Abstract
Electroconvulsive therapy (ECT) and ablative neurosurgical procedures are established interventions for treatment-resistant depression (TRD), but their use may be limited in part by neuropsychological adverse effects. Additional neuromodulation strategies are being developed that aim to match or exceed the efficacy of ECT/ablative surgery with a better neurocognitive side effect profile. In this review, we briefly discuss the neurocognitive effects of ECT and ablative neurosurgical procedures, then synthesize the available neurocognitive information for emerging neuromodulation therapies including repetitive transcranial magnetic stimulation, magnetic seizure therapy, transcranial direct current stimulation, vagus nerve stimulation, and deep brain stimulation. The available evidence suggests these procedures may be more cognitively benign relative to ECT or ablative neurosurgical procedures, though further research is clearly needed to fully evaluate the neurocognitive effects, both positive and negative, of these novel neuromodulation interventions.
Keywords: Major Depression, Neuromodulation, Neuropsychology, Antidepressant Treatment
Introduction
Up to half of patients with Major Depressive Disorder (MDD) do not respond to first-line antidepressant treatment (1), and one third do not respond to two or more treatments (2, 3). Treatment-resistant depression (TRD) is therefore prevalent, resulting in added patient suffering, disability, and suicide risk (4, 5). Established treatments for severe TRD include electroconvulsive therapy (ECT) and ablative neurosurgery – both of which are associated with cognitive side effects that may limit use. Over the past several years, a number of new neuromodulation techniques have been investigated with the goal of achieving or exceeding the efficacy of established TRD treatments with better cognitive safety. In this review, we describe the cognitive effects associated with ECT and ablative neurosurgical procedures and summarize available data on the neurocognitive safety of emerging neuromodulation techniques.
Electroconvulsive Therapy
ECT involves the serial administration of electrical current through the brain under general anesthesia to induce a generalized tonic-clonic seizure (6). ECT is one of the most effective acute treatments for a depressive episode (7), with response and remission rates as high as 79% and 75%, respectively, with brief pulse (1.0 ms) bitemporal (BT) electrode placement (Kellner et al., 2006; Husain et al., 2004). Recently, with the introduction of ultrabrief pulse (0.3 ms) ECT, studies have found varying efficacy results. For instance, Sackeim and colleagues found a high remission rate of 73% when using right-unilateral (RUL) electrode placement relative to 35% for a bilateral electrode configuration (8). However, Loo and colleagues found a modest remission rate of 27% with the use of ultrabrief pulse and RUL electrode placement (9). A possible explanation for this difference is that the latter study included a sample with greater treatment resistance. Seinaert and colleagues showed equivalent efficacy for ultrabrief pulse RUL and bifrontal (BF) ECT: response rate was 78.1% for both groups, remission rate was 43.75% for RUL and 34.38% for BF (10).
Balanced against its antidepressant efficacy, ECT results in significant cognitive sequelae including transient confusion, anterograde amnesia, and retrograde amnesia. Research has suggested that the confusion after each ECT treatment and the anterograde amnesia are time-limited (11-15), but retrograde amnesia has been found to persist up to and past 6-months in some cases (11-15). Patient-specific factors including greater age (16), lower education level, and lower premorbid intelligence (17) may increase the level of cognitive impairment associated with ECT. Also, concurrent use of certain psychotropic medications may either exacerbate (e.g., lithium, venlafaxine) or minimize (e.g., nortriptyline) adverse cognitive effects (18, 19).
Procedural modifications have been developed to minimize the severity of adverse cognitive effects (12). The use of brief or ultra brief pulse width rather than sine wave current has been found to lessen the cognitive impact of ECT (11, 20-22), and ultra brief pulse may be more cognitively advantageous than brief pulse (8). Dose titration – finding the smallest amount of energy required to elicit a seizure, then providing subsequent treatments relative to this threshold – has become a common practice that attempts to maximize efficacy while minimizing cognitive effects by treating at the lowest possible dose (23, 24). Electrode configuration has also been shown to minimize adverse cognitive effects. RUL and BF ECT are associated with less severe retrograde amnesia compared to BT ECT (21, 25). BF ECT has been reported to have a superior cognitive profile to BT ECT (26) and RUL ECT (27); however, these studies focused on memory (i.e., primarily temporal lobe tasks) rather than executive functions (i.e., primarily frontal lobe tasks) that might have been more affected (25).
Ablative Neurosurgery
Ablative neurosurgical procedures represent the earliest surgical attempts to treat TRD. Procedures in use today primarily include anterior capsulotomy, anterior cingulotomy, subcaudate tractotomy, and limbic leucotomy. Ablative surgery may be effective in 30%-70% of patients (28) – rigorous safety and efficacy data are lacking. In addition to the risks inherent to any neurosurgical procedure, undesirable personality changes and cognitive functioning have been reported with each approach. In terms of cognitive change, the processes mediated by the site of ablation are most likely to be affected.
Anterior Capsulotomy
Anterior capsulotomy severs a portion of the white matter tracts in the anterior section of the internal capsule that connects the thalamus to the frontal cortex (29). When combined with anterior cingulotomy it may cause greater reductions in emotion recognition than anterior cingulotomy alone (30). Anterior capsulotomy has primarily been used for treatment-refractory obsessive compulsive disorder (OCD) and is less-studied in TRD patients (31). One study of anterior capsulotomy for treating OCD found a long term (about 10 year) mild impairment in set-shifting and verbal fluency (32). Set shifting and working memory deficits have also been reported in treatment-refractory anxiety disorders patients receiving anterior capsulotomy (33). In sum, available data suggest possible, but not definitive, modest negative effects of anterior capsulotomy on neurocognitive function, particularly executive functioning.
Anterior Cingulotomy
Anterior cingulotomy involves bilateral lesions of the dorsal anterior cingulate gyrus (34). There is conflicting information regarding the neurocognitive effects related to anterior cingulotomy. Two studies have found impaired executive functions (e.g., emotional recognition, response inhibition, and mental image rotation (30, 35)). However, another found no impairment using a computerized neurocognitive battery and showed that patients improved on certain measures of executive functioning and spatial working memory (36).
Stereotactic Subcaudate Tractotomy (SST)
Stereotactic subcaudate tractotomy (SST) severs a portion of nerve fibers anterior to the head of the caudate nucleus connecting prefrontal cortex to hippocampus, amygdala, thalamus, and hypothalamus (37). There are limited neuropsychological data for patients undergoing SST. One group found SST to be associated with widespread frontal impairment at 2 weeks, but not 6 months, after surgery (38). It was concluded that these transient deficits were due to post-operative edema as opposed to the surgical lesions per sé (38).
Limbic Leucotomy
Limbic leucotomy combines anterior cingulotomy with SST (29). Neuropsychological data are limited, but preliminary results from a study of 88 patients showed no impairment on the Wechsler Adult Intelligence Scale (WAIS) 6 weeks after surgery (39). Improvements were noted on the Verbal, Performance and Full Scale IQ scores, but these changes may have been related to practice effects.
Emerging Neuromodulation Techniques
Repetitive Transcranial Magnetic Stimulation (rTMS)
Repetitive transcranial magnetic stimulation (rTMS) uses a focal, rapidly changing magnetic field to induce an electrical current in a targeted brain region. Meta-analyses have shown high-frequency (5-20 Hz) rTMS of the left dorsolateral prefrontal cortex (DLPFC) to be an effective antidepressant therapy with moderate to large effect size (40-43). A recent sham-controlled, multi-site study confirmed the statistically significant antidepressant effects of rTMS (44). A growing database supports the antidepressant efficacy of low-frequency (≤1 Hz) rTMS applied to the right DLPFC (45-47); however, these data are limited relative to left DLPFC studies. Some studies have suggested left DLPFC rTMS might have similar efficacy to ECT (48-50). However, there are conflicting data (51), and it is notable that a secondary analysis of the recent multi-site study showed that active rTMS was statistically more effective than sham rTMS only in patients failing no more than one adequate antidepressant treatment (52).
In general, studies of rTMS have been found it to be safe and well-tolerated (44). The most severe potential adverse effect is seizure (53), but implementation of established safety guidelines (54) has substantially reduced this risk (53). rTMS also appears safe in patients taking concurrent antidepressant medications (55). Based on its novelty and the possibility that rTMS might offer an alternative to ECT, neurocognitive assessment has been common in rTMS treatment trials. The vast majority of studies have found rTMS to have no deleterious effects on cognition (47, 56-60). Some studies have reported minimal deficits in sustained attention (61), spatial planning, and verbal retention (62), but the treatment protocols used in these studies were relatively nonstandard: one utilized a twice-daily treatment schedule instead of a more typical once-daily treatment schedule (61), while the other used bilateral DLPFC rTMS (62). Direct comparison of 10 Hz left DLPFC rTMS to brief-pulse RUL ECT suggests that rTMS has a superior cognitive effect profile (63, 64), though one study using less sensitive neurocognitive measures failed to find such a difference (51).
Many studies have noted improvements in aspects of cognitive functioning over the course of rTMS treatment. Specific areas of improvement have included manual motor speed, simple reaction time, verbal and visual learning, attention, processing speed, verbal fluency, autobiographical memory, working memory, and executive functioning (47, 56-58, 60-62, 65, 66). However these findings must be interpreted with caution due to various methodological factors that could have mediated the findings, including use of select cognitive instruments without alternate forms, practice effects, cohort characteristics (i.e., age, education level), statistical chance, and lack of a control group (58, 65, 67, 68). As a whole, the available neurocognitive safety data for rTMS in TRD are insufficient to formulate conclusive hypotheses regarding the presence or absence of neurocognitive improvements. Future studies with larger sample sizes and stricter methodological controls may clarify this issue more definitively.
Magnetic Seizure Therapy (MST)
Magnetic seizure therapy (MST) uses an rTMS device to administer a series of rTMS pulses delivered at high intensity to induce a single seizure under general anesthesia and muscle relaxation. A series of seizures is given over several weeks, analogous to ECT. Efficacy is supported by two case reports (69, 70), and two small studies comparing MST and ECT (71, 72). These data suggest MST has antidepressant effects, but not necessarily equivalent to ECT (72). Compared to ECT, MST is associated with fewer somatic side effects, most notably headaches and muscle aches (73). A two-center controlled trial comparing MST to RUL ECT (administered with ultra-brief pulse) is ongoing.
MST results in fewer adverse cognitive effects than ECT (69), perhaps due to the more focal nature of acute stimulation (71) and less generalized impact on cortical regions subserving neurocognitive functions. In nonhuman primates, MST (administered at 50 Hz) caused little anterograde or retrograde amnesia relative to electroconvulsive shock (ECS) (74, 75); MST administered at 100 Hz retained its cognitive advantage over ECS and was comparable to sham (anesthesia only) (76). In depressed patients, cognitive data suggest that, compared to ECT, MST is associated with fewer subjective memory complaints and fewer objective cognitive side effects (73). Specifically, cognitive advantages of MST included preserved face recognition (both neutral and affective), sentence recognition, and category fluency (Lisanby, Luber et al., 2003). Only delayed figure reproduction was worse in patients receiving MST than in those receiving ECT. Following a complete course of 50 Hz MST in a controlled trial with 20 patients, there was little to no change in neurocognitive performance on measures of auditory or visuospatial learning and memory, retrograde memory for public information, and global cognitive function (77). A consistent finding with both 50 Hz and 100 Hz MST is rapid recovery of orientation after treatment (73, 78).
Transcranial Direct Current Stimulation (tDCS)
In transcranial direct current stimulation (tDCS), a relatively low intensity, nonconvulsive electrical current is non-invasively applied to the brain (79). tDCS for depression focuses electrical current through the DLPFC with a supraorbital grounding (80), but likely differs from rTMS in mechanism of action (80). Preliminary antidepressant efficacy is supported by two randomized, sham-controlled studies (81, 82). A comparison study of tDCS and fluoxetine found both treatments significantly reduced depressive symptoms, though tDCS showed a faster antidepressant effect (83). Side effects of tDCS have included slight tingling at the electrode sites, headache, fatigue, and nausea (84).
tDCS studies including neurocognitive assessment have focused on global cognitive function, processing speed, working memory, attention, and executive functions (85). Of importance, no decreases were noted in any neurocognitive test following treatment, and a statistically significant improvement was found in the domain of working memory. This improvement was only observed in patients who received active DLPFC stimulation. Improvement in working memory has also been observed following DLPFC tDCS in healthy controls (86) and individuals with idiopathic Parkinson's disease (87). Additionally, improvement in verbal recognition memory has been observed following tDCS in individuals with Alzheimer's disease (88). However, one recent investigation of tDCS in individuals with depression found no effects on verbal working memory, recognition memory, or attention (89). The authors posited the interference effects from medications, differences in stimulation parameters, and cognitive battery administration time as the reason for the lack of improvement in verbal working memory observed in other studies (89).
Vagus Nerve Stimulation (VNS)
In vagus nerve stimulation (VNS), a bipolar electrode wrapped around the cervical vagus nerve (typically left) transmits low-frequency electrical pulses originating from a pulse generator implanted subcutaneously in the anterior chest wall (90). The treatment has been used to treat refractory epilepsy (US FDA approved in 1997) and the US FDA approved its use as an adjunctive treatment of refractory depression in 2005 (91). In a placebo-controlled clinical trial, VNS did not show a statistically significant acute (10-week) antidepressant effect compared to sham treatment. After 12 months of VNS plus treatment-as-usual (TAU), response and remission rates were 27% and 16%, respectively (92), which were statistically significantly higher than a non-randomized observation-only, TAU group (93). Side effects included voice alteration, dyspnea, and neck pain, all of which are generally mild and restricted to time of stimulation (91, 94).
Cognitive safety data on VNS are limited. Only one study of VNS for TRD reported cognitive effects: patients receiving open-label VNS showed no decrements on measures of processing speed, psychomotor function, verbal fluency, attention, memory, or executive functioning (95). Patients showed improved performance on measures of psychomotor speed, language, and executive function. These results were not attributable to practice effects, though may have been associated with mood improvement. In studies of patients treated with VNS for epilepsy, no impairments were found in the domains of attention, motor function, short-term memory, learning and memory, IQ, processing speed, and executive function (96-98). However, Helmstaedter et al. (2001) reported that poor performance on measures of visual-spatial memory and increased time to task completion was related to higher intensity (1-2.5 mA) VNS stimulation.
Deep Brain Stimulation (DBS)
For deep brain stimulation (DBS), electrodes are stereotactically implanted at a specific neuroanatomical target and focal stimulation is delivered. Use is currently widespread for movement disorders, with several subcortical regions including the thalamic ventral intermediate nucleus (VIM) for tremor, the globus pallidus internus (GPi) for dystonia, and the subthalamic nucleus (STN) and GPi for Parkinson's disease (PD) (99). DBS offers a revisable, adjustable, and reversible alternative to ablative procedures. However, DBS is not simply a reversible lesion (100). Therefore, this treatment may reasonably have a different cognitive side effect profile compared to ablation of the same targets (101).
Although the majority of PD DBS patients experience a safe and effective outcome, potential site-related side effects have been reported (102). DBS applied to the STN has been associated with reductions in verbal fluency and, less consistently, verbal memory, conditional associative learning, conceptual reasoning, and global cognitive function (102). DBS applied to the GPi has been associated with relatively mild reductions on measures of verbal fluency and visuospatial construction (102). However, while it may seem from the available literature that DBS applied to the STN relative to the GPi might be associated with more cognitive side effects, methodological differences between studies (e.g. sample size and medication effects) weaken this conclusion (102). For example, a recent study has found that in patients receiving STN DBS for PD, variables such as surgical trajectory and electrode placement may be associated with differences in neurocognitive side effects experienced (103). Importantly, DBS of either the STN or GPi is considered relatively safe from a cognitive standpoint.
For studies of DBS in TRD, the three best-studied targets are the subcallosal cingulate white matter (SCCwm), the ventral caudate/ventral striatum (VC/VS), and the nucleus accumbens (NAc). Open-label DBS of the SCCwm has shown six month response and remission rates of approximately 60% and 35% respectively (104, 105). VC/VS DBS emerged as a potential target for TRD when patients with treatment-resistant OCD also showed an antidepressant response (106). Six month response and remission rates of open-label VC/VS DBS for TRD have been reported to be 40% and 20%, respectively (107). Finally, the antidepressant response rate for twelve months of DBS applied to the NAc (a target anatomically similar to the VC/VS) has recently been reported as 50% (108). The most common side effects of SCCwm DBS treatment for TRD are wound infection, perioperative headache, and worsening/irritable mood (105). Increased suicidality was seen in 13% of TRD patients receiving VC/VS DBS (107), and 20% of patients receiving NAc DBS (108).
McNeely et al. (2008) evaluated the neurocognitive safety of SCCwm DBS for TRD using tests of verbal IQ, attention, psychomotor speed, risk taking, memory, and executive functioning and found no deterioration on any measure. Improvements were noted in verbal and visual memory, manual motor speed, and verbal learning in the subset of patients who were performing these tasks at below-average levels at baseline, moving them closer to average levels after 12 months of treatment (109). These changes did not correlate with changes in mood over the same time period, leading the authors to conclude that there might exist separate neural circuits for cognitive function and depression (109). Limitations of this study included small sample size (six patients) and the fact that patients at below-average baseline levels of cognitive functioning have the most room for improvement from practice effects.
Patients receiving VC/VS DBS for TRD were found to have no negative effects from stimulation on measures of general intellectual ability, language, processing speed, executive function, or learning and memory (107). Notably, the cognitive adverse effects reported with anterior capsulotomy were not seen with acute or chronic stimulation. Similar to the study of SCCwm DBS for TRD, improvement was observed in verbal memory that was statistically unrelated to change in depression severity (107). In the study of patients receiving NAc DBS, stimulation was not associated with any impairment of general intellectual ability, language, processing speed, executive functioning, learning, and memory (108).
Additional DBS targets for TRD are under investigation, though neurocognitive data are limited. A case report of DBS of the inferior thalamic peduncle reported efficacy in TRD, and neurocognitive data showed either no change or improvement in a number of domains (110). Finally, DBS of the white matter adjacent to the lateral habenula has shown an antidepressant effect in a single case report (111). While cognitive effects were not reported, animal data suggest habenular DBS may adversely affect associative learning and spatial working memory in rats (112, 113). Therefore testing in these domains should be considered in future clinical studies of habenular DBS.
In sum, DBS of the SCCwm, the VC/VS, and the NAc for TRD do not appear associated with any adverse cognitive side effects, and slight improvements in certain domains have been reported for SCCwm and VC/VS DBS. However, the available clinical data are quite limited, and results from ongoing large clinical trials will be needed to verify the cognitive safety of these treatments. Data are even more limited for other emerging targets. Given that DBS for TRD is still in the early stages of development, it is strongly recommended that comprehensive neuropsychological evaluation – paying particular attention to which domains might be affected by stimulation at a specific target – should be incorporated into ongoing and future clinical trials.
Summary
Challenges in interpreting cognitive effects related to antidepressant therapies
In interpreting the cognitive effects of antidepressant therapies, three issues must be carefully considered: practice effects, neurocognitive impairments associated with depression (that may or may not improve with treatment), and substantial methodological variations between studies. These issues complicate interpretation of neurocognitive findings to date with various focal brain stimulation therapies.
Practice effects are improvements in test performance solely due to prior test exposure. For example, individuals who complete intelligence tests at multiple time points tend to show improved scores, even with lengthy intervals between test administration (114). Moreover, the use of alternate test forms, a strategy commonly used to minimize practice effects, does not completely remove the influence of these effects (115). Thus, with tests where practice effects are expected to improve performance (e.g., Digit Span, Stroop Color Word Test), the lack of improvement (without obvious worsening) could indicate cognitive impairment. In designing neuropsychological testing for future studies, a potentially beneficial strategy may be to administer the battery to a control group at identical time points to clarify whether the specific battery and testing conditions used were associated with practice effects.
Studies of neurocognitive side effects of antidepressant therapies are additionally complicated by the cognitive impairments associated with depression. A systematic review of data from individuals with MDD published between 1980 and 2008 suggested a pattern of global-diffuse impairment in many cognitive domains including processing speed, attention, memory, and executive function (116). For example, investigations have reported associations between MDD and impairment in divided attention (117), short-term and long-term memory (118), and executive functions including cognitive flexibility, inhibition, and problem solving (119-122).
Collectively, the available evidence suggests that cognitive deficits observed in MDD occur with more demanding (e.g., measures of cognitive flexibility) relative to less challenging tests (e.g., measures of simple reaction time) (123-125). Particularly relevant to treatment studies, these deficits may resolve as patients recover from depression. This has been seen in studies of patients with cyclic illness patterns (e.g., Seasonal Affective Disorder) (126, 127) and medication trials (128-130), though some level of residual cognitive impairment may persist (131-133). Correlations between individuals' changes in neurocognitive performance and their changes in mood can help clarify whether any recovery of neurocognitive function was more likely to have been a direct effect of antidepressant therapy or a secondary effect of response to treatment. Such analyses suggested that improvements in test performance were likely attributable to improvements in mood in the VNS for TRD study, but not in the rTMS, tDCS, or DBS studies.
Lastly, many of the reported neurocognitive findings could be due, in part, to the substantial methodological variations between studies. Differences in study cohorts, treatment parameters, antidepressant effects, and testing conditions have important implications and must be controlled or accounted for when comparing multiple studies. For example, cohort and treatment parameter differences may account for the inconsistency in the tDCS literature regarding a possible improvement in working memory. Similarly, differences in testing conditions and neurocognitive batteries used may be the source of the large variability in reported neurocognitive improvements following rTMS. Future investigations should further characterize the basis of observed neurocognitive changes through improved methodological rigor.
Cognitive safety of emerging neuromodulation techniques: a summary
With the above caveats in mind, the reviewed literature suggests that many of the neuromodulation treatments currently under investigation have a lower risk of adverse neurocognitive side effects compared to ECT. Specifically, two of these novel treatments (MST and rTMS) have been directly compared to ECT and demonstrated greater neurocognitive safety (64, 73). For rTMS, multiple studies have confirmed the lack of significant adverse cognitive effects allowing one to conclude that these treatments are neuropsychologically benign. A smaller database supports the cognitive safety of tDCS. While VNS in general appears to have no concerning neuropsychological adverse effects, data specific to TRD are more limited. Finally, preliminary data for three DBS targets for TRD (SCCwm, VC/VS, and NAc) have not shown any neurocognitive adverse effects associated with treatment; however, more research is clearly needed to confirm this.
Conclusion
Much progress is being made in the search for TRD treatments that can match or exceed the antidepressant efficacy of ECT and ablative surgeries while minimizing adverse neurocognitive effects. Evaluation of cognitive safety has therefore been and continues to be an important component of this effort. As development of these and other therapies moves forward, careful, comprehensive assessment of neurocognitive outcomes should continue – especially for more invasive approaches (such as DBS) where potential benefit must be weighed very carefully against possible adverse effects. Specific to DBS – where multiple potential targets exist – comprehensive neuropsychological assessment insures that comparable data will be obtained across studies.
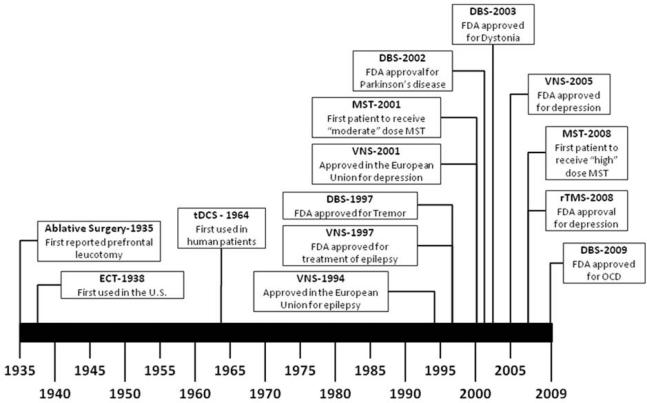
Figure 1.
Timeline of Major Milestones for Established and Emerging Neuromodulation Techniques. DBS=deep brain stimulation, ECT=electroconvulsive therapy, FDA= United States Food and Drug Administration, MST=magnetic seizure therapy, OCD=Obsessive Compulsive Disorder, rTMS=repetitive transcranial magnetic stimulation, tDCS=transcranial direct current stimulation, VNS=vagus nerve stimulation
Table 1.
Neurocognitive Effects of Established and Emerging Neuromodulation Techniques
| Neuromodulation Technique | Neurocognitive Effects |
|---|---|
| Electroconvulsive Therapy | Retrograde amnesia, anterograde amnesia, postictal disorientation |
|
| |
| Ablative Surgery | |
| Anterior Capsulotomy | Possible impairment in emotion recognition, set shifting, verbal fluency, and working memory |
| Anterior Cingulotomy | Mixed reports, with some studies showing impairments in executive functioning, and others reporting improvements in executive functioning |
| Stereotactic Subcaudate Tractotomy | Possible transient wide spread frontal impairment (potentially associated with post-operative edema) |
| Limbic Leucotomy | No neurocognitive impairment on the WAIS; possible improvements in WAIS Verbal, Performance, and Full Scale IQ Scores (potentially associated with practice effects) |
| Repetitive Transcranial Magnetic Stimulation | Mixed reports, with most studies reporting no impairments, but some studies finding mild reductions in sustained attention, spatial planning, and verbal retention; possible improvements in global cognitive awareness, manual motor speed, simple reaction time, verbal learning, attention, processing speed, verbal fluency, autobiographical memory, visual learning, working memory, and executive functioning |
|
| |
| Magnetic Seizure Therapy | Minimal retrograde amnesia, minimal anterograde amnesia, rapid postictal reorientation |
|
| |
| Transcranial Direct Current Stimulation | No neurocognitive impairment in psychomotor speed, working memory, attention, recognition memory, or executive functioning; possible improvement in working memory |
|
| |
| Vagus Nerve Stimulation | No neurocognitive impairment in attention, psychomotor speed, verbal fluency, memory, or executive functioning; possible improvement in psychomotor speed, language, and executive functioning (potentially associated with mood improvement) |
|
| |
| Deep Brain Stimulation | |
| Subcallosal cingulate white matter (SCCwm) | No neurocognitive impairment in verbal IQ, attention, psychomotor speed, risk taking, memory, or executive functioning; possible improvement in verbal and visual memory, manual motor speed, and verbal learning in patients performing below average at baseline (not apparently associated with mood improvement) |
| Ventral capsule/ventral striatum (VC/VS) | No neurocognitive impairment in general intellectual ability, language, processing speed, executive functioning, learning, or memory; possible improvement in verbal learning (not apparently associated with mood improvement) |
| Nucleus Accumbens (NAc) | No neurocognitive impairment in general intellectual ability, language, processing speed, executive functioning, learning, or memory |
| Inferior thalamic peduncle (ITP) | No changes in visual attention, visuoconstructive perception, verbal fluency or abstraction; possible improvements in manual praxis and verbal/nonverbal memory |
| Lateral Habenula (LHb) | No data available |
Acknowledgements and Disclosures
This work was supported in part by the Summer Undergraduate Research Experience (SURE) at Emory program (JLM), KL2 RR024983 (SMM; PI: Milton Packer) and K23 MH077869 (PEH). JLM has received grant funding from Emory College's Scholarly Inquiry and Research at Emory (SIRE) program. SMM has received grant funding from NARSAD, National Institutes of Mental Health (NIMH), and the National Center for Research Resources (NCRR). PEH has received grant funding from the Dana Foundation, Greenwall Foundation, NARSAD, National Institute of Mental Health, National Institutes of Health Loan Repayment Program, Neuronetics, Northstar, Stanley Medical Research Institute, and Woodruff Foundation; he is or has been a consultant for AvaCat Consulting, AstraZeneca, Oppenheimer & Co., St. Jude Medical Neuromodulation, Shaw Science, Tetragenex; he has received honoraria from CME Outfitters, Inc. (Cyberonics), CME LLC, Inc. (Bristol-Myers Squibb), and Letters and Sciences (Bristol-Myers Squibb).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 2.Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;23(354):1231–42. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–52. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 4.Nemeroff CB. Prevalence and Management of Treatment-Resistant Depression. J Clin Psychiatry. 2007;68:17–25. [PubMed] [Google Scholar]
- 5.Crown WH, Finkelstein S, Berndt ER, et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63:963–71. doi: 10.4088/jcp.v63n1102. [DOI] [PubMed] [Google Scholar]
- 6.Rasimas JJ, Stevens SR, Rasmussen KG. Seizure length in electroconvulsive therapy as a function of age, sex, and treatment number. J ECT. 2007;23:14–6. doi: 10.1097/01.yct.0000263254.21668.f0. [DOI] [PubMed] [Google Scholar]
- 7.Kho KH, van Vreeswijk MF, Simpson S, Zwinderman AH. A meta-analysis of electroconvulsive therapy efficacy in depression. J ECT. 2003;19:139–47. doi: 10.1097/00124509-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Sackeim HA, Prudic J, Nobler MS, et al. Effects of Pulse Width and Electrode Placement on the Efficacy and Cognitive Effects of Electroconvulsive Therapy. Brain Stimulat. 2008;1:71–83. doi: 10.1016/j.brs.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loo CK, Sainsbury K, Sheehan P, Lyndon B. A comparison of RUL ultrabrief pulse (0.3 ms) ECT and standard RUL ECT. Int J Neuropsychopharmacol. 2008;11:883–90. doi: 10.1017/S1461145708009292. [DOI] [PubMed] [Google Scholar]
- 10.Sienaert P, Vansteelandt K, Demyttenaere K, Peuskens J. Randomized comparison of ultra-brief bifrontal and unilateral electroconvulsive therapy for major depression: clinical efficacy. J Affect Disord. 2009;116:106–12. doi: 10.1016/j.jad.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Sackeim HA, Prudic J, Fuller R, et al. The Cognitive Effects of Electroconvulsive Therapy in Community Settings. Neuropsychopharmacology. 2007;32:244–54. doi: 10.1038/sj.npp.1301180. [DOI] [PubMed] [Google Scholar]
- 12.Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT. 2000;16:87–96. doi: 10.1097/00124509-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Donahue AB. Electroconvulsive therapy and memory loss: a personal journey. J ECT. 2000;16:133–43. doi: 10.1097/00124509-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Falconer DW, Cleland J, Fielding S, Reid IC. Using the Cambridge Neuropsychological Test Automated Battery (CANTAB) to assess the cognitive impact of electroconvulsive therapy on visual and visuospatial memory. Psychol Med. 2009;24:1–9. doi: 10.1017/S0033291709991243. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor M, Lebowitz BK, Ly J, et al. A dissociation between anterograde and retrograde amnesia after treatment with electroconvulsive therapy: a naturalistic investigation. J ECT. 2008;24:146–51. doi: 10.1097/YCT.0b013e318158792f. [DOI] [PubMed] [Google Scholar]
- 16.Zervas IM, Calev A, Jandorf L, et al. Age-Dependent Effects of Electroconvulsive Therapy on Memory. Convuls Ther. 1993;9:39–42. [PubMed] [Google Scholar]
- 17.Legendre SA, Stern RA, Solomon DA, Furman MJ, Smith KE. The influence of cognitive reserve on memory following electroconvulsive therapy. J Neuropsychiatry Clin Neurosci. 2003;15:333–9. doi: 10.1176/jnp.15.3.333. [DOI] [PubMed] [Google Scholar]
- 18.Dolenc TJ, Rasmussen KG. The safety of electroconvulsive therapy and lithium in combination: a case series and review of the literature. J ECT. 2005;21:165–70. doi: 10.1097/01.yct.0000174383.96517.77. [DOI] [PubMed] [Google Scholar]
- 19.Sackeim HA, Dillingham EM, Prudic J, et al. Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes: short-term efficacy and adverse effects. Arch Gen Psychiatry. 2009;66:729–37. doi: 10.1001/archgenpsychiatry.2009.75. [DOI] [PubMed] [Google Scholar]
- 20.Weiner RD, Coffey CE. Comparison of Brief-Pulse and Sine Wave ECT Stimuli. Convuls Ther. 1989;5:184–5. [PubMed] [Google Scholar]
- 21.Squire LR, Zouzounis JA. ECT and memory: brief pulse versus sine wave. Am J Psychiatry. 1986;143:596–601. doi: 10.1176/ajp.143.5.596. [DOI] [PubMed] [Google Scholar]
- 22.Fujita A, Nakaaki S, Segawa K, et al. Memory, attention, and executive functions before and after sine and pulse wave electroconvulsive therapies for treatment-resistant major depression. J ECT. 2006;22:107–12. doi: 10.1097/00124509-200606000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Coffey CE, Lucke J, Weiner RD, Krystal AD, Aque M. Seizure threshold in electroconvulsive therapy (ECT) II. The anticonvulsant effect of ECT. Biol Psychiatry. 1995;37:777–88. doi: 10.1016/0006-3223(95)00053-J. [DOI] [PubMed] [Google Scholar]
- 24.Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993;328:839–46. doi: 10.1056/NEJM199303253281204. [DOI] [PubMed] [Google Scholar]
- 25.Crowley K, Pickle J, Dale R, Fattal O. A critical examination of bifrontal electroconvulsive therapy: clinical efficacy, cognitive side effects, and directions for future research. J ECT. 2008;24:268–71. doi: 10.1097/YCT.0b013e318168e72c. [DOI] [PubMed] [Google Scholar]
- 26.Bakewell CJ, Russo J, Tanner C, Avery DH, Neumaier JF. Comparison of clinical efficacy and side effects for bitemporal and bifrontal electrode placement in electroconvulsive therapy. J ECT. 2004;20:145–53. doi: 10.1097/00124509-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Ranjkesh F, Barekatain M, Akuchakian S. Bifrontal versus right unilateral and bitemporal electroconvulsive therapy in major depressive disorder. J ECT. 2005;21:207–10. doi: 10.1097/01.yct.0000187041.79087.59. [DOI] [PubMed] [Google Scholar]
- 28.Abosch A, Cosgrove GR. Biological basis for the surgical treatment of depression. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC/2008/25/7/E2. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg BD, Price LH, Rauch SL, et al. Neurosurgery for intractable obsessive-compulsive disorder and depression: critical issues. Neurosurg Clin N Am. 2003;14:199–212. doi: 10.1016/s1042-3680(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 30.Ridout N, O'Carroll RE, Dritschel B, et al. Emotion recognition from dynamic emotional displays following anterior cingulotomy and anterior capsulotomy for chronic depression. Neuropsychologia. 2007;45:1735–43. doi: 10.1016/j.neuropsychologia.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Feldman RP, Alterman RL, Goodrich JT. Contemporary psychosurgery and a look to the future. J Neurosurg. 2001;95:944–56. doi: 10.3171/jns.2001.95.6.0944. [DOI] [PubMed] [Google Scholar]
- 32.Ruck C, Karlsson A, Steele JD, et al. Capsulotomy for obsessive-compulsive disorder: long-term follow-up of 25 patients. Arch Gen Psychiatry. 2008;65:914–21. doi: 10.1001/archpsyc.65.8.914. [DOI] [PubMed] [Google Scholar]
- 33.Ruck C, Andreewitch S, Flyckt K, et al. Capsulotomy for refractory anxiety disorders: long-term follow-up of 26 patients. Am J Psychiatry. 2003;160:513–21. doi: 10.1176/appi.ajp.160.3.513. [DOI] [PubMed] [Google Scholar]
- 34.Cosgrove GR, Rauch SL. Stereotactic cingulotomy. Neurosurg Clin N Am. 2003;14:225–35. doi: 10.1016/s1042-3680(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 35.Ochsner KN, Kosslyn SM, Cosgrove GR, et al. Deficits in visual cognition and attention following bilateral anterior cingulotomy. Neuropsychologia. 2001;39:219–30. doi: 10.1016/s0028-3932(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 36.Steele JD, Christmas D, Eljamel MS, Matthews K. Anterior cingulotomy for major depression: clinical outcome and relationship to lesion characteristics. Biol Psychiatry. 2008;63:670–7. doi: 10.1016/j.biopsych.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Knight G. Stereotactic tractotomy in the surgical treatment of mental illness. J Neurol Neurosurg Psychiatry. 1965;28:304–10. doi: 10.1136/jnnp.28.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kartsounis LD, Poynton A, Bridges PK, Bartlett JR. Neuropsychological correlates of stereotactic subcaudate tractotomy. A prospective study. Brain. 1991;114:2657–73. doi: 10.1093/brain/114.6.2657. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell-Heggs N, Kelly D, Richardson A. Stereotactic limbic leucotomy--a follow-up at 16 months. Br J Psychiatry. 1976;128:226–40. doi: 10.1192/bjp.128.3.226. [DOI] [PubMed] [Google Scholar]
- 40.Holtzheimer PE, 3rd, Russo J, Avery DH. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull. 2001;35:149–69. [PubMed] [Google Scholar]
- 41.Kozel FA, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract. 2002;8:270–5. doi: 10.1097/00131746-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Martin JL, Barbanoj MJ, Schlaepfer TE, et al. Repetitive transcranial magnetic stimulation for the treatment of depression. Systematic review and meta-analysis. Br J Psychiatry. 2003;182:480–91. doi: 10.1192/bjp.182.6.480. [DOI] [PubMed] [Google Scholar]
- 43.Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol. 2002;5:73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- 44.O'Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–16. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 45.Fitzgerald PB, Hoy K, Daskalakis ZJ, Kulkarni J. A randomized trial of the antidepressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26:229–34. doi: 10.1002/da.20454. [DOI] [PubMed] [Google Scholar]
- 46.Klein E, Kreinin I, Chistyakov A, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry. 1999;56:315–20. doi: 10.1001/archpsyc.56.4.315. [DOI] [PubMed] [Google Scholar]
- 47.Fitzgerald PB, Brown TL, Marston NA, et al. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60:1002–8. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- 48.Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry. 2000;47:314–24. doi: 10.1016/s0006-3223(99)00254-1. [DOI] [PubMed] [Google Scholar]
- 49.Grunhaus L, Schreiber S, Dolberg OT, Polak D, Dannon PN. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry. 2003;53:324–31. doi: 10.1016/s0006-3223(02)01499-3. [DOI] [PubMed] [Google Scholar]
- 50.Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry. 2002;51:659–67. doi: 10.1016/s0006-3223(01)01354-3. [DOI] [PubMed] [Google Scholar]
- 51.Eranti S, Mogg A, Pluck G, et al. A randomized, controlled trial with 6-month follow-up of repetitive transcranial magnetic stimulation and electroconvulsive therapy for severe depression. Am J Psychiatry. 2007;164:73–81. doi: 10.1176/ajp.2007.164.1.73. [DOI] [PubMed] [Google Scholar]
- 52.Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34:522–34. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 53.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 55.Hausmann A, Pascual-Leone A, Kemmler G, et al. No deterioration of cognitive performance in an aggressive unilateral and bilateral antidepressant rTMS add-on trial. J Clin Psychiatry. 2004;65:772–82. doi: 10.4088/jcp.v65n0608. [DOI] [PubMed] [Google Scholar]
- 56.Avery DH, Claypoole K, Robinson L, et al. Repetitive transcranial magnetic stimulation in the treatment of medication-resistant depression: preliminary data. J Nerv Ment Dis. 1999;187:114–7. doi: 10.1097/00005053-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 57.George MS, Nahas Z, Molloy M, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry. 2000;48:962–70. doi: 10.1016/s0006-3223(00)01048-9. [DOI] [PubMed] [Google Scholar]
- 58.Loo CK, Sachdev P, Elsayed H, et al. Effects of a 2- to 4-Week Course of Repetitive Transcranial Magnetic Stimulation (rTMS) on Neuropsychological Functioning, Electroencephalogram, and Auditory Threshold in Depressed Patients. Biol Psychiatry. 2001;49:615–23. doi: 10.1016/s0006-3223(00)00996-3. [DOI] [PubMed] [Google Scholar]
- 59.Manes F, Jorge R, Morcuende M, et al. A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderly. Int Psychogeriatr. 2001;13:225–31. doi: 10.1017/s1041610201007608. [DOI] [PubMed] [Google Scholar]
- 60.Holtzheimer PE, 3rd, Russo J, Claypoole KH, Roy-Byrne P, Avery DH. Shorter duration of depressive episode may predict response to repetitive transcranial magnetic stimulation. Depress Anxiety. 2004;19:24–30. doi: 10.1002/da.10147. [DOI] [PubMed] [Google Scholar]
- 61.Loo CK, Mitchell PB, McFarquhar TF, Malhi GS, Sachdev PS. A sham-controlled trial of the efficacy and safety of twice-daily rTMS in major depression. Psychol Med. 2007;37:341–9. doi: 10.1017/S0033291706009597. [DOI] [PubMed] [Google Scholar]
- 62.Loo CK, Mitchell PB, Croker VM, et al. Double-blind controlled investigation of bilateral prefrontal transcranial magnetic stimulation for the treatment of resistant major depression. Psychol Med. 2003;33:33–40. doi: 10.1017/s0033291702006839. [DOI] [PubMed] [Google Scholar]
- 63.O'Connor M, Brenninkmeyer C, Morgan A, et al. Relative effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy on mood and memory: a neurocognitive risk-benefit analysis. Cogn Behav Neurol. 2003;16:118–27. doi: 10.1097/00146965-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Schulze-Rauschenbach SC, Harms U, Schlaepfer TE, et al. Distinctive neurocognitive effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in major depression. Br J Psychiatry. 2005;186:410–6. doi: 10.1192/bjp.186.5.410. [DOI] [PubMed] [Google Scholar]
- 65.Padberg F, Zwanzger P, Thoma H, et al. Repetitive transcranial magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression: comparative study of fast, slow and sham rTMS. Psychiatry Res. 1999;88:163–71. doi: 10.1016/s0165-1781(99)00092-x. [DOI] [PubMed] [Google Scholar]
- 66.Fitzgerald PB, Benitez J, de Castella A, et al. A randomized, controlled trial of sequential bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression. Am J Psychiatry. 2006;163:88–94. doi: 10.1176/appi.ajp.163.1.88. [DOI] [PubMed] [Google Scholar]
- 67.Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125–32. doi: 10.1016/s1388-2457(03)00046-4. [DOI] [PubMed] [Google Scholar]
- 68.Mosimann UP, Schmitt W, Greenberg BD, et al. Repetitive transcranial magnetic stimulation: a putative add-on treatment for major depression in elderly patients. Psychiatry Res. 2004;126:123–33. doi: 10.1016/j.psychres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Kosel M, Frick C, Lisanby SH, Fisch HU, Schlaepfer TE. Magnetic seizure therapy improves mood in refractory major depression. Neuropsychopharmacology. 2003;28:2045–8. doi: 10.1038/sj.npp.1300293. [DOI] [PubMed] [Google Scholar]
- 70.Lisanby SH, Schlaepfer TE, Fisch HU, Sackeim HA. Magnetic seizure therapy of major depression. Arch Gen Psychiatry. 2001;58:303–5. doi: 10.1001/archpsyc.58.3.303. [DOI] [PubMed] [Google Scholar]
- 71.Lisanby SH, Morales O, Payne N, et al. New developments in electroconvulsive therapy and magnetic seizure therapy. CNS Spectr. 2003;8:529–36. doi: 10.1017/s1092852900019003. [DOI] [PubMed] [Google Scholar]
- 72.White PF, Amos Q, Zhang Y, et al. Anesthetic considerations for magnetic seizure therapy: a novel therapy for severe depression. Anesth Analg. 2006;103:76–80. doi: 10.1213/01.ane.0000221182.71648.a3. [DOI] [PubMed] [Google Scholar]
- 73.Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA. Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology. 2003;28:1852–65. doi: 10.1038/sj.npp.1300229. [DOI] [PubMed] [Google Scholar]
- 74.Moscrip TD, Terrace HS, Sackeim HA, Lisanby SH. A primate model of anterograde and retrograde amnesia produced by convulsive treatment. J ECT. 2004;20:26–36. doi: 10.1097/00124509-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 75.Moscrip TD, Terrace HS, Sackeim HA, Lisanby SH. Randomized controlled trial of the cognitive side-effects of magnetic seizure therapy (MST) and electroconvulsive shock (ECS) Int J Neuropsychopharmacol. 2006;9:1–11. doi: 10.1017/S146114570500578X. [DOI] [PubMed] [Google Scholar]
- 76.Spellman T, McClintock SM, Terrace H, et al. Differential effects of high-dose magnetic seizure therapy and electroconvulsive shock on cognitive function. Biol Psychiatry. 2008;63:1163–70. doi: 10.1016/j.biopsych.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McClintock SM, Cullum CM, Husain MM, Luber B, Lisanby SH. Rapid recovery of orientation following magnetic seizure therapy. Journal of the International Neuropsychological Society. 2008;14(Supplement 1):141. [Google Scholar]
- 78.Kirov G, Ebmeier KP, Scott AI, et al. Quick recovery of orientation after magnetic seizure therapy for major depressive disorder. Br J Psychiatry. 2008;193:152–5. doi: 10.1192/bjp.bp.107.044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nitsche MA, Boggio PS, Fregni F, Pascual-Leone A. Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol. 2009;219:14–9. doi: 10.1016/j.expneurol.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 81.Fregni F, Boggio PS, Nitsche MA, et al. Treatment of major depression with transcranial direct current stimulation. Bipolar Disord. 2006;8:203–4. doi: 10.1111/j.1399-5618.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 82.Boggio PS, Rigonatti SP, Ribeiro RB, et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol. 2008;11:249–54. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rigonatti SP, Boggio PS, Myczkowski ML, et al. Transcranial direct stimulation and fluoxetine for the treatment of depression. Eur Psychiatry. 2008;23:74–6. doi: 10.1016/j.eurpsy.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 85.Fregni F, Boggio PS, Nitsche MA, Rigonatti SP, Pascual-Leone A. Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depress Anxiety. 2006;23:482–4. doi: 10.1002/da.20201. [DOI] [PubMed] [Google Scholar]
- 86.Fregni F, Boggio PS, Nitsche M, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 87.Boggio PS, Ferrucci R, Rigonatti SP, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson's disease. J Neurol Sci. 2006;249:31–8. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 88.Ferrucci R, Mameli F, Guidi I, et al. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology. 2008;71:493–8. doi: 10.1212/01.wnl.0000317060.43722.a3. [DOI] [PubMed] [Google Scholar]
- 89.Ferrucci R, Bortolomasi M, Vergari M, et al. Transcranial direct current stimulation in severe, drug-resistant major depression. J Affect Disord. 2009;118:215–9. doi: 10.1016/j.jad.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 90.George MS, Sackeim HA, Marangell LB, et al. Vagus nerve stimulation. A potential therapy for resistant depression? Psychiatr Clin North Am. 2000;23:757–83. doi: 10.1016/s0193-953x(05)70196-9. [DOI] [PubMed] [Google Scholar]
- 91.Nahas Z, Marangell LB, Husain MM, et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J Clin Psychiatry. 2005;66:1097–104. doi: 10.4088/jcp.v66n0902. [DOI] [PubMed] [Google Scholar]
- 92.Rush AJ, Sackeim HA, Marangell LB, et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry. 2005;58:355–63. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 93.George MS, Rush AJ, Marangell LB, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry. 2005;58:364–73. doi: 10.1016/j.biopsych.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 94.Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry. 2002;51:280–7. doi: 10.1016/s0006-3223(01)01343-9. [DOI] [PubMed] [Google Scholar]
- 95.Sackeim HA, Keilp JG, Rush AJ, et al. The Effects of Vagus Nerve Stimulation on Cognitive Performance in Patients with Treatment-Resistant Depression. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:53–62. [PubMed] [Google Scholar]
- 96.Helmstaedter C, Hoppe C, Elger CE. Memory alterations during acute high-intensity vagus nerve stimulation. Epilepsy Res. 2001;47:37–42. doi: 10.1016/s0920-1211(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 97.Hoppe C, Helmstaedter C, Scherrmann J, Elger CE. No Evidence for Cognitive Side Effects after 6 Months of Vagus Nerve Stimulation in Epilepsy Patients. Epilepsy Behav. 2001;2:351–6. doi: 10.1006/ebeh.2001.0219. [DOI] [PubMed] [Google Scholar]
- 98.Dodrill CB, Morris GL. Effects of Vagal Nerve Stimulation on Cognition and Quality of Life in Epilepsy. Epilepsy Behav. 2001;2:46–53. doi: 10.1006/ebeh.2000.0148. [DOI] [PubMed] [Google Scholar]
- 99.Rezai AR, Machado AG, Deogaonkar M, et al. Surgery for movement disorders. Neurosurgery. 2008;62(Suppl 2):809–38. doi: 10.1227/01.neu.0000316285.52865.53. [DOI] [PubMed] [Google Scholar]
- 100.McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–48. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 101.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–9. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Voon V, Kubu C, Krack P, Houeto JL, Troster AI. Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord. 2006;21(Suppl 14):S305–27. doi: 10.1002/mds.20963. [DOI] [PubMed] [Google Scholar]
- 103.York MK, Wilde EA, Simpson R, Jankovic J. Relationship between neuropsychological outcome and DBS surgical trajectory and electrode location. J Neurol Sci. 2009;287:159–71. doi: 10.1016/j.jns.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 105.Lozano AM, Mayberg HS, Giacobbe P, et al. Subcallosal Cingulate Gyrus Deep Brain Stimulation for Treatment-Resistant Depression. Biol Psychiatry. 2008;64:461–7. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 106.Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–93. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 107.Malone DA, Jr., Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–75. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus Accumbens Deep Brain Stimulation Decreases Ratings of Depression and Anxiety in Treatment-Resistant Depression. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.09.013. In press. [DOI] [PubMed] [Google Scholar]
- 109.McNeely HE, Mayberg HS, Lozano AM, Kennedy SH. Neuropsychological Impact of Cg25 Deep Brain Stimulation for Treatment-Resistant Depression: Preliminary Results Over 12 Months. J Nerv Ment Dis. 2008;196:405–10. doi: 10.1097/NMD.0b013e3181710927. [DOI] [PubMed] [Google Scholar]
- 110.Jimenez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57:585–93. doi: 10.1227/01.neu.0000170434.44335.19. [DOI] [PubMed] [Google Scholar]
- 111.Sartorius A, Kiening KL, Kirsch P, et al. Remission of Major Depression Under Deep Brain Stimulation of the Lateral Habenula in a Therapy-Refractory Patient. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.08.027. In press. [DOI] [PubMed] [Google Scholar]
- 112.Lecourtier L, Kelly PH. Bilateral lesions of the habenula induce attentional disturbances in rats. Neuropsychopharmacology. 2005;30:484–96. doi: 10.1038/sj.npp.1300595. [DOI] [PubMed] [Google Scholar]
- 113.Lecourtier L, Neijt HC, Kelly PH. Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. Eur J Neurosci. 2004;19:2551–60. doi: 10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- 114.Basso MR, Carona FD, Lowery N, Axelrod BN. Practice effects on the WAIS-III across 3- and 6-month intervals. Clin Neuropsychol. 2002;16:57–63. doi: 10.1076/clin.16.1.57.8329. [DOI] [PubMed] [Google Scholar]
- 115.Beglinger LJ, Gaydos B, Tangphao-Daniels O, et al. Practice effects and the use of alternate forms in serial neuropsychological testing. Arch Clin Neuropsychol. 2005;20:517–29. doi: 10.1016/j.acn.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 116.McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology. 2010 doi: 10.1037/a0017336. In press. [DOI] [PubMed] [Google Scholar]
- 117.Majer M, Ising M, Kunzel H, et al. Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychol Med. 2004;34:1453–63. doi: 10.1017/s0033291704002697. [DOI] [PubMed] [Google Scholar]
- 118.Wang CE, Halvorsen M, Sundet K, et al. Verbal memory performance of mildly to moderately depressed outpatient younger adults. J Affect Disord. 2006;92:283–6. doi: 10.1016/j.jad.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 119.Landro NI, Stiles TC, Sletvold H. Neuropsychological function in nonpsychotic unipolar major depression. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:233–40. [PubMed] [Google Scholar]
- 120.Langenecker SA, Kennedy SE, Guidotti LM, et al. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol Psychiatry. 2007;62:1272–80. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Okada G, Okamoto Y, Morinobu S, Yamawaki S, Yokota N. Attenuated Left Prefrontal Activation during a Verbal Fluency Task in Patients with Depression. Neuropsychobiology. 2003;47:21–6. doi: 10.1159/000068871. [DOI] [PubMed] [Google Scholar]
- 122.Grant MM, Thase ME, Sweeney JA. Cognitive Disturbance in Outpatient Depressed Younger Adults: Evidence of Modest Impairment. Biol Psychiatry. 2001;50:35–43. doi: 10.1016/s0006-3223(00)01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harvey PO, Le Bastard G, Pochon JB, et al. Executive functions and updating of the contents of working memory in unipolar depression. J Psychiatr Res. 2004;38:567–76. doi: 10.1016/j.jpsychires.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 124.Michopoulos I, Zervas IM, Pantelis C, et al. Neuropsychological and hypothalamic-pituitary-axis function in female patients with melancholic and nonmelancholic depression. Eur Arch Psychiatry Clin Neurosci. 2008;258:217–25. doi: 10.1007/s00406-007-0781-8. [DOI] [PubMed] [Google Scholar]
- 125.Fitzgerald PB, Srithiran A, Benitez J, et al. An fMRI Study of Prefrontal Brain Activation During Multiple Tasks in Patients With Major Depressive Disorder. Hum Brain Mapp. 2008;29:490–501. doi: 10.1002/hbm.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.O'Brien JT, Sahakian BJ, Checkley SA. Cognitive impairments in patients with seasonal affective disorder. Br J Psychiatry. 1993;163:338–43. doi: 10.1192/bjp.163.3.338. [DOI] [PubMed] [Google Scholar]
- 127.Moffoot AP, O'Carroll RE, Bennie J, et al. Diurnal variation of mood and neuropsychological function in major depression with melancholia. J Affect Disord. 1994;32:257–69. doi: 10.1016/0165-0327(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 128.Herrera-Guzman I, Gudayol-Ferre E, Lira-Mandujano J, et al. Cognitive predictors of treatment response to bupropion and cognitive effects of bupropion in patients with major depressive disorder. Psychiatry Res. 2008;160:72–82. doi: 10.1016/j.psychres.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 129.Mandelli L, Serretti A, Colombo C, et al. Improvement of cognitive functioning in mood disorder patients with depressive symptomatic recovery during treatment: an exploratory analysis. Psychiatry Clin Neurosci. 2006;60:598–604. doi: 10.1111/j.1440-1819.2006.01564.x. [DOI] [PubMed] [Google Scholar]
- 130.Castaneda AE, Suvisaari J, Marttunen M, et al. Cognitive functioning in a population-based sample of young adults with a history of non-psychotic unipolar depressive disorders without psychiatric comorbidity. J Affect Disord. 2008;110:36–45. doi: 10.1016/j.jad.2007.12.239. [DOI] [PubMed] [Google Scholar]
- 131.Weiland-Fiedler P, Erickson K, Waldeck T, et al. Evidence for continuing neuropsychological impairments in depression. J Affect Disord. 2004;82:253–8. doi: 10.1016/j.jad.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 132.Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord. 2005;89:125–35. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 133.Hammar A, Lund A, Hugdahl K. Long-lasting cognitive impairment in unipolar major depression: a 6-month follow-up study. Psychiatry Res. 2003;118:189–96. doi: 10.1016/s0165-1781(03)00075-1. [DOI] [PubMed] [Google Scholar]