Abstract
Purpose of review
Immune reconstitution syndrome (IRIS) is the paradoxical worsening or unmasking of an infection or neoplasm in HIV-1 infected patients shortly after antiretroviral therapy (ART) initiation. New insights into the pathogenesis of IRIS may help identify biomarkers that could be useful in predicting or diagnosing IRIS.
Recent findings
Studies of immunopathogenesis have shown a signification activation of both innate and adaptive responses with elevation of plasma or serum chemokines and cytokines. Markers of inflammation such as C-reactive protein, interferon-inducible protein 10 or Interferon γ may be helpful as predictors of IRIS events. In addition, tuberculosis (TB)- associated IRIS is associated with a prominent Th1 response that can be heightened even prior to ART initiation in cases of unmasking TB, and may assist in early diagnosis. Large prospective studies are needed to elucidate the predictive and diagnostic value of IRIS biomarkers and advance them to the clinic.
Summary
Reversal of immunosuppression by ART leads to exaggerated pathogen-specific immune responses (known as IRIS) that appear to be primed prior to therapy. Inflammatory markers, chemokines and cytokines that signify innate and adaptive immune activation are biomarkers that could prove of clinical value after appropriate validation.
Keywords: IRIS, Tuberculosis, Th1 responses, C-reactive protein
Introduction
Immune Reconstitution Inflammatory Syndrome (IRIS) is a term used to describe the paradoxical worsening of a pre-existing infection or the presentation of a previously undiagnosed condition in HIV infected patients soon after commencement of antiretroviral therapy (ART) (1). Most cases of IRIS emerge within the first few weeks to few months of ART, at a time when morbidity and mortality still remain high and frequently in patients with severe CD4 lymphopenia who remain vulnerable to opportunistic diseases. It is thus crucial to distinguish IRIS from acquisition of new opportunistic infections, medication toxicities and non-infectious complications of HIV.
There are currently no widely accepted definitions of IRIS. The International Network for Studies in HIV associated IRIS (INSHI) has published a definition for tuberculosis-IRIS (2) and other clinical trial networks use their own consensus definitions. Paradoxical IRIS is diagnosed in patients with a known infection, who experience clinical improvement after starting appropriate antimicrobial therapy, but after ART commencement they deteriorate clinically despite evidence of successful suppression of HIV plasma viremia. In paradoxical IRIS, viable organisms may not be present at the site of infection and cultures can be sterile. In unmasking IRIS, patients are diagnosed with the infectious condition after starting ART and an inflammatory component either topical or systemic may be prominent in their presentation (for example Mycobacterium avium lymphadenitis in a patient without known mycobacterial infection at prior to ART).
Because of the complexity of clinical presentations in hosts who are still severely immunocompromised, and the fact that IRIS is a diagnosis of exclusion, there is a great need for biomarkers to help in predicting and diagnosing IRIS. In recent years it has become apparent that biomarkers reflecting the immunologic consequences of HIV infection may have additive value to CD4 T cell counts and HIV plasma viremia as prognostic indicators (3). Data from the Strategies in Management of Antiretroviral Therapies (SMART) study indicated that IL-6, D-dimer and C-reactive protein (CRP) could help classify patients at risk for all cause morbidity and mortality (3). For opportunistic diseases specifically, IL-6 and CRP several months prior to the event were of significant prognostic value (4•). Other studies have since confirmed and expanded on these findings including cohorts of anti-retroviral therapy (ART)-naïve patients (5•, 6). Studies on the pathogenesis of IRIS should focus on demonstrating clinically applicable biomarkers that could assist in identifying patients at risk and in differentiating IRIS from alternate diagnoses.
Pathogenesis of IRIS: non-pathogen specific pathways
The immunopathogenesis of IRIS remains unclear but is currently intensely investigated. Based on the clinical observations, it appears that presence of pathogens and severe CD4 lymphopenia with potential contribution of genetic factors can lead to the dysregulated immune response that is recognized as IRIS (7, 8).
Many retrospective and prospective studies have concluded that one of the main clinical predictors of IRIS is severe CD4 T cell lymphopenia prior to ART initiation (9). Animal and human data show that T lymphocytes respond to lymphopenia by enhanced homeostatic proliferation driven by both cytokines such as IL-7 and IL-15 as well as TCR triggering by self or other antigens (10–14). In animal models, profound T cell proliferation in the presence of foreign antigens can significantly skew the T cell regeneration towards pathogen-specific T cells (15). In a study of patients with graft versus host disease (GVHD), elevated serum levels of IL-7 were observed compared to controls without GVHD (16) and higher serum IL-7 levels were also found in a study of IRIS patients compared to their controls with similar CD4 T cell counts (17). The role of IL-7 in IRIS may need to be further investigated.
Another important predictor of IRIS is pre-existing opportunistic infection. Suppressed CD4 T cell function can lead to ineffective pathogen clearance and high antigen load. Reversal of the functional defect, as happens with ART, may lead to dysregulated inflammatory responses. Perhaps the best example of the role of functional T cell defect as opposed to pure immunodeficiency comes from the non-HIV literature. Clifford et al reviewed 28 cases of progressive multifocal leukoencephalopathy (PML) that occurred in patients with multiple sclerosis who received natalizumab monotherapy (18••). Natalizumab is a monoclonal antibody that blocks the α4β7 and α4β1 integrins preventing normal extravasation of leukocytes from the bloodstream and leading to reactivation of JC and BK viruses (19)and clinical PML (20, 21). Patients who developed PML underwent plasma exchange or immunoabsorption and developed PML-IRIS, which required use of corticosteroid therapy (18). This demonstrates that IRIS can occur upon reversal of functional suppression of T lymphocytes even in the absence of lymphopenia.
Innate immune responses may also be playing a role regardless of the specific pathogen involved. This was demonstrated in recent studies in both TB as well as hepatitis B associated IRIS (22••, 23•). Markers of antigen presenting cell activation such as IL-6 that induces C-reactive protein production may prove to be of clinical utility in IRIS. C-reactive protein (CRP) was found in a small study to be elevated prior to ART in patients who developed IRIS (24•) and was higher in patients with TB-IRIS in another study (25). In an older study, plasma levels of IL-6 were found to be elevated in a group of patients with IRIS associated with herpeseviruses (CMV retinitis, cutaneous zoster and peri-anal herpes) and these elevated levels persisted and progressed for up to 4 years (26). In addition, peripheral blood mononuclear cells (PBMC) from HIV subjects with a history of IRIS produced higher amounts of IL-6 and sIL-6R than PBMC of HIV patients who did not develop IRIS following commencement of ART (27).
Recently, it was proposed that interaction of highly activated T cells in HIV with monocytes expressing PD-1 leads to high IL-10 production by monocytes and functional T cell suppression (28••). Antonelli et al (submitted manuscript) showed that patients who develop IRIS have higher PD-1 expression on CD4+ T cells prior to ART initiation. It is feasible that decreases of these PD-1/PD-L interactions after suppression of HIV viremia results in lower IL-10 production and reversal of T cell suppression. Most studies to date though have failed to find a difference of IL-10 levels in IRIS patients compared to their controls.
Pathogenesis of IRIS: pathogen-specific pathways, mycobacteria
The clinico-pathological manifestations of augmented pathogen-specific immune responses in IRIS can vary greatly for each type of pathogen and, therefore, immune responses may be different for each pathogen. Currently, the most comprehensive data are available for IRIS associated with Mycobacterium tuberculosis infection.
Disease associated with M. tuberculosis infection in HIV patients who have recently commenced ART presents in one of two ways. Patients with treated tuberculosis (TB) may experience a recurrence or exacerbation of inflammation at sites of previous disease and/or inflammation at sites previously unaffected by TB. The inflammatory response is often atypical for TB in the context of HIV/AIDS, especially the severity of inflammation in affected tissues and systemically. Consequently, this condition has been referred to as TB-IRIS. In contrast, disease resulting from unrecognized, and therefore untreated, M. tuberculosis infection after commencement of ART usually has a presentation that is more like typical tuberculosis and has been referred to as ART-associated TB (29). Both TB-IRIS and ART-associated TB appear to be a consequence of augmenting immune responses against M. tuberculosis antigens, though other causes of TB presenting after ART should always be considered, including drug-resistant M. tuberculosis infection and persistent immunodeficiency.
Analysis of various biomarkers in patients with TB-IRIS and ART-associated TB has not only provided evidence that these conditions result from augmenting immune responses against M. tuberculosis antigens but also that the type of immune response may differ and be a determinant of disease presentation. Both TB-IRIS and ART-associated TB have been associated with an increase in the number of circulating T cells that produce interferon-γ (IFN-γ) in response to M. tuberculosis antigens, which has been demonstrated using enzyme-linked immunospot (ELISOT) or whole blood IFN-γ release assays (IGRAs) and flow cytometric analysis of intracellular cytokine production in T cells.
Using ELISPOT assays, Bourgarit et al (30) undertook a 24-week longitudinal study on 7 patients who developed TB-IRIS and reported higher numbers of IFN-γ+ T cells reactive with purified protein derivative (PPD) of M. tuberculosis compared with controls. Similar findings were demonstrated in a small number of patients from Malaysia (31). In a follow-up study by Bourgarit et al (32••) on 11 patients with TB-IRIS, it was shown by flow cytometry that the IFN-γ+T cells were activated effector memory CD4+ T cells that also produced TNF-α.
In a study on 35 South African patients with TB-IRIS, Meintjes et al (25) used ELISPOT assays to examine IFN-γ+ T cells. A cross-sectional assessment of data at the time of TB-IRIS demonstrated that median numbers of IFN-γ+ T cells reactive with PPD, the secreted antigen ESAT-6 and the heat shock proteins Acr1 and Acr2 were increased compared with controls. However, responses were very heterogeneous and some controls also had increased numbers of antigen-reactive T cells. Furthermore, longitudinal analyses over 8 weeks showed very variable responses and few differences between cases and controls.
Elliott et al (33••) used whole blood IGRAs to conduct a prospective study over 24 weeks in 15 Cambodian patients with TB-IRIS and demonstrated increased IFN-γ+ T cells reactive with PPD. However, there were also increased responses in controls and a significant difference between cases and controls was not apparent until 24 weeks after ART was commenced. There were no differences in IFN-γ+ T cells reactive with a mixture of the region of difference 1 (RD1) antigens ESAT-6, CFP-10 and TB 7.7, which are relatively specific for M. tuberculosis. A study that was also conducted over 24 weeks on 22 TB-IRIS patients from Thailand by Tieu et al (34) also used a whole blood IGRA and did not demonstrate a difference in IFN-γ+ T cells reactive with PPD between cases and controls at any time point. However, cases did demonstrate an increase in PPD-reactive IFN-γ+ T cells at the time of TB-IRIS compared with baseline. In agreement with Elliott et al (33••), there was no difference between cases and controls in IFN-γ+ T cells reactive with RD1 antigens.
Studies of T cell responses to M. tuberculosis antigens in patients with TB-IRIS therefore suggest that IFN-γ+ T cells reactive with PPD and particular heat shock proteins contribute to the immunopathogenesis of this condition. However, it also appears that the activity of these cells is not a sufficient explanation for the immunopathology that is observed in all patients. There are some data indicating that increased serum levels of type 1 helper T cell (Th1) cytokines, such as IFN-γ, interleukin IL-2 and IL-12, and proinflammatory cytokines, such as IL-6 and tumour necrosis factor (TNF)-α, are associated with the immunopathology (30). In addition, a recently reported study suggests that innate immune responses to M. tuberculosis are involved. Oliver et al (23•) assayed mediators of innate immune responses in plasma from unstimulated whole blood IGRAs obtained from Cambodian patients with TB-IRIS and controls, who had also been assessed for antigen-induced IFN-γ production (33••). TB-IRIS was associated with increased production of IL-18, a macrophage-derived inducer of IFN-γ that contributes to protective immune responses against M. tuberculosis (35, 36), and persistently increased production of CXCL10 (also known as IFN-γ inducible protein-10 [IP-10]), which is chemotactic for effector T cells (37). In addition, production of CCL2 (also known as monocyte chemotactic protein 1 [MCP-1]) was lower in patients with TB-IRIS. These findings require confirmation, but a preliminary interpretation is that impaired monocyte chemotaxis resulting from CCL2 deficiency contributes to a higher mycobacterial load while increased IL-18 and CXCL10 production augment effector T cell responses against M. tuberculosis antigens. Further evidence suggesting that lower immune responses against M. tuberculosis may be a risk factor for TB-IRIS is provided by observations that plasma levels of antibodies to PGL-Tb1 (38) and the frequency of subpopulations of γδ+ T cells (32••) are both lower before ART is commenced.
The immune response in ART-associated differs from TB-IRIS in that an adaptive immune response mediated primarily against RD1 antigens during the first few weeks of ART appears to predominate (33••). In addition, Oliver et al (23•) demonstrated that IL-18 production was higher in ART-associated TB than in TB-IRIS and that CXCL10 or CCL2 production was not significantly different from controls. One interpretation of these data is that ART-associated TB is characterized by a predominantly adaptive immune response against secreted antigens of live mycobacteria, such as ESAT-6, whereas TB-IRIS is characterized by a combination of innate and adaptive immune responses against antigens of non-viable mycobacteria, such as PPD and heat shock proteins.
Immunological studies in patients with IRIS associated with non-tuberculous mycobacterial infection also indicate that T cell responses against mycobacterial antigens are augmented (39, 40).
Pathogenesis of IRIS: pathogen-specific pathways, cryptococcal disease
Disseminated cryptococcal disease is frequently associated with immune reconstitution inflammatory syndrome (IRIS). The pathogenesis of the inflammatory response in cryptococcal IRIS has been investigated mainly in patients who develop cryptococcal meningitis IRIS (CM-IRIS).
A small study found that plasma IgG antibody responses and T-cell IFN-γ responses to cryptococcal mannonprotein peaked at the time of CM-IRIS and were 10 fold higher than that seen in non-IRIS patients or healthy controls (31). Higher proportions of regulatory T cells also peaked at the time of IRIS and were higher than in non-IRIS patients.
Boulware et al (41••) prospectively studied 85 HIV+ Ugandan patients who survived to commence ART a median of 5 weeks after diagnosis of CM, and examined markers of inflammation in cerebrospinal fluid (CSF) of subjects with and without CM-IRIS. Twenty seven patients (32.5%) developed CM-IRIS after a median of 8 weeks on ART and 5 developed culture positive CM relapse. The 27 subjects who developed IRIS had less inflammation in their CSF at CM diagnosis than subjects who did not subsequently develop IRIS, with decreased levels of CSF leucocytes and protein and lower levels of IFN-γ and the pro inflammatory cytokines IL-6, IL-8 and TNF-α. Initial CSF cryptococcal antigen titers, quantitative cryptococcal cultures and glucose were comparable. Only 25% of those with prominent CSF inflammation (WBC>25 cells/µL, protein>50mg/dL) at time of initial diagnosis developed CM-IRIS compared to 71% of those below both thresholds (41••).
A strong CSF Th1 response was subsequently seen in those who developed IRIS, with a 3 fold elevation in IFN-γ and TNF-α levels from baseline as well as 2 fold increases in vascular endothelial growth factor (VEGF) and eotaxin (CCL11) levels. In terms of differentiating CM-IRIS from CM relapse, CSF levels of pro-inflammatory cytokines [IFN-γ, TNFα, G-CSF, VEGF and eotaxin (CCL11)] were increased significantly from baseline in those with CM-IRIS but not in those with culture positive CM relapse (41••). The results of this study suggested that CM-IRIS pathogenesis principally involved a proinflammatory cytokine response including Th1 cytokines (41••).
In another study from Cape Town in 65 patients of whom 11 (17%) developed CM-IRIS at a median of 29 days from start of ART (42), CSF pro-inflammatory cytokines such as IFN-γ, TNF-α and IL-6 did not differ significantly between first diagnosis with CM and subsequent CM-IRIS. Patients who developed IRIS did however have a more rapid rise of CD4 count from baseline to 6 months (42).
Pathogenesis of IRIS: pathogen-specific pathways, viral diseases
Hepatotoxicty as manifested by elevated liver function tests is not uncommon after initiation of ART in HIV/HBV infected patients (43). In many cases this is likely to be due to IRIS although the pathogenesis is often not clear. One study of 36 HIV/HBV co-infected patients who were commenced on a HBV active ART regimen as part of a randomized controlled trial, found that hepatic flare (HF) -defined as ALT levels >5 times upper limit of normal or >200 IU/mL higher than baseline- occurred in 8 patients (22.2%) and was associated with increased plasma levels of the immune mediators CXCL-10, sCD30, IL-18 and CCL-2 (22••). The flare was also associated with higher levels of HBV-DNA and HIV-RNA prior to ART initiation and elevated levels of CXCL-10 and IL-18 compared to controls. The authors suggested that the association of CXCL-10 and IL-18 with HF provides evidence that IFN-γ may mediate HBV related IRIS in a manner similar to TB and cryptococcal IRIS (22••).
IRIS has also been reported in association with all of the herpesviruses that are opportunistic pathogens in patients with HIV infection. Recent data are available mainly for Kaposi’s sarcoma associated herpes virus (KSHV, also known as human herpes virus 8) from studies conducted in Africa. The seroprevalence of KSHV infection is particularly high in Sub-Saharan Africa and paradoxical worsening of stable KS or new presentation of KS is a well-recognized presentation of IRIS (KS-IRIS).
Letang et al (44) prospectively followed 69 consecutive HIV infected adults, 64 of whom were KSHV sero-positive and 5 KSHV sero-negative but with clinical KS. Of these, 8 (11.6%) developed KS-IRIS (4 unmasking and 4 paradoxical) at a median time of 13.8 weeks after commencing ART. The 8 KS-IRIS cases showed a more pronounced decrease in plasma KSHV DNA levels post ART as well as an increase in median KSHV-specific antibody levels from baseline compared to the KS patients who did not develop IRIS (44). These findings support the hypothesis that KS-IRIS may be partly explained by a KSHV-specific immune response that reduces KSHV replication while promoting paradoxical cellular proliferation with KS progression (44•). The same study identified pre-ART markers predictive of KS-IRIS including detectable KSHV DNA, higher plasma HIV viremia and haematocrit <30%.
Another study was conducted on 33 ART-naive clade C HIV infected subjects with biopsy confirmed KS randomized to receive ART alone or ART plus chemotherapy. KSHV-specific T cell responses became increasingly stronger in both groups after 5 months of ART. In those treated with ART and chemotherapy a pronounced decline in KSHV DNA levels was reported compared to those just treated with ART suggesting a direct effect on KSHV by the chemotherapeutic agents (45).
Conclusion
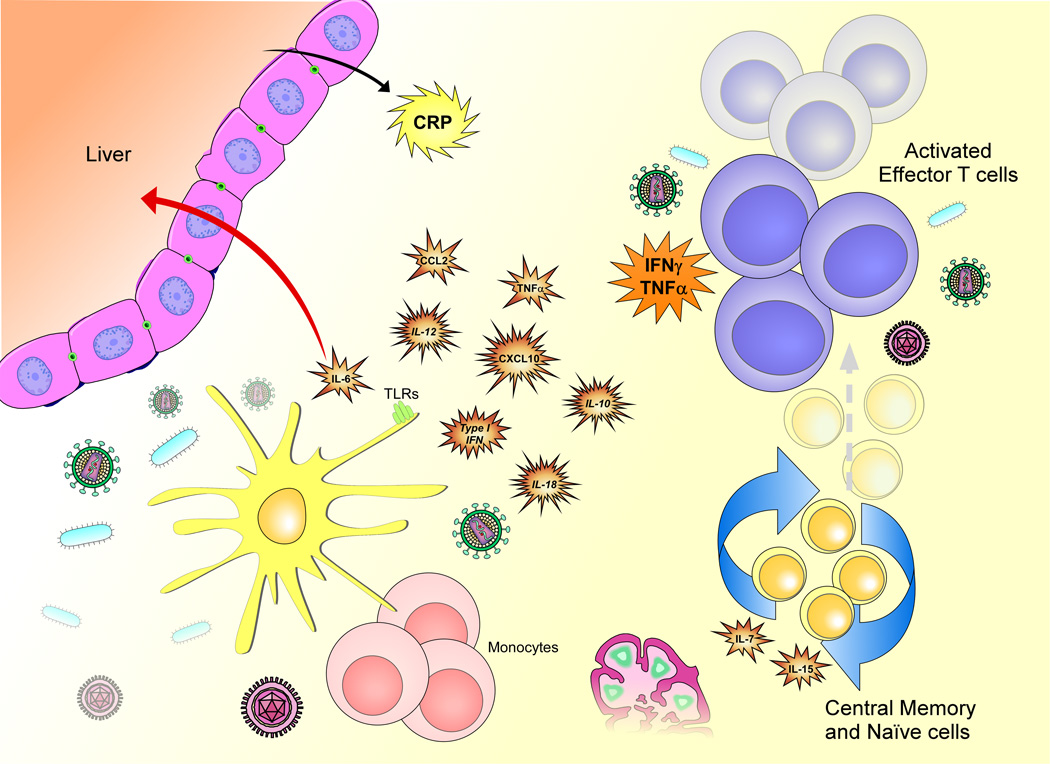
Studies of IRIS immunopathogenesis highlight a component of both innate and adaptive immune activation with converging as well as distinct pathways depending on the underlying pathogen ( Figure 1). Better understanding of IRIS pathogenesis could help identify biomarkers that may assist in identifying patients at risk for paradoxical IRIS who may require closer monitoring, as well as patients with occult infections who may benefit from further diagnostic work up to prevent unmasking IRIS (Table 1). In addition, biomarkers, such as CRP, INF-γ and IP-10 may prove useful in the diagnosis of IRIS episodes.
Figure 1.
The immune reconstitution inflammatory syndrome (IRIS) results from the reversal of immunosuppression caused by HIV infection. It is characterized by innate and adaptive immune responses to dead or live pathogens that lead to the release of cytokines and chemokines, which may differ for different types of pathogen. The effects of the cytokines and chemokines produced may include enhancement of Th1 responses (IL-18, IFNγ), recruitment of T cells and NK cells to sites of inflammation (CXCL-10) and production of inflammatory molecules such as CRP (IL-6). One or more of these molecules could be candidate biomarkers for IRIS.
Table 1.
Biomarkers of interest for potential application in IRIS prediction or diagnosis
|
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH. The authors would like to thank Anita Mora from Visual Medical Arts, Rocky Mountain Laboratories, Division of Intramural Research, NIAID, for her assistance with the figure design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript th cat has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101–107. doi: 10.1086/595006. [DOI] [PubMed] [Google Scholar]
- 2.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 May;:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D, et al. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. 2009;200:973–983. doi: 10.1086/605447. • Case control study in SMART participants looking at opportunistic disease (OD) events and showing that C-reactive protein and IL-6 were predictors of OD when measured several months prior to the event.
- 5. Kalayjian RC, Machekano RN, Rizk N, Robbins GK, Gandhi RT, Rodriguez BA, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201:1796–1805. doi: 10.1086/652750. • Case control study in ART-naïve subjects showing that higher pre-ART levels of Interleukin 6, sTNFR-1 and sCD40L were associated with AIDS or death events that developed at a median of 51 weeks of ART.
- 6.Redd AD, Eaton KP, Kong X, Laeyendecker O, Lutalo T, Wawer MJ, et al. C-Reactive Protein Levels Increase During HIV-1 Disease Progression in Rakai, Uganda, Despite the Absence of Microbial Translocation. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3181e0cdea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French M, Colebunders R. Immune restoration disease. Curr Opin HIV AIDS. 2008;3:417–418. doi: 10.1097/COH.0b013e32830341fc. [DOI] [PubMed] [Google Scholar]
- 8.Price P, Murdoch DM, Agarwal U, Lewin SR, Elliott JH, French MA. Immune restoration diseases reflect diverse immunopathological mechanisms. Clin Microbiol Rev. 2009;22:651–663. doi: 10.1128/CMR.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 10.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 12.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 13.van Leeuwen EM, Sprent J, Surh CD. Generation and maintenance of memory CD4(+) T Cells. Curr Opin Immunol. 2009;21:167–172. doi: 10.1016/j.coi.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci U S A. 2008;105:19851–19856. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieper WC, Troy A, Burghardt JT, Ramsey C, Lee JY, Jiang HQ, et al. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 16.Dean RM, Fry T, Mackall C, Steinberg SM, Hakim F, Fowler D, et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol. 2008;26:5735–5741. doi: 10.1200/JCO.2008.17.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonelli L, Mahnke Y, Hodge J, Porter B, DerSimonian R, Roby G, et al. Elevated Serum IL-7 Levels, Expansion of Memory CD4+ T Cells, Augmented T Cell Activation and Inflammation in Patients Developing IRIS after ART Initiation. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, USa. 2010. [Google Scholar]
- 18. Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. •• PML-IRIS occurred in HIV negative patients after withdrawal of natalizumab therapy that was given to treat multiple sclerosis. Natalizumab affects the function and not the numbers of CD4 T cells by blocking the a4b7 integrin. This shows that reversal of functional suppression can lead to IRIS.
- 19.Chen Y, Bord E, Tompkins T, Miller J, Tan CS, Kinkel RP, et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med. 2009;361:1067–1074. doi: 10.1056/NEJMoa0904267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 21.Linda H, von Heijne A, Major EO, Ryschkewitsch C, Berg J, Olsson T, et al. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N Engl J Med. 2009;361:1081–1087. doi: 10.1056/NEJMoa0810316. [DOI] [PubMed] [Google Scholar]
- 22. Crane M, Oliver B, Matthews G, Avihingsanon A, Ubolyam S, Markovska V, et al. Immunopathogenesis of hepatic flare in HIV/hepatitis B virus (HBV)-coinfected individuals after the initiation of HBV-active antiretroviral therapy. J Infect Dis. 2009;199:974–981. doi: 10.1086/597276. •• Prospective study of hepatitis flares (HF) following ART initiation in hepatitis B and HIV co-infection. HF patients (8/36) had higher HBV DNA and ALT levels pre-ART and had persistence of high sCD30 and CXCL10 after ART initiation.
- 23. Oliver BG, Elliott JH, Price P, Phillips M, Saphonn V, Chhi Vun M, et al. Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning ART. J Infect Dis. 2010 doi: 10.1086/657082. In press. •• Patients who developed paradoxical TB-IRIS had higher levels of CXCL10 (IP-10) and IL-18 and lower levels of CCL2 highlighting the involvement of innate system responses in IRIS pathogenesis and the possible monocyte defects in HIV-TB co-infected patients. ART-associated TB patients had higher levels of IL-18.
- 24. Porter BO, Ouedraogo GL, Hodge JN, Smith MA, Pau A, Roby G, et al. d-Dimer and CRP levels are elevated prior to antiretroviral treatment in patients who develop IRIS. Clin Immunol. 2010 doi: 10.1016/j.clim.2010.02.010. • C-reactive protein and D-dimer levels were found to be elevated pre-ART in patients who developed IRIS from a variety of pathogens.
- 25.Meintjes G, Wilkinson KA, Rangaka MX, Skolimowska K, van Veen K, Abrahams M, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2008;178:1083–1089. doi: 10.1164/rccm.200806-858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone SF, Price P, Brochier J, French MA. Plasma bioavailable interleukin-6 is elevated in human immunodeficiency virus-infected patients who experience herpesvirus-associated immune restoration disease after start of highly active antiretroviral therapy. J Infect Dis. 2001;184:1073–1077. doi: 10.1086/323599. [DOI] [PubMed] [Google Scholar]
- 27.Stone SF, Price P, Keane NM, Murray RJ, French MA. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002;3:21–27. doi: 10.1046/j.1464-2662.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 28. Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–459. doi: 10.1038/nm.2106. •• Microbial products and inflammatory cytokines lead to upregulation of PD-1 on monocytes. Interaction of PD-1 with PD-L1 expressed on various cell types, leads to increased IL-10 production by monocytes and reversible CD4 T cell dysfunction.
- 29.Meintjes G, Rabie H, Wilkinson RJ, Cotton MF. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin Chest Med. 2009;30:797–810. doi: 10.1016/j.ccm.2009.08.013. x. [DOI] [PubMed] [Google Scholar]
- 30.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 31.Tan DB, Yong YK, Tan HY, Kamarulzaman A, Tan LH, Lim A, et al. Immunological profiles of immune restoration disease presenting as mycobacterial lymphadenitis and cryptococcal meningitis. HIV Med. 2008;9:307–316. doi: 10.1111/j.1468-1293.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- 32. Bourgarit A, Carcelain G, Samri A, Parizot C, Lafaurie M, Abgrall S, et al. Tuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculin-specific CD4 Th1 cells and KIR-negative gammadelta T cells. J Immunol. 2009;183:3915–3923. doi: 10.4049/jimmunol.0804020. •• Patients who developed TB-IRIS had pre-ART a higher proportion of TCRγδ+Vδ2+ T cells compared to non-IRIS TB patients and expansion of KIR-TCRγδ+Vδ2+ T cells occurred during IRIS events.
- 33. Elliott JH, Vohith K, Saramony S, Savuth C, Dara C, Sarim C, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis. 2009;200:1736–1745. doi: 10.1086/644784. •• In a prospective study, ART-naïve patients who subsequently developed ART-associated TB had higher raw and corrected by baseline CD4 count IFNγ production in response to RD-1 when studied with Interferon γ release assays in response to purified protein derivative (PPD) and region of difference 1 (RD-1). This suggests priming of the T cells prior to ART initiation.
- 34.Tieu HV, Ananworanich J, Avihingsanon A, Apateerapong W, Sirivichayakul S, Siangphoe U, et al. Immunologic markers as predictors of tuberculosis-associated immune reconstitution inflammatory syndrome in HIV and tuberculosis coinfected persons in Thailand. AIDS Res Hum Retroviruses. 2009;25:1083–1089. doi: 10.1089/aid.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maxwell JR, Yadav R, Rossi RJ, Ruby CE, Weinberg AD, Aguila HL, et al. IL-18 bridges innate and adaptive immunity through IFN-gamma and the CD134 pathway. J Immunol. 2006;177:234–245. doi: 10.4049/jimmunol.177.1.234. [DOI] [PubMed] [Google Scholar]
- 36.Schneider BE, Korbel D, Hagens K, Koch M, Raupach B, Enders J, et al. A role for IL-18 in protective immunity against Mycobacterium tuberculosis. Eur J Immunol. 2010;40:396–405. doi: 10.1002/eji.200939583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 38.Simonney N, Dewulf G, Herrmann JL, Gutierrez MC, Vicaut E, Boutron C, et al. Anti-PGL-Tb1 responses as an indicator of the immune restoration syndrome in HIV-TB patients. Tuberculosis (Edinb) 2008;88:453–461. doi: 10.1016/j.tube.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Seddiki N, Sasson SC, Santner-Nanan B, Munier M, van Bockel D, Ip S, et al. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur J Immunol. 2009;39:391–403. doi: 10.1002/eji.200838630. [DOI] [PubMed] [Google Scholar]
- 40.Foudraine NA, Hovenkamp E, Notermans DW, Meenhorst PL, Klein MR, Lange JM, et al. Immunopathology as a result of highly active antiretroviral therapy in HIV-1-infected patients. AIDS. 1999;13:177–184. doi: 10.1097/00002030-199902040-00005. [DOI] [PubMed] [Google Scholar]
- 41. Boulware DR, Bonham SC, Meya DB, Wiesner DL, Park GS, Kambugu A, et al. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis. 2010 doi: 10.1086/655785. in press. •• Evaluation of inflammatory markers at the site of infection (cerebrospinal fluid, CSF). Patients who developed cryptococcal IRIS had less CSF inflammatory response prior to ART compared to those who did not. In contrast, cryptococcal IRIS had a significant inflammatory component that was distinct from cryptococcal meningitis relapses.
- 42.Bicanic T, Meintjes G, Rebe K, Williams A, Loyse A, Wood R, et al. Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis: A Prospective Study. J Acquir Immune Defic Syndr. 2009 doi: 10.1097/QAI.0b013e3181a56f2e. [DOI] [PubMed] [Google Scholar]
- 43.Crane M, Matthews G, Lewin SR. Hepatitis virus immune restoration disease of the liver. Curr Opin HIV AIDS. 2008;3:446–452. doi: 10.1097/COH.0b013e3282fdc953. [DOI] [PubMed] [Google Scholar]
- 44. Letang E, Almeida JM, Miro JM, Ayala E, White IE, Carrilho C, et al. Predictors of immune reconstitution inflammatory syndrome-associated with kaposi sarcoma in mozambique: a prospective study. J Acquir Immune Defic Syndr. 2010;53:589–597. doi: 10.1097/QAI.0b013e3181bc476f. • Prospective study of Kaposi’s sarcoma (KS) IRIS showing that pre-ART clinical KS and KS-associated herpes virus DNA are important predictors.
- 45.Bihl F, Mosam A, Henry LN, Chisholm JV, 3rd, Dollard S, Gumbi P, et al. Kaposi's sarcoma-associated herpesvirus-specific immune reconstitution and antiviral effect of combined HAART/chemotherapy in HIV clade C-infected individuals with Kaposi's sarcoma. AIDS. 2007;21:1245–1252. doi: 10.1097/QAD.0b013e328182df03. [DOI] [PubMed] [Google Scholar]