Abstract
Background
Aspergillus species are ubiquitous. We hypothesized that climatic variables that affect airborne mold counts affect the incidence of invasive aspergillosis (IA).
Methods
Patients who received hematopoietic stem cell transplants (HSCTs) in geographically and climatically diverse regions (Seattle, WA, and Houston, TX) were examined. Cumulative incidence function, Kaplan-Meier analysis, and Cox proportional hazards regression were performed to examine the association between IA and season. Poisson regression analysis was performed to evaluate the seasonal patterns in IA rates and association with spore counts and climate.
Results
In Seattle, the 3-month incidence of IA was 4.6% (5.7% in allograft recipients and 0.8% in autograft recipients). During the 10-year study period, there was a decrease in the incidence of IA among allogeneic HSCT recipients, corresponding to decreased risks during the nonsummer months; receipt of HSCTs during the summer months was associated with an increased hazard for IA (hazard ratio, 1.87; 95% confidence interval, 1.25–2.81) after adjustment for other known risks. The person-month IA rate in Seattle was positively associated with environmental spore counts, which increased with high temperature and low precipitation. No seasonal effect on IA was observed in Houston, where total spore counts were lower and not variable by climate.
Conclusions
Climatic variables differentially affect airborne spore counts and IA risk in geographically disparate centers.
Invasive aspergillosis (IA) occurs in <5% of autologous and 5%–10% of allogeneic hematopoietic stem cell transplant (HSCT) recipients [1-4]. In view of its high associated mortality rate, prevention is an important goal. Our current incomplete understanding of the precise sources and modes of acquisition of Aspergillus species limits the development of effective prevention strategies. Historically, this infection was considered to be primarily nosocomial in acquisition, largely because diagnosis was confirmed during prolonged periods of hospitalization before engraftment. In more recent years, infection has been documented to occur late after allogeneic HSCT, suggesting the potential for environmental acquisition [2, 4, 5]. Infection may be acquired by both airborne and waterborne environmental sources [6, 7]. Because Aspergillus species exhibit a high degree of genetic heterogeneity and exposure may be a nonclonal event, determining either nosocomial or environmental sources of infection has been difficult, especially in outside infection outbreak situations [8, 9]. Moreover, the required inocula and incubation period appear highly variable and difficult to define, limiting our knowledge of the natural history of infection [10].
Seasonal influences on meteorologic conditions and changes in warming patterns affect the epidemiology of bacterial, viral, and fungal infections. This largely occurs because changes in weather conditions affect the prevalence or virulence of pathogens, host behaviors, and variation in host susceptibility, with alterations in light-dark cycles and melatonin secretion [11, 12]. The epidemiology of environmental fungal infections is largely influenced by meteorologic conditions, which vary in regions of endemicity. For example, Coccidioides immitis is a geographically restricted fungus that proliferates in the environment in wet years and becomes more widely dispersed during dry years; oscillations in climate influence rates of infection in areas of exposure and human activity [13-15]. Indeed, Comrie [13] noted a bimodal disease incidence seasonality—on the basis of a variably lagged time series model—that mirrored the known bimodal annual precipitation pattern of southern Arizona. Also, the recent emergence of other pathogenic fungi, such as Cryptococcus gattii in British Columbia (Canada) and the Pacific Northwest United States, has been suggested to correspond with environmental changes, with climate warming potentially creating a new microniche conducive to organism growth and propagation [16-18]. Results of studies focused largely on allergic diseases suggest that airborne mold spore concentrations may be dependent on meteorologic conditions; however, the impact of environmental variables on the incidence of IA is not well defined and likely varies according to geography, host risks, and other factors that lead to IA infection outbreak situations, such as demolition [19-22]. We used data collected from 2 large cohorts in geographically disparate transplantation centers to evaluate the hypothesis that the incidence of IA is associated with variations in environmental acquisition and climatic variables that are likely to influence airborne spore counts.
METHODS
Environmental data
Mean temperature and total precipitation data were obtained from the National Weather Service [23]. Mean airborne mold concentrations during each month were calculated from data collected by the Northwest Asthma and Allergy Center (Seattle, WA; 1992–1998) [24] and the Houston Department of Health and Human Services (Houston, TX; 1993–2003) [25].
Cohort data
This study was approved by the Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA) institutional review board. From January 1, 1992, through December 31, 2003, a total of 4213 patients who received 1 HSCT in Seattle contributed to the analysis. Baseline demographics and host and transplant variables were analyzed as contributors to risk. Demographic information included age, sex, and race. Putative transplant-related risk factors for IA included stem cell source (bone marrow, cord blood, or peripheral blood stem cells), transplant type, type of conditioning (nonmyeloablative vs conventional myeloablative), human leukocyte antigen match and relatedness, and cytomegalovirus (CMV) serostatus. We defined a “malignancy group at high risk for IA” to include acute lymphocytic leukemia, myelodysplastic syndrome, acute nonlymphocytic leukemia, and chronic granulomatous disease. Time of transplantation was coded by date, month of year, quarter of year (first quarter, January–March; second quarter, April–June; third quarter, July–September; and fourth quarter, October–December), and season (dry, April through September; wet, October through March). Patients who developed IA after HSCT were identified by review of clinical records, microbiology and pathology records, long-term follow-up data, and infection control data; prospective surveillance contributed to case identification after 1998. Proven and probable IA were defined in accordance with the European Organisation for Research and Treatment of Cancer Mycoses Study Group criteria that were available at the time of cohort construction [26]. Patients with cutaneous or mixed mold infections were excluded.
A separate limited analysis was approved by the M. D. Anderson Cancer Center (MDACC) institutional review board. This was performed to determine whether date and season of transplantation are associated with the risk of IA at the MDACC (Houston, TX) and to compare associations among environmental variables. This analysis included 4292 patients who received 1 HSCT from January 1, 1993, through December 31, 2003. Infections were identified by review of microbiology and infection control records.
Statistical analysis
Overall cumulative IA incidence within 18 months of follow-up was examined using cumulative incidence function with the event defined as IA and the competing risk of IA-unrelated death [27]. On the basis of the patterns of weather and cumulative IA incidence rate, analyses of seasonal impact were focused on the first 3 months after HSCT. Time to IA was defined as the number of days from date of transplantation to date of IA diagnosis; patients who had IA after 3 months or remained IA free after 3 months were censored at 3 months. Time to death from any cause was calculated from date of transplantation to date of death; patients who died after 3 months or were still alive after 3 months were censored at 3 months. Person-month IA rate was calculated as the number of IA cases in the month divided by the total number of patients at risk during that month. Patients who developed IA, died, or were lost to follow-up were removed from the risk group in subsequent months.
The cumulative IA incidence within the first 3 months was estimated, taking into consideration the competing risk of IA-unrelated death within 3 months [27]. The Kaplan-Meier method and Cox regression were used to examine potential associations of 3-month IA with season (yearly quarter) at HSCT. Independent predictors of IA were identified using a stepwise model selection procedure. Adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated from the final models. Predicted HRs for the 4 seasonal categories were computed at the means of identified risk factors.
The seasonal variation of person-month IA incidence was examined using a generalized additive model with Poisson distribution. Briefly, association between IA and season was evaluated by treating seasonal effect as a nonparametric function of month. We used a cubic spline model with a smoothing parameter chosen by a generalized cross-validation method [28]. To determine the association between person-month IA rate and its corresponding monthly environmental spore count, the IA rate was modeled using the Poisson distribution [29]. In the model, patients at risk at each month served as the offset variable to normalize the fitted IA mean in the corresponding month. Correlations between monthly spore count and climatic variables were examined using the Spearman correlation coefficient.
All reported P values are 2-sided, and P < .05 was considered to be statistically significant. The analyses were performed using SAS statistical software, version 9.1 (SAS Institute).
RESULTS
FHCRC cohort analysis
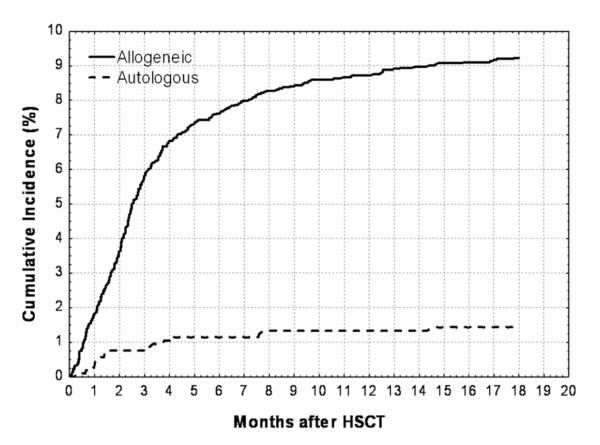
Among 4213 patients who underwent HSCT in Seattle, 290 patients developed IA within 12 months, for a cumulative annual incidence of 6.9% (8.8% in allograft recipients and 1.3% in autograft recipients). A total of 192 cases of incident IA were diagnosed within the first 3 months after transplantation, providing a 3-month cumulative incidence of 4.6% (5.7% in allograft recipients and 0.8% in autograft recipients; Figure 1).
Figure 1.
Estimated cumulative incidence of invasive aspergillosis in allogeneic and autologous hematopoietic stem cell transplant (HSCT) recipients at the Fred Hutchinson Cancer Research Center (1992–2003).
Characteristics of these patients and their associations with IA that was diagnosed in the first 3 months are given in Table 1. Risk factors for IA within 3 months are given in Table 2 as unadjusted and adjusted HRs. In these analyses, a warmer season at time of transplantation (April through September) appeared to confer a higher risk of IA. Other factors that remained important in the multivariable model included donor type and human leukocyte antigen match (autologous HSCT recipients having the lowest risk), relapsed disease at HSCT, stem cell source (peripheral blood stem cells showing a protection over either bone marrow or cord blood), and HSCT recipient CMV seropositivity. Importantly, patients who underwent transplantation during the warm, dry months (April through September) did not differ from others with respect to those factors shown to be important in either univariate or multivariable modeling; this included age, transplant type, underlying disease, CMV serostatus, and stem cell source (data not shown). Patients did not differ with regard to time until neutrophil engraftment or duration of secondary (after engraftment) cytopenias (neutrophils or lymphocytes; data not shown).
Table 1.
Characteristics of Hematopoietic Stem Cell Transplant Recipients at the Fred Hutchinson Cancer Research Center, 1992–2003
| Factor | Patients, no. (%) | Cumulative IA at 3 months, no. (% [95% CI]) |
P, log rank |
|---|---|---|---|
| Overall | 4213 (100) | 192 (4.6 [4.0–5.3]) | |
| Year of HSCT | .08 | ||
| 1992–1997 | 2248 (53.4) | 112 (5.0 [4.2–6.0]) | |
| 1998–2003 | 1965 (46.6) | 80 (4.1 [3.3–5.1]) | |
| Month of HSCT | .002 | ||
| 1–3 | 967 (23.0) | 31 (3.2 [2.3–4.5]) | |
| 4–6 | 1070 (25.4) | 59 (5.5 [4.3–7.1]) | |
| 7–9 | 1105 (26.2) | 66 (6.0 [4.7–7.6]) | |
| 10–12 | 1071 (25.4) | 36 (3.4 [2.4–4.6]) | |
| Season of transplantationa | <.001 | ||
| Cold | 2038 (48.4) | 67 (3.3 [2.6–4.2]) | |
| Warm | 2175 (51.6) | 125 (5.8 [4.9–6.8]) | |
| Race | .85 | ||
| White | 3543 (84.1) | 160 (4.5 [3.9–5.3]) | |
| Black | 80 (1.9) | 5 (6.3 [2.7–14.7]) | |
| Hispanic | 195 (4.6) | 10 (5.1 [2.8–9.4]) | |
| Other | 395 (9.3) | 19 (4.3 [2.7–6.9]) | |
| Sex | .41 | ||
| Female | 1928 (45.8) | 83 (4.3 [3.5–5.3]) | |
| Male | 2285 (54.2) | 109 (4.8 [4.0–5.7]) | |
| Age, years | .02 | ||
| <18 | 584 (13.9) | 25 (4.3 [2.9–6.3]) | |
| 18–39 | 1362 (32.3) | 46 (3.4 [2.5–4.5]) | |
| 40–59 | 1958 (46.5) | 109 (5.6 [4.6–6.7]) | |
| ≥60 | 309 (7.3) | 12 (3.9 [2.2–6.8]) | |
| Donor and HLA typeb | <.001 | ||
| Autologous | 1050 (24.9) | 8 (0.8 [0.4–1.4]) | |
| Allogeneic, HLA matched | 1371 (32.8) | 73 (5.3 [4.3–6.7]) | |
| Allogeneic, HLA mismatched or unrelated | 1762 (42.2) | 399 (6.2 [5.2–7.5]) | |
| Underlying disease risk | <.001 | ||
| High | 1785 (42.2) | 108 (6.1 [5.0–7.3]) | |
| Low | 2428 (57.6) | 84 (3.5 [2.8–4.3]) | |
| Disease status | .007 | ||
| New, chronic, accelerated | 978 (23.2) | 47 (4.8 [3.6–6.4]) | |
| Remission | 1053 (25.0) | 29 (2.8 [1.9–3.9]) | |
| Relapse | 1454 (34.5) | 77 (5.3 [4.3–6.6]) | |
| Unknown | 728 (17.3) | 39 (5.4 [4.0–7.3]) | |
| Stem cell typec | <.001 | ||
| Bone marrow | 2304 (55.5) | 136 (6.0 [5.1–7.0]) | |
| PBSC | 1812 (43.6) | 51 (2.8 [2.2–3.7]) | |
| Cord | 37 (0.9) | 4 (10.8 [4.3–27.3]) | |
| CMV serostatus (recipient/donor)d | <.001 | ||
| Positive/positive | 1466 (34.9) | 64 (4.4 [3.4–5.6]) | |
| Positive/negative | 751 (17.9) | 53 (7.1 [5.4–9.2]) | |
| Negative/positive | 464 (11.1) | 23 (5.0 [3.3–7.4]) | |
| Negative/negative | 1516 (36.1) | 51 (3.4 [2.6–4.4]) |
NOTE. CI, confidence interval; CMV, cytomegalovirus; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplantation; IA, invasive aspergillosis; PBSC, peripheral blood stem cell.
Cold months defined as 1–3 and 10–12; warm months are 4–9.
Thirty patients with unknown HLA match are not listed.
Sixty patients with unknown stem cell source are not listed.
Sixteen patients with unknown serostatus are not listed.
Table 2.
Factors Associated with 3-Month Invasive Aspergillosis at the Fred Hutchinson Cancer Research Center, 1992–2003
| Unadjusted HR |
Adjusted HR |
|||
|---|---|---|---|---|
| Factor | Mean (95% CI) | P | Mean (95% CI) | P |
| Year of HSCT | .08 | |||
| 1992–1997 | 1.29 (0.97–1.72) | |||
| 1998–2003 | Reference | |||
| Month of transplantation | ||||
| 1–3 | 0.98 (0.61–1.58) | .91 | 1.03 (0.64–1.66) | .89 |
| 4–6 | 1.69 (1.12–2.56) | .01 | 1.67 (1.1–2.53) | .02 |
| 7–9 | 1.82 (1.22–2.74) | .004 | 1.87 (1.25–2.81) | .003 |
| 10–12 | Reference | Reference | ||
| Age, years | ||||
| <18 | 1.12 (0.56–2.23) | .75 | ||
| 18–39 | 0.89 (0.46–1.64) | .66 | ||
| 40–59 | 1.49 (0.8–2.7) | .19 | ||
| ≥60 | Reference | |||
| Donor and HLA type | ||||
| Autologous | Reference | Reference | ||
| Allogeneic, HLA matched | 7.26 (3.50–15.1) | <.001 | 7.07 (3.3–15.1) | <.001 |
| Allogeneic, HLA mismatched or unrelated | 8.93 (4.36–18.1) | <.001 | 9.20 (4.26–19.9) | <.001 |
| Underlying disease risk | ||||
| High | 1.87 (1.41–2.49) | <.001 | ||
| Low | Reference | |||
| Disease status | ||||
| New, chronic, accelerated | Reference | Reference | ||
| Remission | 0.58 (0.37–0.93) | .02 | 0.78 (0.48–1.26) | .30 |
| Relapse | 1.20 (0.83–1.72) | .34 | 2.27(1.55–3.33) | <.001 |
| Unknown | 1.27 (0.77–1.79) | .47 | 1.66 (1.08–2.56) | .02 |
| Stem cell type | ||||
| PBSC | Reference | Reference | ||
| Bone marrow (or bone marrow plus PBSC) | 2.22 (1.62–3.06) | <.001 | 1.46 (1.04–2.08) | .02 |
| Cord | 5.04 (1.82–13.9) | .002 | 3.15 (0.96–10.3) | .06 |
| CMV serostatus (recipient/donor) | ||||
| Positive/positive | 1.33 (0.92–1.92) | .13 | 1.56 (1.07–2.27) | .02 |
| Positive/negative | 2.23 (1.52–3.27) | <.001 | 1.53 (1.0–2.26) | .03 |
| Negative/positive | 1.53 (0.93–2.50) | .09 | 1.15 (0.70–1.99) | .59 |
| Negative/negative | Reference | Reference | ||
NOTE. CI, confidence interval; CMV, cytomegalovirus; HLA, human leukocyte antigen; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; PBSC, peripheral blood stem cell.
The percentage of patients free of IA at 3 months after HSCT is given in Table 3, according to month and type of HSCT. The seasonal difference in IA was predominantly observed in patients who received allogeneic HSCTs and not patients who received autologous HSCTs, who had relatively lower rates of IA.
Table 3.
Patients Free of Invasive Aspergillosis and Alive at 3 Months After Hematopoietic Stem Cell Transplantation, According to Month and Type of Transplant at the Fred Hutchinson Cancer Research Center, 1992–2003
| Season of transplantation |
No. (%) of patients (n = 4213) |
||
|---|---|---|---|
| Overall | Allogeneic | Autologous | |
| Overall | 4021 (95.4) | 2950 (94.2) | 1042 (99.2) |
| Month 1–3 | 936 (96.7) | 671 (95.7) | 255 (99.6) |
| Month 4–6 | 1011 (94.5) | 757 (93.3) | 249 (98.0) |
| Month 7–9 | 1039 (94.0) | 753 (92.2) | 283 (99.7) |
| Month 10–12 | 1035 (96.6) | 769 (95.7) | 255 (99.6) |
| P a | .002 | .004 | .09 |
Log rank, testing seasonal effects.
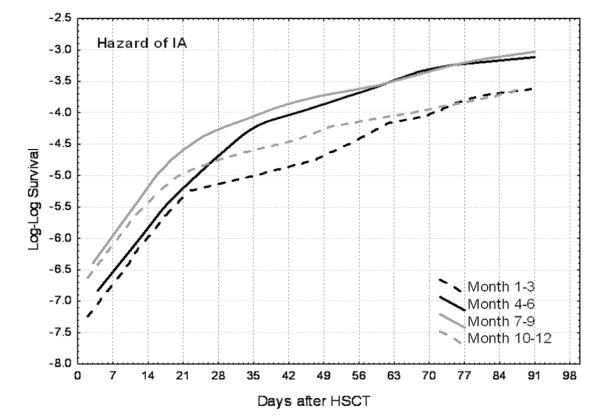
Baseline hazards of IA were evaluated at the means of known risks and plotted according to season (Figure 2). All curves appear to increase at a decreasing rate (slope), suggesting a nonconstant hazard. During the first 3 weeks, the hazard of IA was high, with a sharp slope, and it had a similar pattern in all seasons. After these early weeks, the slopes decreased in months 1–3 and 10–12 but continued to increase at a relatively sharper slope during the summer months (months 4–6 and 7–9). Thus, the higher risks during the summer months were largely due to sustained high risks relatively later after HSCT, corresponding with the period that most patients are cared for outside the hospital.
Figure 2.
Hazard of invasive aspergillosis (IA) in the first 3 months after hematopoietic stem cell transplantation (HSCT) at the Fred Hutchinson Cancer Research Center (1992–2003) according to month of transplantation (1 indicates January and 12 indicates December).
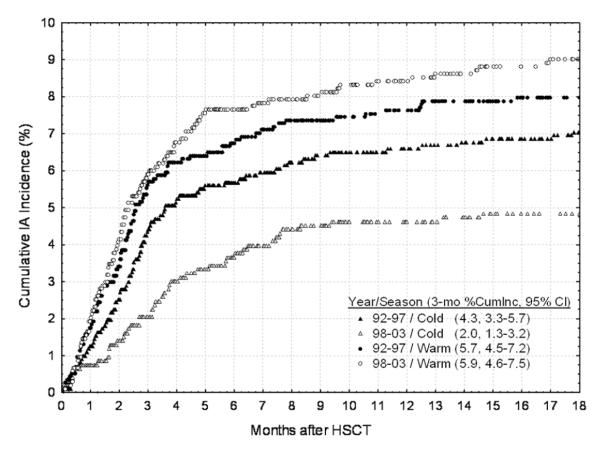
We noted that the 3-month cumulative incidence of IA among allogeneic HSCT recipients decreased in patients who received allogeneic HSCT in the later years (1998–2003), compared with the corresponding incidence in the earlier years (1992–1997), with curves that separated beyond 2 months after HSCT; the incidence of IA among autologous HSCT recipients did not change (data not shown). Hazard for IA was thus evaluated by season in allogeneic HSCT recipients during two 5-year periods (1992–1997 and 1998–2003; Figure 3). Figure 3 shows that the IA risk within the first 3 months after HSCT was higher (sharp slope) in the warm season during both the early (1992–1997) and later (1998–2003) years (5.7% vs 5.9%). However, the 3-month IA cumulative incidence decreased in the later years during the cold season (from 4.3% to 2.0%). Therefore, the difference in incidence was primarily witnessed among allogeneic HSCT recipients who underwent transplantation during nonspring and nonsummer months. Differences observed according to season suggest an impact of environmental sources of exposure. To further understand the influence of environment on risk for IA, an alternative cohort with differences in geographic exposure was examined.
Figure 3.
Estimated cumulative incidence (CumInc) of invasive aspergillosis (IA) by season and year of hematopoietic stem cell transplantation (HSCT) in allogeneic HSCT recipients at the Fred Hutchinson Cancer Research Center. CI, confidence interval.
MDACC cohort analysis
A more limited analysis was performed on the cohort of patients who received HSCTs at the MDACC in Houston. This site was chosen for comparison primarily because the weather trends markedly contrast with those found in Seattle; specifically, weather is much warmer during the spring and summer, but unlike Seattle, precipitation is increased during the warmer weather. From 1993 through 2003, 56 of 4292 patients who received HSCTs at the MDACC developed IA within 3 months after receiving the first HSCT, yielding a cumulative incidence rate of 1.4% (2.1% in allograft recipients and 0.6% in autograft recipients). Clinical risks are not directly comparable because more patients at the MDACC received autologous HSCTs (51.5%) than at the FHCRC (24.9%). As expected, allogeneic HSCT recipients had >3-fold increased hazard for IA at 3 months after transplantation (HR, 3.35; 95% CI, 1.83–6.15; P = .007), compared with the corresponding hazard for autologous HSCT recipients. Because host data were not available in the MDACC cohort, detailed risk analyses were not possible.
Because more autologous HSCTs were performed at the MDACC (51.5%) than at the FHCRC (24.9%), descriptive effects of season were performed only among patients who received allogeneic grafts. Season had no significant impact on IA rate at the MDACC, with a 3-month IA incidence of 1.6% during cold months and a 2.4% 3-month incidence during warm months (HR, 0.68; 95% CI, 0.36–1.24; P = .20; data not shown).
Environmental analysis
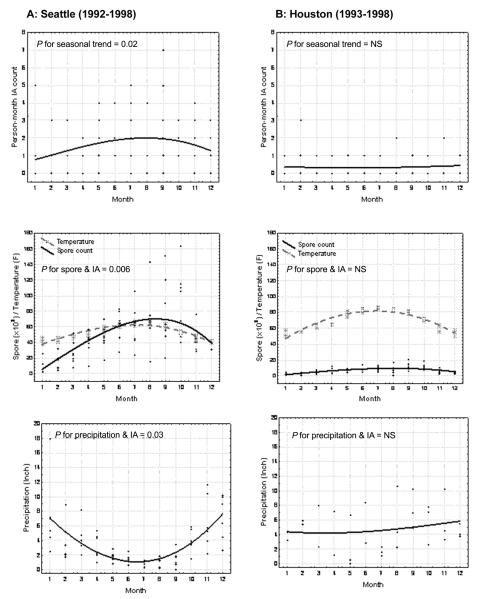
Evaluation of the spore counts according to climatic variables in the 2 cities could be informative, given differences in temperature associations with precipitation. Monthly person-month IA incidence and environmental spore count are illustrated in Figure 4. Because of limitations in the availability of environmental spore and climatic information, analyses of person-month IA incidence were restricted to 1992–1998 in Seattle and 1993–1998 in Houston. Elevated person-month IA incidence (P = .02 for seasonal trend), which corresponded with high spore counts and low precipitation, was observed during summer months in Seattle (Figure 4A) but not in Houston (Figure 4B). In Seattle, periods of low precipitation corresponded to high spore counts (Spearman ρ = −0.28, P = .02), whereas precipitation, remaining relatively high and uniform across months in Houston, was not associated with spore counts (Spearman ρ = 0.08, P = .72). In Seattle, the person-month IA incidence was positively associated with spore count (P = .006) and negatively associated with precipitation (P = .03); no climatic associations were apparent on person-month IA rate at the MDACC in Houston.
Figure 4.
Person-time invasive aspergillosis (IA) count and total spore count according to season and precipitation in Seattle, Washington (A), and Houston, Texas (B). NS, not significant.
DISCUSSION
IA, an infection previously considered to be primarily nosocomial in origin, has become a late complication after HSCT, with acquisition likely from environmental sources. Here, data suggest a seasonal pattern much like that observed for other environmentally acquired fungal infections (eg, coccidioidomycosis). Data demonstrate a high incidence of IA occurring just after seasonal periods of low precipitation and high temperatures, which coincide with high environmental spore counts in Seattle, suggesting a complex and variable nature of exposure and disease.
A previously unexplained seasonal pattern of IA incidence was noted in prior risk factor analyses that included patients in Seattle, but these trends have not been demonstrated in all studies [1-4]. Recently, another study performed during a period of demolition in Germany noted that spore counts correlated with temperature and humidity, with highest counts recovered in outdoor air in the summer and autumn [22, 30]. However, our results demonstrate that climatic conditions affect spore counts and, potentially, rates of infection differently, because increased counts were not noted in the warm, wet weather that characterizes the summer in Houston. It is tempting to speculate that warm, dry weather allows for more avid dispersal of hydrophobic Aspergillus conidia, as has been observed for other fungal propagules. It also seems logical that similar seasonal patterns are observed in relation to spore exposure for other disease states associated with mold exposure, such as those associated with allergy [19-22].
Although most IA cases appear to be sporadic, outbreaks of IA infection associated with construction, particularly in the health care setting, constitute a subset of disease that has been successfully targeted with host and environmental prophylaxis. Indeed, the Healthcare Infection Control Practices Advisory Committee has recommended the use of high-efficiency particulate air filtration with appropriate pressure differentials or air exchanges and Environmental Protection Agency–registered antifungal biocides (eg, copper-8-quinolinolate) for decontaminating structural materials as environmental protective measures in the scenario of health care–associated IA infection outbreaks [31]. Such techniques have reduced the incidence of health care–associated IA among HSCT recipients during construction activity, but it is unclear whether these strategies are independently beneficial [32, 33]. Our findings of a sustained elevated hazard for IA during warm periods that correlate with high ambient exposure to molds provide a compelling argument that preventive strategies need to focus on nonnosocomial sources of Aspergillus species, especially in allogeneic HSCT recipients. For an outpatient, the most likely sources of exposure are community associated, although acquisition in outpatient health care facilities cannot be ruled out. The feasibility of nonnosocomial environmental prevention efforts is questionable, given the ubiquity of fungi in the air and/or water. One approach might be to target the host by increasing antifungal prophylaxis use during high incidence periods among HSCT recipients, especially those possessing other known independent risk factors for IA.
Comparison across periods suggests that the contribution of high hazards during the spring to summer months remained constant, whereas hazards during other months decreased in the most recent years. Overall risks for IA involve differences in hosts and preventive strategies, and these variables have changed during the 10-year period; however, these variables remain constant during the seasons, supporting a true impact of season on risks for IA.
We examined the effect of season at time of transplantation. The primary reason that we limited the analysis to the month of transplantation and risks according to transplant variables is because these risks do not change over time as do typical time-dependent factors that affect immune reconstitution. Also, analyzing the impact of environmental factors that occur >3 months after HSCT would not be feasible in patients treated in these reference centers, because patients are typically discharged to geographically diverse sites later (ie, >3 months after the procedure). Also, one can assume that climatic changes within 3-month periods are relatively small, at least when classified within typical seasons. In addition, we analyzed the effect of the current season (season at IA diagnosis), and a similar seasonal effect was found, with warm season having a high HR relative to cold season (HR, 1.97; 95% CI, 1.44–2.71; P < .001).
A direct comparison of IA incidence between transplantation centers is not valid with the use of this data set, because cases were not identified using similar methods and the incidence of reported IA can vary widely on the basis of differences in diagnostics, including use of non–culture-based assays, and differences in use of early antifungal treatment strategies. Also, the cohorts examined differed widely in underlying risks, with proportionally more autologous HSCT recipients in the MDACC, compared with the proportion in the FHCRC. Hence, differences in IA incidence or hazard should be interpreted only over seasons within and not between the transplantation centers.
This study has several limitations. With any observational study, especially a retrospective one, residual confounding is certain to be present. For example, we lack information regarding detailed risks for IA, which include multiple variables that affect immune reconstitution. There is difficulty defining or interpreting time to event in this disease, because of the uncertainties in latency period, the relative lack of sensitive diagnostic tests, and clinician-dependent differences in diagnostic aggressiveness. Further investigation is needed to explore how the finding of seasonal influence on IA may apply in other geographic settings and among different patient populations, by means of standardized diagnostic and case identification methods.
The association between environmental spore counts and IA rates in Seattle supports the notion that a substantial proportion of IA is acquired outside the hospital. Also, weather variables that affect environmental spore counts appear to differ in different geographic locales. Optimizing host and environmental prevention strategies during these high-risk periods may be an efficient means to decrease disease burden.
Acknowledgments
We thank Chris Davis of FHCRC and personnel at Northwest Asthma and Allergy Center (Seattle, WA) and the Houston Department of Health and Human Services (Houston, TX) for data acquisition.
Financial support. National Institutes of Health (grants AI51468, AI54736, CA 18029, and CA15704; and UL1 RR024140 to the Oregon Clinical and Translational Research Institute, Portland); Knight Cancer Institute, Oregon Health & Science University, Portland (grant P30CA069533).
Footnotes
Potential conflicts of interest. M.B. has served as a consultant for Pfizer. K.A.M. has served as a consultant for Pfizer, Astellas, Basilea, Merck, and Schering-Plough. D.P.K. has received research support and honoraria from Schering-Plough, Pfizer, Astellas, Enzon, and Merck. All other authors: no conflicts.
References
- 1.Marr K, Carter R, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 2.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 3.Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 4.Grow W, Moreb J, Roque D, et al. Late onset of invasive Aspergillus infection in bone marrow transplant patients at a university hospital. Bone Marrow Transplant. 2002;29:15–19. doi: 10.1038/sj.bmt.1703332. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda T, Boeckh M, Carter RA, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102:827–833. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 6.Mullins J, Harvey R, Seaton A. Sources and incidence of airborne Aspergillus fumigatus (Fres) Clin Allergy. 1976;6:209–217. doi: 10.1111/j.1365-2222.1976.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 7.Warris A, Verweij PE. Clinical implications of environmental sources for Aspergillus. Med Mycol. 2005;43(suppl 1):S59–S65. doi: 10.1080/13693780400025260. [DOI] [PubMed] [Google Scholar]
- 8.Symoens F, Burnod J, Lebeau B, et al. Hospital-acquired Aspergillus fumigatus infection: can molecular typing methods identify an environmental source? J Hosp Infect. 2002;52:60–67. doi: 10.1053/jhin.2002.1263. [DOI] [PubMed] [Google Scholar]
- 9.Lasker BA. Evaluation of performance of four genotypic methods for studying the genetic epidemiology of Aspergillus fumigatus isolates. J Clin Microbiol. 2002;40:2886–2892. doi: 10.1128/JCM.40.8.2886-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patterson JE, Zidouh A, Miniter P, Andriole VT, Patterson TF. Hospital epidemiologic surveillance for invasive aspergillosis: patient demographics and the utility of antigen detection. Infect Control Hosp Epidemiol. 1997;18:104–108. doi: 10.1086/647563. [DOI] [PubMed] [Google Scholar]
- 11.Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis. 2001;7:369–374. doi: 10.3201/eid0703.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roenneberg T, Merrow M. Seasonality and photoperiodism in fungi. J Biol Rhythms. 2001;16:403–414. doi: 10.1177/074873001129001999. [DOI] [PubMed] [Google Scholar]
- 13.Comrie AC. Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ Health Perspect. 2005;113:688–692. doi: 10.1289/ehp.7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park BJ, Sigel K, Vaz V, et al. An epidemic of coccidioidomycosis in Arizona associated with climatic changes, 1998–2001. J Infect Dis. 2005;191:1981–1987. doi: 10.1086/430092. [DOI] [PubMed] [Google Scholar]
- 15.Zender CS, Talamantes J. Climate controls on valley fever incidence in Kern County, California. Int J Biometeorol. 2006;50:174–182. doi: 10.1007/s00484-005-0007-6. [DOI] [PubMed] [Google Scholar]
- 16.MacDougall L, Kidd SE, Galanis E, et al. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg Infect Dis. 2007;13:42–50. doi: 10.3201/eid1301.060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser JA, Giles SS, Wenink EC, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature. 2005;437:1360–1364. doi: 10.1038/nature04220. [DOI] [PubMed] [Google Scholar]
- 18.Byrnes EJ, Iii, Bildfell RJ, Frank SA, Mitchell TG, Marr KA, Heitman J. Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J Infect Dis. 2009;199(7):1081–1086. doi: 10.1086/597306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodley JM, Clayton YM, Hay RJ. Environmental sampling for aspergilli during building construction on a hospital site. J Hosp Infect. 1994;26:27–35. doi: 10.1016/0195-6701(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 20.Jones BL, Cookson JT. Natural atmospheric microbial conditions in a typical suburban area. Appl Environ Microbiol. 1983;45:919–934. doi: 10.1128/aem.45.3.919-934.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rath PM, Ansorg R. Value of environmental sampling and molecular typing of aspergilli to assess nosocomial sources of aspergillosis. J Hosp Infect. 1997;37:47–53. doi: 10.1016/s0195-6701(97)90072-4. [DOI] [PubMed] [Google Scholar]
- 22.Hansen D, Blahout B, Benner D, Popp W. Environmental sampling of particulate matter and fungal spores during demolition of a building on a hospital area. J Hosp Infect. 2008;70(3):259–264. doi: 10.1016/j.jhin.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 23.NOAA’s national weather service. US Department of Commerce [Updated 20 April 2010. Accessed 21 April 2010];National Oceanic and Atmospheric Administration Web site. http://www.nws.noaa.gov/
- 24.Northwest Asthma [Accessed 21 April 2010];Northwest Asthma & Allergy Center Web site. http://www.asthmainc.org/index.asp. Published 2002.
- 25.City of Houston eGovernment Center [Updated 2010. Accessed 21 April 2010];City of Houston Web site. http://www.houstontx.gov/health/Pollen-Mold/pollenarchive.html.
- 26.Ascioglu A, Rex J, DePauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 27.Kalbfleisch J, Prentice R. The statistical analysis of failure time data. 2nd ed Wiley; Hoboken, NJ: 2002. [Google Scholar]
- 28.Hastie T, Tibshirani R. Generalized additive models. Chapman and Hall; New York: 1990. [DOI] [PubMed] [Google Scholar]
- 29.Aitkin M, Anderson D, Francis B, Hinde J. Statistical modeling in GLIM. Clarendon Press; Oxford: 1989. [Google Scholar]
- 30.Koch A, Heilemann KJ, Bischof W, Heinrich J, Wichmann HE. Indoor viable mold spores: a comparison between two cities, Erfurt (eastern Germany) and Hamburg (western Germany) Allergy. 2000;55:176–180. doi: 10.1034/j.1398-9995.2000.00233.x. [DOI] [PubMed] [Google Scholar]
- 31.Sehulster L, Chinn RY. Guidelines for environmental infection control in health care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 2003;52:1–42. [PubMed] [Google Scholar]
- 32.Hahn T, Cummings KM, Michalek AM, Lipman BJ, Segal BH, McCarthy PL., Jr Efficacy of high-efficiency particulate air filtration in preventing aspergillosis in immunocompromised patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2002;23:525–531. doi: 10.1086/502101. [DOI] [PubMed] [Google Scholar]
- 33.Humphreys H. Positive-pressure isolation and the prevention of invasive aspergillosis: what is the evidence? J Hosp Infect. 2004;56:93–100. doi: 10.1016/j.jhin.2003.10.011. [DOI] [PubMed] [Google Scholar]