Abstract
The copying of chromosomal DNA initiates from a single nucleoprotein assembly called the prereplication complex. New findings in a recent issue of Molecular Cell (Yardimci et al., 2010) reveal that this complex dissolves into two independent replisomes that move away from each other as DNA synthesis ensues.
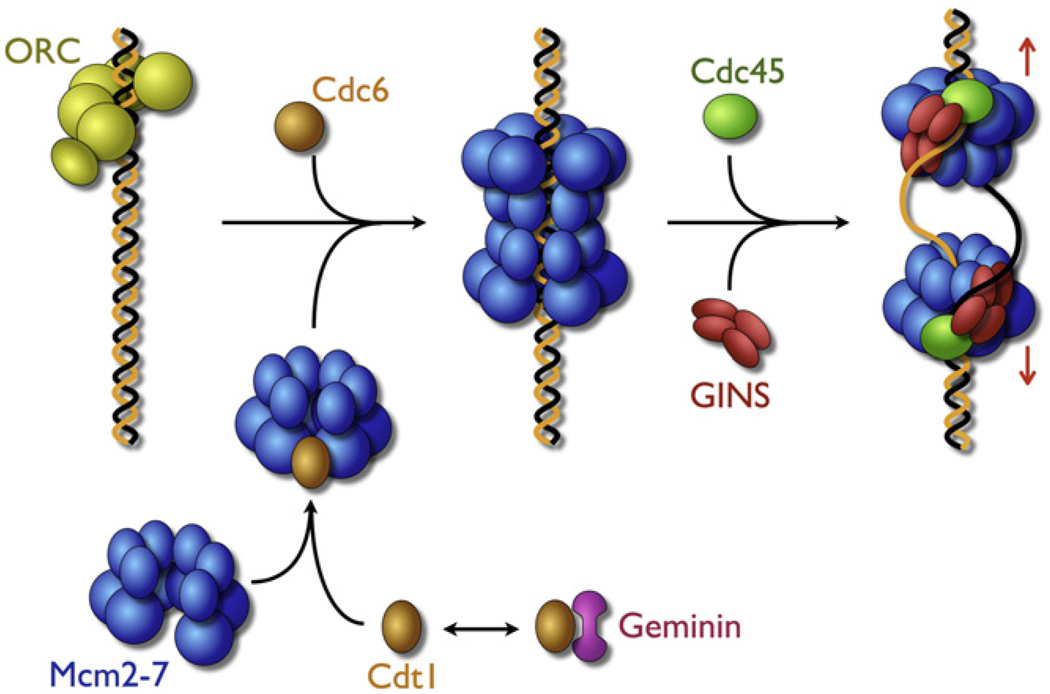
The bidirectional initiation of chromosomal DNA replication, though a universal event in all cells, is established in different ways in different organisms. In bacteria, the initiator protein DnaA melts specialized DNA start sites (termed origins) and assists in the loading of two hexameric helicases in opposing directions on the two separated strands (Sutton et al., 1998; Mott et al., 2008). Each DnaB hexamer, which is uncoupled from its partner, is loaded in an active state and destined to form an independent replication fork accompanied with the synthetic machinery including two DNA polymerases and various accessory factors (Breier et al., 2005). In eukaryotes, the Mcm2-7 heterohexameric helicase provides the unwinding functions at the fork. The eukaryotic origin recognition complex (ORC) works with Cdc6 and the loading factor Ctd1 to recruit Mcm2-7 to origins, forming an intermediate known as the prereplication complex (pre-RC). In contrast to observations for DnaB, recent work in S. cerevisiae has shown that pre-RC formation is linked to the concerted deposition of two Mcm2-7 copies into an inactive, head-to-head double hexamer around unmelted duplex DNA (Remus and Diffley, 2009) (Figure 1).
Figure 1. Model for Formation of Bidirectional Replication Forks.
The Mcm2-7 complex assumes an open form that may be closed by Ctd1, a molecular matchmaker that brings the helicase to ORC and Cdc6 bound to a chromosomal origin. In a concerted reaction, ORC/Cdc6/Cdt1 load two Mcm rings onto duplex DNA in an inactive, head-to-head complex. In a second concerted reaction, assisted by the Dbf4-dependent kinase and the binding of GINS/Cdc45, the double hexamer is converted into a functioning CMG assembly that melts the duplex and separates into two independent replisomes.
Electron micrographs of the Mcm2-7 double hexamer bear a striking resemblance to images of the SV40 and papilloma virus replicative helicases assembled at viral preinitiation origins. These superfamily 3 viral helicases are loaded in an active state competent to unwind DNA, whereas Mcm2-7 activation requires additional factors, such as the GINS complex and Cdc45 (Ilves et al., 2010). In archaeons, which possess homologs of most eukaryotic replication proteins, less is known about the formation of preinitiation complexes, but Mcm double hexamers have been observed, formed by head-to-head interactions between the amino-terminal domains of the motor (Brewster and Chen, 2010). These views likely recapitulate some of the molecular details of how the eukaryotic Mcm2-7 double hexamer appears when initially loaded onto an origin. Thus, archaeal/eukaryl replicative helicases, as well as those of certain viruses, appear to contain the seeds for forming a bidirectional replication complex as an integral part of their assembly mechanism. This capacity in turn has frequently been used to suggest that the action of functioning replisomes is linked through a doubly hexameric motor. Now, recent studies published in Molecular Cell (Yardimci et al., 2010) stand to counter this view, in that they demonstrate that the single pre-RCs formed during initiation actually convert into two uncoupled sister replisomes.
Some history behind this breakthrough is in order. In 1980, Sundin and Varshavsky (1980) first proposed that sister replisomes in eukaryotes might be held tightly together through helicases as an efficient way to circumvent strand entanglement. These ideas received strong support from the EM pictures of the SV40 T-antigen unwinding duplex, where a fraction of unwound molecules showed double hexamers with “rabbit ears” of single-stranded DNA spooling out from the body of the particle (Wessel et al., 1992). Subsequent biochemical studies suggested that double hexamers might have more robust helicase activity than single hexamers, and have led to models in which the template becomes unwound through the pumping of duplex DNA into a central vestibule connecting the two hexamers that separates single DNA strands. By contrast, studies on bacterial DnaB (as well as its phage T7 and T4 equivalents) have indicated that only one DNA strand is engaged by the hexameric helicase, and that DNA unwinding occurs by a steric occlusion mechanism in which the ring-shaped motor splits the duplex as it travels forward. These two models have shared a somewhat uneasy coexistence in the replication field, with their distinctions largely papered over by evoking the evolutionary differences between bacterial and archaeal/eukaryl replication systems. However, recent work on the papilloma virus replicative helicase, a close relative of SV40, has indicated that the superfamily 3 enzymes also encircle only one strand in their active unwinding state (Enemark and Joshua-Tor, 2006). This latter finding has raised the possibility that SV40 T-antigen double hexamers might not represent the active replicating state of the enzyme and might instead be the result of fortuitous collisions of individual hexamers (which outnumber the double hexamers by nearly three to one) during their deposition on EM grids.
The recent elegant single molecule studies from the labs of Walter and Van Oijen help cut through this “Gordian knot” and answer directly the question as to whether forks are linked on the chromosome. Xenopus egg extracts, which support initiation in the absence of any specific origin sequence, will readily replicate lambda DNA in vitro. Tethering one end of this phage DNA to a surface in a microfluidic chamber, Yardimci et al. (2010) first load the DNA with the Mcm2-7 to create pre-RCs and then introduce factors required for DNA synthesis. Modified nucleotides are used to visualize the products with TIRF microscopy. The average number of initiations seen is about four to five per molecule, which in turn corresponds to an average ori-to-ori distance of 10 Kb, a value in good agreement with previous bulk measurements. Remarkably, the same experiment can be carried out with DNA stretched close to its maximal B form length to create a counterforce against any predisposition for two sister forks to remain together. In both instances, the average number of initiation events per DNA stays constant, as does the ability of initiation foci to separate. Replication fork speed also is unaffected, nor do sister fork growth rates from a single pre-RC appear at all correlated. While these observations do not rule out the possibility that cells may loosely cluster DNA metabolism factors into local neighborhoods as a means of maximizing replication efficiency, they do establish that sister replisomes have no direct biochemical need for coupling per se.
The data established by Yardimci et al. (2010) are difficult to reconcile with the pump model of DNA unwinding by the Mcms. What possibilities remain? A second prevailing model envisions the existence of a molecular “plough,” which can be dragged through DNA strands behind a translocating motor to drive unwinding. It is known that to be activated in eukaryotes, Mcm2-7 must associate with five additional factors, the four-subunit GINS complex and Cdc45. GINS, which can bind single-stranded DNA, has been one candidate for such a plough. However, these activating factors may bind neither in front of nor behind Mcm2-7 but to its periphery, parallel with the central axis of the motor. Moreover, the CMG—in contrast to isolated Mcm complexes—cannot bind duplex DNA (Ilves et al., 2010).
Together, the available data appear to best fit a “classic” steric exclusion model, such as that invoked for DnaB-like helicases, wherein only one strand of DNA passes through the middle of the ring-shaped motor as it tracks forward. But how do linked double hexamers, which can slide on DNA in an ATP-independent manner, convert and dissociate into two independently functioning replisomes? Schwacha and colleagues (Bochman et al., 2008) have proposed that an opening between Mcm 2 and 5 subunits may serve as a gate for DNA binding, and we propose that the activating factors Cdc45 and the GINS serve to close and seal this gate and that this may allow for better intersubunit communication around the Mcms. Although EM models of pre-RC loaded double hexamers do not show evidence of such a gap, the averaging methods used to improve the quality of these models may have blurred out such a feature, or the gap may be sufficiently small to have been indistinguishable at the resolution of the models. Binding of GINS and Cdc45 could serve to both promote DNA untwisting by Mcm2-7 and capture one of the two strands as it egresses from the motor interior. The molecular details of the pathway that leads to the higher-order assembly toward the CMG in the cell are not understood but are dependent upon S phase-promoting kinases, which along with other factors may help dissociate the two linked Mcms into the mature, separated forks observed by Walter and colleagues. Clearly there are still many fundamental mysteries to be solved in this oft-considered “mature” field.
REFERENCES
- Bochman ML, Bell SP, Schwacha A. Mol. Cell Biol. 2008;28:5865–5873. doi: 10.1128/MCB.00161-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier AM, Weier HG, Cozzarelli NR. Proc. Natl. Acad. Sci. USA. 2005;102:3942–3947. doi: 10.1073/pnas.0500812102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AS, Chen XS. Crit. Rev. Biochem. Mol. Biol. 2010;45:243–256. doi: 10.3109/10409238.2010.484836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enemark EJ, Joshua-Tor L. Nature. 2006;442:270–275. doi: 10.1038/nature04943. [DOI] [PubMed] [Google Scholar]
- Ilves I, Petojevic T, Pesavento JJ, Botchan MR. Mol. Cell. 2010;37:247–258. doi: 10.1016/j.molcel.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott ML, Erzberger JP, Coons MM, Berger JM. Cell. 2008;135:623–634. doi: 10.1016/j.cell.2008.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remus D, Diffley JF. Curr. Opin. Cell Biol. 2009;21:771–777. doi: 10.1016/j.ceb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Sundin O, Varshavsky A. Cell. 1980;21:103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Sutton MD, Carr KM, Vicente M, Kaguni JM. J. Biol. Chem. 1998;273:34255–34262. doi: 10.1074/jbc.273.51.34255. [DOI] [PubMed] [Google Scholar]
- Wessel R, Schweizer J, Stahl H. J. Virol. 1992;66:804–815. doi: 10.1128/jvi.66.2.804-815.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardimci H, Loveland AB, Habuchi S, van Oijen AM, Walter JC. Mol. Cell. 2010;40:834–840. doi: 10.1016/j.molcel.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]