Abstract
Amyloid-β (Aβ) peptides, derived from the amyloid precursor protein, and the microtubule-associated protein tau are key pathogenic factors in Alzheimer’s disease (AD). How exactly they impair cognitive functions is unknown. Here we assessed the effects of Aβ and tau on axonal transport of mitochondria and the neurotrophin receptor TrkA, cargoes that are critical for neuronal function and survival and whose distributions are altered in AD. Aβ oligomers rapidly inhibited axonal transport of these cargoes in wildtype neurons. Lowering tau levels prevented these defects without affecting baseline axonal transport. Thus, Aβ requires tau to impair axonal transport and tau reduction protects against Aβ-induced axonal transport defects.
Amyloid-β (Aβ) peptides, derived from the amyloid precursor protein (APP), and the microtubule-associated protein tau are key pathogenic factors in Alzheimer’s disease (AD). However, the exact mechanisms by which they impair cognitive functions are unknown. Human APP (hAPP) transgenic mice have high Aβ levels in the brain and develop aberrant neuronal activity and behavioral deficits (1, 2). Lowering endogenous tau prevents these abnormalities without affecting baseline neuronal functions (1, 2). The mechanisms of this rescue are unknown. Axonal transport is critical for neuronal function and is impaired by Aβ (3–6). Whether tau also affects axonal transport is controversial (7–9).
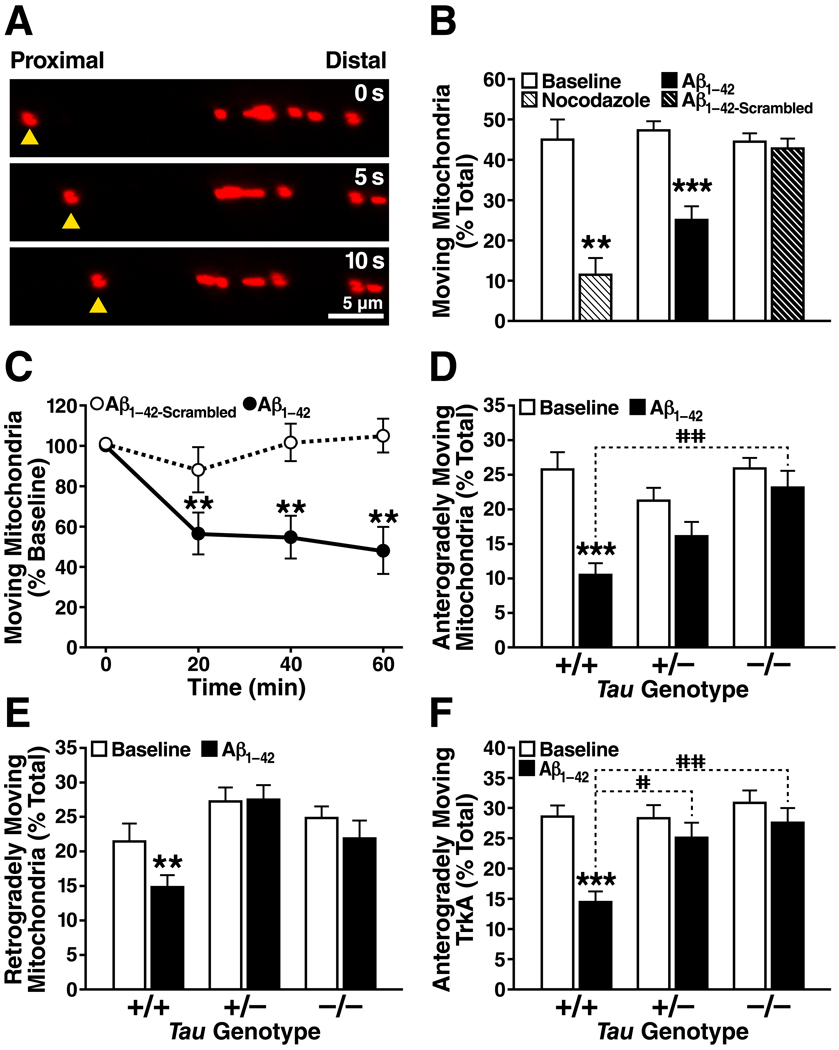
To explore whether tau reduction prevents Aβ-induced defects in axonal transport, we studied axonal transport of mitochondria and the neurotrophin receptor TrkA, whose neuronal distributions are altered in AD (10, 11). Hippocampal neuronal cultures from tau-deficient (Tau−/− or Tau+/−) mice (1) and wildtype (Tau+/+) controls were transfected with plasmids expressing fluorescent markers of mitochondria (mito-RFP) or TrkA (TrkA-mCherry). At 10–14 days in vitro, cargo motility (moving cargoes/total cargoes) and velocity (µm/s) were assessed before and after treatment with Aβ1-42 oligomers (2 µM) (12). Aβ rapidly inhibited axonal motility of mitochondria and TrkA in wildtype neurons. The effect was stronger for anterograde (mitochondria: P<0.001; TrkA: P<0.001) than retrograde (mitochondria: P<0.01; TrkA: non-significant) transport. Complete or partial tau reduction prevented these defects without affecting axonal transport at baseline (Fig. 1; Movies S1–6). Moving cargoes had similar velocities across all Tau genotypes at baseline and after Aβ treatment.
Figure 1.
(A) Anterograde axonal movement of a single mitochondrion (yellow triangles) is shown in successive 5-s image frames. (B) The microtubule-depolymerizing agent nocodazole (10 µg/ml) and oligomeric Aβ1-42 (2 µM) inhibited mitochondrial movements in Tau+/+ axons within 60 min. Aβ with a scrambled amino acid sequence (Aβ1-42-Scrambled, 2 µM) had no effect. n = 7–24 axons per condition. **P<0.01, ***P<0.001 versus corresponding baseline (paired t tests with Bonferroni correction). (C) Aβ1-42 inhibited mitochondrial movements in Tau+/+ axons within 20 min. n = 7–8 axons for each data point. **P<0.01 versus corresponding baseline (paired t tests, Bonferroni). (D–F) Aβ1-42 inhibited mitochondrial (D, E) and TrkA (F) motility in Tau+/+ axons within 60 min but not in Tau+/− or Tau−/− axons. n = 24–45 axons for each genotype and condition. **P<0.01, ***P<0.001 versus corresponding baseline (paired t tests, Bonferroni). The percent reduction in anterograde transport in the presence of Aβ was greater in Tau+/+ axons than in Tau+/− or Tau−/− axons (D, F). #P<0.05, ##P<0.01 (Kruskal-Wallis ANOVA, Dunn). Error bars are SEM. See also Table S1.
Tau reduction did not enhance axonal transport under physiological conditions. However, tau levels were clearly more critical for axonal transport in the presence of Aβ. Aβ oligomers impair axonal motility of cargoes through complex mechanisms involving NMDA receptor signaling (3), activation of glycogen synthase kinase 3β (3, 4) and casein kinase 2 (5), and actin polymerization (6). Why Aβ requires tau to impair axonal transport is uncertain; tau might interact directly or indirectly with any of these pathways or enhance the effects independently by competing with motor proteins for microtubule access (7). Although most concentrated in axons, tau may have Aβ-enabling activities also in dendrites (2).
Tau ablation induces axonal spheroids in Tg2576 APP transgenic mice (13), but the functional significance is unknown. Tau reduction prevents Aβ-induced neuronal and behavioral deficits in hAPPJ20 mice (1) and APP23 mice (2). Protection against Aβ-induced defects in axonal transport is one of several possible mechanisms for this rescue.
Although tau did not affect axonal transport under baseline untreated conditions in vitro (this study) or in vivo (9), it may still be important under physiologic conditions. For example, local tau gradients may promote cargo detachment at strategic points (7, 13). Tau may also regulate cellular transport of its binding partners (2), and tau reduction might have affected axonal transport of cargoes we did not assess. Partial tau reduction may strike a balance between therapeutic safety and efficacy because it prevented Aβ-induced axonal transport defects as well as aberrant neuronal activity (1), cognitive deficits (1), and premature mortality (1, 2) in hAPP mice. In addition to tau reduction strategies, components of the axonal transport machinery and of Aβ- or tau-related signaling cascades are potential therapeutic targets warranting further investigation.
Supplementary Material
Acknowledgments
We thank K. Ho, E. LaDow, H. Solanoy, X. Wang, and G. Yu for excellent technical support and advice, Y. Huang for helpful discussions on mitochondrial transport, Y. Yoon for the mito-RFP plasmid, H.M. Brown for the TrkA-mCherry plasmid, M. Vitek and H. Dawson for tau knockout mice, A. Holloway for statistical support, S. Ordway for editorial review, J. Carroll for graphics support, and M. Dela Cruz for administrative assistance. L.M. has received research funding from Elan Pharmaceuticals for other projects and serves on the Scientific Advisory Boards of AgeneBio, iPierian, and Probiodrug. This work was supported by NIH grants AG011385, NS041787 (L.M) and NS057906 (B.C.) and the McBean Family Foundation (K.A.V.). The animal care facility was partly supported by an NIH Extramural Research Facilities Improvement Program Project (C06 RR018928).
Footnotes
SUMMARY
Tau reduction prevents amyloid-β-induced impairments in axonal transport, providing a possible mechanism for the protective effects of tau reduction in Alzheimer’s disease mouse models.
References and Notes
- 1.Roberson ED, et al. Science. 2007;316:750. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 2.Ittner LM, et al. Cell. 2010;142:387. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Decker H, Lo KY, Unger SM, Ferreira ST, Silverman MA. J. Neurosci. 2010;30:9166. doi: 10.1523/JNEUROSCI.1074-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rui Y, Tiwari P, Xie Z, Zheng JQ. J. Neurosci. 2006;26:10480. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pigino G, et al. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5907. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiruma H, Katakura T, Takahashi S, Ichikawa T, Kawakami T. J. Neurosci. 2003;23:8967. doi: 10.1523/JNEUROSCI.23-26-08967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixit R, Ross JL, Goldman YE, Holzbaur EL. Science. 2008;319:1086. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebneth A, et al. J. Cell Biol. 1998;143:777. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan A, Kumar A, Peterhoff C, Duff K, Nixon R-A. J. Neurosci. 2008;28:1682. doi: 10.1523/JNEUROSCI.5242-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirai K, et al. J. Neurosci. 2001;21:3017. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schindowski K, Belarbi K, Buee L. Genes Brain Behav. 2008;7:43. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Materials and methods are available as supporting material on Science Online.
- 13.Dawson HN, et al. Neuroscience. 2010;169:516. doi: 10.1016/j.neuroscience.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.