Abstract
Prior studies have indicated that effects of cholera enterotoxin (CT) on the small intestine are delayed in onset and involve an interaction with adenyl cyclase in the mucosa. It has also been shown that the administration of cycloheximide to rabbits in doses which inhibit crypt cell mitoses (20 mg/kg), diminishes CT-induced fluid production in jejunal loops. These latter studies have been interpreted as indications that CT-related intestinal secretion is a crypt cell function and that it is mediated by a CT-induced protein.
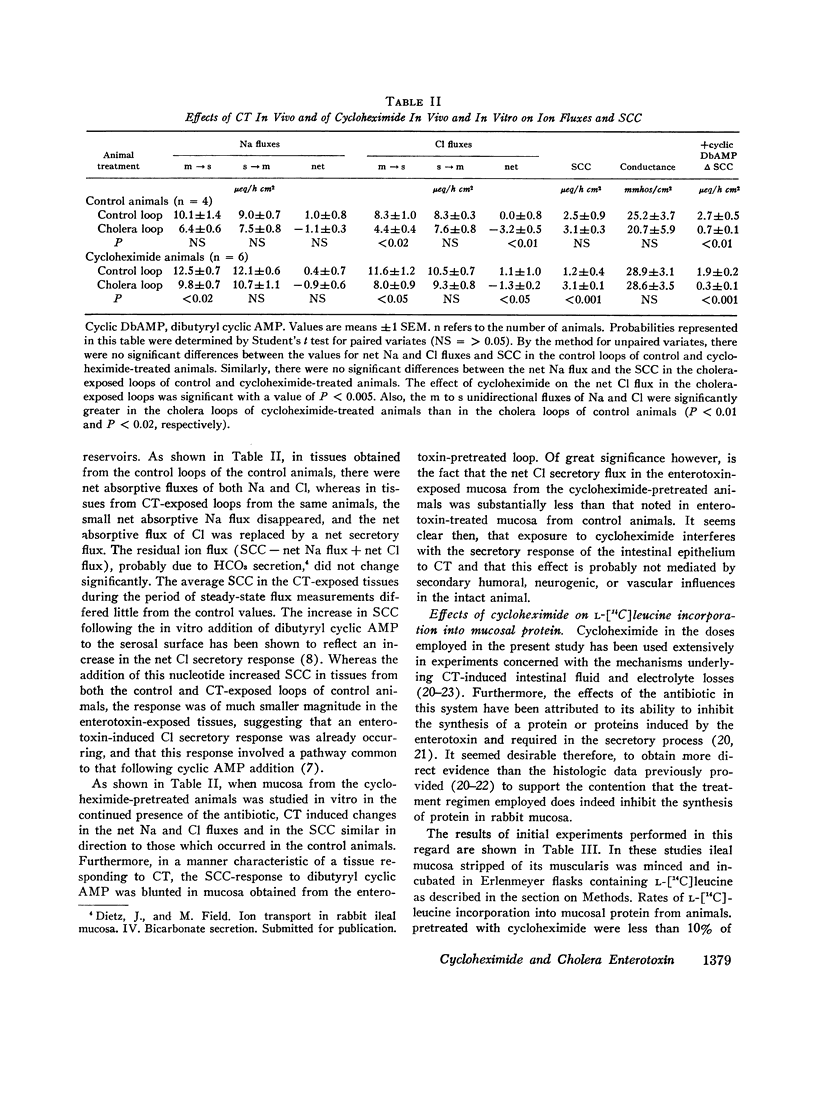
The present study was undertaken to delineate more precisely the nature of the interaction in the intestine between cycloheximide and cholera toxin. Pretreatment of rabbits with cycloheximide reduced by 60% the secretory response to CT in isolated ileal loops with intact blood supply. Sodium and chloride flux measurements on mucosa isolated from these and control loops indicated that this antisecretory effect of cycloheximide persists in vitro. Measurements of radioactive leucine incorporation into mucosal protein indicated that the dose of cycloheximide employed inhibited protein synthesis by 90%. This inhibitory effect was shown to be independent of any effect of cycloheximide on amino acid uptake across the brush border. Measurements of adenyl cyclase activity and cyclic AMP levels in ileal mucosa of cycloheximide pretreated and control animals indicated that cycloheximide did not diminish the CT-induced increases in these parameters.
These observations demonstrate that cycloheximide reduces CT-induced intestinal fluid production without interfering with the CT-induced augmentation of adenyl cyclase activity or the consequent rise in cyclic. AMP concentration. Since the antisecretory effect of cycloheximide persists in vitro, it probably involves a direct interaction of the antibiotic with mucosal cell ion transport mechanisms rather than an indirect effect mediated by other humoral or neurogenic factors. The present observations also suggest that the secretory response of the intestine to CT involves neither the synthesis of new adenyl cyclase nor that of a protein modifying its activity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banwell J. G., Pierce N. F., Mitra R. C., Brigham K. L., Caranasos G. J., Keimowitz R. I., Fedson D. S., Thomas J., Gorbach S. L., Sack R. B. Intestinal fluid and electrolyte transport in human cholera. J Clin Invest. 1970 Jan;49(1):183–195. doi: 10.1172/JCI106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. C., Jr Cholera enterotoxin--recent investigations yield insights into transport processes. Am J Med. 1971 Jan;50(1):1–7. doi: 10.1016/0002-9343(71)90198-7. [DOI] [PubMed] [Google Scholar]
- Carpenter C. C., Sack R. B., Feeley J. C., Steenberg R. W. Site and characteristics of electrolyte loss and effect of intraluminal glucose in experimental canine cholera. J Clin Invest. 1968 May;47(5):1210–1220. doi: 10.1172/JCI105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Evans W. R. The effect of cycloheximide on membrane transport in Euglena. A comparative study with nigericin. J Biol Chem. 1971 Oct 25;246(20):6144–6151. [PubMed] [Google Scholar]
- Field M: Intestinal secretion: effect of cyclic AMP and its role in cholera. N Engl J Med. 1971 May 20;284(20):1137–1144. doi: 10.1056/NEJM197105202842008. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., al-Awqati Q., Greenough W. B., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972 Apr;51(4):796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Ion transport in rabbit ileal mucosa. II. Effects of cyclic 3', 5'-AMP. Am J Physiol. 1971 Oct;221(4):992–997. doi: 10.1152/ajplegacy.1971.221.4.992. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Nellans H. N., Acheson L. S., Schultz S. G. Effect of cycloheximide on influx across the brush border of rabbit small intestine. Biochim Biophys Acta. 1973 Jan 2;291(1):302–307. doi: 10.1016/0005-2736(73)90422-7. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr, Brown R., Kurnick J. T. Studies on the mode of action of diphtheria toxin. VII. Toxin-stimulated hydrolysis of nicotinamide adenine dinucleotide in mammalian cell extracts. J Exp Med. 1969 Jan 1;129(1):1–21. doi: 10.1084/jem.129.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayer D. T., Serebro H. A., Iber F. L., Hendrix T. R. Effect of cycloheximide on unidirectional sodium fluxes in the jejunum after cholera exotoxin exposure. Gastroenterology. 1970 Jun;58(6):815–819. [PubMed] [Google Scholar]
- Guerrant R. L., Chen L. C., Sharp G. W. Intestinal adenyl-cyclase activity in canine cholera: correlation with fluid accumulation. J Infect Dis. 1972 Apr;125(4):377–381. doi: 10.1093/infdis/125.4.377. [DOI] [PubMed] [Google Scholar]
- Harper D. T., Jr, Yardley J. H., Hendrix T. R. Reversal of cholera exotoxin-induced jejunal secretion by cycloheximide. Johns Hopkins Med J. 1970 May;126(5):258–266. [PubMed] [Google Scholar]
- Hendrix T. R., Bayless T. M. Digestion: intestinal secretion. Annu Rev Physiol. 1970;32:139–164. doi: 10.1146/annurev.ph.32.030170.001035. [DOI] [PubMed] [Google Scholar]
- Iber F. L., McGonagle T., Serebro H. A., Luebbers E., Bayless T. M., Hendrix T. R. Unidirectional sodium flux in small intestine in experimental canine cholera. Am J Med Sci. 1969 Nov;258(5):340–350. doi: 10.1097/00000441-196911000-00005. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moritz M., Iber F. L., Moore E. W. Rabbit cholera: effects of cycloheximide on net water and ion fluxes and transmural electric potentials. Gastroenterology. 1972 Jul;63(1):76–82. [PubMed] [Google Scholar]
- Pierce N. F., Carpenter C. C., Jr, Elliott H. L., Greenough W. B., 3rd Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971 Jan;60(1):22–32. [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEKEVITZ P. Uptake of radioactive alanine in vitro into the proteins of rat liver fractions. J Biol Chem. 1952 Apr;195(2):549–565. [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebro H. A., Iber F. L., Yardley J. H., Hendrix T. R. Inhibition of cholera toxin action in the rabbit by cycloheximide. Gastroenterology. 1969 Mar;56(3):506–511. [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Vaughan M., Pierce N. F., Greenough W. B., 3rd Stimulation of glycerol production in fat cells by cholera toxin. Nature. 1970 May 16;226(5246):658–659. doi: 10.1038/226658a0. [DOI] [PubMed] [Google Scholar]



