Abstract
Wnt/β-catenin signaling is essential for tooth development beyond the bud stage, but little is known about the role of non-canonical Wnt signaling in odontogenesis. Here we compared the expression of Wnt5a, a representative of noncanonical Wnts, with that of Ror2, the Wnt5a receptor for non-canonical signaling, in the developing tooth, and analyzed tooth phenotype in Wnt5a mutants. Wnt5a deficient mice exhibit retarded tooth development beginning from E16.5, leading to the formation of smaller and abnormally patterned teeth with a delayed odontoblast differentiation at birth. These defects are associated with upregulated Axin2 and Shh expression in the dental epithelium and reduced levels of cell proliferation in the dental epithelium and mesenchyme. Retarded tooth development and defective odontoblast differentiation were also observed in Ror2 mutant mice. Our results suggest that Wnt5a regulates growth, patterning, and odontoblast differentiation during odontogenesis, at least partially by modulating Wnt/β-catenin canonical signaling.
Keywords: Wnt5a, tooth development, patterning, growth
INTRODUCTION
Mammalian tooth development begins with determination of tooth forming sites and tooth types, followed by progression through distinct morphological stages, including the lamina, the bud, the cap, and the bell stages, to tooth root formation and tooth eruption. Reciprocal and sequential epithelial-mesenchymal interactions govern each step of tooth development, regulating growth, differentiation, and pattern formation (Zhang et al., 2005). Members of several growth factor families, including Bmp, Fgf, Hh, and Wnt, play pivotal in mediating such tissue interactions. During tooth development, these factors are often expressed in either the same tissue layer or in the adjacent one, and act synergistically or antagonistically to regulate gene expression. Different signaling pathways also often talk each other and form regulatory networks to control many aspects of tooth development.
The Wnt signaling molecules have been implicated in the patterning, proliferation, and differentiation of a variety of organs and cell types during embryonic development (Cadigan and Nusse, 1997). Wnt proteins signal through the Frizzled trans-membrane receptors (Fz) and LRP5/6 co-receptors, activating either the β-catenin dependent canonical pathway or β-catenin independent noncanonical pathways (Veeman et al., 2003; van Amerongen and Nusse, 2009). The latter includes the planar cell polarity pathway and the Wnt/Ca2+ pathway. The “Wnt5a class” Wnt proteins, including Wnts -4, -5a, and -11, are classified as noncanonical Wnt family members and signals via noncanonical pathways. The noncanonical Wnt pathways have been known to inhibit the canonical Wnt/β-catenin signaling, possibly by promoting degradation of β-catenin in a Gsk3-independent manner (Topol et al., 2003).
Many lines of evidence have supported an essential role for the canonical Wnt signaling in tooth development (Liu and Millar, 2010). During determination of tooth forming site and tooth types at embryonic day 10.5 (E10.5), Wnt7b is expressed in the oral epithelium to interact with Shh signaling to set up the ectodermal boundaries between oral and dental ectoderm (Sarkar et al., 2000). From E11.5 on, several Wnt ligands, receptors, and effectors for the canonical Wnt signaling, including Wnts -3, -4, -6, -7b, -10a, -10b, and Lef1, are expressed in the developing mouse tooth germ (Kratochwil et al., 1996; Dassule and McMahon, 1998; Sarkar and Sharpe, 1999). Targeted inactivation of Lef1 in mice leads to an arrest of tooth development at the bud stage, and overexpression of the canonical Wnt signaling inhibitor Dkk arrests tooth development at the lamina stage (Kratochwil et al., 1996; Andl et al., 2002). The absolute requirement of β-catenin in both the dental epithelial and mesenchymal compartments for early tooth development has been demonstrated recently by tissue specific inactivation of Catnb, the gene encoding β-catenin (Liu et al., 2008; Chen et al., 2009). In addition, mutations in Wise (Ectodin) that encodes an inhibitor of Wnt canonical signaling result in formation of supernumerary tooth in the diastemal region (Kassai et al., 2005; Ahn et al., 2010). Despite numerous studies on the Wnt/β-catenin signaling in tooth development, little is known regarding an involvement of noncanonical Wnt signaling in the regulation of odontogenesis.
Wnt5a is a representative of the noncanonical Wnts, having been shown to regulate convergent extension movement in vertebrates (Moon et al., 1993; Kilian et al., 2003), to inhibit secondary axis induction by Wnt8 in Xenopus (Torres et al., 1996), and to block the canonical Wnt signaling in the developing limbs (Topol et al., 2003). Ror2, an orphan tyrosine kinase that serves as an alternative Wnt receptor, has been demonstrated to mediate Wnt5a-initiated noncanonical signaling and to be required for Wnt5a-mediated inhibition of the Wnt canonical signaling (Oishi et al., 2003; Mikels and Nusse, 2006). In the developing palate, Ror2-mediated Wnt5a noncanonical signaling regulates cell proliferation and directional cell movement (He et al., 2008). Additionally, the orhphan receptor Ryk was also shown to bind to Wnt5a as well as other Wnt ligands (Lu et al., 2004; Keeble et al., 2006). Interestingly, in the presence of Fz4 and Lrp5, Wnt5a can also activate Wnt/β-catenin signaling (Mikels and Nusse, 2006), suggesting a dual role for Wnt5a signaling in a receptor dependent manner.
Despite previous report on Wnt5a expression in the developing tooth (Sarkar and Sharpe, 1999), it has not yet been documented that if the absence of Wnt5a would cause defective tooth development. In the present study, we compared the expression of Wnt5a and its receptor Ror2 in the developing tooth and analyzed tooth phenotype in Wnt5a mutant mice. Consistent with a strong Wnt5a expression in the dental mesenchyme and differentiating odontoblasts, Wnt5a mutant teeth appear smaller and mis-patterned, and show a delayed odontoblast differentiation. Associated with the retarded tooth growth are reduced levels of cell proliferation in both dental epithelium and mesenchyme. Gene expression analyses showed an upregulated expression of Axin2 and Shh in the dental epithelium of Wnt5a mutants. Consistent with the expression of Ror2 in the developing tooth and its role as Wnt5a receptor, retarded tooth development was also observed in Ror2 mutants. Our results suggest that during tooth development, one of Wnt5a’s roles is to modulate the Wnt/β-catenin canonical signaling, possibly mediated by Ror2.
RESULTS AND DISCUSSION
Expression of Wnt5a and its receptor Ror2 in the developing tooth
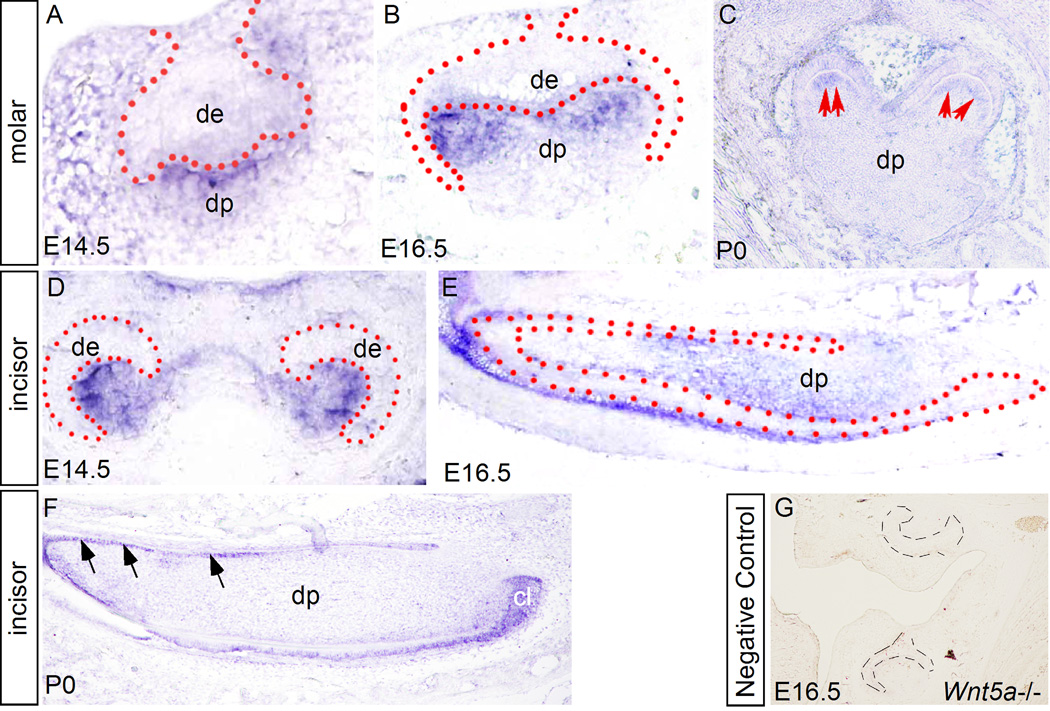
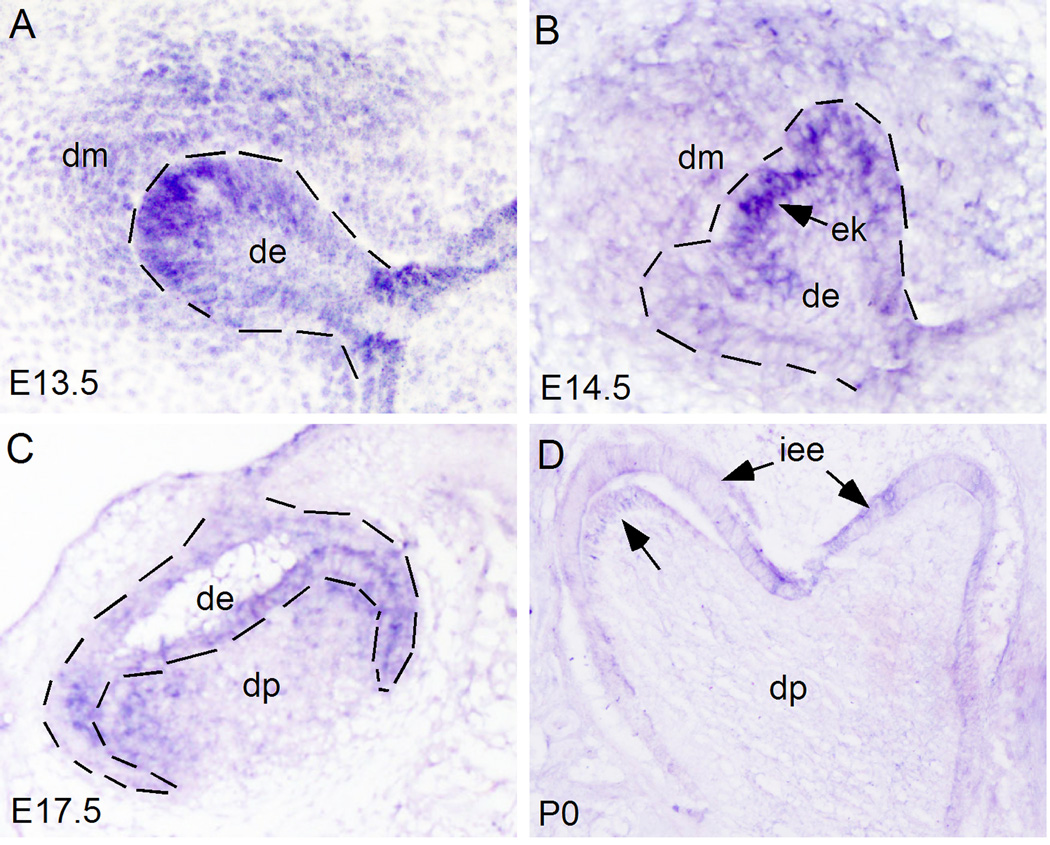
While Wnt5a expression in the early developing mouse teeth has been reported previously (Sarker and Sharpe, 1999), we re-examined Wnt5a expression, particularly at later stages that were not covered in the previous studies. Since Ror2 is known to mediate Wnt5a-initiated noncanonical signaling (Oishi et al., 2003; Mikels and Nusse, 2006), we also examined Ror2 expression in parallel. Consistent with the previous report (Sarkar and Sharpe, 1999), Wnt5a expression was initially detected in the dental mesenchyme at E13.5 (data not shown), and remained in the dental papilla at E14.5 in both incisors and molars (Fig. 1A, 1D). At E16.5, Wnt5a transcripts were continuously present in the dental papilla of incisor and molar teeth (Fig. 1B, 1E). Strong expression was found in the papilla tissue adjacent to the forming secondary enamel knots in the molar germ (Fig.1B). At P0, Wnt5a expression was observed specifically in the differentiating odontoblasts in both molar and incisor (Fig. 1C, 1F), however, in the incisor, Wnt5a expression was also detected in the enamel epithelium including the cervical loop (Fig. 1F). While Ror2 expression was reported previously in the molar at E16.5 (Schwabe et al., 2004), detailed expression patterns in the developing tooth have not yet been documented. We therefore examined Ror2 expression in the developing molars at the bud, the cap, the bell, and the differentiating stages. Our in situ hybridization studies showed that at the E13.5 bud stage, Ror2 is expressed in the dental epithelium and the condensed dental mesenchyme, with stronger expression in the tip of dental epithelium where the enamel knot will form (Fig. 2A). At the E14.5 cap stage, Ror2 expression remains in both the epithelial and mesenchymal compartments, and again with a higher level of expression in the enamel knot (Fig. 2B). At E17.5 bell stage, Ror2 transcripts were continuously detected in the dental epithelium and dental papilla (Fig. 2C). At P0, Ror2 expression became downregulated in the developing tooth, but was still detectable in the inner enamel epithelium and the differentiating odontoblasts (Fig. 2D). The presence of Ror2 expression in the developing tooth implicates a role of Wnt5a-mediated noncanonical signaling in tooth development.
Figure 1.
Expression of Wnt5a in the developing tooth. (A, D) Wnt5a expression is detected by in situ hybridization mainly in the dental papilla of molar (A) and incisor (D) at E14.5. (B, E) Expression of Wnt5a is found in dental papilla of E16.5 molar (B) and incisor (E). Note in the molar (B) the expression is strongly localized in the areas immediately adjacent to the epithelial sites where the secondary enamel is forming. (C, F) At P0, Wnt5a expression is found restrictedly in the differentiating odontoblasts (arrows) of the molar (C) and incisor (F). Wnt5a expression is also detected in enamel epithelium, particularly in the cervical loop in the incisor at this stage (F). (G) Lack of Wnt5a expression in an E16.5 Wnt5a mutant molar, which serves as a negative control for in situ hybridization. All sections shown were made through coronal plane, except that in panels E and F were made through sagittal plane. Dot lines demarcate the boundary of dental epithelium. cl, cervical loop; De, dental epithelium; dp, dental papilla.
Figure 2.
Expression of Ror2 in the developing molar. Ror2 expression is observed in both the dental epithelium and dental mesenchyme at E13.5 (A), E14.5 (B), E17.5 (C), and P0 (D). Note strong Ror2 expression in the site of future enamel knot at E13.5 (A) and the enamel knot at E14.5 (B). At P0 (D), Ror2 expression becomes downregulated, but remains detectable in the inner enamel epithelium and the differentiating odontoblasts (arrow). Dot lines demarcate the dental epithelial boundary. de, dental epithelium; dm, dental mesenchyme; dp, dental papilla; iee, inner enamel epithelium.
Wnt5a mutant mice exhibit retarded tooth growth
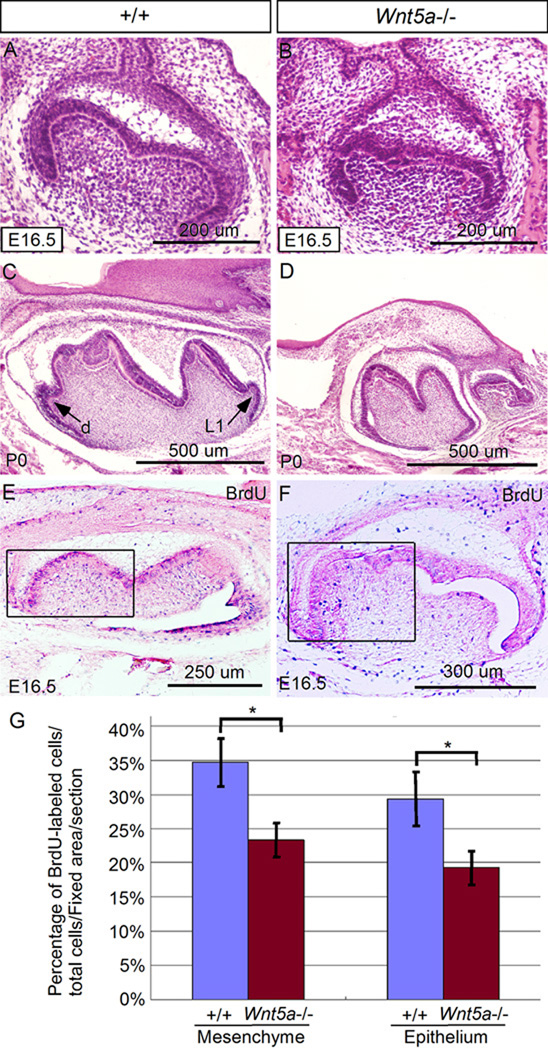
Histological examination of developing tooth germs in Wnt5a mutants revealed normal tooth development until the E14.5 cap stage (data not shown). However, delayed development of tooth with obviously reduced size could be initially identified at E16.5, compared to the wild type control (Fig.3A, 3B). At P0, the mutant teeth became significantly smaller compared to the controls (Fig. 3C, 3D; Fig. 4A, 4B, 4E, and 4F). Decreased level of cell proliferation and induction of excessive cell death are two major cellular processes contributing to reduced size of a developing organ. Since the absence of Wnt5a alters the rates of cell proliferation but does not cause excessive cell death in the developing palate (He et al., 2008), we performed cell proliferation assays on Wnt5a−/− teeth by BrdU labeling. Our results demonstrated a significantly reduced level (P < 0.01) of cell proliferation in both the dental epithelium and mesenchyme at E16.5 compared to the littermate controls (Fig. 3E, 3F, 3G), consistent with a reduced size of Wnt5a mutant tooth at this stage. Gross examination of extracted Wnt5a mutant teeth at P0 revealed not only reduced size, but also deformed morphology (Fig. 4B, 4F). The mutant molars exhibited abnormally patterned cusps, including blunted cusps and lack of lingual 1 (L1) and distal (d) cusps (Fig. 3C), consistent with the observations at histological level (Fig. 3C, 3D). Similarly, the incisors from mutants manifested an extremely reduced proximal-distal length and blunted end, compared to the controls (Fig. 4E, 4F). While BrdU labeling assay was not performed on incisors, given the similar Wnt5a expression pattern in both incisor and molar, we would assume that the reduced size of incisor could also be attributed to a decreased level of cell proliferation in the mutant incisor.
Figure 3.
Retarded tooth development and reduced cell proliferation rate in Wnt5a mutant. (A, B) Histological comparison of molars from the wild type control (A) and Wnt5a mutant (B) at E16.5 reveals a slightly smaller molar in the mutant. (C, D) At P0, the mutant molar (D) appears much smaller than its wild type counterpart (C). Note that the mutant molar has less and blunted cusps. The lingual 1 (L1) and distal (d) cusps are missing in the mutant molar. (E, F) BrdU labeling of wild type (E) and mutant molar (F) at E16.5. Boxes indicate the area where labeled cells and total cell number were counted and compared. (G) Comparison of percentage of BrdU-labeled cells in the fixed areas of molars from the wild type control and Wnt5a mutant. Panels A and B are coronal sections, and panels C–F are sagittal sections. *: P < 0.01.
Figure 4.
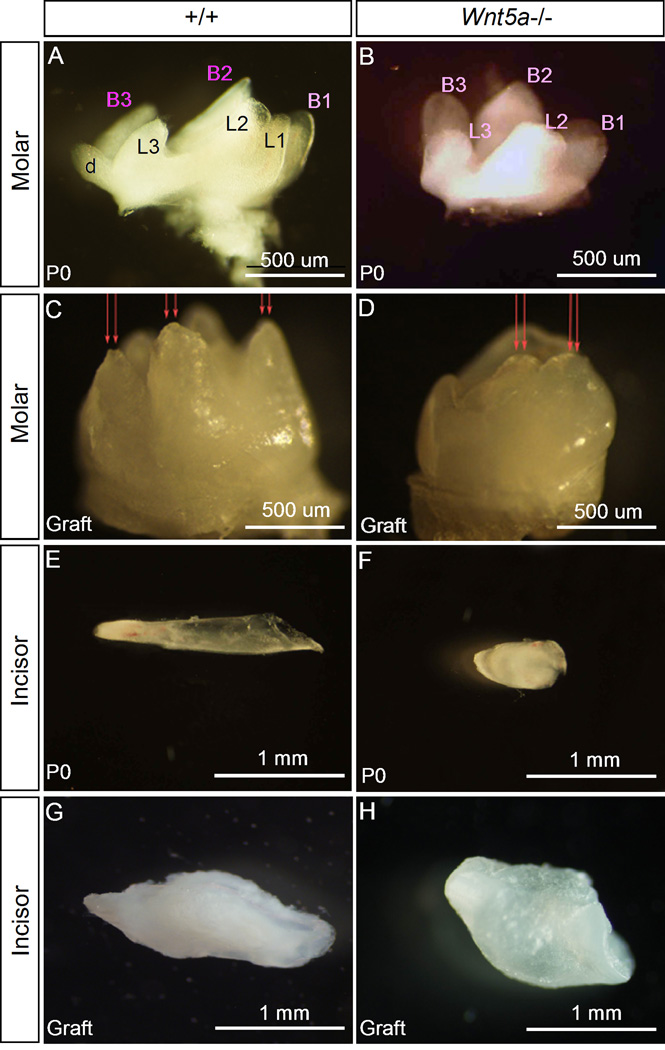
Wnt5a is required for tooth development and patterning. (A, B) Whole mount view of the first molars from P0 wild type (A) and P0 Wnt5a mutant (B) reveals retarded tooth development and dis-regulated cusp patterning in the mutant. The mutant molar appears smaller and misses the lingual 1 (L1) and distal (d) cusps. All remaining cusps in the mutant are blunted. (C, D) After four weeks in subrenal culture, the mutant molar remains smaller and shows blunted cusps (arrows) (D), as compared to the wild type controls (C). (E–H) Wnt5a mutant incisor becomes largely shortened in proximal-distal length bluntly ended at P0 (F), and remains similarly morphologically after 4-week in subrenal culture (H), as compared to the wild type controls (E, G). B1-3 represents buccal side cusps; and L1-3 indicate lingual site cusps.
Since Wnt5a−/− mice develop a cleft palate phenotype and die a few hours after birth (He et al., 2008), we wondered if the reduced size and mis-patterned phenotype observed in Wnt5−/− teeth was simply a consequence of delayed development. In addition, Wnt5a mutant mice have truncated snout and mandible, which could potentially restrain tooth growth. To test these possibilities, we performed subrenal culture experiments by grafting incisor germs from E14.5 and molars from P0 of wild type controls and mutants, respectively. Similar to the defects identified at P0, grafted mutant teeth, after 4-week in subrenal culture, remained a smaller size and aberrantly patterned cusps, as compared to the wild type control (Fig. 4C, 4D, 4G, 4H). Thus, delayed development and hypoplastic mandible and maxilla do not represent the causatives of the reduced tooth size and dys-regulated tooth patterning in Wnt5a mutants. We conclude that Wnt5a regulates tooth growth and cusp patterning during odontogenesis.
Enhanced expression of Axin2 and Wnt/β-catenin signaling targeted genes in Wnt5a mutant tooth
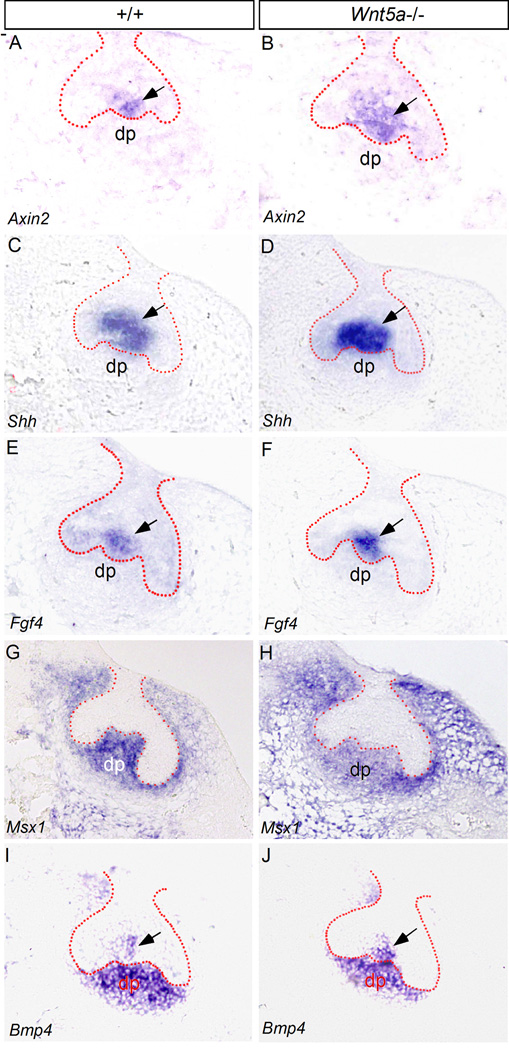
Given the fact that Ror2 expression overlaps with that of Wnt5a in the developing tooth and Ror2’s function in mediating Wnt5a initiated noncanonical signaling to modulate the Wnt canonical signaling, we set to determine if the absence of Wnt5a would alter the activity of Wnt/β-catenin signaling in the tooth. It was shown previously that active Wnt/β-catenin canonical signaling is initially present in the dental epithelium before the bud stage, and then subsequently becomes restricted in the enamel knot which is known to serve as a signaling center to regulate tooth patterning (Liu et al., 2008). This Wnt/β-catenin signaling activity in the enamel knot and late in the dental epithelium was recently confirmed by an Axin2-LacZ reporter mouse line, although Axin2 expression was also found in the dental mesenchyme and differentiating odontoblasts (Lohi et al., 2010). Since Axin2 is a direct target and reliable indicator of Wnt/β-catenin signaling activity (Jho et al., 2002; Ontiveros et al., 2008; Wang et al., 2008), we chose to examine Axin2 expression in the mutant tooth. We also examined the expression of several tooth developmental genes, particularly the tooth patterning genes Shh and Fgf4 that are expressed in the enamel knot and are known to be regulated by the Wnt canonical signaling (Jernvall et al., 1994; Dassule et al., 2000; Gritli-Linde et al., 2002; Kratochwil et al., 2002; Liu et al., 2008). At E14.5 when the primary enamel knot forms in the wild type molar, we detected Axin2 expression in the enamel knot (Fig. 5A), consistent with the observation in the previous report using the TOPGAL transgenic allele as the indicator of Wnt/β-catenin signaling and the Axin2-LacZ reporter line (Liu et al., 2008; Lohi et al., 2010). In Wnt5a mutant at the same stage, we observed an upregulated and expanded Axin2 expression in the enamel knot area (Fig. 5B), indicating an enhanced Wnt/β-catenin signaling activity. The Wnt/β-catenin signaling acts as a positive upstream regulator of Shh and Fgf4 expression in the enamel knot (Kratochwil et al., 2002; Liu et al., 2008). Coinciding with an increased Wnt/β-catenin signaling in the Wnt5a mutant enamel knot, we also found an upregulated level of Shh and Fgf4 expression, as compared to their littermate controls (Fig. 5C–F).
Figure 5.
Altered gene expression in Wnt5a−/− molar at E14.5. (A–F) In situ hybridization assays reveal up-regulated expression of Axin2, Shh, and Fgf4 in the enamel knot of Wnt5a−/− molar (B, D, F), as compared to the wild type controls (A, C, E). (G–J) At the same stage, expression of Msx1 and Bmp4 is slightly reduced in the dental papilla of Wnt5a−/− molar (H, J), as compared to the controls (G, I). In contrast, Bmp4 expression appears to be slightly up-regulated in the enamel knot of Wnt5a mutant (J). Dotted lines demarcate the boundary of dental epithelium. dp, dental papilla. Arrows point to the enamel knot.
Since Wnt5a expression is restricted in the dental papilla at this stage and the presence of Ror2 in this tissue compartment, we also examined the expression of Msx1, which is expressed exclusively in the dental mesenchyme, and Bmp4, which is expressed in the dental mesenchyme and the enamel knot at this stage (E14.5) (Fig. 5G, 5I). Our results showed a slightly reduced expression of these two genes in the dental papilla of the mutant (Fig. 5H, 5J). Interestingly, Bmp4 expression in the enamel knot of mutants appeared to be up-regulated (Fig. 5J), suggesting differential gene expression regulation by Wnt5a in a tissue specific manner in the developing tooth. In fact, this differential regulation of Bmp4 expression by Wnt5a has been observed in the palatal mesenchyme that has the same cranial neural crest origin as the dental mesenchyme (Chai et al., 2000; Ito et al., 2003; He et al., 2008). Slight downregulation of Msx1 was also observed in the palatal mesenchyme of Wnt5a mutant (He et al., 2008). These results suggest a conserved role for Wnt5a in the regulation of Msx1 and Bmp4 expression in the developing tooth and palatal shelves.
Wnt5a−/− tooth exhibits a delayed odontoblast differentiation
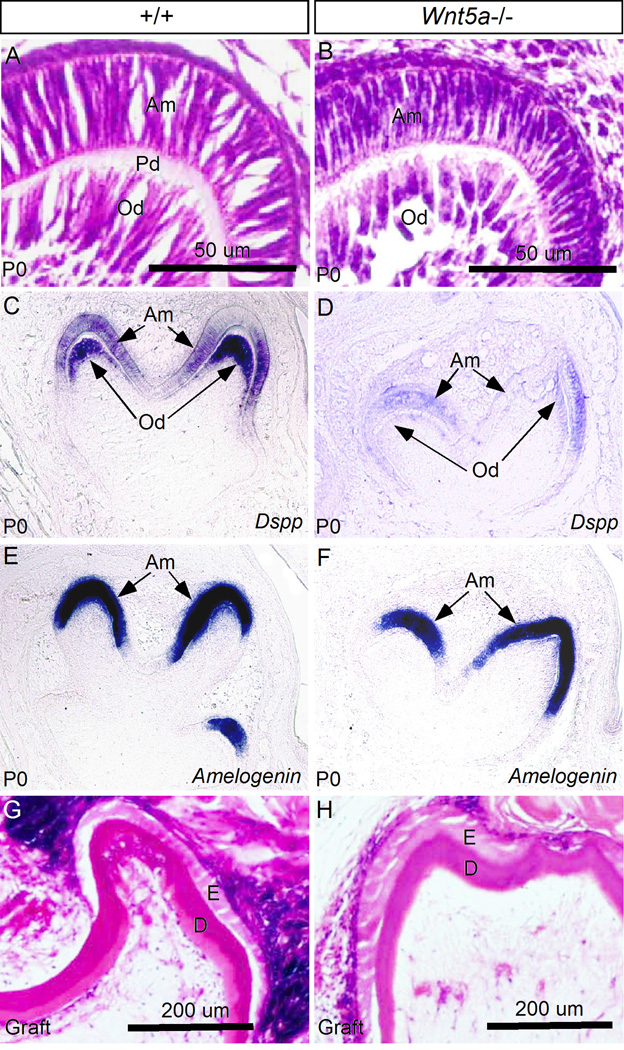
During tooth development, odontoblast differentiation begins at the late bell stage, as assessed morphologically by polarized cell shape. In wild type control at P0, differentiated pre-odontoblasts were present at the tip of the cusp (Fig. 6A), and had begun to produce pre-dentin matrix molecules, including Dspp which could be strongly detected in the pre-odontoblasts but at a relatively low level in the ameloblasts as well (Fig. 6C). At the same time, the ameloblast differentiation marker Amelogenin is also strongly expressed in the polarized pre-ameloblasts (Fig. 6E). Since Wnt5a is exclusively expressed in the differentiating odontoblasts at this stage, we took a closer histological examination of the molars in Wnt5a mutants. In the mutants, while the odontoblasts indeed became elongated, they appeared to be relatively shorter in length but thicker in width, as compared to their wild type counterparts (Fig. 6B). Pre-dentin formation was not found in the mutant molar. However, the mutant ameloblasts appeared more or less comparable to the controls morphologically. Examination of differentiation markers revealed that in the mutant, while obvious Dspp expression could be observed in the pre-ameloblasts, the level of Dspp expression was extremely low in the odontoblasts (Fig. 6D), indicating a defective odontoblast differentiation. Consistent with the relatively normal morphology of the pre-ameloblasts, Amelogenin expression in the mutant was found at a level comparable to the controls (Fig. 6G, 6H). Histological examination of grafted teeth after 4- week subrenal culture, as shown in Fig. 2, revealed dentin formation in the mutants (Fig. 6H) comparable to the controls (Fig. 6G), indicating a delayed odontoblast differentiation in Wnt5a mutants.
Figure 6.
Wnt5a−/− tooth displays delayed odontoblast differentiation. (A, B) Histological sections show abnormal morphology of differentiating odontoblasts and lack of pre-dentin formation in Wnt5a−/− molar at P0, as compared to the control (A). (C–F) In situ hybridization shows significantly down-regulated expression of Dspp in the differentiating odontoblasts but not the expression of Amelogenin in the ameloblasts in Wnt5a−/− molars (D, F), as compared to the wild type controls (C, E). (G, H) Histological sections through wild type (G) and Wnt5a−/− molar grafts (H) show deposition of dentin in both wild type control and mutant. D, dentin; E, enamel; Am, ameloblasts; Od, odontoblasts; Pd, pre-dentin.
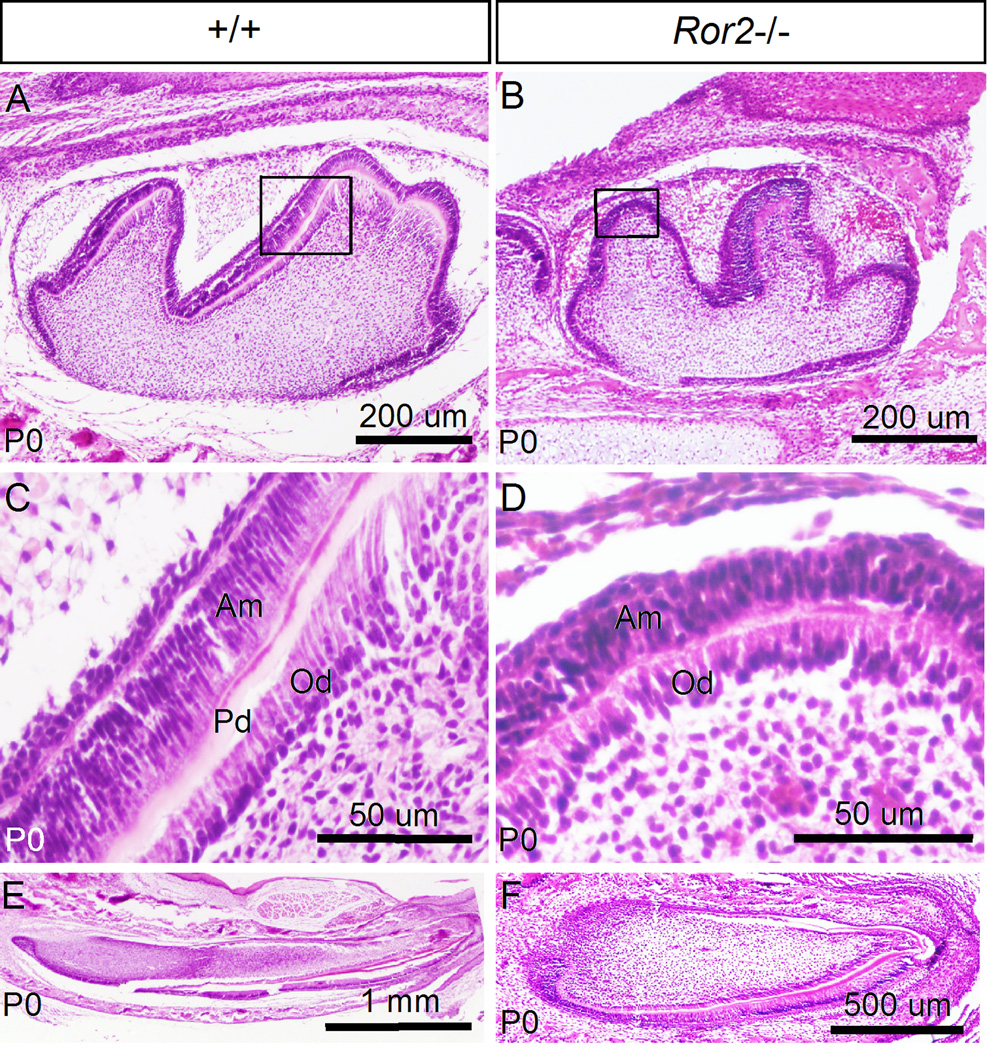
We have demonstrated previously that Wnt5a functions through Ror2 to regulate cell proliferation in the developing palatal shelves (He et al., 2008). The reduced cell proliferation rate in the Wnt5a mutant tooth prompted us to examine tooth phenotype in Ror2 mutant mice. Since it was reported previously that Ror2 mutants exhibited normal size, shape, and number of molar at E16.5 (Schwabe et al., 2004), we examined Ror2−/− teeth at E17.5 and P0 (Ror2 mutants die soon after birth due to a cleft palate defect). At E17.5, Ror2−/− molar appeared comparable to the littermate controls (data not shown). At P0, however, Ror2−/− molar exhibited retarded growth, as assessed by its smaller size, as compared to the wild type controls (Fig. 7A, 7B). Similar to the Wnt5a−/− molar, close examination revealed a lack of pre-dentin formation in Ror2−/− tooth at this stage, as shown in Fig. 7D. In line with this defect, the pre-odontoblasts, while polarized, appear relatively shorter as compared to the control (Fig. 7C, 7D). Interestingly, pre-ameloblasts in the mutant were also shorter than that in the control. Similarly, the mutant incisor also exhibited reduced length along the proximal-distal axis at P0, as compared to the control (Fig. 7E, 7F). These observations indicate retarded tooth growth and defective odontoblast and ameloblast development in Ror2−/− mice.
Figure 7.
Ror2−/− mice exhibits retarded development and defective differentiation of tooth. (A, B) Histological sections reveal retarded molar development in Ror2 mutant at P0 (B), as compared to the control (A). (C) A higher magnification of wild type molar from the box in (A) shows formation of pre-dentin (Pd) and normal morphology of ameloblasts (Am) and odontoblasts (Od). (D) A higher magnification of Ror2 mutant molar from the box in (B) shows absent pre-dentin formation and abnormal morphology of ameloblasts (Am) and odontoblasts (Od). (E, F) Sagittal sections reveal the normal size of a wild type incisor (E) and a shortened incisor in Ror2 mutant (F) at P0.
While Wnt5a expression is restricted in the dental mesenchyme and differentiating odontoblasts, its receptors are expressed in the dental epithelium and mesenchyme, suggesting an impact of Wnt5a on the development of both epithelial and mesenchymal compartments. Indeed, the absence of Wnt5a causes a significantly reduced level of cell proliferation in the dental epithelium as well as the mesenchyme, which contributes to a retarded tooth growth. It has been demonstrated previously that Ror2 mediated Wnt5a signaling regulates cell proliferation in the developing palatal shelves (He et al., 2008). Given the fact that Ror2 is expressed in both the dental epithelium and mesenchyme, and retarded tooth development is seen in Ror2 mutants, it is conceivable that this Wnt5a/Ror2-rmediated cell proliferation regulatory pathway is conserved in the developing tooth, but at the late stage. This is because a retarded tooth development in Ror2 mutants occurs later in development than that observed in Wnt5a mutant. Other receptors must play key role in mediating Wnt5a’s signaling in the regulation of cell proliferation during early tooth development. While we do not know how Wnt5a regulates the expression of Bmp4 and Msx1 and what exact impact that the slightly down-regulated Bmp4 and Msx1 in the dental mesenchyme would have on tooth development in Wnt5a mutants, this slightly reduced Msx1 expression may also contribute to the altered cell proliferation rate in Wnt5a−/− teeth, given the fact that the absence of Msx1 causes significantly decreased level of cell proliferation in the palate and tooth (Zhang et al., 2002; Han et al., 2003). Nevertheless, it appears that the Wnt5a-Bmp4-Msx1 regulatory pathway is also conserved in both developing palate and tooth (He et al., 2008; this study).
One function of Wnt5a-initiated noncanonical signaling is to inhibit the Wnt/β-catenin canonical signaling (Topol et al., 2003; Mikels and Nusse, 2006), which is known to be mediated by Ror2 (Oishi et al., 2003). Consistent with the expression of Ror2 in the dental epithelium where active Wnt/β-catenin signaling is present, the absence of Wnt5a leads to an enhanced Wnt/β-catenin signaling activity in the dental epithelium, as evidenced by the upregulated Axin2 expression. Along with the enhanced canonical Wnt signaling is the upregulation of Shh and Fgf4 expression in the enamel knot. This is not a surprise because the canonical Wnt signaling is known to act upstream of Shh and Fgf4 expression in the enamel knot (Kratochwil et al., 2002; Liu et al., 2008). Given the importance of Shh and Fgf4 in tooth patterning (Jernvall et al., 1994; Dassule et al., 2000; Gritli-Linde et al., 2002), the altered Shh and Fgf4 expression in the enamel knot appears to be responsible for the patterning defects found in Wnt5a mutants.
The delayed odontoblast differentiation observed in Wnt5a mutant teeth could represent a cell autonomous effect. This is evidenced by the facts that Wnt5a is specifically expressed in the differentiating odontoblasts in mice and humans (Peng et al., 2010a; this study), and overexpression of Wnt5a was shown to promote differentiation of human dental papilla cells (Peng et al., 2010b). While the underlying mechanism warrants future investigation, the overlapped expression of Ror2 with Wnt5a in the differentiating odontoblasts and similar defective odontoblast differentiation in both Wnt5a and Ror2 mutants strongly support a role for Ror2 in mediating Wnt5a’s role in the regulation of odontoblast differentiation. However, the possibility exist that altered expression of signaling molecules in the differentiating ameloblasts, such as an enhanced Shh expression (data not shown), in the absence of Wnt5a may exert regulatory effects on odontoblast differentiation. Despite a defective odontoblast differentiation, ameloblast differentiation appears normal in Wnt5a mutants. Thus Wnt5a acts on the enamel epithelium to regulate cell proliferation but does not have an impact on ameloblast differentiation.
In sum, our results presented here demonstrate a role for Wnt5a, a noncanonical Wnt signaling molecule, in the regulation of growth, patterning, and differentiation of tooth. Given its expression in the developing tooth and its property as the receptor for Wnt5s-initiated non-canonical signaling, Ror2 may participate in mediating Wnt5a’s signaling in these processes by modulating, at least partially, the Wnt/β-catenin canonical signaling.
EXPERIMENTAL PROCEDURES
Animals
Wnt5a+/− mice (Yamaguchi et al., 1999) were obtained from the Jackson Laboratories, and Ror2+/− mice (Takeuchi et al., 2000) were provided by Dr. Yasuhiro Minami of Kobe University, Japan. PCR-based genotyping was performed to determine genotypes of mutant mice as described previously (Yamaguchi et al., 1999; Takeuchi et al., 2000). All animal studies were approved by the Tulane University Institutional Animal Care and Use Committee.
Histology, in situ hybridization, BrdU labeling assay, and subrenal culture
For histological and in situ hybridization analyses, embryos collected from timed pregnant females or tissue grafts were fixed in 4% paraformaldehyde (PFA) at 4°C for overnight, followed by dehydration, paraffin embedding and sectioning at 10-µm. Postnatal teeth (including the tooth grafts) were decalcified in 10% EDTA (pH 7.4) after fixation for 1 to 7 days depending on the ages. Sections were subjected to standard Hematoxylin/Eosin staining and non-radioactive in situ hybridization, as described previously (St. Amand et al, 2000). BrdU labeling assay was performed to determine cell proliferation rate, as described previously (Zhang et al., 2002). Timed pregnant mice were injected with BrdU at a dose of 1.5ml of labeling reagent/100 g body weight using the BrdU labeling and Detection kit II from Roche for 1 hour. Samples were fixed in Carnoy’s fixative and paraffin sections were made at 5-µm. Three individual embryos of wild type and mutant were included, and three adjacent sections from each sample were counted for BrdU labeling. BrdU-positive cells were counted and were presented as percentage of labeled cells among total cells within a defined arbitrary area, and Student’s t-test was applied to determine the significance of difference, as described previously. For subrenal culture, lower molars from P0 and lower incisor germs from E14.5 embryos were isolated respectively, and subjected for subrenal culture for 4-week as described previously (Zhang et al., 2003).
ACKNOWLEDGMENTS
Grant sponsor: NIH; Grant number: DE12329; Grant number: DE15123; Grant sponsor: National Natural Science Foundation of China; Grant number: 30771132; Grant Sponsor: Ministry of Science and Technology of China; Grant Number: “973” Project 2010CB944800.
The authors thank Dr. Yasuhiro Minami of Kobe University, Japan for providing Ror2 mutant mice, and members of the Chen Lab for advice and assistance.
REFERENCES
- Ahn Y, Sanderson BW, Klein OD, Krumlauf R. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development. 2010;137:3221–3231. doi: 10.1242/dev.054668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch D, Soriano P, McMahon A, Sucov H. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chen J, Lan Y, Baek JA, Gao Y, Jiang R. Wnt-beta-catenin signaling plays an esential role in activation of odontogenic mesenchyme during early tooth development. Dev Biol. 2009;334:174–185. doi: 10.1016/j.ydbio.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassule HR, McMahon AP. Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev Biol. 1998;202:215–227. doi: 10.1006/dbio.1998.8992. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Leiws P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the teeth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Bei M, Maas R, Zhang XM, Linde A, McMahon AP. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development. 2002;129:5323–5337. doi: 10.1242/dev.00100. [DOI] [PubMed] [Google Scholar]
- Han J, Ito Y, Yeo JY, Sucov HM, Maas R, Chai Y. Cranial neural crest-derived mesenchymal proliferation is regulated by Msx1-mediated p19(INK4d) expression during odontogenesis. Dev Biol. 2003;261:183–196. doi: 10.1016/s0012-1606(03)00300-2. [DOI] [PubMed] [Google Scholar]
- He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, Nishita M, Suzuki K, Yamada G, Minami Y, Chen YP. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development. 2008;135:3871–3879. doi: 10.1242/dev.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Jr, Namkajima A, Shuler C, Moses HL, Chai Y. Conditional inactivation of Tgfbr2 in cranial crest causes cleft palate and calvaria defects. Development. 2003;130:5269–5280. doi: 10.1242/dev.00708. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev. Biol. 1994;38:463–469. [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/β-Catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassai Y, Munne P, Hotta Y, Penttila E, Kavanaghagh K, Ohbayashi N, Takada S, Thesleff I, Jernvall J, Itoh N. Regulation of mammalian tooth cusp patterning by ectodin. Science. 2005;309:2067–2070. doi: 10.1126/science.1116848. [DOI] [PubMed] [Google Scholar]
- Keeble TR, Halford MM, Seaman C, Kee N, Macheda M, Anderson RB, Stacker SA, Cooper HM. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci. 2006;26:5840–5848. doi: 10.1523/JNEUROSCI.1175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5a in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Dull M, Farinas I, Galceran J, Grosschedl R. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–1394. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. FGF4, a direct target of LEF1 and Wnt signaling can rescue the arrest of tooth organogenesis in Lef1−/− mice. Genes Dev. 2002;16:3173–3185. doi: 10.1101/gad.1035602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Millar SE. Wnt/β-catenin signaling in oral tissue development and disease. J Dent Res. 2010;89:318–330. doi: 10.1177/0022034510363373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, Taketo MM, Morrisey EE, Atit R, Dlugosz AA, Millar SE. Wnt/β-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313:210–224. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi M, Tucker AS, Sharpe PT. Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev Dyn. 2010;239:160–167. doi: 10.1002/dvdy.22047. [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin/TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Campbell RM, Christian JL, McGrew LL, Shih J, Fraser S. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development. 1993;119:97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signaling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Ontiveros CS, Salm SN, Wilson EL. Axin2 expression identifies projenitor cells in the murine prostate. Prostate. 2008;68:1263–1272. doi: 10.1002/pros.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Dong G, Xu P, Ren LB, Wang CL, Aragon M, Zhou XD, Ye V. Expression of Wnt5a in tooth germs and the related signal transduction analysis. Arch Oral Biol. 2010a;55:108–114. doi: 10.1016/j.archoralbio.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Peng L, Ren LB, Dong G, Wang CL, Xu P, Ye L, Zhou XD. Wnt5a promotes differentiation of human dental papilla cells. Int Endod J. 2010b;43:404–412. doi: 10.1111/j.1365-2591.2010.01693.x. [DOI] [PubMed] [Google Scholar]
- Sarkar L, Sharpe PT. Expression of Wnt signalling pathways genes during tooth development. Mech Dev. 1999;85:197–200. doi: 10.1016/s0925-4773(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Sarkar L, Cobourne M, Naylor S, Smalley M, Dale T, Sharpe PT. Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc Natl Acad Sci USA. 2000;97:4520–4524. doi: 10.1073/pnas.97.9.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe GC, Trepczik B, Süring K, Brieske N, Tucker AS, Sharpe PT, Minami Y, Mundlos S. Ror2 knockout mouse as a model for the developmental pathology of autosomal recessive Robinow syndrome. Dev. Dyn. 2004;229:400–410. doi: 10.1002/dvdy.10466. [DOI] [PubMed] [Google Scholar]
- St. Amand TR, Zhang YD, Semina EV, Zhao X, Hu YP, Nguyen L, Murray JC, Chen YP. Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev Biol. 2000;217:323–332. doi: 10.1006/dbio.1999.9547. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, Tamura S, Ueda T, Hatta T, Otani H, Terashima T, Takada S, Yamamura H, Akira S, Minami Y. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes to Cells. 2000;5:71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;133:1123–1137. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon: Functions and mechanisms of β-catnein-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Wang BE, Wang XD, Ernst JA, Polakis P, Gao WQ. Regulation of epithelial branching morphogenesis and cancer cell growth of the prostate by Wnt signaling. PLoSOne. 2008;3(5):e2186. doi: 10.1371/journal.pone.0002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Zhang YD, Wang S, Song Y, Han J, Chai Y, Chen YP. Timing of odontogenic neural crest cell migration and tooth-forming capability in mice. Dev Dyn. 2003;226:713–718. doi: 10.1002/dvdy.10274. [DOI] [PubMed] [Google Scholar]
- Zhang YD, Chen Z, Song YQ, Liu C, Chen YP. Making a tooth: growth factors, transcription factors, and stem cells. Cell Res. 2005;15:301–316. doi: 10.1038/sj.cr.7290299. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Song Y, Zhao X, Zhang X, Fermin C, Chen YP. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 2002;129:4135–4146. doi: 10.1242/dev.129.17.4135. [DOI] [PubMed] [Google Scholar]