Abstract
Inhibition of gap junctional intercellular communication (GJIC) and the activation of intracellular mitogenic pathways are common hallmarks of epithelial derived cancer cells. We previously determined that the 1‐methyl and not the 2‐methyl isomer of anthracene, which are prominent cigarette smoke components, activated extracellular receptor kinase, and inhibited GJIC in WB‐F344 rat liver epithelial cells. Using these same cells, we show that an immediate upstream response to 1‐methylanthracene was a rapid (<1 min) release of arachidonic acid. Inhibition of phosphatidylcholine‐specific phospholipase C prevented the inhibition of GJIC by 1‐methylanthracene. In contrast, inhibition of phosphatidylinositol specific phospholipase C, phospholipase A2, diacylglycerol lipase, phospholipase D, protein kinase C, and tyrosine protein kinases had no effect on 1‐methylanthracene‐induced inhibition of GJIC. Inhibition of protein kinase A also prevented inhibition of GJIC by 1‐methylanthracene. Direct measurement of phosphatidylcholine‐specific phospholipase C and sphingomyelinase indicated that only phosphatidylcholine‐specific phospholipase C was activated in response to 1‐methylanthracene, while 2‐methylanthracene had no effect. 1‐methylanthracene also activated p38‐mitogen activated protein kinase; however, like extracellular kinase, its activation was not involved in 1‐methylanthracene‐induced regulation of GJIC, and this activation was independent of phosphatidylcholine‐specific phospholipase C. Although mitogen activated protein kinases were activated, Western blot analyzes indicated no change in connexin43 phosphorylation status. Our results indicate that phosphatidylcholine‐specific phospholipase C is an important enzyme in the induction of a tumorigenic phenotype, namely the inhibition of GJIC; whereas mitogen activated protein kinases triggered in response to 1‐methylanthracene, were not involved in the deregulation of GJIC. (Cancer Sci 2008; 99: 696–705)
Abbreviations:
- GJIC
gap junctional intercellular communication
- Cx43
connexin43
- MAPK
mitogen activated protein kinase
- ERK
extracellular receptor kinase
- PC
phosphatidylcholine
- PLC
phospholipase C
- PC‐PLC
phosphatidylcholine specific phospholipase C
- PI‐PLC
phosphatidylinositol specific PLC
- SM
sphingomyelin
- SMase
sphingomyelinase
- PKA
protein kinase A
- PKC
protein kinase C
- SL‐DT
scrape loading‐dye transfer
- PAH
polycyclic aromatic hydrocarbon
- PAHs
polycyclic aromatic hydrocarbons
- 1‐MeA
1‐methylanthracene
- 2‐MeA
2‐methylanthracene
- PCBs
polychlorinated biphenyls
- TPA
12‐O‐tetradecanoylphorbol‐13‐acetate
- PBS
phosphate buffered saline
- H2O2
hydrogen peroxide
- BSA
bovine serum albumin
Considerable toxicological and carcinogenic research on polycyclic aromatic hydrocarbons (PAH) has focused on the genotoxic attributes of these compounds.( 1 ) However, many human diseases, such as cancer, are not solely the consequence of non‐reversible mutagenic events, but also include reversible, epigenetic events (altered expression of genes at transcriptional, translational and post‐translational levels) that alter the normal phenotype of a cell.( 2 ) Thus, there is a need to reassess the toxicity and carcinogenicity of environmental toxicants, including PAH, at the epigenetic level. Rosenkranz et al. ( 3 ) showed that out of a group of 251 chemicals, there was actually a much stronger correlation of tumorigenicity with the inhibition of gap junctional intercellular communication (GJIC), an epigenetic event, than with mutagenicity. Cell proliferative diseases such as cancer include crucial epigenetic events that ultimately remove quiescent initiated‐cells from the growth suppressive control of neighboring cells.( 2 , 4 ) Intercellular communication between contiguous cells via protein complexes, known as gap junctions, also alters, epigenetically, the expression of genes and strongly suggests that intercellular signaling can further modulate the intracellular signal transduction pathways.( 5 , 6 , 7 )
Cancer cells have been characterized as cells that lose their ability to regulate growth through contact inhibition,( 8 ) and that lack the ability to terminally differentiate,( 9 ) which implies a breakdown in one of the communicating mechanisms.( 2 ) Alteration of cell‐to‐cell communication via gap junctions has been implicated in the carcinogenic process and is supported by considerable evidence.( 7 , 10 ) Most cancer cells have defective GJIC.( 11 ) Chemical tumor promoters and oncogenes inhibit GJIC, while tumor suppressor genes and chemopreventative compounds enhance GJIC.( 10 , 12 ) Inactivation of gap junction genes in normal cells result in cancer‐like cells,( 13 ) and gap junction genes transfected into cancer cells restore their normal growth regulation.( 10 ) The knockout of the gap gene connexin (Cx32), in mice that were administered with a single dose of the tumor initiator, diethylnitrosamine (DEN) had a 3.3–12.8 times increase of preneoplastic foci as compared to the DEN‐treated wild‐type mice. These resultes indicate that the deletion of Cx32 promoted the carcinogenic effects of diethylnitrosamine.( 14 ) Cx32 knockout mice also exhibit increased levels of radiation and chemical‐induced liver and lung tumor formation.( 15 , 16 ) Tumor induction utilizing X‐ray radiation resulted in a statistically significant increase in overall tumor burden in Cx32‐deficient mice compared with wild‐type mice due to tumorigenesis in several tissues such as the lung, liver, adrenal, lymph and small intestine.( 16 ) Increased levels of activated mitogen‐activated protein kinases (MAPK) were also observed in these Cx32‐deficient mouse model systems compared with wild‐type counterparts implicating that MAPK‐related pathways may be preferentially activated or conversely that tumors harboring activated MAPK pathways may selectively progressed towards more advanced tumor states in the absence of Cx32‐mediated GJIC.( 16 ) These results collectively indicate that Cx32 potentially play a tumor suppressive role.
Cell proliferative diseases such as cancer not only require the release of a quiescent cell from growth suppression via down‐regulation of GJIC and/or changes in extracellular components (i.e. integrins), but also need to activate mitogenic signaling pathways. The MAPK pathways are the major intracellular signaling mechanisms by which a cell activates, via phosphorylation, transcription factors involved in mitogenesis.( 17 , 18 , 19 ) Four major mitogenic cascade pathways are the following: extracellular receptor kinase (ERK), stress activated protein kinase/Jun N‐terminal kinase, p38,( 19 ) and ERK5/big MAPK1.( 20 ) The ERK‐pathway has been extensively characterized and is the most understood of the MAPK pathways.( 19 ) The relationship between MAPK and GJIC has not been well characterized. The disruption of GJIC by epidermal growth factor, a mitogen, might be mediated in part by the phosphorylation of three serine sites of connexin43 (Cx43) via MAPK.( 21 ) However the phosphorylation of Cx43 alone is insufficient in the closure of gap junction channels,( 22 ) but phosphorylation does play multiple roles in gap junction function and expression.( 23 )
We have previously reported that PAH with strict structural criteria, specifically the existence of a bay or bay‐like region, activate ERK‐MAPK and inhibit GJIC.( 24 , 25 , 26 , 27 ) PAH, like polychlorinated biphenyls (PCB), do not regulate GJIC through a MEK‐dependent mechanism,( 25 , 28 ) which is unlike growth factors and protein kinase C (PKC)‐dependent promoters, such as 12‐O‐tetradecanoylphorbol‐13‐acetate (TPA).( 29 ) We previously reported that phosphatidylcholine‐specific phospholipase C (PC‐PLC) is involved in PCB‐induced inhibition of GJIC.( 28 ) Thus, we tested the hypothesis that PC‐PLC and not MAPK is directly involved in the regulation of GJIC by PAH. We specifically chose 1‐methylanathracene (1‐MeA) and 2‐methylanthracene (2‐MeA) for this study for the following reasons. Our previous studies indicated that the 1‐methyl and not the 2‐methyl isomer of anthracene inhibit GJIC and activate ERK‐MAPK,( 24 , 26 , 27 ) which gives us an excellent model system of comparing biological effects of two similar compounds, yet one isomer (2‐MeA), can serve as a negative control. These compounds are also environmentally relevant,( 30 ) and in particular represent a major component of the PAH fraction of cigarette smoke.( 31 ) In this report, we demonstrate that 1‐MeA inhibited GJIC through a PC‐PLC‐dependent mechanism, and released arachidonic acid. In addition to activating ERK‐MAPK,( 25 ) 1‐MeA also activated p38‐MAPK, yet the phosphorylation patterns of Cx43 were not altered as determined by Western blot analyzes. In contrast, the 2‐MeA, did not inhibit GJIC, and did not activate ERK, p38 and PC‐PLC, nor induced the release of arachidonic acid.
Methods and Materials
Chemicals. The following are the commercial sources for the reagents used in this study: Lucifer Yellow (Molecular Probes, Eugene, OR, USA), sodium dodecyl sulfate (SDS), Tween 20, TRIS, glycine, acrylamide, and TEMED (Bio‐Rad Laboratories, Hercules, CA, USA); anthracene, 1‐MeA, 2‐MeA, 9‐MeA, RHC80267, 1‐butanol, ET‐18‐OCH3, 4′,6‐diamidino‐2‐phenylindole (DAPI), p‐bromophenacy/bromide (BPB) and genistein (Sigma Chemical Co., St. Louis, MO, USA); acetonitrile (EM Science, Gibstown, NJ, USA); PP2 (Calbiochem, LA Jolla, CA, USA); D609 (Tocris Bioscience, Ellisville, MO, USA); H‐89, BEL (Biomol International, Plymouth Meeting, PA, USA); AACOCF3 and MAFP (Cayman Chemical, Ann Arbor, MI, USA), all electrophoresis reagents and the DC protein kit (Bio‐Rad laboratories); phospho‐specific polyclonal antibodies directed to ERK‐1 phosphorylated at Thr 202/Tyr204, and ERK‐2 directed to phosphorylated Thr183/Tyr185 (New England Biolabs, Ipswich, MA, USA) and to Cx43 (Zymed Laboratories Inc., San Francisco, CA, USA), GADPH (Chemicon, Temecula, CA, USA), and to α‐tubulin (Abcam, Cambridge, MA, USA), phosphospecific polyclonal antip38 antibody directed to phosphorylated Thr 180/Tyr 182 (Invitrogen/Zymed, Carlsbad, CA, USA). Secondary antibodies used were antirabbit and antimouse Ig‐G conjugated with horse radish peroxidase (Amersham, Life Science, Denver CO, USA). The final volume of the exposure medium for each independent culture plate was 2 mL. The concentration of each chemical is indicated in each figure or figure legend and the vehicle for each experiment is equivalent to the volume added with the test compounds.
Cell culture. The WB‐F344 rat liver epithelial cell line was obtained from Drs J. W. Grisham and M. S. Tsao of the University of North Carolina (Chapel Hill, NC, USA),( 32 ) and cultured in D‐medium (Formula no. 78–5470EF, Gibco Laboratories, Grand Island, NY, USA) on 35 mm tissue culture plates (Corning Inc., Corning, NY, USA), supplemented with 5% fetal bovine serum (Gibco Laboratories), and incubated at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. Bioassays were conducted with confluent cultures that were obtained after two to three days of growth.
This cell line expresses primarily Cx43 and serves as an in vitro cell model system for regenerative epithelial cell types that expresses Cx43. The following are some further attributes of this cell line. They are diploid and non‐tumorigenic,( 32 ) and have been extensively characterized for its expressed gap junction genes and functional GJIC using all available techniques in the absence and presence of well‐known tumor promoters, growth factors, tumor suppressor genes and oncogenes to modulate GJIC.( 10 ) Intrahepatic transplantation of WB‐cells into adult syngenic F344 rats results in the morphological differentiation of these cells into hepatocytes and incorporation into hepatic plates.( 33 ) The pluripotency of these cells was further demonstrated by their differentiation into functional‐contracting cardiomyocytes when transplanted into the heart tissue of adult syngenic F344 rats.( 34 )
Bioassay of GJIC. The scrape loading‐dye transfer (SL‐DT) technique was adapted after the method of El‐Fouly et al.( 35 ) The test chemicals were added directly to the cell culture medium from concentrated stock solutions and appropriate solvent controls were run at all times, which did not differ from untreated cells. After treatment, cells were washed with phosphate buffered saline (PBS) followed by the addition of 1 mg/mL of Lucifer Yellow dissolved in PBS. The dye was introduced into the cells with three different scrapes through the monolayer of confluent cells using a surgical steel scalpel blade. The transfer of dye through gap junctions was for three minutes, followed by a thorough rinse with PBS to remove extracellular dye, and then fixed with a 4% formalin solution in PBS. Migration of the dye in the cells was observed at 200X using a Nikon epifluorescence microscope equipped with a Nikon Cool Snap EZ CCD camera and the images digitally acquired using a Nikon NIS‐Elements F2.2 imaging system. The fluorescence area of the dye migration from the scrape line was quantified using ‘Gel Expert’ image analysis program (NucleoTech Corp, San Mateo, CA). The data was reported as a fraction of the dye spread of the vehicle control.
Western blot analysis. Cells were grown in 35 mm diameter tissue culture plates from Corning (Corning, NY) to the same confluency as the SL‐DT assay. The cells treated by the test chemicals were done under the same conditions as those used in the SL‐DT assay. The proteins were extracted with 20% SDS solution containing 1 mM phenylmethylsulfonyl fluoride, 100 µM Na3VO4 to the PBS, and 100 nM aprotinin, 1.0 µM leupeptin and 1.0 µM antipain, 100 µM Na3VO4, 5.0 mM NaF. The protein content was determined with the Bio‐Rad DC assay kit. The proteins (15 µg) were separated on 12.5% SDS‐PAGE according to the method of Laemmli.( 36 ) The proteins were electrophoretically transferred from the gel to polyvinylidene fluoride (PVDF) membranes (Millipore Corp, Bedford, MA, USA). Detection of the Western Blot signals for Cx43, p38 and ERK used enhanced chemiluminescent (ECL) detection kit and HyperFilm™‐MP (Amersham, Life Science, Denver, CO, USA), except for p38, we used SuperSignal West Pico Stable Peroxide Solution and Luminol/Enhancer Solution (Pierce, Rockford, IL, USA), film HyBlot™‐CL (Denville Scientific, Metuchen, NJ, USA).
Assay of PC‐PLC and sphingomyelinase (SMase) activity. Confluent cells in 100 mm plates, serum‐deprived for 4 h, were exposed to 1‐MeA and 2‐MeA at the desired concentrations for 5 and 15 min at 37°C (solvent control did not exceed 0.5% v/v). After exposure, cells were briefly washed with ice‐cold PBS, scraped and centrifuged. The pellets (about 2.5 × 106 cells) were lyzed for 60 min on ice with 0.6 mL of an ice cold buffer (pH 7.5) containing 50 mM TRIS, 150 mM sodium chloride, 2 mM ethylene diamine tetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 1% v/v, Triton‐X, 1 µg/mL aprotinin and 100 µg/mL phenylmethylsulfonyl fluoride. We used about 200 µL of the lysis buffer per plate. The lyzed‐suspension was centrifuged at 17 000 × g for 10 min, and the supernatant fraction was collected and stored at –80°C for SMase and PLC activity assays.
SMase and PC‐PLC activities were determined using Amplex Red reaction kits (Molecular Probes, Invitrogen, Eugene, OR, USA). The assay according to Zhou et al. ( 37 ) worked as follows. SMase and PC‐PLC hydrolytic activities were determined by the exogenous addition of their respective substrates, sphingomyelin (SM) or phosphatidylcholine (PC), to the thawed supernatant fraction. Phosphocholine, the hydrolysis product of either SM/SMase or PC/PC‐PLC, was dephosphorylated with alkaline phosphatase to choline, which was further oxidized with choline oxidase and ambient oxygen to betaine aldehyde and hydrogen peroxide (H2O2). The H2O2, in the presence of horseradish peroxidase, oxidizes Amplex Red to the fluorescent resorufin. Reaction mixtures contained the buffer, which for SMase determination was 100 mM TRIS HCl and 10 mM magnesium chloride, pH 7.4, and for PC‐PLC activity was 50 mM TRIS HCl, 140 mM NaCl, 2 mM CaCl2, pH 7.4; plus the substrates (0.5 mM SM or PC, respectively), 0.1 unit/mL choline oxidase, 5 µM Amplex Red, 4 units/mL alkaline phosphatase, 1 unit/mL horseradish peroxidase. The kinetics of the fluorescence intensity was recorded at 590 nm (excitation at 544 nm) using Gemini microplate fluorescence reader (Molecular Devices, Sunnyvale, CA, USA), at 37°C. The slopes were derived from the initial 10 min of the kinetic reaction.
Assay of Arachidonic acid release. Arachidonic acid‐release was measured according to the method of Tithof et al.( 38 ) Cells were grown to 75% confluency in 35 mm culture dishes and then treated for 24 h with 1.00 µCi 3[H]‐arachidonic acid (specific activity = 180–240 Ci/mM) at normal cell culture conditions. At the end of the labeling period in which the cells have attained 95–100% confluency, the extracellular 3[H]‐arachidonic acid was removed with three washings of PBS and resuspended in growth medium containing 0.1% bovine serum albumin (BSA) that is arachidonic acid and phospholipid free. The BSA served as a sink for the arachidonic acid released into the medium.( 38 ) The incorporation of 3[H]‐arachidonic acids into the cells was typically greater than 80%. These cells were treated with the PAH and at the end of the treatment time the culture medium was added to 14 mL of scintillation cocktail and counted for radioactivity in a scintillation counter. The remaining cells were trypsinized and solubilized in 14 mL of scintillation cocktail and counted for radioactivity in a scintillation counter. The data were expressed as a percentage of total cellular radioactivity released into the medium.
Assay of immunostaining of Cx43. WB‐F344 cells were cultured to confluency in eight‐well chamber slides (Fisher Scientific, Pittsburgh, PA, USA). Subsequently, cells were gently rinsed three times with PBS and fixed with 5% acetic acid in methanol for 10 min. The non‐specific binding sites were blocked with 10% goat serum (Zymed, Carlsbad, CA, USA) for 2 h at room temperature (RT). Rabbit polyclonal Cx43 antibody (Zymed, South San Francisco, CA, USA) was diluted 1:150 in 1% BSA +0.1% Tween‐20 in PBS at 4°C for 2 h, washed three times with 1% BSA +0.1% Tween‐20 in PBS, then incubated with Alexa Fluor 488 goat antirabbit secondary antibody (Molecular Probes, Eugene, OR, USA) for 30 min at RT. Negative controls were prepared by eliminating the primary/ secondary antibody. Slides were washed with PBS and counterstained with DAPI for 5 min before finally embedding with Prolong Antifade kit (Molecular Probes). Fluorescent images were obtained using a Nikon Epi‐fluorescent microscope equipped with a SPOTRT digital camera (Diagnostic Instruments, Detroit, MI, USA) and analyzed with Spot Advanced analysis software (Diagnostic Instruments).
Results
The structures of 1‐MeA and 2‐MeA are illustrated in Figure 1(a). Inhibition of GJIC by 1‐MeA was dose and time‐dependent (Fig. 1b). A shift of the methyl group from the 1 position, which forms a bay‐like region, to the 2 position on the anthracene ring, which does not form a bay‐like region, renders this isomer of methylanthracene as fairly inactive relative to the inhibition of GJIC up to 3 h, although slight inhibition was seen at 8 h. A slight recovery was observed after a 4 h exposure time to 1‐MeA, which was maintained up to 8 h. We previously reported that 1‐MeA and not 2‐MeA activated ERK‐MAPK,( 25 ) and the inhibition of GJIC was independent of this MAPK. Similarly, 1‐MeA activated p38‐MAPK, while 2‐MeA did not activate p38 (Fig. 1c). Like the previous data on ERK‐MAPK,( 25 ) activation occurred after the time period needed to inhibit GJIC, 5 min versus 10 min (Fig. 1b vs Fig. 1c). Figure 1(d) illustrates the data for the densitometry analyzes of the p38 band. Although there was a slight trend of 2‐MeA induced phospho‐p38, these results were not significant, while the increase of the p38 bands in 1‐MeA treated cells were significant (Fig. 1d). Confirmation that the inhibition of GJIC by 1‐MeA was p38‐independent is indicated in Figure 1(e) where the significant inactivation of p38 with SB202190, a selective inhibitor of p38, did not prevent 1‐MeA from inhibiting GJIC.
Figure 1.
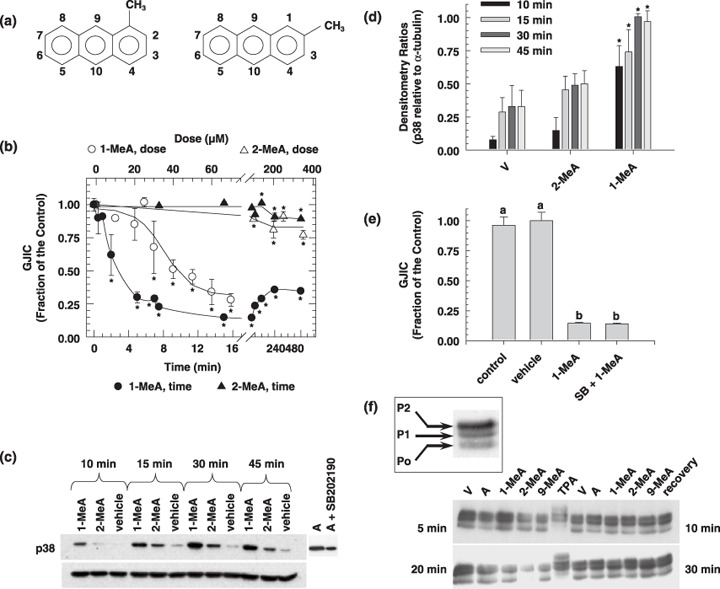
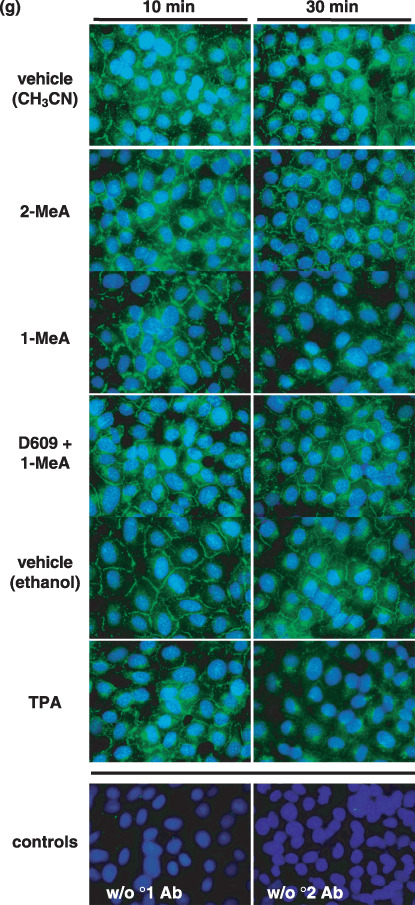
The effects of 1‐methylanthracene (1‐MeA) versus 2‐methylanthracene (2‐MeA) on gap junction biology. (a) Structures of 1‐MeA and 2‐MeA; (b) Dose and time effect of 1‐MeA versus 2‐MeA on gap junctional intercellular communication (GJIC) activity. The data for the dose–response were obtained from Rummel et al. in which WB‐F344 rat liver epithelial cell line were treated with the indicated polycyclic aromatic hydrocarbons (PAH) for 15 min. The dose for the time response data of each PAH was 75 µM, and the WB‐F344 rat liver epithelial cell line was used for this and all subsequent experiments. The scrape load‐dye transfer assay was used to assess GJIC for both the time and dose–response experiments. Average of the data (n = 3) ±standard deviation at the 95% confidence level. An anova indicated significance at P < 0.001 for 1‐MeA dose (F = 29.1), 1‐MeA time (F = 87.0), 2‐MeA dose (F = 18.2), 2‐MeA time (F = 15.9), and *indicated significance from the vehicle control using a Holm Sidak posthoc T‐test at the P = 0.05 level. (c) The effect of 1‐MeA versus 2‐MeA on the mitogen activated protein kinase, p38, as determined by Western blot analysis of p38 using phospho‐specific antibodies (top gel) and the house keeping protein α‐tubulin (bottom gel). The proteins were extracted from cells treated with 75 µM of the indicated polycyclic aromatic hydrocarbon. Anisomycin (A) at 0.5 µM and 30 min was used as a positive control. (d) Densitometry analysis of Western blots of p38 from proteins extracted from three independently treated cell culture plates, including the gel presented in (c). The values are the densitometry ratios of the p38 relative to α‐tubulin. anovas of the 10 min group (F = 23.5, P < 0.001), 15 min group (F = 9.4, P = 0.014), 30 min group (F = 34.1, P < 0.001), 45 min group (F = 31.2, P < 0.001), indicated significance between the vehicle, 2‐MeA and 1‐MeA. *Specifically indicates a difference as compared to the vehicle within each time group at P = 0.05 using a Holm‐Sidak post hoc test. (e) The effect of a p38 inhibitor on 1‐MeA‐induced inhibition of GJIC. The scrape loading‐dye transfer assay was used to measure GJIC. The dose and preincubation time for the p38 inhibitor, SB202190 (4‐(4‐Fluorophenyl)‐2‐(4‐hydroxyphenyl)‐5‐(4‐pyridyl)‐1H‐imidazole), were 20 µM and 20 min. The GJIC data are an average of the data (n = 3) ±standard deviation at the 95% confidence level. An anova indicated significance at P < 0.001, F = 285, and the different letters indicated significance using an all pair multiple comparison‐Holm Sidak posthoc T‐test at the P = 0.05 level. (f) The effect of methylanthracene isomers on the phosphorylation status of connexin43 (Cx43). A Western blot analysis using antiCx43 antibodies was used to identify the phosphorylated states of Cx43. The duration of exposure times to the various PAH and vehicle were 5, 10, 20, and 30 min as indicated in the four quadrants of the figure. The quadrants are separated by the 12‐O‐tetradecanoylphorbol‐13‐acetate (TPA) data, in which these cells were treated with TPA for 10 min. Lanes TPA = 500 ng/mL, V = vehicle (0.3% v/v acetonitrile), A = 60 µM anthracene, 1‐MeA = 60 µM 1‐methylanthracene, 2‐MeA = 60 µM 2‐methylanthracene, 9‐MeA = 60 µM 9‐methylanthracene, Recovery for top gel = 6 h recovery from cells treated with 60 µM 1‐MeA for 30 min, Recovery for bottom gel = 6 h recovery from cells treated with 60 µM 9‐MeA for 30 min. The 1st (bottom), 2nd and 3rd bands are typically designated P0, P1 and P2 as indicated in the boxed inset, and the addition of alkaline phosphatase to the protein samples results in only a P0 band, using this Western blot analysis protocol. The experiment was done in triplicate and the densitometry analyzes of the data are presented in Table 1. (g) Cytological localization of Cx43 in cells treated with 1‐MeA, 2‐MeA and TPA. The concentrations of 1‐MeA and 2‐MeA were both 70 µM, and for TPA 50 ng/mL. The vehicle was 0.3% (v/v) acetonitrile. An antiCx43 antibody was used to label this protein. The magnification of the images was 1000×. Ab, antibody; w/o, without.
Changes in the phosphorylation status of the connexin proteins, which make up the gap junction channels, has been implicated in the inhibition of GJIC. Our results indicate that the various isomers of methylanthracene, including 1‐, 2‐ and 9‐methylanthracene, did not alter the phosphorylation status of Cx43, the major connexin type in this cell line (Fig. 1f). TPA was used as the positive control for altering the phosphorylation status of Cx43, as indicated in a shift of the bands to higher molecular weights and the disappearance of the P0 band. The P0 band is the unphosphorylated form of Cx43 as determined by all bands collapsing to this position after treatment with alkaline phosphatase.( 39 ) This lack of an effect on the phosphorylation of Cx43 continued even after the known activation of ERK,( 25 ) and p38 (Fig. 1c,d). The densitometry analysis of the three Cx43 bands of three independent experiments, including data from Figure 1(f) is presented in Table 1. ANOVA analyzes indicated that there were no significant changes in the ratios of the P1 and P2 bands relative to P0 of all cells treated by the methylated anthracenes, while the TPA treated cells had a significant decrease in the P0 band relative to P1 and P2.
Table 1.
Densitometry scans of the phosphorylated (P1 and P2) Cx43‐bands relative to the unphosphorylated (P0) band from Western blots of proteins extracted from three different cell treatments in which one blot is presented in Figure 1(f)
| Treatment | Densitometry Ratios of P1 and P2 Relative to P0 (FOC) | |||
|---|---|---|---|---|
| 5 min | 10 min | 20 min | 30 min | |
| P0/P1 | ||||
| V | 1.00 ± 0.04 | 1.00 ± 0.18 | 1.00 ± 0.08 | 1.00 ± 0.08 |
| A | 0.90 ± 0.12 | 0.99 ± 0.28 | 0.94 ± 0.05 | 0.91 ± 0.23 |
| 1‐MeA | 1.01 ± 0.03 | 1.04 ± 0.12 | 1.05 ± 0.12 | 0.97 ± 0.15 |
| 2‐MeA | 0.93 ± 0.05 | 1.05 ± 0.25 | 0.62 ± 0.28 | 0.93 ± 0.02 |
| 9‐MeA | 1.00 ± 0.07 | 1.01 ± 0.12 | 0.91 ± 0.01 | 1.12 ± 0.05 |
| TPA | ND | † 0.34 ± 0.16 | ND | ND |
| ANOVA (F, P‐value) | (1.73, 0.218) | (4.718, 0.013) | (3.468, 0.05) | (1.233, 0.357) |
| P0/P2 | ||||
| V | 1.00 ± 0.04 | 1.00 ± 0.19 | 1.00 ± 0.14 | 1.00 ± 0.17 |
| A | 0.93 ± 0.13 | 0.94 ± 0.28 | 0.95 ± 0.13 | 0.86 ± 0.22 |
| 1‐MeA | 1.03 ± 0.06 | 1.00 ± 0.13 | 1.13 ± 0.07 | 1.16 ± 0.25 |
| 2‐MeA | 0.97 ± 0.16 | 1.01 ± 0.26 | 0.54 ± 0.25 | 1.00 ± 0.24 |
| 9‐MeA | 1.05 ± 0.09 | 0.94 ± 0.08 | 1.03 ± 0.15 | 1.26 ± 0.33 |
| TPA | ND | † 0.27 ± 0.07 | ND | ND |
| ANOVA (F, P‐value) | (0.613, 0.663) | (6.584, 0.004) | (2.642, 097) | (1.484, 0.278) |
Significant from the vehicle as determined by a Holm‐Sidak post hoc test. The ANOVA was conducted on the densitometry data, including the vehicle. The fraction of the control (FOC) is the densitometry value of each treatment divided by the densitometry value of the vehicle within each time group. 1‐MeA, 1‐methylanthracene; 2‐MeA, 2‐methylanthracene; 9‐MeA, 9‐methylanthracene; A, anthracene; Cx43, connexin43; ND, not determined; TPA, 12‐O‐tetradecanoylphorbol‐13‐acetate; V, vehicle.
Connexins are commonly internalized into the cytoplasm in response to a tumor promoter such as TPA. Similar to TPA, Cx43 was internalized in response to 1‐MeA, but not in response to 2‐MeA (Fig. 1g). However, this trafficking event of Cx43 does not occur within 10 min, which is after the inhibition of GJIC by either 1‐MeA or TPA. Thus, inhibition of GJIC precedes the trafficking of Cx43. However, inhibition of PC‐PLC by D609, a selective inhibitor of PC‐PLC, partially prevented the internalization of Cx43.
Phospholipases have been implicated in the regulation of GJIC,( 28 ) thus we measured the release of arachidonic acid from the membranes of the cells in response to exposure to the methyl‐isomers of anthracene as an indicator of phospholipase activation (Fig. 2a and 2b). The 1‐MeA isomer induced the release of arachidonic acid in a time and dose dependent manner, while the 2‐MeA isomer had no effect (Fig. 2a and 2b). Significant release of arachidonic acid began at 10 µM, which is a dose significantly lower than the dose needed to inhibit GJIC. This release of arachidonic acid began within one minute of treatment. Although the dose–response data suggested that the release of arachidonic acid might not be directly involved in the inhibition of GJIC, these data indicated that phospholipases are activated in the same isomer‐specific mode and precedes the inhibitory events of GJIC. We have also measured the release of arachidonic acid from WB‐F344 cells by a non‐radioactive high performance liquid chromatography‐fluorescence detection method, which does not require pretreatment with 3[H]‐arachidonic acid. Our aim was to confirm that this effect of 1‐MeA was not affected by increased levels of arachidonic acid incorporated in the membrane. In this assay, only 1‐MeA was active, thus confirming the results of the release of 3[H]‐arachidonic acid (data not shown). PLC, which hydrolyzes the polar group of phosphoglycerol lipids, was one class of phospholipases that we investigated for involvement in the control of GJIC. Inhibition of phosphatidylinositol specific PLC (PI‐PLC) by ET‐18‐OCH3, a selective inhibitor of PI‐PLC, did not prevent the inhibition of GJIC by 1‐MeA (Fig. 2c,d and Table 2), however, inhibition of PC‐PLC by D609, did prevent the inhibition of GJIC by 1‐MeA (Fig. 2c,d and Table 2). Similarly, inhibition of PC‐PLC with D609 also prevented the internalization of Cx43 in response to 1‐MeA treatment (Fig. 1g), a downstream event to the inhibition of GJIC.
Figure 2.
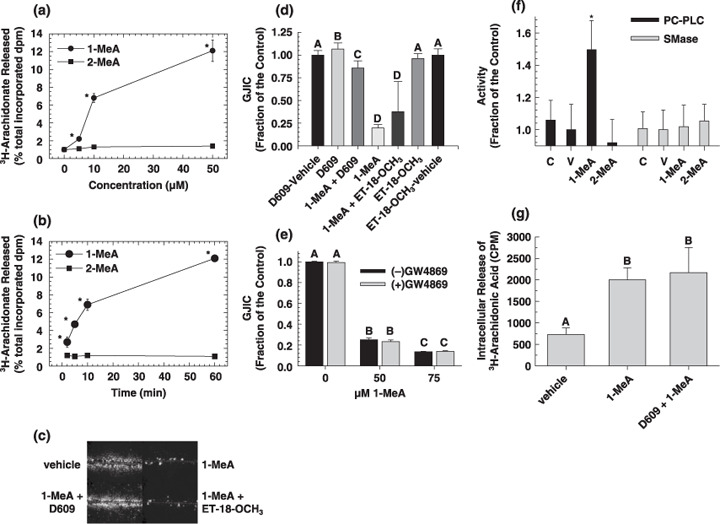
The role of phospholipid signaling in 1‐methylanthracene (1‐MeA) versus 2‐methylanthracene (2‐MeA) induced effects on gap junctional intercellular communication (GJIC). (a) The effect of 1‐MeA versus 2‐MeA doses on the release of arachidonic acid. (b) The effect of 1‐MeA versus 2‐MeA exposure times on the release of arachidonic acid. (a,b) 0.1% albumin was added to the medium to inhibit reacylation and metabolism of released 3[H]‐arachidonic acid therefore the data reflected cumulative deacylation from membrane phospholipids. At the end of the incubation period, the medium was collected into scintillation cocktail solution and radioactivity determined by scintillation counting of disintegrations per minute (dpm). Radioactivity in the cellular fraction was also determined, and 3[H]‐fatty acid release was expressed as a percentage of total cellular radioactivity. The data are an average of at least four independent treatments, and *indicated significant differences paired between 1‐MeA and 2‐MeA for each dose and time as determined by a two‐tailed paired t‐test at the 95% confidence interval, P < 0.001. (c) The effects of phospholipase C (PLC) inhibitors on 1‐MeA‐induced inhibition of GJIC. The scrape load‐dye transfer (SL‐DT) assay was used to assess GJIC at 200×. (d) Presents a summary of the averaged data of n = 3, including those shown in (c). (c,d), Phosphatidylcholine(PC)‐ and phosphatidylinositol(PI)‐specific‐PLC were inhibited by preincubating the cells for 15 min with either 50 µM D609 or 30 µM ET‐18‐OCH3, respectively. The inhibitors were left on the cells for an additional 15 min, which was the exposure time of 1‐MeA. The dose of 1‐MeA was 70 µM. The data are an average of the data (n = 3) ± standard deviation at the 95% confidence level. An anova indicated significance at P < 0.001, F = 420.1, and the different letters (A, B, C and D) indicated significance using an all pair multiple comparison‐Holm Sidak posthoc T‐test at the P = 0.05 level. (e) The effect of a sphingomyelinase inhibitor on 1‐MeA‐induced inhibition of GJIC. The SL‐DT assay was used to determine GJIC. The incubation time of 1‐MeA was 15 min, and the concentration and preincubation time of the sphingomyelinase inhibitor, GW4869 (N,N′‐Bis[4‐(4,5‐dihydro‐1H‐imidazol‐2‐yl)phenyl]‐3,3′‐p‐phenylene‐bis‐acrylamide dihydrochloride) was 40 µM and 30 min. The results are an average of the data (n = 3) ±standard deviation at the 95% confidence level. An anova indicated significance at P < 0.001, F = 3743.6, and the different letters (A, B and C) indicated significance using an all pair multiple comparison‐Holm Sidak posthoc T‐test at the P = 0.05 level. (f) The effects of 1‐MeA versus 2‐MeA on the activation of either PC‐PLC or sphingomyelinase (SMase). An AmplexRed assay system as described in the methods and material section was used to measure the activities of the indicated lipases. The concentration and incubation times of the PAH were 100 µM and 15 min. The results are an average of the data (n = 5) ±standard deviation at the 95% confidence level. An ANOVA indicated significance at P = 0.003, F = 5.153, and the *indicated significance from all other groups using an all pair multiple comparison‐Holm Sidak posthoc T‐test at the P = 0.05 level. (g) Intracellular release of 3[H]‐arachidonic acid from cells treated with 1‐MeA and a phosphatidylcholine‐specific PLC inhibitor. The extracellular media was decanted, cells rinsed with phosphate buffered saline and then 1 mL of 0.5% bovine serum albumin in water was added to the cells for 10 min to extract the intracellular arachidonic acid and the radioactivity was determined by scintillation counting of counts per minute (CPM). The results are an average of the data (n = 3) ± standard deviation at the 95% confidence level. An anova indicated significance at P < 0.001, F = 25.2, and the different letters indicated significance using an all pair multiple comparison‐Holm Sidak posthoc T‐test at the P = 0.05 level.
Table 2.
Modulation of 1‐MeA‐induced inhibition of GJIC by various inhibitors of phospholipases and protein kinase signaling proteins
| † Inhibitor | Target signaling protein | ‡ Effect | § % inhibition of GJIC |
|---|---|---|---|
| None (+1‐MeA) | Gap Junction | ↓↓↓ | 100 |
| +D609 (50 µM, 15 min) | PC‐PLC | ↑↑↑ | *10 |
| +H‐89 (40 µM, 30 min) | PKA, GPCR | ↑↑ | ¶ 28 |
| +PKI (50 µM, 2 h) | PKA | ↑↑↑ | ¶ 16 |
| +BEL (2.5 µM, 30 min) | iPLA2 | ↑ | ¶ 74 |
| +BPB (5.0 µM, 20 min) | sPLA2 | ↓↓↓ | 97 |
| +AACOF3 (10 µM, 30 min) | cPLA2, iPLA2 | ↓↓↓ | 115 |
| +MAFP (1.25 µM, 30 min) | cPLA2, iPLA2 | ↓↓↓ | 101 |
| +MJ33 (10 µM, 30 min) | acidic iPLA2 | ↓↓↓ | 104 |
| +ET‐18‐OCH3 (30 µM, 30 min) | PI‐PLC | ↓↓↓ | 90 |
| +1‐butanol (0.5% v/v, 15 min) | PLD | ↓↓↓ | 101 |
| +RHC80267 (50 µM, 20 min) | DAG lipase | ↓↓↓ | 97 |
| +GF109203X (20 µM) | PKC (pan‐specific) | ↓↓↓ | 100 |
| +PP2 (40–100 µM) | Src | ↓↓↓ | 102 |
| +Genistein (100 µM) | Protein Tyr kinases (pan‐specific) | ↓↓↓ | 102 |
Inhibitors were added before the addition of 1‐MeA, which was incubated for 15 min at 70 µM, except for the BEL experiment where the time of 1‐MeA treatment was 30 min. The inhibitors were added at concentrations and times indicated in the parentheses, and remained in the medium for the duration of the experiment. D609, tricyclodecan‐9‐yl‐xanthate; H‐89, N‐(2‐(4‐bromocinnamylamino)ethyl)‐5‐isoquinolinesulfonamide; BPB, p‐bromophenacyl bromide; AACOCF3, arachidonyl trifluoromethylketone; PKI, myristoylated PKI (14–22) amide; BEL, bromoenol lactone; MAFP, methyl arachidonyl fluorophosphates; MJ33, 1‐hexadecyl‐3‐(trifluoroethyl)‐sn‐glycero‐2‐phosphomethanol; ET‐18‐OCH3, 1‐O‐Octadecyl‐2‐O‐methyl‐sn‐glycero‐3‐phosphorylcholine; RHC80267, 1,6‐bis‐(cyclohexyloximinocarbonylamino)hexane; GF109203X, bisindoylmaleimide I; PP2, 4‐amino‐5‐(4‐chloro‐phenyl)‐7‐(t‐butyl)pyrazolo[3,4‐d]pyrimidine).
↓↓↓– complete inhibition of GJIC; ↑, ↑↑– partial reversal of 1‐MeA‐induced inhibition of GJIC; ↑↑↑– complete reversal of 1‐MeA‐induced inhibition of GJIC.
An average with n = 3, % Inhibition = [(1 – FOC of Inhibitor)/(1 – FOC of vehicle)] × 100.
Significant as determined by a two‐tailed paired t‐test at the 95% confidence interval, P < 0.001 between the (1‐MeA) versus (1‐MeA + inhibitor) treated cells.
1‐MeA, 1‐methylanthracene; DAG, diacylglycerol; GJIC, gap junctional intercellular communication; GPCR, G‐protein coupled receptor; PC‐PLC, phosphatidylcholine specific phospholipase C; PI‐PLC, phosphatidylinositol specific phospholipase C; PKA, protein kinase A; PLD, phospholipase D.
The PC‐PLC inhibitor used, D609, has also been implicated in the inhibition of SMase. Thus, we inhibited SMase with an inhibitor specific to this enzyme (GW4869), which did not reverse the inhibition of GJIC (Fig. 2e). Further evidence of PC‐PLC involvement is indicated by the activation of this specific phospholipase by 1‐MeA, while 1‐MeA did not increase the activity of SMase (Fig. 2f). These results indicate that D609 probably reversed the 1‐MeA‐induced inhibition of GJIC by acting upon PC‐PLC and not SMase. However, D609 did not reverse the effects of 1‐MeA induced release of arachidonic acid (Fig. 2g). This suggests that the release of arachidonic acid was not dependent on PC‐PLC but rather from another phospholipase. Western blot analyzes of ERK and p38 (Fig. 3a,b) demonstrated that D609 did not reverse the activation of these MAPKs in response to 1‐MeA. Densitometry analyzes of the bands were normalized to the house keeping protein glyceraldehyde 3‐phosphate dehydrogenase and t‐tests were conducted comparing (1‐MeA) versus (1‐MeA + D609) for each of the MAPK bands.
Figure 3.
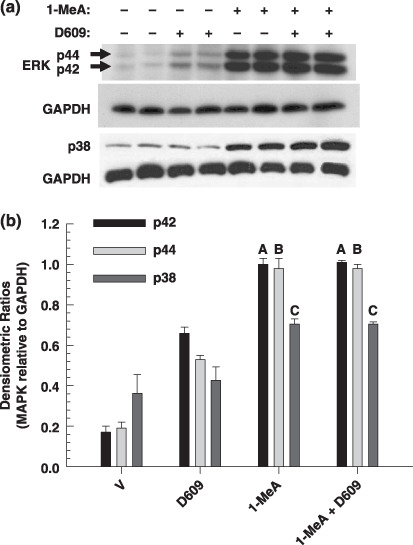
The effect of a phosphatidyl choline specific phospholipase C (PC‐PLC) inhibitor on 1‐methylanthracene (1‐MeA)‐induced activation of extracellular receptor kinase (ERK) and p38. (a) The activation of ERK and p38 was determined by Western blot analysis using phosphospecific antibodies. (b) The densitometry analyzes of the three independent Western blot experiments including those shown in (a) of the mitogen activated protein kinase (MAPK) bands relative to the house keeping protein, GAPDH. (a,b) The dose and time of 1‐MeA was 75 µM and 30 min. The PC‐PLC inhibitor, 50 µM of D609, was added 20 min prior to 1‐MeA and remained on the cells for an additional 30 min after the 1‐MeA was added. The D609 control was 50 min. The first two lanes was the vehicle. Using a two‐tailed t‐test, the letter A in (b) indicates no significant difference in the p42 band between the 1‐MeA and 1‐MeA + D609 treatment (t = –0.548, P = 0.613). Similarly, the letter B indicated no difference in the p44 band (t = –0.000, P = 1.000) and the letter C indicated no difference in the p38 band (t = –0.0418, P = 0.969) between the 1‐MeA and 1‐MeA + D609 treatment.
Inhibitors of other signal transduction proteins were also preincubated with the cells before the addition of 1‐MeA (Table 2). Most significant, was inhibition of protein kinase A (PKA) where a 72–84% recovery was observed with the following inhibitors H89 and PKI, respectively. Several inhibitors of PLA2 were used against the various PLA2 isoforms; particularly sPLA2 (p‐bromophenacyl bromide), cPLA2 (AACOF3 and MAFP), iPLA2 (bromoenol lactone [BEL] and MAFPP) and acidic iPLA2 (MJ33). BEL was the only PLA2 inhibitor that partially reversed (26%) 1‐MeA‐induced closure of gap junctions, while all other PLA2 inhibitors had no significant effect, which suggests that the small BEL effect was not specific to PLA2. Inhibitors of diacylglycerol (DAG) lipases, PLA2, PI‐PLC, phospholipase D (PLD), sphingomyelinase, PKC, Src kinases, and protein tyrosine kinases did not prevent the inhibition of GJIC by 1‐MeA (Table 2).
Discussion
The mechanisms by which exogenous and endogenous chemicals modulate GJIC have only been recently explored. There are examples of modulation of GJIC at all three levels of control: transcriptional, translational and post‐translational.( 40 ) Phosphorylation of gap junction proteins has been the most extensively studied post‐translational modification of gap junctions.( 40 ) Compounds, such as phorbol esters, which activate PKC are also known to inhibit GJIC and cause a hyperphosphorylation of gap junction proteins via a Mek‐dependent mechanism.( 40 ) The hyperphosphorylation of gap junction proteins can be detected by Western blot analysis using 1‐dimensional gel systems in which shifts in the electrophoretic bands can be readily observed.( 41 ) However, phosphorylation alone has been shown to be insufficient in the regulation of GJIC.( 22 ) Apparently, non‐phosphorylating mechanisms are also involved in the control of GJIC,( 40 ) which is evidently how 1‐MeA is regulating gap junctions. Our Western blot results clearly indicated that the activation of MAPKs, such as ERK and p38, does not necessarily result in the hyperphosphorylation of Cx43, even though Cx43 has known consensus sequences for ERK‐MAPK.( 23 ) We previously demonstrated that the addition of a Mek inhibitor does not prevent the closure of gap junction channels,( 25 , 28 ) and in this report, we similarly showed that inhibition of p38 did not reverse the effects of 1‐MeA on GJIC. Thus, activation of MAPKs, although typically required in cell proliferation and is always accompanied with inhibition of GJIC, does not always result in their direct control of gap junction function.
Lipid signaling has been implicated in the control of GJIC.( 42 ) Lipid metabolites, such as arachidonic acid, are known to inhibit GJIC.( 43 , 44 ) Therefore, we explored the potential role of phospholipases. The release of arachidonic acid was significantly detected within 2 min indicating that phospholipases are immediately activated in response to 1‐MeA. The employment of a consortium of phospholipase inhibitors indicated that PC‐PLC was involved in 1‐MeA‐induced inhibition of GJIC. Inhibition of PC‐PLC by the inhibitor, D609, has also been implicated in the inhibition of sphingomyelinase SMase, but our results showed that a more specific SMase inhibitor did not prevent inhibition of GJIC by 1‐MeA, and the activation of PC‐PLC and not SMase by 1‐MeA strongly suggested that PC‐PLC was the key regulator of GJIC in response to 1‐MeA. However, PC‐PLC was involved in neither the release of arachidonic acid nor the activation of ERK and p38 MAPKs indicating that other phospholipases are involved in these two important signaling events. Apparently 1‐MeA either initiated a general activator or repressed a general inhibitor of phospholipases. In contrast to PI‐PLC, the function of PC‐PLC in tumorigenesis has not been extensively studied, yet there are reports indicating that PC‐PLC does play a very significant role in cancer.( 45 ) Our results implicate that one significant role PC‐PLC in cancer could be the potential regulation of GJIC by environmental agents such as certain polycyclic aromatic hydrocarbons known to be prevalent in cigarette smoke.
Although PC‐PLC was an essential component of the signaling pathway controlling GJIC, this phospholipase was not involved in arachidonic acid release indicating that other phospholipases were activated. However, inhibition of other phospholipases such as PI‐PLC, various PLA2 isozymes, PLD and diacylglycerol lipase did not prevent the inhibition of GJIC by 1‐MeA indicating that these lipid signaling enzymes were not regulating GJIC. In addition, inhibition of Src kinases and PKC, both common signaling enzymes, also did not prevent the inhibition of GJIC by 1‐MeA. The concentrations and times used for these inhibitors of signaling enzymes were similar to those reported in literature where an inhibitor‐induced effect was observed, but there is always the possibility of a false negative. However, the use of several PLA2 inhibitors strongly suggested that this class of signal transduction enzymes were not involved in the regulation of GJIC. When cells were preincubated with a PKA inhibitor, H89, a 72% recovery from 1‐MeA‐induced inhibition of GJIC was seen, indicating a potential role of the cAMP dependent kinase, PKA, in the regulation of GJIC. Although H89 is known to inhibit at least eight other kinases such as MAPKAP‐K1b, S6K1, MSK1, KBα, SGK, ROCK II, AMPK and CHK 1,( 46 ) further experiments with the cell permeable specific inhibitor of PKA, myristoylated PKI (14–22) amide, also indicated the role of PKA in the down‐regulation of GJIC after exposure to 1‐MeA (84% recovery of GJIC). Another class of compounds, PCB, also inhibit GJIC through a PC‐PLC dependent mechanism, but1‐MeA, unlike the PCB did not involve Src or DAG lipase.( 28 )
Most research on PAH toxicity has focused on the mutagenic and DNA damaging properties of the higher molecular weight compounds but clearly the lower molecular weight‐PAH are capable of inducing biological effects relevant to tumor promotion. We previously reported the effects of 41 different PAH on GJIC including 16 with five or more rings.( 24 , 27 , 47 , 48 ) Most of these PAH, including the high molecular weight PAH, inhibited GJIC with varying potencies, but the potencies of the lower molecular weight PAH (four rings or less) tended to be much stronger if they contained a bay or bay‐like region.( 6 , 24 , 27 ) The low molecular weight PAH also tend to be the most abundant in the environment.
One example is cigarette smoke, where the low molecular weight fractions, particularly the three‐ and four‐ringed PAH predominate in which the methylated anthracenes and phenanthrenes are 62 times higher than benzo(a)pyrene and benzo(e)pyrene.( 31 ) Considering that the fraction of cigarette smoke containing the three‐ and four‐ringed‐PAH is highly cocarcinogenic when applied to the skin of mice treated with benzo(a)pyrene,( 49 ) and that cigarette smoke is a strong promoter and weak complete carcinogen,( 50 , 51 , 52 , 53 ) suggest that this fraction could significantly contribute to cancer. Ten to 15 years after giving up smoking, the ex‐smoker faces the same low risk of developing cancer of the upper digestive tract, the lung, the pancreas, and the urinary tract as the non‐smoker.( 54 ) This fact strongly implicates that cigarette smoke contributes to the non‐genotoxic and reversible phases of cancer. Contribution of cigarette smoke to the non‐genotoxic phase of cancer is particularly important. Early work on the carcinogenicity of cigarette smoke condensates strongly indicated that the neutral fractions, which contained primarily PAHs were the most carcinogenic fractions, but that the concentrations of the most prominent complete carcinogens, that is benzo(a)pyrene, was far too low to account, by themselves, for the carcinogenic activity of the condensates.( 50 , 55 ) In addition, we have shown that the 1‐MeA, but not the 2‐MeA, inhibited GJIC and prevented the induction of differentiation of normal human pancreatic cells using an in vitro system at non‐cytotoxic doses.( 56 ) Cigarette smoking has been one of the suspected risk factors for pancreatic cancer. Furthermore, the much less studied area of cocarcinogenesis is a closer fit to the extended exposure of human smoking than the complete carcinogenic nature of selected PAH from cigarette smoke condensates.( 50 ) Therefore, understanding the biological effects of these three‐ and four‐ringed PAH, which are very prevalent in cigarette smoke and possess cocarcinogenic activity, on cell signaling pathways relevant to the epigenetic, non‐genotoxic phase of cancer is important. In particular, functional GJIC offers a very central signaling system to assess risk.
The homeostasis of a tissue requires functional GJIC. Although, the transient closure of gap junction channels during proliferation is a normal response to mitogens, the chronic inhibition of GJIC by repeated exposures to toxicants, toxins, or cytokines released during compensatory hyperplasia could contribute to cancer. Chronic inhibition of GJIC has been correlated with enhanced proliferation, and inhibition of apoptosis and differentiation. Determining the effect of PAH on key signaling and gene expression events would provide invaluable mechanistically based information on the epigenetic toxicity of these compounds, thereby aiding in the development of preventative strategies of controlling human diseases such as cancer.
Conclusion
Inhibition of GJIC and the activation of intracellular mitogenic pathways are characteristic of epithelial derived cancer cells. Our results indicate that PC‐PLC is an important signaling enzyme needed for the inhibition of GJIC in response to a cigarette smoke relevant polycyclic aromatic hydrocarbon. This report clearly indicates that specific phospholipid signaling is involved in the regulation of GJIC, and that, in addition to reported Mek‐dependent regulation of GJIC, the regulation of GJIC can be Mek‐independent even though this MAPK pathway is activated. Thus, not all tumor promoters inhibit GJIC through the same signaling pathway, and this implicates that chemoprevention strategies relative to up‐regulating GJIC activity probably can not be universally effective.
Acknowledgments
This research was supported by National Institute of Environmental Health Sciences (NIEHS) grants #R01 ES013268‐01A2 to Upham and it's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS; and #5D43 TW00641‐10 Fogarty International Center, National Institutes of Health, USA, to the Institute of International Health at Michigan State University for the funding of M. Gužviæ; and Czech Science Foundation (grant no. 524/05/0595) and the Czech Ministry of Agriculture (No. MZE0002716201) to M. Machala and J. Vondráček.
References
- 1. Baird WM. Carcinogenic polycyclic aromatic hydrocarbon‐DNA adducts and mechanism of action. Environ Mol Mutagenesis 2005; 45: 106–14. [DOI] [PubMed] [Google Scholar]
- 2. Trosko JE, Upham BL. The emperor wears no clothes in the field of carcinogen risk assessment: ignored concepts in cancer risk assessment. Mutagenesis 2005; 20: 81–92. [DOI] [PubMed] [Google Scholar]
- 3. Rosenkranz M, Rosenkranz HS, Klopman G. Intercellular communication, tumor promotion and non‐genotoxic carcinogenesis: relationships based upon structural considerations. Mutat Res 1997; 381: 171–88. [DOI] [PubMed] [Google Scholar]
- 4. Fagotto F. Cell contact‐dependent signaling. Developmental-Biology 1996; 180: 445–54. [DOI] [PubMed] [Google Scholar]
- 5. Upham BL, Trosko JE. A paradigm shift in the understanding of oxidative stress and it's implications to exposure of low‐level ionizing radiation. Acta Med Nagasakiensia 2006; 50: 63–8. [Google Scholar]
- 6. Upham BL, Weis LM, Trosko JE. Modulated gap junctional intercellular communication as a biomarker of PAH epigenetic toxicity: structure‐function relationship. Environ Health Perspect 1998; 106: 975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamasaki H, Krutovskikh V, Mesnil M, Tanaka T, Zaidan DM, Omori Y. Role of connexin (gap junction) genes in cell growth control and carcinogenesis. C R Acad Sci III 1999; 322: 151–9. [DOI] [PubMed] [Google Scholar]
- 8. Borek C, Sachs L. The difference in contact inhibition of cell replication between normal cells and cells transformed by different carcinogens. Proc Natl Acad Sci USA 1966; 56: 1705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Potter VR. Phenotypic diversity in experimental hepatomas: The concept of partially blocked ontogeny. Br J Cancer 1978; 38: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trosko JE, Ruch RJ. Gap junctions as targets for cancer chemoprevention and chemotherapy. Curr Drug Targets 2002; 3: 465–82. [DOI] [PubMed] [Google Scholar]
- 11. Yamasaki H, Naus CCG. Role of connexin genes in growth control. Carcinogenesis 1996; 17: 1199–213. [DOI] [PubMed] [Google Scholar]
- 12. King TJ. Connexins as targets for cancer chemoprevention and chemotherapy. Biochimica Biophys Acta 2005; 1719: 146–60. [DOI] [PubMed] [Google Scholar]
- 13. Ruch RJ, Guan X, Sigler K. Inhibition of gap junctional intercellular communication and enhancement of growth in BALB/c 3T3 cells treated with connexin43 antisense oligonucleotides. Mol Carcinog 1995; 14: 269–74. [DOI] [PubMed] [Google Scholar]
- 14. Evert M. Morphology and morphometric investigation of hepatocellular preneoplastic lesions and neoplasms in connexin32‐deficient mice. Carcinogenesis‐ 2002; 23: 697–703. [DOI] [PubMed] [Google Scholar]
- 15. King TJ. Deficiency in the gap junction protein connexin32 alters p27Kip1 tumor suppression and MAPK activation in a tissue‐specific manner. Oncogene‐ 2005; 24: 1718–26. [DOI] [PubMed] [Google Scholar]
- 16. King TJ. Mice deficient for the gap junction protein Connexin32 exhibit increased radiation‐induced tumorigenesis associated with elevated mitogen‐activated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis 2004; 25: 669–80. [DOI] [PubMed] [Google Scholar]
- 17. Wright JH, Munar E, Jameson DR et al . Mitogen‐activated protein kinase kinase activity is required for the G(2)/M transition of the cell cycle in mammalian fibroblasts. Proc Natl Acad Sci USA 1999; 96: 11335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seger R, Krebs EG. The MAPK signaling cascade. FASEB J 1995; 9: 726–35. [PubMed] [Google Scholar]
- 19. Denhardt DT. Signal‐transducing protein phosphorylation cascades mediated by Ras/Rho proteins in the mammalian cell: the potential for multiplex signalling. Biochem J 1996; 318: 729–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukuhara S, Marinissen MJ, Chiariello M, Gutkind JS. Signaling from G protein‐coupled receptors to ERK5/Big MAPK 1 involves Galpha q and Galpha 12/13 families of heterotrimeric G proteins. Evidence for the existence of a novel Ras and Rho‐independent pathway. J Biol Chem 2000; 275: 21730–6. [DOI] [PubMed] [Google Scholar]
- 21. Warn CB, Lampe PD, Kurata WE et al . Characterization of the mitogen‐activated protein kinase phosphorylation sites on the connexin‐43 gap junction protein. J Biol Chem 1996; 271: 3779–86. [DOI] [PubMed] [Google Scholar]
- 22. Hossain MZ, Jagdale AB, Ao P, Boynton AL. Mitogen‐activated protein kinase and phosphorylation of connexin43 are not sufficient for the disruption of gap junctional communication by platelet‐derived growth factor and tetradecanoylphorbol acetate. J Cell Physiol 1999; 179: 87–96. [DOI] [PubMed] [Google Scholar]
- 23. Lampe PD. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 2004; 36: 1171–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bláha L, Kapplová P, Vondráček J, Upham B, Machala M. Inhibition of gap‐junctional intercellular communication by environmentally occurring polycyclic aromatic hydrocarbons. Toxicol Sci 2002; 65: 43–51. [DOI] [PubMed] [Google Scholar]
- 25. Rummel AM, Trosko JE, Wilson MR, Upham BL. Polycyclic aromatic hydrocarbons with bay‐like regions inhibited gap junctional intercellular communication and stimulated MAPK activity. Toxicol Sci 1999; 49: 232–40. [DOI] [PubMed] [Google Scholar]
- 26. Upham BL, Weis LM, Rummel AM, Masten SJ, Trosko JE. The effects of anthracene and methylated anthracenes on gap junctional intercellular communication in rat liver epithelial cells. Fundam Appl Toxicol 1996; 34: 260–4. [DOI] [PubMed] [Google Scholar]
- 27. Weis LM, Rummel AM, Masten SJ, Trosko JE, Upham BL. Bay or baylike regions of polycyclic aromatic hydrocarbons were potent inhibitors of Gap junctional intercellular communication. Environ Health Perspect 1998; 106: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Machala M, Bláha L, Vondráček J, Trosko JE, Scott J, Upham BL. Inhibition of gap junctional intercellular communication by noncoplanar polychlorinated biphenyls. inhibitory potencies and screening for potential mode(s) of action. Toxicol Sci 2003; 76: 102–11. [DOI] [PubMed] [Google Scholar]
- 29. Rivedal E, Opsahl H. Role of PKC and MAP kinase in EGF‐ and TPA‐induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis 2001; 22: 1543–50. [DOI] [PubMed] [Google Scholar]
- 30. Vondráček J, Švihálková‐Šindlerová L, Pěnčiková K et al . Concentrations of methylated naphthalenes, anthracenes, and phenathrenes occurring in Czech river sediments and their effects on toxic events associated with carcinogenesis in rat liver cells. Environ Toxicol Chem 2007; 26: 2308–16. [DOI] [PubMed] [Google Scholar]
- 31. Severson RF, Snook ME, Higman HC, Chortyk OT, Akin FJ. Isolation, identification, and quantification of polynuclear aromatic hydrocarbons in tobacco smoke. In: Freudenthal RI, Jones PW, eds. Carcinogenesis‐a Comprehensive Survey. Vol. 1. Polynuclear Aromatic Hydrocarbons: Chemistry, Metabolism, and Carcinogenesis. New York (NY): Raven Press, 1976: 253–70. [Google Scholar]
- 32. Tsao MS, Smith JD, Nelson KG, Grisham JW. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of ‘oval’ cells. Exp Cell Res 1984; 154: 38–52. [DOI] [PubMed] [Google Scholar]
- 33. Coleman WB, Wennerberg AE, Smith GJ, Grisham JW. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol 1993; 142: 1373–82. [PMC free article] [PubMed] [Google Scholar]
- 34. Malouf NN, Coleman WB, Grisham JW et al . Adult‐derived stem cells from the liver become myocytes in the heart in vivo. Am J Pathol 2001; 158: 1929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. El‐Fouly MH, Trosko JE, Chang CC. Scrape‐loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res 1987; 168: 422–30. [DOI] [PubMed] [Google Scholar]
- 36. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–5. [DOI] [PubMed] [Google Scholar]
- 37. Zhou M, Diwu Z, Panchuk Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem 1997; 253: 162–8. [DOI] [PubMed] [Google Scholar]
- 38. Tithof PK, Schiamberg E, Peters‐Golden M, Ganey PE. Phospholipase A2 is involved in the mechanism of activation of neutrophils by polychlorinated biphenyls. Environ Health Perspect 1996; 104: 52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trosko JE, Chang CC, Wilson MR, Upham B, Hayashi T, Wade M. Gap junctions and the regulation of cellular functions of stem cells during development and differentiation. Methods 2000; 20: 245–64. [DOI] [PubMed] [Google Scholar]
- 40. Saez JC, Berthoud VM, Moreno AP, Spray DC. Gap junctions. Multiplicity of controls in differentiated and undifferentiated cells and possible functional implications. Adv Second Messenger Phosphoprotein Res 1993; 27: 163–98. [PubMed] [Google Scholar]
- 41. Matesic DF, Rupp HL, Bonney WJ, Ruch RJ, Trosko JE. Changes in gap‐junction permeability, phosphorylation, and number mediated by phorbol ester and non‐phorbol‐ester tumor promoters in rat liver epithelial cells. Mol Carcinog 1994; 10: 226–36. [DOI] [PubMed] [Google Scholar]
- 42. Aylsworth CF. Effects of fatty acids on gap junctional communication: possible role in tumor promotion by dietary fat. Lipids 1987; 22: 445–54. [DOI] [PubMed] [Google Scholar]
- 43. Lazrak A. Ca‐mediated and independent effects of arachidonic acid on gap junctions and Ca‐independent effects of oleic acid and halothane. Biophysical-Journal 1994; 67: 1052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gainer HS. Diacylglycerol inhibits gap junctional communication in cultured epidermal cells: evidence for a role of protein kinase C. Biochem Biophys Res Commun 1985; 126: 1109–13. [DOI] [PubMed] [Google Scholar]
- 45. Cheng J, Weber JD, Baldassare JJ, Raben DM. Ablation of Go alpha‐subunit results in a transformed phenotype and constitutively active phosphatidylcholine‐specific phospholipase C. J Biol Chem 1997; 272: 17312–9. [DOI] [PubMed] [Google Scholar]
- 46. Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev 2006; 24: 261–74. [DOI] [PubMed] [Google Scholar]
- 47. Ghoshal S, Weber WJ, Rummel AM, Trosko JE, Upham BL. Epigenetic toxicity of a mixture of polycyclic aromatic hydrocarbons on gap junctional intercellular communication before and after biodegradation. Environ Sci Technol 1999; 33: 1044–50. [Google Scholar]
- 48. Upham BL, Masten SJ, Lockwood BR, Trosko JE. Nongenotoxic effects of polycyclic aromatic hydrocarbons and their ozonation by‐products on the intercellular communication of rat liver epithelial cells. Fundam Appl Toxicol 1994; 23: 470–5. [DOI] [PubMed] [Google Scholar]
- 49. Hoffmann D, Schmeltz SS, Hecht SS, Wynder EL. Tobacco carcinogenesis. In: Gelboin HV, Ts’o PO, eds. Polycyclic Aromatic Hydrocarbonds and Cancer. Vol. 1. Environment, Chemistry, and Metabolism. New York, NY: Academic Press, Inc., 1978: 85–117. [Google Scholar]
- 50. Rubin H. Selective clonal expansion and microenvironmental permissiveness in tobacco carcinogenesis. Oncogene 2002; 21: 7392–411. [DOI] [PubMed] [Google Scholar]
- 51. Van Duuren BL, Sivak A, Langseth L. The tumor‐promoting activity of tobacco leaf extract and whole cigarette tar. Br J Cancer 1967; 21: 460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Duuren BL, Sivak A, Katz C, Melchionne S. Cigarette smoke carcinogenesis: importance of tumor promoters. J Natl Cancer Inst 1971; 47: 235–40. [PubMed] [Google Scholar]
- 53. Bock FG. Tumor promoters in tobacco and cigarette‐smoke condensate. J Natl Cancer Inst 1972; 48: 1849–53. [PubMed] [Google Scholar]
- 54. Wynder EL, Hoffmann D. Tobacco and tobacco smoke. Semin Oncol 1976; 3: 5–15. [PubMed] [Google Scholar]
- 55. Hoffmann D, Hoffmann I, El Bayoumy K. The less harmful cigarette: a controversial issue. a tribute to Ernst L. Wynder Chem Res Toxicol 2001; 14: 767–90. [DOI] [PubMed] [Google Scholar]
- 56. Tai MH, Upham BL, Olson LK, Tsao MS, Reed DN Jr, Trosko JE. Cigarette smoke components inhibited intercellular communication and differentiation in human pancreatic ductal epithelial cells. Int J Cancer 2007; 120: 1855–62. [DOI] [PubMed] [Google Scholar]


