Abstract
Human skin pigmentation is the product of two clines produced by natural selection to adjust levels of constitutive pigmentation to levels of UV radiation (UVR). One cline was generated by high UVR near the equator and led to the evolution of dark, photoprotective, eumelanin-rich pigmentation. The other was produced by the requirement for UVB photons to sustain cutaneous photosynthesis of vitamin D3 in low-UVB environments, and resulted in the evolution of depigmented skin. As hominins dispersed outside of the tropics, they experienced different intensities and seasonal mixtures of UVA and UVB. Extreme UVA throughout the year and two equinoctial peaks of UVB prevail within the tropics. Under these conditions, the primary selective pressure was to protect folate by maintaining dark pigmentation. Photolysis of folate and its main serum form of 5-methylhydrofolate is caused by UVR and by reactive oxygen species generated by UVA. Competition for folate between the needs for cell division, DNA repair, and melanogenesis is severe under stressful, high-UVR conditions and is exacerbated by dietary insufficiency. Outside of tropical latitudes, UVB levels are generally low and peak only once during the year. The populations exhibiting maximally depigmented skin are those inhabiting environments with the lowest annual and summer peak levels of UVB. Development of facultative pigmentation (tanning) was important to populations settling between roughly 23° and 46° , where levels of UVB varied strongly according to season. Depigmented and tannable skin evolved numerous times in hominin evolution via independent genetic pathways under positive selection.
Keywords: folate, vitamin D, melanin, tanning, UVB
Variation in skin color is the most noticeable of human polymorphisms. As visually dominant mammals, we readily notice differences in skin color in each other. As primates who uniquely use language to create categories, we readily give names to these differences. Since the mid-18th century, skin color has been the single most important physical trait used to define human groups, including variously named varieties, races, subspecies, and species. Charles Darwin observed variation in human skin color while abroad during the voyage of the H.M.S. Beagle (1831–1836), but he soundly rejected the notion that physical differences such as skin color constituted the basis for distinguishing separate human species (1). Darwin’s rejection of the existence of distinct human species was based upon his observation that human groups “graduate into each other, and that it is hardly possible to discover clear distinctive character between them” (1, p. 226). His aversion to the separation of humans into discrete species was also motivated by his vehement aversion to slavery, which in his lifetime was defended and promoted on the basis of the superiority and inferiority of allegedly distinct human species (2). It is also well known that early in his career, Darwin collected copious notes on human origins and descent (3), but “without any intention of publishing on the subject, but rather with a determination not to publish, as I thought that I should thus only add to the prejudices against my views” (1, p. 1). Darwin thus deflected potential criticism of natural selection in the first decade after publication of The Origin by avoiding almost entirely discussion of humans in an evolutionary context.
The causes of variation in human skin pigmentation were much discussed long before Darwin's time. Observers beginning with Hippocrates in the fifth century associated human traits and temperament with the environment and recognized that skin color was part of this package (4). The association of dark skin pigmentation with intense sunshine and heat was further developed by Aristotle and his followers as part of a comprehensive “climatic theory,” which related human features, dispositions, and cultures to the environment. By the mid-18th century, naturalists such as John Mitchell and, later, Samuel Stanhope Smith recognized a pronounced latitudinal gradient of skin pigmentation among the world's peoples—from dark near the equator to light toward the poles—and related it mainly to differences in sunshine heat experienced by people at different latitudes (5, 6). “This general uniformity in the effect,” Smith wrote, “indicates an influence in climate, that, under the same circumstances, will always operate in the same manner” (5, p. 34). It is thus surprising that Darwin, who was so keen to identify adaptations of organisms to “different conditions of life,” rejected a causal association of skin pigmentation with climate in favor of the notion that variations in skin color had evolved primarily through the agency of sexual selection (1). Writing in 1871 in The Descent of Man, and Selection in Relation to Sex (1), he stated:
“If, however, we look to the races of man, as distributed over the world, we must infer that their characteristic differences cannot be accounted for by the direct action of different conditions of life, even after exposure to them for an enormous period of time” (p 246). “It can further be shewn that the differences between the races of man, as in colour, hairyness, form of features, &c., are of the nature which it might have been expected would have been acted on by sexual selection” (p 250).
Darwin's preference for sexual selection in matters of human variation blinded him to the importance of natural selection in producing the attributes of human skin.* Human skin is functionally naked and as such served for hundreds of thousands of years as the sole interface between our bodies and the environment. Lacking the covering of dense body hair that protects other mammals and exposed to the myriad physical, chemical, and biological challenges of the environment, human skin evolved under intense pressures of natural selection. The hairless condition itself did not evolve because of a partiality for smooth skin, as averred by Darwin, but primarily because of the need to lose body heat from the skin's surface during exertion and under hot environmental conditions (7, 8). Cooling by evaporation of eccrine sweat is impeded by thick body hair (9); the primary selective pressure promoting the evolution of hair loss in humans was thermoregulation. The loss of body hair in humans was accompanied by enhanced barrier functions of the stratum corneum (10, 11), including the evolution of other epidermal keratins (12, 13), which reduced the skin's permeability and improved its abilities to resist abrasion and microbial attack. The rapid divergence of genes responsible for epidermal differentiation was one of the most significant results to emerge from the initial comparison of human and chimpanzee genomes (12). Changes in skin pigmentation also accompanied loss of body hair, and multiple lines of evidence indicate that permanent, dark, eumelanin-based pigmentation evolved soon after the emergence of the genus Homo in Africa (7, 14). Sexual selection was not the primary, or even a major, determinant of skin pigmentation, although the preference by males in some cultures for females of lighter color probably has heightened sexual dimorphism in skin pigmentation in some populations (7). Rather, it was natural selection that produced the conspicuous gradient of skin tones groups observed in our species.
Samuel Stanhope Smith's observation of a correlation between latitude, solar processes, and human skin pigmentation was refined in the latter part of the 20th century when it was demonstrated that human skin reflectance (as a measure of skin pigmentation) was more highly correlated with latitude as a surrogate for UVR than with temperature, humidity, or altitude (15, 16). The introduction of geographic information systems (GIS) technology and the availability of remotely sensed environmental data permitted accurate and precise testing of the strength of the relationship between physical parameters of the environment and skin pigmentation, and demonstrated conclusively the high correlation between skin pigmentation and UVR (7, 17). Among the most notable findings from these studies was the demonstration that skin reflectance was more highly correlated with autumn levels of UVR than with annual average, summer, or maximum levels (17). Establishment of UVR as the cause, not simply the correlate, of variations in human skin pigmentation has involved elucidation of the probable selective mechanisms involved.
During most of 20th century, arguments about the selective value of dark pigmentation focused on the protective effects of melanin against sunburn, skin cancer, and overproduction of vitamin D (18, 19). These factors can no longer be considered significant selective pressures. Sunburn and skin cancer have negligible effects on reproductive success (7, 18). Nonmelanoma skin cancers are common in older individuals from modern lightly pigmented populations inhabiting sunny climes, but they are rarely fatal or incapacitating (20). Melanoma afflicts younger individuals and is often fatal, but it is much rarer than nonmelanoma skin cancers. Modern statistics on skin cancer prevalence must be viewed with caution, however, in considering the evolutionary importance of skin cancers. The prevalence of skin cancers is highest in lightly pigmented people who experience chronic or intense episodic exposures to strong UVR in places far from their ancestral homelands (21). Skin cancers are mostly a consequence of modern human migrations and resulting mismatches between skin pigmentation and geography or lifestyle. The effects of skin cancers on reproductive success in humans today are modest, and were probably statistically inconsequential in the centuries before rapid, long-distance travel and migration. This inference is further supported by genetic evidence indicating no significant association of 15 SNPs and skin cancer risk (22). Overproduction of vitamin D was refuted as the primary cause of the evolution of dark pigmentation by the discovery that hypervitamosis D due to sun exposure is physiologically impossible because of photochemical regulation (23). With the traditional agents of skin pigmentation evolution rendered untenable, we undertook a reexamination of possible selective agents.
The possibility that photolysis of folate by sunlight was a determining factor in the evolution of dark pigmentation was first explored (24) before the full importance of folate in DNA biosynthesis, repair, DNA methylation, amino acid metabolism, and melanin production was recognized. In 2000, we advanced the theory that dark skin pigmentation in humans had evolved primarily to prevent reduction of fertility due to the photolysis of folate present in cutaneous blood vessels (7). We presented evidence that folate depletion by UVR would precipitate folate deficiencies that would, in turn, lead to potentially fatal birth defects such as neural tube defects (NTDs). Since then, investigations of the photosensitivity of folate under different conditions in vitro and in vivo have demonstrated that the relationship between skin pigmentation and folate metabolism is complicated, and involves direct photodegradation of folate (in its main form of 5-methyltetrahydrofolate or 5-MTHF) as well as its photodegradation in the presence of flavins and porphyrins by reactive oxygen species (ROS) (25–28). Considerable epidemiological work is needed to investigate the relationship between skin pigmentation, folate metabolism, and the prevalence of NTDs, but a protective effect of dark pigmentation against folate depletion (29, 30) and NTDs (31, 32) is apparent. Folate is important especially in rapidly dividing cells, such as those of the embryo and seminiferous tubules. Thus folate deficiencies caused by UVR would potentially affect both female and male fertility. Low folate levels cause derangements of folate-mediated 1-carbon metabolism that lead to serious diseases and birth defects. Folate deficiencies cause faulty DNA replication due to strand breaks caused by misincorporation of excessive uracil into DNA (33, 34). Maintenance of adequate folate levels is associated with a 72% reduction in NTDs, which is the most common class of human birth defects (35). This is due to the direct action of folate on the normalization of neural tube development due to its role in the division of rapidly dividing cells (36, 37). Folate deficiency also impairs nucleotide excision repair, which is the primary mechanism for removing UVR-induced DNA photoproducts (33).
Competition for folate can be severe, especially when the body is stressed by UVR exposure and insufficient dietary folate. Recent research has demonstrated that folate regulates melanin production because it is required for the synthesis of GTP, which is a substrate for de novo production of tetrahydrobiopterin (BH4) and 6BH4 in melanocytes and keratinocytes (38, 39). 6BH4 in turn regulates tyrosinase activity in the melanosome (40). Because of the manifold importance of folate and its derivatives in cell division, DNA repair, and melanin production, and because of the sensitivity of these compounds to break down by UVR and ROS, natural selection to protect folate levels has been intense. Maintaining the integrity of folate metabolism has a high evolutionary valence because it directly affects reproductive success and survival early in life. The mechanisms operating to prioritize the utilization of folate under conditions of environmental and cellular stress caused by high UVR levels are not yet known.
The near absence in African populations of functionally significant variation in the coding region of the melanocortin 1 receptor (MC1R), one of the major genes regulating human eumelanin production, indicates the action of purifying selection maintaining dark pigmentation under intense selective pressure (41–43). The evidence of functional constraint on MC1R in African populations is unusual in light of the high levels of polymorphism observed at other loci in African populations (42). Evidence is mounting that darkly pigmented skin, or the potential for facultative development of dark pigmentation through tanning, evolved secondarily under positive selection in populations moving from low- to high-UVR environments. Pigmentary changes such as these appear to have occurred following the dispersal of lightly and moderately pigmented “Ancestral North Indians” into high-UVR reaches of the Indian subcontinent (44) and in lightly and moderately pigmented east Asians moving into the high-UVR environments of Central and mountainous South America (45). Further study of the genomic signatures of selection on pigmentation genes in human population is much needed.
The evolution of light pigmentation at high latitudes has long been related to the significance of production of vitamin D in the skin under conditions of reduced sunlight (19, 46). Vitamin D3 is made in the skin when UVR penetrates the skin and is absorbed by 7-dehydrocholesterol (7-DHC) in the epidermis and dermis to form previtamin D3. This reaction only occurs in the presence of wavelengths of 290–310 nm in the UVB range, with peak conversion occurring at 295–297 nm. Photosynthesis of vitamin D3 in the skin depends upon the solar zenith angle, which changes with season, latitude, and time of day, and is further controlled by the amount of pigment and thickness of the skin (47, 48). The importance of vitamin D3 as a selective force in the evolution of skin pigmentation is related to the manifold effects of the vitamin on fitness as reviewed in earlier papers (7, 49). The vitamin D endocrine system is involved in the regulation of many independent biological processes including bone metabolism, the innate immune response, cell proliferation, and differentiation (50, 51). The roles of vitamin D3 in the regulation of intestinal calcium absorption, and in bone formation and remodeling, have been known for decades, but only recently has the importance of vitamin D3 in the establishment and maintenance of innate immunity, and in the normal functioning of the pancreas, brain, and heart, been recognized (51, 52). Reduction of fertility due to vitamin D3 deficiencies is greatest in cases of nutritional rickets, but is also significant because of increased prevalence of bacterial and viral infections and increased risk of autoimmune diseases such as multiple sclerosis and Type 1 diabetes (53). Natural selection to promote continued vitamin D production through loss of constitutive pigmentation under conditions reduced UVR was strong, and its independent action on hominin populations dispersing to low UVR habitats was inferred before genetic evidence demonstrating positive selection for depigmentation became known (7). Generally low and highly seasonally variable levels for UVB created a selective environment favoring the capture of UVB photons required for vitamin D3 photosynthesis through loss of melanin pigmentation. Genetic verification of three independent occurrences of evolution of depigmented skin in hominin populations has been documented in the lineages leading to modern northern Europeans and modern east Asians (54, 55) and in Homo neanderthalensis (56). It is significant that the genetic and physiological mechanisms causing light-skinned phenotypes in each group were different from one another. Regulatory mechanisms involve the control of the formation of melanosomes (the organelles in which melanins are produced and stored) (54, 55), and the production of the different types and mixtures of melanins. The mechanisms whereby similar phenotypic ends have been reached by different genetic means have been reviewed recently (57). One of the most interesting questions remaining to be answered about the physiology of vitamin D is humans concerns the nature of variation in the vitamin D receptor (VDR), specifically whether the pattern and nature of polymorphisms in the VDR is related to UVB levels and/or length of habitation under specific UVB regimes.
Geographic Variation in UV Radiation
Mounting genetic evidence demonstrating the role of natural selection in establishing and maintaining darkly and lightly pigmented cutaneous phenotypes near the equator and poles, respectively, prompts a closer look at the nature of the prime selective agent, UVR. Differences in the strength, seasonal distribution, and bioactivity of UVA and UVB have been recognized for a long time (58, 59), but the relevance of these variables to the evolution of human skin pigmentation has not been fully appreciated. The dispersals of hominins out of Africa that occurred about 1.9 Ma and 80 ka, respectively, involved the movement of people out of highly UVR-rich environments into habitats that were much more mixed with respect to the seasonal pattern, intensity, and wavelength mixture of UVR. Although two distinct forms of hominin (early Homo and Homo sapiens, respectively) were involved in these dispersals, neither form made or used clothing or used other portable methods of sun protection. Thus, apart from the time they spent in the shade, their bodies were subjected to the full force of UVR wherever they went.
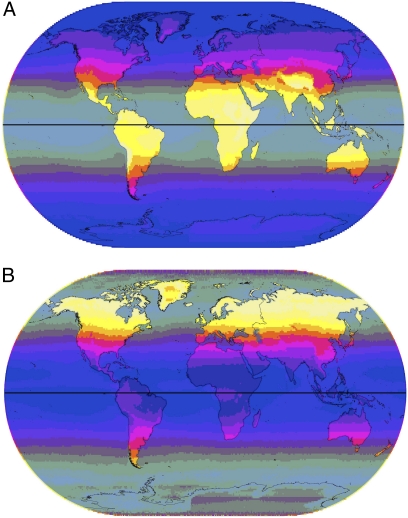
The Earth's surface receives much less UVB than UVA because most UVB reaching the Earth is scattered and absorbed by oxygen, ozone, and water molecules in the atmosphere. Because of this and the geometry of sunlight reaching different places in different seasons, UVB is much more variable in its intensity and distribution than UVA. Levels UVB are highest near the equator in more arid regions, and in high altitude areas such as the Tibetan Plateau and the Altiplano (Fig. 1A). North or south of about 46°, levels of UVB are insufficient to initiate cutaneous production of previtamin D3 for much of the year (7, 17). The pattern of distribution of UVB is most strongly influenced by latitude because of atmospheric scattering and absorption. Africa receives high and uniform amounts, whereas northern Eurasia receives negligible amounts. The coefficient of variation (CoV) for UVB (Fig. 1B) is strongly associated with its seasonal nature outside of the tropics, and is lowest in the equatorial zone and highest in northern Eurasia and North America. Humidity and monsoon precipitation reduce average UVB, and the CoV is higher relative to the mean level in moisture-rich regions.
Fig. 1.
(A) Annual mean UVB (305 nm). Intensity is indicated by gradations from dark to light varying from 1 to 135 Jm−2 in 10 steps with oceans partially grayed-out. (B) Annual CoV for UVB (305 nm). Gradations of dark to light varying from 10 to 300 in 10 steps, with oceans area partially grayed-out.
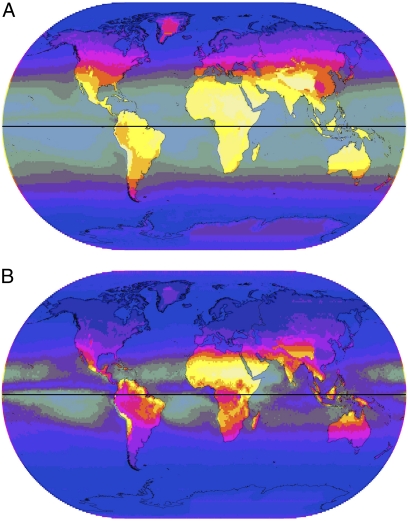
Levels of UVA (Fig. 2A) are considerably higher than those for UVB. The latitudinal bands of UVA distribution are wider than those of UVB and higher levels of UVA exist toward the poles. UVA at 380 nm is about fifteen times more plentiful than UVB at 305 nm, with Western Europe receiving an average 283–570 Jm−2 of UVA compared to only 20–40 Jm−2 of UVB. Equatorial regions received slightly less UVA than tropical and subtropical areas. Albedo from lighter-colored ground and, especially, from snow increased the level of UVA; levels elsewhere were unchanged by atmospheric moisture. The pattern for the coefficient of variation in annual UVA (Fig. 2B) is nearly the inverse of that of UVB, and the rate of variation is approximately 1/200th of that of UVB. UVA varies mostly in dry tropical regions and exhibits low levels of variation away from the equator.
Fig. 2.
(A) Annual mean UVA (380 nm). Intensity is indicated by gradations from dark to light varying from 65 to 930 Jm−2 in 10 steps with oceans partially grayed-out. (B) Annual CoV for UVA (380 nm). Gradations of dark to light varying from 1 to 13 in 10 steps, with oceans area partially grayed-out.
As a summary, it is useful to compare the patterns of UVR at the equator and within the tropics to those outside of the tropics. At the equator and within the tropics, average UVB is high, with two seasonal peaks at the equinoxes (49). Average UVA at the equator and within the tropics is also extremely high, but shows more variation in its intensity throughout the year. Outside of the tropics, average UVB levels are much lower and exhibit a single peak at the summer solstice (49). The extremely low average UVB in northern Eurasia and North America is matched with a high CoV. [Note that there is no comparable, habitable, low-UVB land mass in the Southern Hemisphere (SH) except for the southern tip of South America (60).] Average UVA outside of the tropics is lower, but exhibits much less variation in its pattern. This indicates that loads of UVA throughout the year at these latitudes are lower but are more uniform throughout the year.
Within the time frames of hominin lifespans and dispersals, solar regimes have been in flux. Solar irradiance and insolation are not static but vary according to different temporal cycles and scales. Insolation is tied to the degree and pattern of solar magnetic flux at or near the Sun's surface. Shorter wave insolation varies mostly as a result of energetic changes in the Sun's plage network and faculae (61, 62). UVB levels vary by minute and day, as well as according to cycles of solar rotation (27 days) and the 11-year cycle of sunspots (61, 63); longer-term fluctuations occur over multiple decades and centuries. Total solar production varies only by 0.1–0.3%, but that of shorter wavelength UVR can vary by orders of magnitude more (61, 64). For instance, during the Little Ice Age (A.D. 1500–1800) the UVR levels are estimated to have been ten to four times less than those of the present day (64). Orbital parameters change the pattern of insolation in line with Milankovitch cycles of 22,000 years. Currently, SH experiences greater extremes, with intense insolation in the summer, and the Northern Hemisphere (NH) experiences less extremes and receives more insolation in the winter (60, 65). However, the land masses of the SH and NH have unequal annual means of insolation. Further, the distribution of land masses in the two hemispheres is such that a greater fraction of the SH receives high levels of UVR, whereas a greater portion of the NH receives extremely low levels (60).
UV Radiation as a Selective Force in the Evolution of Pigmentation
UV radiation has been a potent and creative force in the evolution of life on Earth (66), and organisms have evolved a range of defenses against specific UVR wavelengths (58, 59, 67). As previously stated, naked skin was the primary interface between the human body and solar radiation throughout most of the history of the genus Homo. In equatorial Africa, members of the early genus Homo and, later, Homo sapiens were subjected to the potent mixture of UVA and UVB that prevails within the tropics throughout the year. UVA is plentiful and is capable of penetrating deeply into the dermis of skin. UVB is more energetic and is less plentiful; it generally does not penetrate the dermis because it is absorbed and scattered. High-UVR environments generated strong selective pressures on the skin and human body (Table 1), leading to the evolution of permanently dark constitutive pigmentation, and the ability to increase eumelanin production in response to seasonal increases in UVB. This was accomplished genetically by positive selection leading to elimination of polymorphism at the MC1R locus and continued purifying selection acting on the same locus.
Table 1.
Summary of the effects of UVA and UVB on human body and the selective mechanisms involved in the evolution of pigmentation. The estimated strength of natural selection operating to darken and lighten pigmentation is indicated by numbers of “+” and “−” signs, respectively. See text for references for proposed mechanisms.
| Agent | Strength and direction of selection | Proposed selective mechanism(s) |
| UVA | +++ | Photolysis of folate (as 5-methyltetrahydrofolate [5MTHF] in serum) directly and by ROS in the presence of flavins and porphyrins, resulting in reduction of folate available for cell division |
| UVA | ++ | Competition for folate: increased folate needs for DNA damage repair and as 1-carbon donor in methylation of DNA competing with folate needed for melanogenesis |
| UVA | ++ | Disruption of melanin production because of sensitivity of tyrosinase to high levels of ROS |
| UVA | + | Malignant melanoma (as the only skin cancer that causes death to individuals of reproductive age) |
| UVA | + | Photoconversion of excess vitamin D3 to inactive metabolites |
| UVB | +++ | Production of cyblobutane pyrimidine dimers and damaged nucleotides requiring repair resulting from DNA absorption of photons; activation of folate-dependent DNA repair processes |
| UVB | + | Direct photolysis of folate (as 5MTHF in serum), reducing the amount of folate available for cell division and regulation of tyrosinase activity in melanogenesis |
| UVB | + | Competition for folate: increased folate needs for DNA damage repair and as 1-carbon donor in methylation of DNA competing with folate needed for melanogenesis |
| UVB | No effect | Sunburn |
| UVB | No effect | Damage to DNA and its repair system and alterations of the immune system lead to progressive genetic alterations and the formation of nonmelanoma skin cancers |
| UVB | − | Cutaneous photosynthesis of vitamin D3 |
| UVB | − | Greater need for vitamin D in females probably causing increasing sexual dimorphism in pigmentation; exaggerated by sexual selection in some populations |
As humans dispersed away from the tropics, into southern Africa, and out of Africa entirely, they entered regions with lower annual average UVR (7, 49) and significantly different seasonal mixtures of UVA and UVB. Two major changes in UVR regime were experienced by humans outside of the tropics. The first was the shift from high annual UVB with twin peaks of intensity at the equinoxes to generally lower annual UVB with a single annual peak at the summer solstice. The second was the steep decline in the duration and intensity of UVB exposure for every degree of increasing latitude (17, 49), rendering much of the NH free of UVB for over 6 months per year. (The only exception to this rule was and is the Tibetan Plateau, which, due to its extreme altitude and thin atmosphere, receives much higher annual and summer levels of UVB than land masses at equivalent latitudes.) Reduced UVB levels and concomitantly reduced potential for cutaneous vitamin D biosynthesis generated positive selection for depigmentation. Hominins and modern humans dispersed independently many times into nontropical latitudes and evolved depigmented phenotypes by numerous and different genetically based means, some of which remain to be illuminated. It is important to stress that habitation of middle latitudes between approximately 23° and 46° involved the evolution of partially depigmented phenotypes capable of tanning.
UV Radiation and the Evolution of Tanning
Constitutive pigmentation is modified by tanning to produce facultative pigmentation. Tanning is an adaptation to seasonally high UVR, especially UVB, levels. Tanning phenotypes evolved many times in human history, probably as the combined result of independently acquired mutations on genes controlling the pigmentary system and of gene flow. Tanning comprises two mechanisms, immediate pigment darkening (IPD) and the delayed tanning reaction (DTR). IPD involves an immediate darkening of the skin following exposure to UVA, with maximum induction at 340 nm (68). The effect produced by IPD is transient and is visible on light skin as blotchy gray or bluish gray coloration appearing on sun-exposed surfaces. The cellular mechanisms of IPD are still poorly understood, but appear to involve both a spatial rearrangement of melanosomes within keratinocytes and melanocytes, along with photooxidation of existing eumelanin (68). Darker constitutive pigment phenotypes exhibit higher and more intense development of IPD (68). We suggest that the net effect of IPD is immediate absorption or scattering of UVR photons at a superficial level within the skin, thus sparing some damage to deeper layers.
Delayed tanning is what is normally thought of as tanning and is the process that results in facultative pigmentation. The DTR develops gradually over the course of several hours to several days or longer, depending on the duration of UVR exposure. UVA and UVB both induce delayed tanning, but the tans produced develop over different time courses and persist for different lengths of time (69). Delayed tanning involves the redistribution of melanin more toward the surface of the skin as in IPD, changes in the shape and intracellular location of melanin [such as the development of protective supranuclear caps of melanosomes over the nuclei of keratinocytes (70)], and increased de novo synthesis of eumelanin (71–73). Tanning affords only moderate protection against cellular damage from UVR (74, 75) and, in fact, appears to be triggered by signals from UVR-damaged DNA. This is because of a Protein 53 (p53)-mediated response to DNA damage caused by UVR leading to increased melanin production through increased synthesis of α-melanocyte stimulating hormone (α-Msh) (76). From an evolutionary perspective, the importance of delayed tanning is that it is delayed, and that a “base tan” is slow to develop. Outside of tropical latitudes, the slow ramp-up of UVB in the spring to levels capable of inducing photosynthesis of previtamin D3 provides a head start for vitamin D3 production and storage before facultative pigmentation developed by the DTR competes for UVB photons in the skin. Under conditions of slowly increasing UVB, sunburns would have been rare and would not have posed a risk to survival or reproductive success. Early humans spent considerable time outdoors without clothing and were subject to gradual changes in UVR intensity and wavelength mixture with the seasons. They did not travel long distances away from home to go on vacation to sunny places nor did they go to tanning parlors. Tanning is viewed by modern clinicians as an imperfect adaptation to UVR because it damages the skin's connective tissues, immune system, and DNA, and thus leads to progressive changes resulting in skin cancer (76). This is an appropriate statement for vagile and longevous 21st century humans but not for those of the 18th century or earlier who lived before the advent of widely available, rapid long-distance transportation. With early reproduction and before the extension of the average human lifespan through improvements in diet and medicine, skin cancer had no effect on reproductive success. Further, the genetic pattern of skin cancer risk does not accord with predictions based on selection for resistance to skin cancer (22). In the context of human evolution, the evolution of tanning was a superb evolutionary compromise.
Conclusions
The visualizations of UVB and UVA levels and variation presented here permit elaboration of the nature of the selective mechanisms involved in the evolution of variation in skin pigmentation and, notably, the evolution of tanning phenotypes in relation to seasonably variable levels of UVR. Skin pigmentation is probably one of the best examples of natural selection acting on a human trait. It is the product of two opposing clines, one emphasizing dark constitutive pigmentation and photoprotection against high loads of UVA and UVB near the equator (Figs. 1 and 2), and the other favoring light constitutive pigmentation to promote seasonal, UVB-induced photosynthesis of vitamin D3 near the poles (7, 49). Intermediate latitudes with their seasonally high loads of UVB favored the evolution of people with moderate constitutive pigmentation who are capable of tanning.
The time course for the elaboration of pigmentation within a human lifetime reflects its importance in human reproduction and, thus, in evolution. Human infants are born more lightly pigmented than adults and develop their genetically determined maximum level of constitutive pigmentation only in their late teens or early 20s (77) when they enter their period of peak fertility. The potential for development of facultative pigmentation also peaks during early adulthood. In middle and old age, constitutive pigmentation fades and the potential for tanning decreases due to a decline in the number of active melanocytes (78).
Skin pigmentation provides an attractive model system for understanding and teaching evolution and should be promoted as such. It is readily visible, and the basic mechanisms contributing to it are easily understood. Skin pigmentation fulfills the criteria for a successful evolutionary model. First, it was produced by an imperfect replicator. Pigmentation is determined by germ-line DNA, which is subject to mutation. Pigmentation is also subject to heritable variations in epigenetic transmission due to differential methylation of DNA and to extracorporeal memetic patterns of inheritance because of different cultural traditions. Second, there must be selection through differential survival of phenotypes. For skin pigmentation, this implies differential survival and reproduction rates of different phenotypes under different solar regimes. Lastly, natural selection must occur uniquely in time and space to give rise to isolating mechanisms. In the evolution of skin pigmentation, isolation was produced by distance and dispersion rather than sexual selection or other mechanisms. Thus, human skin is a perfect model to demonstrate the mechanism of evolution by natural selection in each of its required parts.
Considerable antagonism toward evolution is based on the common understanding of the word “theory” in its colloquial sense as a hunch. That the separate parts of the theory can be shown to apply fully to an easily understandable human trait should help further the acceptance of the “theory of evolution.” Darwin's theory of natural selection can be likened to Newton's attempt to explain the movement of the planets in his “On the Motion of Bodies in an Orbit.” Newton's effort gave rise to the Principia Mathematica and eventually to the Laws of Motion.
Methods
The UVR data used in this study were derived from readings taken from the NASA Total Ozone Mapping Spectrometer (TOMS), which was flown aboard the Nimbus-7 satellite between 1978 and 1993 (79) http://toms.gsfc.nasa.gov/ery_uv/new_uv/. The data were collected and computed for pixels of 1° longitude by 1° latitude each; the solar flux was measured at or near local noon (79). The readings were computed to account for the total ozone column and scene reflectivities (cloud and snow cover) in the same latitude-longitude pixel. The measurements were then combined with the results of radiative transfer calculations, terrain height, and solar zenith angle (79). Single wavelengths representative of long wave and medium wave UVR were sampled by the TOMS. These were 380 nm for UVA (range 315–400 nm) and 305 nm for UVB (range 280–315 nm). The original data set was very large and comprised over 64,800 readings taken each day from 1979 to 1992. Abridged data sets for each wavelength were produced by taking an average for all years, of the average of the 19th through the 23rd days of each month. The abridged data sets and coefficients of variation for both 380 nm UVA and 305 nm UVB were then mapped using ArcGIS. The strength of UVR at the ground varies by elevation, scene reflectivities, time of day, time of year, and factors influencing UVR absorption such as clouds. The TOMS apparatus takes all of this into account in calculating its readings. This means that many of the correcting factors that were used in latitude-based studies of estimated UVR at the Earth's surface were all accounted for in the one measure. Use of TOMS data thus obviated the need to correct for humidity, rainfall, temperature, or elevation, as was done in previous studies (15, 80). Using TOMS data has some advantages over using data collected by terrestrial-based recorders. This is because the TOMS apparatus does not “see” modern pollutants and so its records are more representative of the environment before industrialization than ground-based units that are “blinded” by low altitude pollution. Further, the TOMS data used here were collected mostly before the 1990s, when widespread ozone depletion seriously affected UVR at the Earth's surface. The amount of ozone in the atmosphere decreased through the 1980s and caused a 3–5% increase in UVR in northern latitudes. This amount is insignificant for our purposes because it is comparable to natural variation in ozone concentration before ozone depletion. It is not necessary to model orbital parameters for the TOMS dataset because the data are direct readings and automatically compensate for orbital effects, something that latitude studies do not (65).
Footnotes
*Darwin's development of sexual selection as the primary mechanism for producing visible human variation is curious and inconsistent with his views on the production of variation in other organisms. His unwillingness to implicate natural selection in connection with human variation suggests that he wanted to continue to deflect criticism of natural selection by avoiding discussion of humans being acted upon by this agency, or because he wanted to encourage discourse on human variation as being due to human preferences rather than being shaped by inanimate forces of nature. It is possible that he was motivated by both of these reasons.
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution IV: The Human Condition,” held December 10–12, 2009, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/SACKLER_Human_Condition.
This article is a PNAS Direct Submission.
References
- 1.Darwin C. The Descent of Man, and Selection in Relation to Sex. London: John Murray; 1871. [Google Scholar]
- 2.Desmond AJ, Moore JR. Darwin's Sacred Cause: How a Hatred of Slavery Shaped Darwin's Views on Human Evolution. Boston, MA: Houghton Mifflin Harcourt; 2009. [Google Scholar]
- 3.van Wyhe J. Mind the gap: Did Darwin avoid publishing his theory for many years? Notes and Records of the Royal Society. 2007;61:177–205. [Google Scholar]
- 4.Isaac B. The Invention of Racism in Classical Antiquity. Princeton, NJ: Princeton University Press; 2004. [Google Scholar]
- 5.Smith SS, Jordan W-D. An Essay on the Causes of the Variety of Complexion and Figure in the Human Species. Cambridge, MA: The Belknap Press of Harvard University Press; 1965. [Google Scholar]
- 6.Mitchell J, Collinson P. An essay upon the causes of the different colours of people in different climates. Philosophical Transactions(1683–1775. 1744;43:102–150. [Google Scholar]
- 7.Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 8.Zihlman A, Cohn BA. The adaptive response of human skin to the savanna. Hum Evol. 1988;3:397–409. [Google Scholar]
- 9.Folk GEJ, Jr, Semken HAJ., Jr The evolution of sweat glands. Int J Biometeorol. 1991;35:180–186. doi: 10.1007/BF01049065. [DOI] [PubMed] [Google Scholar]
- 10.Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 11.Montagna W. The consequences of having a naked skin. Birth Defects Orig Artic Ser. 1981;17:1–7. [PubMed] [Google Scholar]
- 12.Chimpanzee Sequencing and Analysis Consortium. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- 13.Moll R, Divo M, Langbein L. The human keratins: Biology and pathology. Histochem Cell Biol. 2008;129:705–733. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers AR, Iltis D, Wooding S. Genetic variation at the MC1R locus and the time since loss of human body hair. Curr Anthropol. 2004;45:105–124. [Google Scholar]
- 15.Roberts DF, Kahlon DPS. Environmental correlations of skin colour. Ann Hum Biol. 1976;3:11–22. doi: 10.1080/03014467600001101. [DOI] [PubMed] [Google Scholar]
- 16.Walter H. Remarks on the environmental adaptation of man. Humangenetik. 1971;13:85–97. doi: 10.1007/BF00295790. [DOI] [PubMed] [Google Scholar]
- 17.Chaplin G. Geographic distribution of environmental factors influencing human skin coloration. Am J Phys Anthropol. 2004;125:292–302. doi: 10.1002/ajpa.10263. [DOI] [PubMed] [Google Scholar]
- 18.Blum HF. Does the melanin pigment of human skin have adaptive value? An essay in human skin have adaptive value? An essay in human ecology and the evolution of race. Q Rev Biol. 1961;36:50–63. doi: 10.1086/403275. [DOI] [PubMed] [Google Scholar]
- 19.Loomis WF. Skin-pigment regulation of vitamin-D biosynthesis in man. Science. 1967;157:501–506. doi: 10.1126/science.157.3788.501. [DOI] [PubMed] [Google Scholar]
- 20.Ricotti C, Bouzari N, Agadi A, Cockerell CJ. Malignant skin neoplasms. Med Clin North Am. 2009;93:1241–1264. doi: 10.1016/j.mcna.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 21.MacKie RM, Hauschild A, Eggermont AMM. Epidemiology of invasive cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi1–vi7. doi: 10.1093/annonc/mdp252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nan H, Kraft P, Hunter DJ, Han J. Genetic variants in pigmentation genes, pigmentary phenotypes, and risk of skin cancer in Caucasians. Int J Cancer. 2009;125:909–917. doi: 10.1002/ijc.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: Skin pigment is not an essential regulator. Science. 1981;211:590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 24.Branda RF, Eaton JW. Skin color and nutrient photolysis: an evolutionary hypothesis. Science. 1978;201:625–626. doi: 10.1126/science.675247. [DOI] [PubMed] [Google Scholar]
- 25.Off MK, et al. Ultraviolet photodegradation of folic acid. J Photochem Photobiol B. 2005;80:47–55. doi: 10.1016/j.jphotobiol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Steindal AH, Juzeniene A, Johnsson A, Moan J. Photodegradation of 5-methyltetrahydrofolate: biophysical aspects. Photochem Photobiol. 2006;82:1651–1655. doi: 10.1562/2006-06-09-RA-915. [DOI] [PubMed] [Google Scholar]
- 27.Tam TTT, Juzeniene A, Steindal AH, Iani V, Moan J. Photodegradation of 5-methyltetrahydrofolate in the presence of Uroporphyrin. J Photochem Photobiol B. 2009;94:201–204. doi: 10.1016/j.jphotobiol.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Steindal AH, Tam TTT, Lu XY, Juzeniene A, Moan J. 5-Methyltetrahydrofolate is photosensitive in the presence of riboflavin. Photochem Photobiol Sci. 2008;7:814–818. doi: 10.1039/b718907a. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence VA. Demographic analysis of serum folate and folate-binding capacity in hospitalized patients. Acta Haematol. 1983;69:289–293. doi: 10.1159/000206909. [DOI] [PubMed] [Google Scholar]
- 30.Lamparelli RD, et al. Nutritional anaemia in pregnant coloured women in Johannesburg. S Afr Med J. 1988;73:477–481. [PubMed] [Google Scholar]
- 31.Buccimazza SS, Molteno CD, Dunne TT, Viljoen DL. Prevalence of neural tube defects in Cape Town, South Africa. Teratology. 1994;50:194–199. doi: 10.1002/tera.1420500304. [DOI] [PubMed] [Google Scholar]
- 32.Leck IAN. The geographical distribution of neural tube defects and oral clefts. Br Med Bull. 1984;40:390–395. doi: 10.1093/oxfordjournals.bmb.a072010. [DOI] [PubMed] [Google Scholar]
- 33.Han J, Colditz GA, Hunter DJ. Polymorphisms in the MTHFR and VDR genes and skin cancer risk. Carcinogenesis. 2007;28:390–397. doi: 10.1093/carcin/bgl156. [DOI] [PubMed] [Google Scholar]
- 34.Stover PJ. One-carbon metabolism-genome interactions in folate-associated pathologies. J Nutr. 2009;139:2402–2405. doi: 10.3945/jn.109.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Group MVSR MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 36.Fleming A, Copp AJ. Embryonic folate metabolism and mouse neural tube defects. Science. 1998;280:2107–2109. doi: 10.1126/science.280.5372.2107. [DOI] [PubMed] [Google Scholar]
- 37.Blom HJ, Shaw GM, den Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nat Rev Neurosci. 2006;7:724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem Biophys. 2004;41:415–434. doi: 10.1385/CBB:41:3:415. [DOI] [PubMed] [Google Scholar]
- 39.Schallreuter KU, Kothari S, Chavan B, Spencer JD. Regulation of melanogenesis—controversies and new concepts. Exp Dermatol. 2008;17:395–404. doi: 10.1111/j.1600-0625.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 40.Schallreuter KU. Advances in melanocyte basic science research. Dermatol Clin. 2007;25:283–291, vii. doi: 10.1016/j.det.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 41.John PR, Makova K, Li WH, Jenkins T, Ramsay M. DNA polymorphism and selection at the melanocortin-1 receptor gene in normally pigmented southern African individuals. Ann N Y Acad Sci. 2003;994:299–306. doi: 10.1111/j.1749-6632.2003.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 42.Makova K, Norton HL. Worldwide polymorphism at the MC1R locus and normal pigmentation variation in humans. Peptides. 2005;26:1901–1908. doi: 10.1016/j.peptides.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 43.Harding RM, et al. Evidence for variable selective pressures at MC1R. Am J Hum Genet. 2000;66:1351–1361. doi: 10.1086/302863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reich D, Thangaraj K, Patterson N, Price AL, Singh L. Reconstructing Indian population history. Nature. 2009;461:489–494. doi: 10.1038/nature08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonilla C, Gutiérrez G, Parra EJ, Kline C, Shriver MD. Admixture analysis of a rural population of the state of Guerrero, Mexico. Am J Phys Anthropol. 2005;128:861–869. doi: 10.1002/ajpa.20227. [DOI] [PubMed] [Google Scholar]
- 46.Murray FG. Pigmentation, sunlight, and nutritional disease. Am Anthropol. 1934;36:438–445. [Google Scholar]
- 47.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Mawer EB, Davies M. Vitamin D nutrition and bone disease in adults. Rev Endocr Metab Disord. 2001;2:153–164. doi: 10.1023/a:1010002710485. [DOI] [PubMed] [Google Scholar]
- 49.Chaplin G, Jablonski NG. Vitamin D and the evolution of human depigmentation. Am J Phys Anthropol. 2009;139:451–461. doi: 10.1002/ajpa.21079. [DOI] [PubMed] [Google Scholar]
- 50.Köstner K, et al. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009;29:3511–3536. [PubMed] [Google Scholar]
- 51.Norman AW. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 52.Holick MF. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 53.Yuen AWC, Jablonski NG. Vitamin D: In the evolution of human skin colour. Med Hypotheses. 2010;74:39–44. doi: 10.1016/j.mehy.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Lamason RL, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 55.Norton HL, et al. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol Biol Evol. 2007;24:710–722. doi: 10.1093/molbev/msl203. [DOI] [PubMed] [Google Scholar]
- 56.Lalueza-Fox C, et al. A melanocortin 1 receptor allele suggests varying pigmentation among Neanderthals. Science. 2007;318:1453–1455. doi: 10.1126/science.1147417. [DOI] [PubMed] [Google Scholar]
- 57.Sturm RA. Molecular genetics of human pigmentation diversity. Hum Mol Genet. 2009;18(R1):R9–R17. doi: 10.1093/hmg/ddp003. [DOI] [PubMed] [Google Scholar]
- 58.Caldwell MM, et al. Effects of increased solar ultraviolet radiation on terrestrial ecosystems. J Photochem Photobiol B. 1998;46:40–52. [Google Scholar]
- 59.Madronich S, McKenzie RL, Björn LO, Caldwell MM. Changes in biologically active ultraviolet radiation reaching the Earth's surface. J Photochem Photobiol B. 1998;46:5–19. doi: 10.1016/s1011-1344(98)00182-1. [DOI] [PubMed] [Google Scholar]
- 60.Chaplin G, Jablonski NG. Hemispheric difference in human skin color. Am J Phys Anthropol. 1998;107:221–223, discussion 223–224. doi: 10.1002/(SICI)1096-8644(199810)107:2<221::AID-AJPA8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 61.Brueckner GE. The variability of the sun’s ultraviolet radiation. Adv Space Res. 1981;1:101–115. [Google Scholar]
- 62.Fligge M, Solanki SK. The solar spectral irradiance since 1700. Geophys Res Lett. 2000;27:2157–2160. [Google Scholar]
- 63.Rottman G. In: Solar Variability and Planetary Climates. Calisesi Y, Bonnet R, Gray L, Langen J, Lockwood M, editors. New York: Springer; 2007. pp. 39–51. [Google Scholar]
- 64.Solanki SK, Usoskin IG, Kromer B, Schüssler M, Beer J. Unusual activity of the Sun during recent decades compared to the previous 11,000 years. Nature. 2004;431:1084–1087. doi: 10.1038/nature02995. [DOI] [PubMed] [Google Scholar]
- 65.Relethford JH. Hemispheric difference in human skin color. Am J Phys Anthropol. 1997;104:449–457. doi: 10.1002/(SICI)1096-8644(199712)104:4<449::AID-AJPA2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 66.Rothschild LJ. The influence of UV radiation on protistan evolution. J Eukaryot Microbiol. 1999;46:548–555. doi: 10.1111/j.1550-7408.1999.tb06074.x. [DOI] [PubMed] [Google Scholar]
- 67.Agar N, Young AR. Melanogenesis: A photoprotective response to DNA damage? Mutat Res. 2005;571:121–132. doi: 10.1016/j.mrfmmm.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 68.Routaboul C, Denis A, Vinche A. Immediate pigment darkening: Description, kinetic and biological function. Eur J Dermatol. 1999;9:95–99. [PubMed] [Google Scholar]
- 69.Suh KS, et al. A long-term evaluation of erythema and pigmentation induced by ultraviolet radiations of different wavelengths. Skin Res Technol. 2007;13:360–368. doi: 10.1111/j.1600-0846.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 70.Gibbs S, et al. Melanosome capping of keratinocytes in pigmented reconstructed epidermis—effect of ultraviolet radiation and 3-isobutyl-1-methyl-xanthine on melanogenesis. Pigment Cell Res. 2000;13:458–466. doi: 10.1034/j.1600-0749.2000.130608.x. [DOI] [PubMed] [Google Scholar]
- 71.Kollias N, et al. Erythema and melanogenesis action spectra in heavily pigmented individuals as compared to fair-skinned Caucasians. Photodermatol Photoimmunol Photomed. 1996;12:183–188. doi: 10.1111/j.1600-0781.1996.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 72.Tadokoro T, et al. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124:1326–1332. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- 73.Yamaguchi Y, et al. Human skin responses to UV radiation: Pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
- 74.Sheehan JM, Potten CS, Young AR. Tanning in human skin types II and III offers modest photoprotection against erythema. Photochem Photobiol. 1998;68:588–592. [PubMed] [Google Scholar]
- 75.Sheehan JM, Cragg N, Chadwick CA, Potten CS, Young AR. Repeated ultraviolet exposure affords the same protection against DNA photodamage and erythema in human skin types II and IV but is associated with faster DNA repair in skin type IV. J Invest Dermatol. 2002;118:825–829. doi: 10.1046/j.1523-1747.2002.01681.x. [DOI] [PubMed] [Google Scholar]
- 76.Miller AJ, Tsao H. New insights into pigmentary pathways and skin cancer. Br J Dermatol. 2009;162:22–28. doi: 10.1111/j.1365-2133.2009.09565.x. [DOI] [PubMed] [Google Scholar]
- 77.Robins AH. Biological Perspectives on Human Pigmentation. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- 78.Quevedo WC, Szabó G, Virks J. Influence of age and UV on the populations of dopa-positive melanocytes in human skin. J Invest Dermatol. 1969;52:287–290. [PubMed] [Google Scholar]
- 79.Herman J, Celarier E. TOMS Version 7 UV-erythemal exposure: 1978-1993. 1996 [Google Scholar]
- 80.Roberts DF. Human pigmentation: Its geographical and racial distribution and biological significance. J Soc Cosmet Chem. 1977;28:329–342. [Google Scholar]