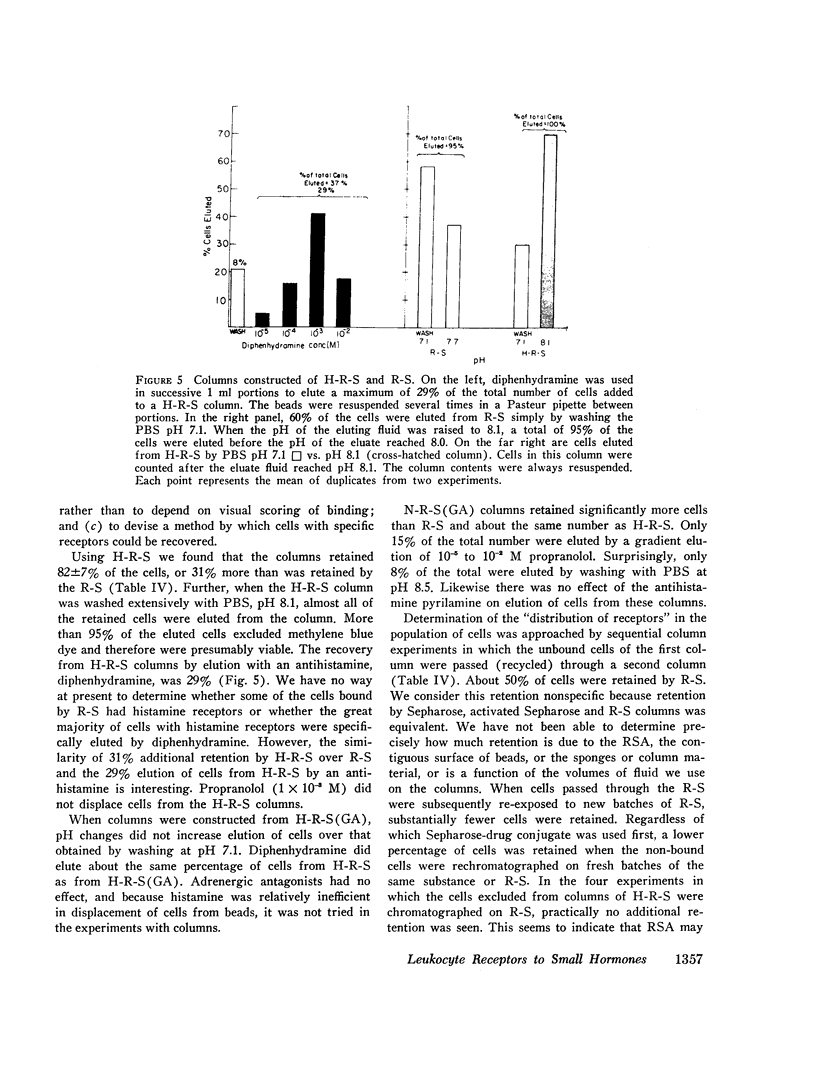
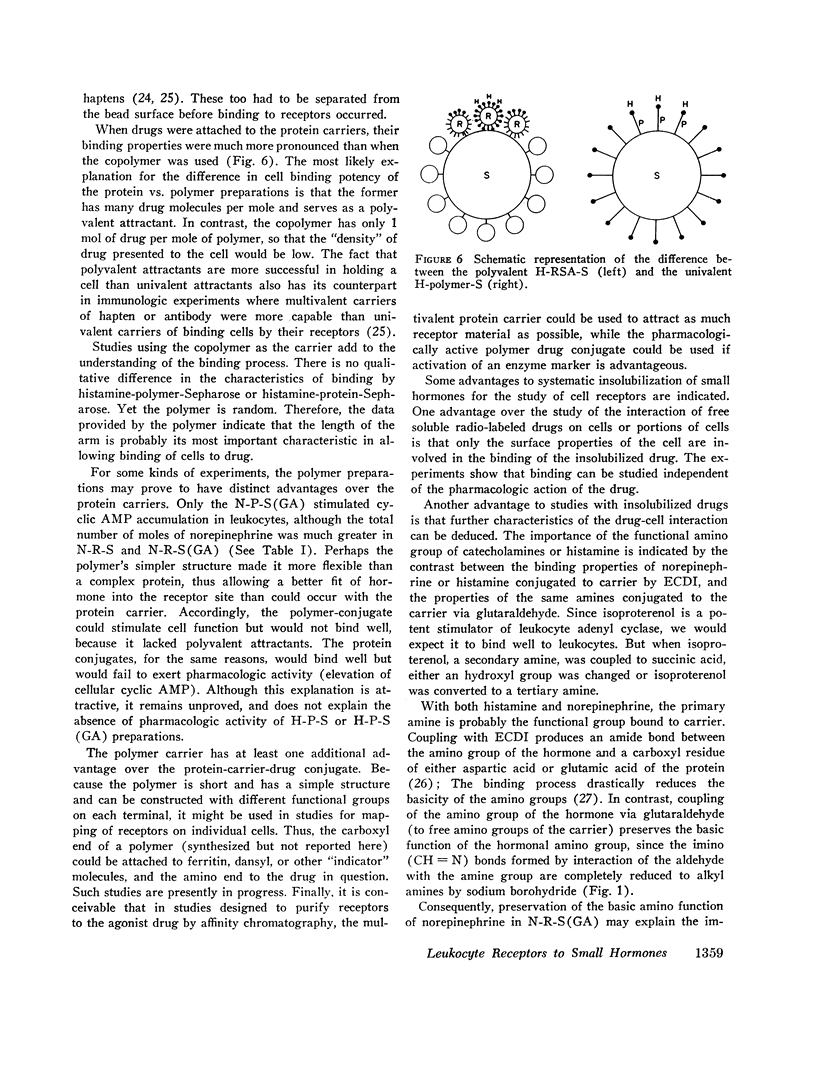
Receptors for small endogenous hormones on human leukocytes were studied by insolubilizing the hormones and incubating them with the cells. Histamine, norepinephrine, and prostaglandin E2 (PGE2) were conjugated to either of two types of carrier: (bovine or rabbit) serum albumin or a random copolymer of DL-alanine and L-tyrosine. The conjugates were linked to agarose beads (Sepharose) and the resultant drug-conjugate-beads were incubated with leukocytes. Norepinephrine (when linked to its carrier via glutaraldehyde) and histamine preparations bound the majority of leukocytes. The binding appeared to be specific for the hormones tested. For example, the binding by histamine-rabbit serum albumin-Sepharose was prevented or reversed by high concentrations of histamine and histamine antagonists, but not by catecholamines or their pharmacologic antagonists. Similarly, binding of cells to the norepinephrine conjugate was inhibited by some catecholamines and propranolol, but not by histamine or histamine antagonists. Conjugates of norepinephrine linked via carbodiimide did not bind cells. The protein or copolymer carriers did not contribute to binding per se. The hormone-protein-conjugates bound more cells than the hormone-polymer conjugates. The former (unlike the free amines) failed to stimulate accumulation of cyclic AMP in leukocytes. The norepinephrine linked to polymer via glutaraldehyde, however, did stimulate leukocyte cyclic AMP accumulation, possibly because of the flexibility of the polymer. Columns of the various Sepharoses were used to determine the distribution of receptors to each hormone in mixed leukocyte populations. The majority of cells appeared to have receptors for both histamine and norepinephrine (bound through glutaraldehyde). Receptors to prostaglandins may have been detected by the column procedure, but their distribution could not be quantitated. The approach described provides a means to separate leukocytes on the basis of what are likely to be preformed receptors to small endogenous hormones, and to study the physiologic importance and function of the receptors.
Full text
PDF


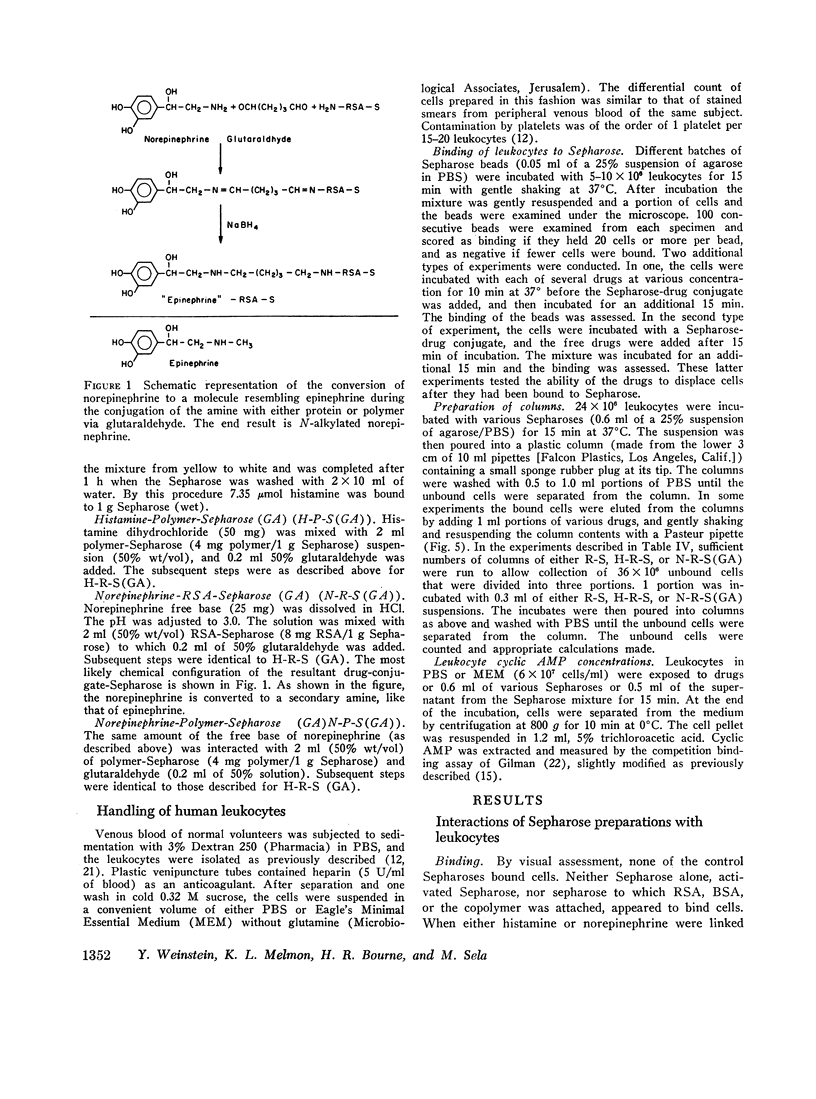
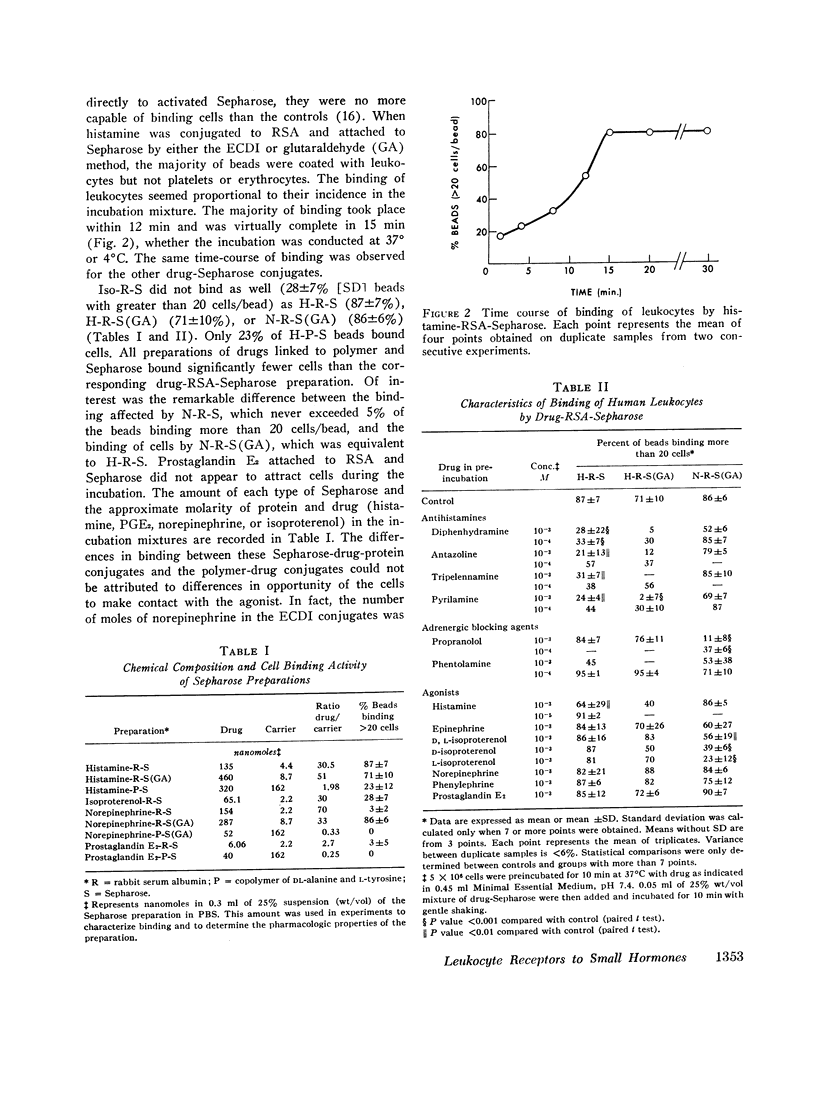
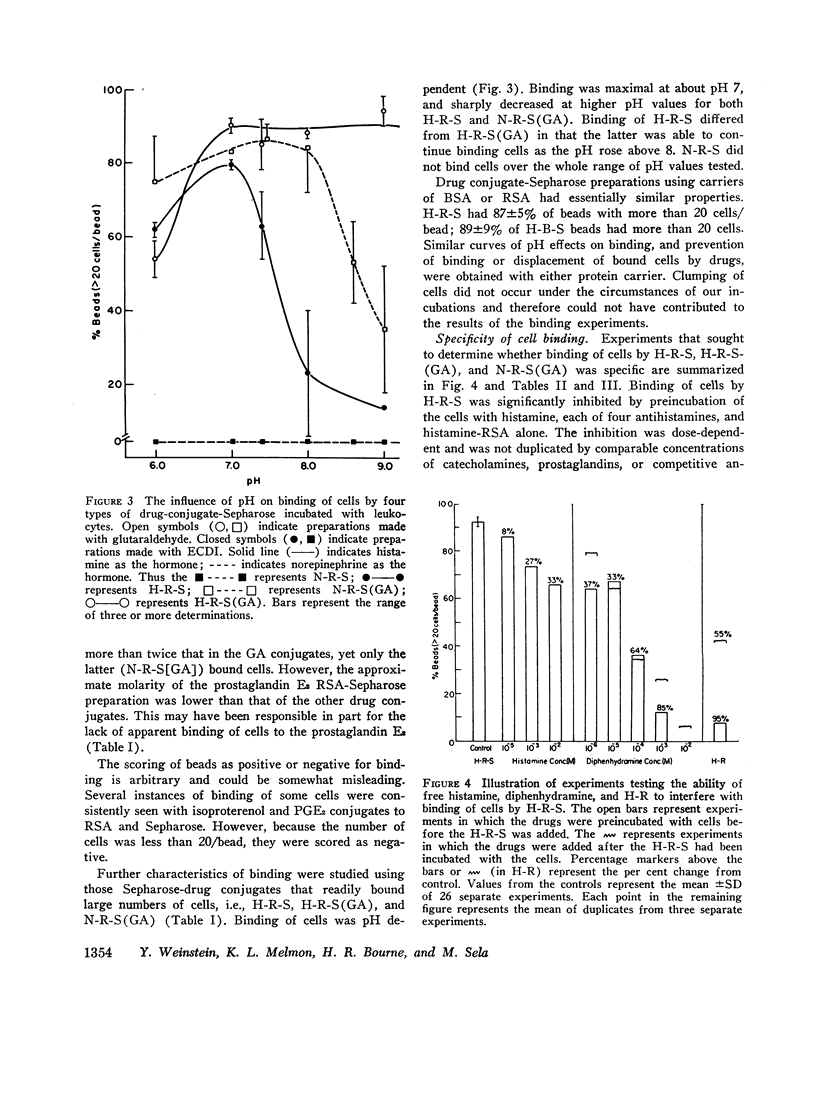



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R., Lehrer R. I., Cline M. J., Melmon K. L. Cyclic 3',5'-adenosine monophosphate in the human lukocyte: synthesis, degradation, andeffects n neutrophil candidacidal activity. J Clin Invest. 1971 Apr;50(4):920–929. doi: 10.1172/JCI106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L. Pharmacologic control of allergic histamine release in vitro: evidence for an inhibitory role of 3',5'-adenosine monophosphate in human leukocytes. J Immunol. 1972 Mar;108(3):695–705. [PubMed] [Google Scholar]
- Bourne H. R., Melmon K. L. Adenyl cyclase in human leukocytes: evidence for activation by separate beta adrenergic and prostaglandin receptors. J Pharmacol Exp Ther. 1971 Jul;178(1):1–7. [PubMed] [Google Scholar]
- Bourne H. R., Melmon K. L., Lichtenstein L. M. Histamine augments leukocyte adenosine 3',5'-monophosphate and blocks antigenic histamine release. Science. 1971 Aug 20;173(3998):743–745. doi: 10.1126/science.173.3998.743. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography. Annu Rev Biochem. 1971;40:259–278. doi: 10.1146/annurev.bi.40.070171.001355. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor of isolated fat cell membranes. J Biol Chem. 1971 Dec 10;246(23):7265–7274. [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick J. K., Marinetti G. V. Hormone action at the membrane level. 3. Epinephrine interaction with the rat liver plasma membrane. Biochim Biophys Acta. 1971 Oct 12;249(1):122–134. doi: 10.1016/0005-2736(71)90089-7. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht J. P., Dellacha J. M., Santome J. A., Paladini A. C., Hurwitz E., Sela M. Lipolytic activity of bovine growth hormone bound to sepharose beads. FEBS Lett. 1972 Jan 15;20(1):83–86. doi: 10.1016/0014-5793(72)80022-x. [DOI] [PubMed] [Google Scholar]
- KAY R. E., HARRIS D. C., ENTENMAN C. Quantification of the ninhydrin color reaction as applied to paper chromatography. Arch Biochem Biophys. 1956 Jul;63(1):14–25. doi: 10.1016/0003-9861(56)90004-2. [DOI] [PubMed] [Google Scholar]
- Krug F., Desbuquois B., Cuatrecasas P. Glucagon affinity absorbents: selective binding of receptors of liver cell membranes. Nat New Biol. 1971 Dec 29;234(52):268–270. doi: 10.1038/newbio234268a0. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Haber E. A fraction of the ventricular myocardium that has the specificity of the cardiac beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1773–1777. doi: 10.1073/pnas.68.8.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pastan I. Effects of calcium on ACTH stimulation of the adrenal: separation of hormone binding from adenyl cyclase activation. Nature. 1970 Nov 28;228(5274):864–866. doi: 10.1038/228864a0. [DOI] [PubMed] [Google Scholar]
- Melmon K. L., Bourne H. R., Weinstein J., Sela M. Receptors for histamine can be detected on the surface of selected leukocytes. Science. 1972 Aug 25;177(4050):707–709. doi: 10.1126/science.177.4050.707. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Olsen R. W., Menez A., Fromageot P., Boquet P., Changeux J. P. Some physical properties of the cholinergic receptor protein from Electrophorus electricus revealed by a tritiated alpha-toxin from Naja nigricollis venom. Biochemistry. 1972 Mar 28;11(7):1200–1210. doi: 10.1021/bi00757a014. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Masouredis S. P., Singer S. J. Quantitative two-dimensional ultrastructural distribution of Rh o (D) antigenic sites on human erythrocyte membranes. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1416–1420. doi: 10.1073/pnas.68.7.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Truffa-Bachi P., Wofsy L. Specific separation of cells on affinity columns. Proc Natl Acad Sci U S A. 1970 Jul;66(3):685–692. doi: 10.1073/pnas.66.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Andersson B. Cell separation on antigen-coated columns. Elimination of high rate antibody-forming cells and immunological memory cells. J Exp Med. 1969 Jan 1;129(1):23–36. doi: 10.1084/jem.129.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANSTIS D. A. Rapid determination of molecular weights of peptides and preteins. Ann N Y Acad Sci. 1960 Aug 31;88:586–601. doi: 10.1111/j.1749-6632.1960.tb20055.x. [DOI] [PubMed] [Google Scholar]