Abstract
Darwinian evolution of humans from our common ancestors with nonhuman primates involved many gene–environment interactions at the population level, and the resulting human-specific genetic changes must contribute to the “Human Condition.” Recent data indicate that the biology of sialic acids (which directly involves less than 60 genes) shows more than 10 uniquely human genetic changes in comparison with our closest evolutionary relatives. Known outcomes are tissue-specific changes in abundant cell-surface glycans, changes in specificity and/or expression of multiple proteins that recognize these glycans, and novel pathogen regimes. Specific events include Alu-mediated inactivation of the CMAH gene, resulting in loss of synthesis of the Sia N-glycolylneuraminic acid (Neu5Gc) and increase in expression of the precursor N-acetylneuraminic acid (Neu5Ac); increased expression of α2–6-linked Sias (likely because of changed expression of ST6GALI); and multiple changes in SIGLEC genes encoding Sia-recognizing Ig-like lectins (Siglecs). The last includes binding specificity changes (in Siglecs -5, -7, -9, -11, and -12); expression pattern changes (in Siglecs -1, -5, -6, and -11); gene conversion (SIGLEC11); and deletion or pseudogenization (SIGLEC13, SIGLEC14, and SIGLEC16). A nongenetic outcome of the CMAH mutation is human metabolic incorporation of foreign dietary Neu5Gc, in the face of circulating anti-Neu5Gc antibodies, generating a novel “xeno-auto-antigen” situation. Taken together, these data suggest that both the genes associated with Sia biology and the related impacts of the environment comprise a relative “hot spot” of genetic and physiological changes in human evolution, with implications for uniquely human features both in health and disease.
Keywords: genomics, Siglec, Neu5Gc, Xeno-auto-antigen
The theory of evolution via descent by natural selection explains the diversity of life on earth (1). Huxley (2) and Darwin (3) correctly predicted that the “Great Apes” (chimpanzees, bonobos, gorillas and orangutans, i.e., Non-Human Hominids, NHHs*) are our closest evolutionary cousins. Indeed, chimpanzees were once considered good models for human disease. However, there are major differences between humans and NHHs in the incidence and severity of various diseases, beyond those explained by anatomical reasons (4–6).
Scholars of mathematical, physical, and chemical sciences sometimes ask why biology does not have the kinds of universal laws that underpin their disciplines. The reason is that although biological systems operate under mathematical, physical and chemical principles, evolutionary mechanisms of random mutation and deterministic selection do not generate consistent nor universal outcomes. Of course, a single origin of life combined with physical constraints resulted in some near-universals, such as the paradigm that nucleic acid sequences encode protein sequences (7). Another apparent biological universal is that all nucleated cells in nature are covered with a dense and complex coating of sugar chains (glycans) (8), which have numerous biological roles (9). Thus, natural selection repeatedly recruited glycans as being the best molecules for decorating the cell surface. Here I focus on one aspect of cellular glycan coating that changed during human evolution, potentially explaining aspects of human uniqueness, in health and in disease.
Sialic Acids Decorate the Canopy of the Cell-Surface Glycan Forest and Have Multiple Biological Roles
In the Deuterostome lineage (vertebrates and so-called “higher” invertebrates) the outer ends of glycan chains are often capped by sialic acids (Sias) (10, 11). Biosynthetic pathways for these nine-carbon backbone molecules likely evolved from those for ancestral nonulosonic acids (12). Although Sias are rare in other taxa (with the exception of certain pathogenic/commensal bacteria, as discussed later) they are ubiquitous on all vertebrate cell surfaces and are essential for embryonic development (13). Indeed, they mediate many critical endogenous functions by virtue of physical properties and via recognition by intrinsic receptors (10, 11). Also, cell-surface Sias are used by complement factor H (14) and by Sia-binding Ig-like lectins (Siglecs) (15, 16) as signals for “self” recognition in the vertebrate innate immune system. However, given their location and abundance (dozens to hundreds of millions of copies on each cell), Sias also are targets for extrinsic receptors of numerous pathogens (10). Meanwhile, Sias have been “re-invented” repeatedly via convergent evolution by microbes that interact with vertebrates (12, 17). Such “molecular mimicry” allows microorganisms to use Sias not only to mask themselves from the complement and adaptive immune systems (11, 14) but also to engage the Siglecs (as discussed later), dampening the innate immune response (18). For all these reasons, Sias are at the nexus of an evolutionary arms race between vertebrate hosts and their pathogens, interactions characterized by many “Red Queen” processes (8, 15). This competition may also explain why there are so many kinds of Sias, each presented in several different linkages to the underlying monosaccharide, on a variety of different types of glycans (10, 11).
“Serum Sickness” as a Clue to Human Uniqueness
Given the considerations discussed in the previous section, it is not surprising that differences in Sia expression are common between different taxa, even closely related ones. However, on closer inspection, such differences tend to be relative rather than absolute (19). One classic exception was a difficulty in finding the Sia N-glycolylneuraminic acid (Neu5Gc) in human tissues (20). Indeed, humans make antibody responses against Neu5Gc during “serum sickness reactions” induced by animal serum infusion, characterized by antibodies agglutinating animal red blood cells bearing Neu5Gc (21–23). However, Neu5Gc was detected in human cancers and fetal tissues (23).
A Sia Difference Between Humans and NHHs
Besides Neu5Gc, the other major Sia on most mammalian cell types is N-acetylneuraminic acid (Neu5Ac). These molecules differ by one oxygen atom, which is added to CMP-Neu5Ac in the cytosol, in a reaction catalyzed by the enzyme cytidine monophosphate N-acetylneuraminic acid hydroxylase (CMAH) (24, 25). Both CMP-Neu5Ac and CMP-Neu5Gc are transported into the Golgi, where they are donors for addition of these Sias to many glycoconjugates. Thus, most mammalian tissues contain both Sias. In contrast, Neu5Gc was claimed to be missing in normal human tissues (20). We showed that whereas all NHHs had easily detectable Neu5Gc in erythrocytes and plasma proteins, it was indeed missing from normal human blood samples (26). This human-specific difference was explained by deletion of a critical 92-base pair exon in the CMAH gene (27, 28)† encoding key amino acids required for enzymatic function. This single Alu-mediated mutation (29) occurred in one ancestral hominin CMAH gene, an allele now universal to humans. Timing was estimated to be ∼2–3 Mya (30), which is, interestingly, just before emergence of the genus Homo (31). Of course, any genomic signatures of selection are erased by such depths of evolutionary time.
Human-Specific Neu5Gc Loss Affects Pathogen Regimes
The loss of Neu5Gc and resulting excess of Neu5Ac (Fig. 1, step 1) would have affected relative efficacy of interactions of various pathogens with humans. Humans should be resistant to pathogens binding Neu5Gc (32–36) and more susceptible to pathogens preferring to bind Neu5Ac. Particularly interesting is a difference in erythrocyte Sia-binding preference between malarial parasites of humans and African NHHs (36). Indeed, we and others suggested that ancestral hominins escaped the prevailing NHH malaria by eliminating Neu5Gc production and that Plasmodium falciparum (today’s human “malignant malaria”) arose later, when a strain of the NHH malaria evolved to be able to bind preferentially to Neu5Ac-rich erythrocytes of humans (37, 38). Further studies of Neu5Gc and Neu5Ac preferences of human and nonhuman pathogens are warranted.
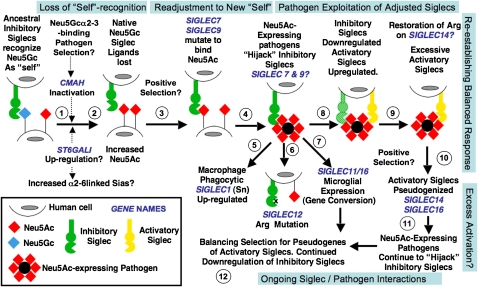
Fig. 1.
Proposed evolutionary scenario linking human-specific changes in Sia-related genes. It is impossible to conclusively prove evolutionary events and selection factors affecting Sia biology before the origin of modern humans. The speculative scenario presented here is based on available information and takes the parsimonious view that the events are related to one another. The first event may have been loss of Neu5Gc expression via CMAH inactivation and fixation (steps 1 and 2). A possible selection mechanism was a Neu5Gc-binding pathogen such as an NHH malaria, combined with genetic drift caused by ancestral demography. Because such organisms prefer binding α2–3-linked Sias, the increased human expression of α2–6-linked Sias may have been related. Human pathogen regimes would also have changed because of loss of Neu5Gc and excess of Neu5Ac. Some outcomes may have been positive (i.e., temporary escape from preexisting pathogens), and others may have been negative (e.g., increase susceptibility to Neu5Ac-binding pathogens and inability to modulate Neu5Gc/Neu5Ac ratios). Meanwhile, the loss of Neu5Gc should have resulted in loss of CD33rSiglec ligands needed for “self-recognition” (step 3). The likely hyperimmune state following Siglec ligand loss would have been followed by positive selection to allow multiple Siglecs (e.g., Siglec-9 and -7) to recognize Neu5Ac (step 4). Following this readjustment to the new “self,” a new risk would emerge. Although microbes appear incapable of synthesizing Neu5Gc, they have repeatedly reinvented Neu5Ac in multiple ways. Such pathogens would now be able to “hijack” inhibitory Siglecs such as Siglec-7 and -9, dampening the innate immune response of hominins (step 4). Indeed, several such organisms tend to be human-specific commensals. Notably, this proposed phase of pathogen exploitation of adjusted Siglecs is also the period of human evolution when newborns were becoming increasingly immature and more susceptible to these types of pathogens, especially those involved in brain invasion. Macrophage Siglec-1 might have then been up-regulated to enhance phagocytosis of Neu5Ac-expressing pathogens (step 5). Consequences of this proposed episode of pathogen exploitation of adjusted Siglecs could have been mutations of the Arg residue required for Sia recognition (Siglec-12, step 6) and the gene conversion event in Siglec-11 associated with recruitment to brain microglia (step 7). Eventually, immune cells would have down-regulated inhibitory Siglecs to escape the Neu5Ac-expressing pathogens while also up-regulating activatory Siglecs to respond to them (step 8). Perhaps this process explains why the critical Arg residue of the activatory Siglec-14 may have been restored in humans. This attempted reestablishment of a balanced response may have resulted in excessive activatory Siglecs (step 9), perhaps explaining the tendency of activatory Siglecs to be pseudogenized in modern humans (step 10). Of course, pathogens always evolve faster, and Neu5Ac-expressing pathogens are likely continuing to evolve to “hijack” our inhibitory Siglecs (step 11). Thus we likely have ongoing adjustments, with balancing selection for pseudogenes of the remaining activatory Siglecs and continued down-regulation of inhibitory Siglecs (step 12). It is also possible that these complex episodes of selection resulted in a changed profile of Siglec expression and function, not only in the innate immune system but also in other organs such as the placenta and the brain. Note that the human-specific changes in SIGLEC6 (placental trophoblast expression) and SIGLEC13 (deletion) are not incorporated into this model.
Differential Expression of α2–6-Linked Sias Between Humans and NHHs
Influenza viruses use Sias as binding targets, and strains infecting some other species do not easily “jump” into humans. However, this difference is not primarily explained by human Neu5Gc deficiency, because these viruses show only relative preferences for the two Sias (39). A bigger difference lies in the finding that although avian influenza viruses preferentially recognize Sias α2–3-linked to the underlying sugar chain, human viruses prefer the α2–6-linked variety (40). This difference corroborates with α2–6–linked Sia expression on human upper airways (41). Meanwhile, chimpanzees challenged with human influenza virus did not show severe infections (42). In keeping with this finding, we found low expression of α2–6-linked Sias in upper airways of NHHs (i.e., more similar to their expression in mice and birds) (43). This difference likely results from preferential human up-regulation of the enzyme ST6Gal-I, which determines expression of α2–6-linked Sias (44) in humans. One possibility is that malarial parasites that preferentially bind α2–3-linked Sias (45) could have selected for up-regulation of α2–6-linked Sias on ancestral hominin erythrocytes (43) and thus, secondarily, in other tissues (Fig. 1, step 1).
Siglecs Differences Between Humans and Non-Human Hominids
Siglecs are a family of Sia-binding proteins characterized by amino-terminal V-set Ig-like domains with a Sia-binding site (15, 16) followed by variable numbers of C2-set domains, a single transmembrane domain, and varying lengths of cytosolic tails that may or may not have signaling domains—typically immunoreceptor inhibitory tyrosine-based motifs (ITIMs), which can recruit the tyrosine phosphatases SHP-1 or SHP-2 and down-regulate cellular activation by antagonizing tyrosine kinase action (15, 16). Siglec homologs are present in most vertebrates (46) and seem prominent in primates (47). One subclass called “CD33-related Siglecs” (CD33rSiglecs) is rapidly evolving via multiple genomic processes (47). Multispecies genomic BAC sequencing of the CD33-related Siglec gene cluster (47) followed by chimpanzee genome sequencing made it possible to clone and characterize what may be all 16 hominid Siglecs. Remarkably, as discussed later, human-specific differences from other NHHs have been found in many CD33rSiglecs.
Human-Specific Adjustments in Sia Recognition by Siglecs
The ancestral condition of Hominid Siglecs appears to have been to recognize Neu5Gc preferentially (48). This supposition fits with the function of CD33rSiglecs to recognize Sias as “self” and send dampening signals to immune cells via cytosolic tail ITIMs (15, 16, 18). Because no pathogen has been reported to synthesize Neu5Gc, and many can synthesize Neu5Ac, Neu5Gc should indeed be the preferred molecule for “self” recognition. Thus, when human ancestral hominins lost Neu5Gc, many CD33rSiglecs would have lost their preferred ligand (Fig. 1, step 2), likely causing excessive innate immune cell activation. Although this loss may even have been beneficial in short-term defense, it would be eventually detrimental for reproductive fitness because of disease processes related to excessive immune responses. In keeping with this reasoning, human Siglecs studied show a preference for Neu5Ac over Neu5Gc (48). For this adjustment to occur, the V-set domain Sia-binding pockets of the CD33rSiglecs in ancestral hominins would have to be selected for multiple amino acid changes, switching either to specifically binding Neu5Ac or simply to accommodating it (Fig. 1, step 3). Indeed, sequence analyses indicate that this domain of CD33rSiglecs has undergone very rapid evolution in humans, even in comparison with relatively high rates in other taxa (49). Taken together, the data suggest (Fig. 1) that lethality caused by Neu5Gc-binding pathogen (perhaps α2–3-linked Sia preferring) first selected the CMAH-null mutation, eliminating host Neu5Gc production. The resulting loss of “self” ligands for the CD33rSiglecs would have likely caused a hyperimmune state, perhaps with a temporary advantage. The next stage would have involved selection for amino acid changes to allow binding of Neu5Ac, restoring CD33rSiglec inhibitory function (Fig. 1, step 3).
Many Human Pathogens Express Neu5Ac, Potentially Engaging CD33rSiglecs and Attenuating Innate Immune Responses
The switch of human Siglecs toward binding Neu5Ac (presumably selected to restore proper “self” recognition) would have exposed humans to pathogens that could “reinvent” Neu5Ac via convergent evolution, thus “hijacking” inhibitory Siglec function to dampen innate immune responses (Fig. 1, step 4, and later discussion). Indeed, many microorganisms that express Neu5Ac appear to be human-specific commensals, becoming pathogenic when circumstances allow (17). For example, Group B Streptococcus expresses a Sia-containing capsule that engages human neutrophil Siglec-9, dampening responses (18). Other sialylated pathogens are recognized by Siglecs (50), likely with similar outcomes (51). Notably, such pathogens would have been a strong selective force, because they often affect fetuses, infants and young adults and frequently cause lethal brain infections (17).
Human-Specific Changes in Sialoadhesin on Macrophages
Sialoadhesin (Sialec-1, Sn) is a Siglec with 17 extracellular Ig-like domains, all conserved from mouse to human (52). Also conserved is the amino terminal V-set domain, which (even in the mouse) does not recognize Neu5Gc but binds only Neu5Ac, and only in α2–3-and α2–8 linkages (52). Notably, Neu5Ac in α2–3 and α2–8 linkages are also the structures typically found on pathogens (17). Furthermore, Sn is found primarily on macrophages, does not have a cytosolic signaling motif, and phagocytoses sialylated bacteria (50). Thus, although Sn has a role in modulating adaptive immunity (53), a likely conserved function is to eliminate sialylated pathogens. Indeed, Sn in rodents is found at sites such as the sinuses of lymph nodes, spleen, and bone marrow that would first encounter bacteria invading extracellular fluids (52) which filter blood or lymph-borne pathogens. In keeping with the human propensity for invasion by Neu5Ac-expressing pathogens, Sn is up-regulated in the human spleen compared with the chimpanzee (54). In the chimpanzee, as in the rodent, only a subset of splenic macrophages is Sn positive, whereas in humans the distribution is more widespread (54). Although more work is needed, current data suggest that Sn expression was up-regulated in humans, perhaps to deal with sialylated pathogens taking advantage of the Neu5Ac-preferring human CD33rSiglecs (Fig. 1, step 5). Interestingly, Sn is also up-regulated following inflammatory responses and in autoimmune diseases (55) and has an additional role as a capture mechanism for certain viruses that have heavily sialylated envelope glycoproteins (56). In keeping with this notion, Sn-positive circulating monocytes may facilitate HIV entry into mac-rophages (57), a viral invasion process prominent in humans.
Human-Specific Changes in a Conserved Arginine Residue Required for Siglec Recognition of Sias
All Siglecs studied to date have a conserved arginine (Arg) residue in the V-set domain, essential for Sia binding (15, 16). This Arg residue underwent a human-specific mutation in Siglec-12, a CD33rSiglec found on macrophages and epithelial surfaces (58). Interestingly, restoration of the Arg residue regenerates binding with a preference for Neu5Gc (58), suggesting that this Siglec may have been “retired” following human loss of Neu5Gc (Fig. 1, step 6). In the second instance, as discussed later, the Arg residue of Siglec-5 and Siglec-14 appears to be mutated in all NHHs, but restored in humans (59).
Human-Specific Gene Conversion Involving Siglec-11
The gene encoding Siglec-11 is ∼1 megabase away from the CD33rSiglec gene cluster on chromosome 19 (60) but has features of a CD33rSiglec, with a Sia-binding amino-terminal V-set domain and ITIM motifs in the cytosolic tail (60). The 5′ sequences of the SIGLEC11 encoding the first two Ig-like domains showed a >99% similarity to the corresponding 5′ end of a nearby Siglec pseudogene SIGLECP16 (61). There is far less similarity in the rest of the sequences. Based on these and other data, we concluded that the SIGLEC11 gene underwent a gene conversion by the 5′ sequences of SIGLECP16, generating a protein with a human-specific amino acid sequence (61). Indeed, this gene conversion is not seen in the NHH Siglec-11 orthologs (61). Moreover, it is human universal, indicating possible selection following gene conversion (Fig. 1, step 7). One consequence is a change in binding specificity toward a preference for Neu5Ac over the ancestral preference for Neu5Gc. Another consequence is that, although Siglec-11 is expressed in both human and chimpanzee tissue macrophages, it is selectively expressed in brain microglia only in humans (61). This unusual brain expression could be related to the propensity of sialylated pathogens to invade the human brain and/or the fact that microglia have multiple roles in the brain beyond innate immunity (62).
In some humans the pseudogene SIGLEC16P locus can instead encode the functional gene sequence SIGLEC16 (63), a molecule with potential activatory properties (as discussed later). Thus, some humans may have an activatory Siglec in brain microglia, and others may not. The population distribution of this segregating pseudo(gene) deserves further study. Consequences for microglia in human brain function and/or disease also need study.
Human-Specific Expression of Siglec-6 in the Placental Trophoblast with Up-Regulation in Preeclampsia
Siglec-6 is an inhibitory CD33rSiglec expressed on B cells of both humans and NHHs (64). However, it also shows human-specific placental expression, not in immune cells but in the trophoblast (64). Placental expression is maximal following human labor and delivery (64), suggesting a possible role in modulating the unusual tempo of human labor, which lasts much longer in humans than in the NHHs (64).
Preeclampsia is a human-specific pregnancy complication of unknown cause, characterized by hypertension, proteinuria, and vascular abnormalities in the placenta leading to fetal dysfunction and early labor (65). In a microarray comparison of placental mRNAs, one of the genes showing the highest expression increase in preeclampsia was SIGLEC6 (65). It is interesting that both placental expression of Siglec-6 and preeclampsia itself are uniquely human phenomena. Many functional studies are needed, including analyses of placental Siglec-6 ligands (64).
CD33rSiglecs Are Expressed at Low Levels on Human T Cells Associated with Overreactive Responses to Activation
Although CD33rSiglecs are found on most human immune cells, essentially no expression was found on CD4+ T cells, and only low expression of Siglec-7 and -9 was found on CD8+ T cells (15, 16, 66). In contrast, there was easily detectable expression of multiple CD33rSiglecs (particularly Siglec-5) on all NHH T cells examined (67). Thus, suppression of inhibitory CD33rSiglec expression is a human-specific condition, perhaps related to the need to escape Neu5Ac-expressing pathogens (Fig. 1, step 8). Regardless of the reason, we found that human T cells reacted more strongly to stimulation (67, 68). Down-regulation of Siglec-5 on the chimpanzee T cells allowed more proliferation, and forced expression in human T cells dampened responses (67). Thus, the human T cell is in a relatively overreactive state, a least partly because of lack of Siglec-5 expression. In this regard, humans seem more prone to diseases involving T-cell activation, including AIDS (69), chronic hepatitis (70), rheumatoid arthritis, and bronchial asthma (4). This relative overreactivity may also explain T-cell activation and excessive release of cytokines (a “cytokine storm”) reported in human volunteers given a superactive anti-CD28 antibody (71) and the excessive human immune reactions in viral vector-based gene therapy trials (72). More recently, we have found that human B cells are also relatively overreactive, compared with chimpanzee cells (68). Further studies are obviously needed, including any roles of activatory Siglecs (as discussed later).
Human-Specific Pseudogenization of Activatory Siglecs
Some primate Siglecs have a charged residue in the transmembrane domain and lack a major cytosolic tail. In at least two known instances (Siglecs -14 and -16) (59, 63) these molecules associate with the adaptor DAP-12, recruiting its immunoreceptor tyrosine-based activatory motifs (ITAMs) and effectively converting them into activatory Siglecs. Interestingly, Siglec-14 is undergoing repeated 5′-end gene conversions with Siglec-5, so that its Sia-binding specificity remains the same (59). This feature is also true of Siglec-16, because of gene conversion with Siglec-11 (61). Analogous to “paired” inhibitory and activatory killer Ig-like receptors (KIR) (73), the most likely explanation is that activatory Siglecs were originally selected to respond against Sia-expressing pathogens that were using inhibitory Siglecs to suppress immune responses (Fig. 1, steps 8 and 9). Interestingly, both Siglec-14 and Siglec-16 are pseudogenized in some humans (63, 74). Additionally, Siglec-13 has potential for being an activatory Siglec and has been deleted in the human genome (47). Overall, there were apparently multiple human-unique pseudogenization events involving activatory Siglecs. Perhaps an evolutionary episode of excessive CD33Siglec-mediated activation resulted in the need to re-establish a balanced response (Fig. 1, step 10). Of course, pathogens are always ahead in an evolutionary arms race, and humans may still be in a period of ongoing adjustments, involving continued “hijacking” of inhibitory Siglecs (Fig. 1, step 11) and balancing selection for pseudogenization of activatory Siglecs (Fig. 1, step 12).
Was Sia-Related Biology a “Hotspot” of Genetic and Physiological Changes in Human Evolution?
The high frequency of human-specific genetic changes associated with Sia biology is unexpected. Although some of these genes (e.g., Siglecs) are rapidly evolving in all taxa, the frequency of uniquely human changes seems unusually high compared with other species. For example, mouse and rat Siglecs appear nearly identical, and differences among NHHs and other old world primates seem limited so far (47). Secondly, less than 60 genes are known to be directly involved in Sia biology (49). Thus, one biochemical/biological pathway has almost 20% of its genes showing human-specific evolution. Overall, it is reasonable to suggest that Sia biology and Sia-related genes are a “hotspot” for genetic and physiological changes in human evolution. It is parsimonious to assume initially that these all of these genetic changes are related to one another, as suggested in the scenario in Fig. 1. Although several aspects are clearly speculative, the scenario is supported by available facts and includes testable concepts and hypotheses.
Metabolic Incorporation of Neu5Gc into Human Cells and a Dietary Source of Neu5Gc in Human Tissues
We also discovered an unusual nongenetic consequence of CMAH loss (Fig. 2). Although Neu5Gc was reported in human cancers and fetal samples (suggesting an “oncofetal” antigen) (23), the CMAH mutation damages the enzyme’s active site (27, 28), and which cannot be repaired. Also, a mouse with a human-like Cmah mutation showed no endogenous Neu5Gc (75). Absent an alternate pathway for Neu5Gc synthesis, the sugar must enter from external sources. Indeed, cultured human cells express Neu5Gc because of uptake and metabolic incorporation from animal products in the medium (e.g., FCS) (76, 77). This process involves macropinocytosis, delivery to the lysosome, and export of free Neu5Gc to the cytosol via the sialin transporter (77). Once Neu5Gc reaches the human cytosol, it is a molecular “Trojan horse.” Differing by only one oxygen atom from endogenous Neu5Ac and having been eliminated only recently in evolutionary time, Neu5Gc is handled by human biochemical pathways as if it were native. Indeed, one can feed Neu5Gc to human cells and make them look like NHH cells (77, 78).
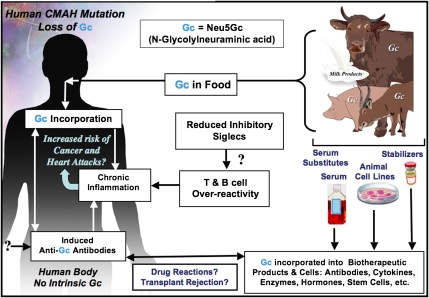
Fig. 2.
Two mechanisms for enhanced chronic inflammation and immune reactions in humans. Metabolic incorporation of dietary Neu5Gc (Gc) from mammalian foods in the face of circulating anti-Neu5Gc antibodies may contribute to chronic inflammation in endothelia lining blood vessels and in epithelia lining hollow organs, perhaps contributing to the increased risks of cardiovascular disease and carcinomas associated with these foods. The apparent T- and B-cell overreactivity of humans associated with decreased inhibitory Siglec expression may contribute further toward chronic inflammation. Also shown is that the fact that some molecular and cellular products of biotechnology are likely contaminated with Neu5Gc from multiple sources, potentially contributing to untoward reactions in some individuals.
Classic studies showed that chickens generate a strong IgY antibody response against Neu5Gc (23). Using a more specific version of such polyclonal antibodies and adding mass spectrometry to be certain (79), we confirmed the presence of Neu5Gc in human tumors and in fetal tissues (76). Surprisingly, we also found smaller amounts in normal human tissues (76). The likely explanation is a dietary origin. Voluntary Neu5Gc ingestion studies confirmed that humans could indeed take up Neu5Gc (76).
Anti-Neu5Gc Antibodies in Humans Are of Broad and Highly Variable Specificities
Why should it matter that human tissues express small amounts of Neu5Gc derived from dietary sources? Although human biochemical pathways do not see Neu5Gc as foreign, it is detected as such by the immune system. Thus, contrary to prior work that used limited methodologies, we find anti-Neu5Gc antibodies circulating in all normal humans. In fact, some individuals have very high levels (80), including complement-fixing IgGs capable of activating and/or killing cells expressing Neu5Gc (78). In this situation, a xeno-antigen can become metabolically incorporated into tissues, even while it is detected as being foreign by B cells. Thus, we call Neu5Gc a “xeno-auto-antigen” in humans (81).
Limited Distribution of Neu5Gc in Foods and Disease Risks Associated with Red Meat
Because Sias are not found in plants, and Neu5Gc is not synthesized by microbes, the dietary source of Neu5Gc must be foods of animal origin. Major sources appear to be red meats (i.e., lamb, pork, and beef) and, to a lesser extent, milk products (76). In contrast, Neu5Gc is not found in poultry, and amounts in fish seem to be low (76). Thus, within limits of current analyses, the primary source of human tissue Neu5Gc appears to be foods of mammalian origin. In this regard, many epidemiological studies have shown an association of red meat ingestion with increased risk for various diseases, including carcinomas (82–84), atherosclerosis (82, 84), type-2 diabetes (85), and age-dependent macular degeneration (86). Although there are other theories for how red meat consumption aggravates these diseases, most of these notions (other than the role of saturated fats in atherosclerosis) are unproven. We suggest that metabolic incorporation of dietary Neu5Gc in the face of anti-Neu5Gc antibodies contributes to red meat aggravation of diseases by stimulating chronic inflammation (79, 81).
Anti-Neu5Gc Antibodies Enhance Growth of Neu5Gc-Positive Tumors in Neu5Gc-Null Mice
Human carcinomas efficiently accumulate dietary Neu5Gc for multiple reasons, including up-regulation of lysosomal Sia transport by hypoxia (87) and enhanced macropinocytosis caused by growth factor activation. This accumulation occurs in the face of anti-Neu5Gc antibody responses, which are enhanced in such patients (23). This combination suggests an immune reaction insufficient to kill the tumor that may, instead, stimulate it. Indeed, antibody-mediated inflammation is known to facilitate tumor progression by recruiting inflammatory cells, which stimulate angiogenesis and provide growth factors (88). We mimicked the human situation using Neu5Gc-null mice bearing a syngeneic mouse tumor line that expresses low levels of Neu5Gc, similar to human tumors. Indeed, passively transferred anti-Neu5Gc immune serum from syngeneic Neu5Gc-null mice increased tumor growth rates associated with inflammation and angiogenesis (79), and these effects were blocked by a COX-2 inhibitor, a drug type that reduces human tumor incidence (79). Of course high levels of these antibodies may instead kill tumor cells, and it is possible that persons with very high anti-Neu5Gc antibodies are protected from some cancers. Indeed, can we harness human anti-Neu5Gc antibodies to target human cancers specifically?
Surprising Differences Between Human and Chimpanzee Heart Disease
The commonest cause of death in both humans and captive chimpanzees is “heart disease,” manifested either as sudden “heart attacks” or as progressive heart failure (89, 90). However, early case reports suggested that the diseases in humans and chimpanzees are different, and recent studies have confirmed this notion (89, 90). Chimpanzees and other NHHs develop a progressive fibrotic replacement of the heart muscle (interstitial myocardial fibrosis), which can cause sudden death by altering heart rhythm or slower death by progressive cardiac failure. “Heart disease” in humans is different, caused by deposition of cholesterol in atherosclerotic plaques in the walls of large blood vessels, including coronary arteries (81, 90). This deposition results in sudden or progressive loss of blood supply, explaining the common “heart attack” of humans (“myocardial infarction”) or progressive heart failure caused by “ischemic heart disease.” Although captive chimpanzees and others NHHs do have atherosclerosis (90), myocardial infarction and ischemic heart disease are rare, despite risk factors such as hypertension (91) and high levels of LDL cholesterol and lipoprotein a (90). Why do NHHs not often have the kind of heart disease common in humans? Conversely, why do humans not often suffer from the fibrotic heart disease so common in our closest evolutionary cousins?
Human-Specific Xeno-Auto-Antibody Reaction Against Endothelium: A Contributing Role in Atherosclerosis?
For unclear reasons, accumulation of dietary Neu5Gc in human tissues is not uniform, and it tends to accumulate particularly in epithelial cells lining hollow organs (where carcinomas develop) or in the endothelium lining blood vessels (where atherosclerosis occurs). In fact, cultured endothelial cells fed with Neu5Gc (with Neu5Ac as a negative control) bind anti-Neu5Gc antibodies and deposit complement from human serum, resulting in cellular activation, expression of adhesion molecules, and binding of monocytes (81). Thus, although underlying mechanisms exist for many vascular diseases, we suggest that endothelial incorporation of Neu5Gc combines with circulating anti-Neu5Gc antibodies to aggravate processes such as atherosclerosis (81). Indeed, human atherosclerotic lesions show Neu5Gc accumulation not just in overlying endothelium but also inside the plaque (81). This Neu5Gc accumulation may facilitate production of anti-Neu5Gc antibodies and further aggravate chronic inflammation in atherosclerosis progression. Thus, this xeno-auto-antigen/antibody process may be an additional explanation for the increased atherosclerosis risk of consuming red meats and milk products.
A Role for Neu5Gc in Red Meat-Related Food Poisoning?
Because Neu5Gc is present in some human cells, are we really resistant to Neu5Gc-binding pathogens? The typical low-affinity, high-avidity binding of pathogens to glycans seems unlikely to succeed when Neu5Gc molecules are rare on a human cell surface. An exception may arise when Neu5Gc is targeted by a multivalent toxin with relatively high affinity (92). Dietary Neu5Gc loads up epithelial and endothelial cells over time. Subsequent exposure to meat or milk products contaminated with SubAB toxin-expressing Escherichia coli would then allow the toxin to bind to gut epithelium, gain access to the blood stream, and target the kidney endothelium, giving a hemolytic-uremic syndrome (92). The process may be facilitated by the fact that (unlike the cows in which this toxin is usually found) humans do not have circulating Neu5Gc-containing glycoproteins to act as natural toxin inhibitors (92). Thus, we speculate that individuals who consume large amounts of red meat and milk may not only increase their risk for this type of food poisoning but also preprepare their tissues for attack by the toxin (93).
Was the Neu5Gc Xeno-Auto-Antigen Phenomenon Significant in Human Evolution?
Hunting and red meat consumption along with cooking very likely played a supporting role in the emergence of the genus Homo (94, 95), and milk consumption was positively selected in some human civilizations (96). Indeed, these foods continue to be a vital source of important nutrients for currently undernourished populations. It should be noted that most diseases associated with red meat and/or milk consumption would not have affected natural selection in times past, because they are manifest primarily after the age of peak reproductive fitness. We now live much longer and have much greater access to red meat and milk, thus transforming these once beneficial foods into likely culprits for exacerbating diseases of older humans (94).
Potential Roles of Sia-Related Changes in Uniquely Human Disease Propensities
We have here discussed multiple potential mechanisms by which uniquely human changes in Sia biology could contribute to such uniquely human disease phenotypes. Although many of the hypotheses are speculative and need further exploration, most are testable either by modeling in Neu5Gc-deficient and/or Siglec-modified mice or by studies in human subjects and human populations. Some of these issues are summarized in Fig. 2, along with reference to another area that deserves attention—the contamination of molecular and cellular biotherapeutic products by Neu5Gc derived from nonhuman sources.
Future Directions
This work has generated even more questions than answers. Apart from issues already discussed, some others are briefly discussed below.
Population Genetics and Polymorphisms of Siglecs.
Siglecs -12, -14, and -16 are partially pseudogenized (i.e., expressed as active and inactive alleles) in the human population (58, 63, 74). Do any of these instances represent balanced polymorphisms, and are there more examples? Further studies must address allele distribution in various populations and consider associations with risk of diseases. Additional population-level studies of all Siglecs in NHHs are also warranted, not only to reaffirm that some changes are human specific but also to see whether additional differences and/or polymorphisms exist.
Siglecs in Bacterial Pathogenesis.
Details of how Neu5Ac-expressing pathogens suppress immune responses via inhibitory Siglecs (18) are as yet unknown. Protein–protein interactions between bacteria and human Siglecs can also mediate similar processes (97). Meanwhile, the role of the activatory Siglecs in bacterial pathogenesis is postulated to be the opposite, but this notion needs proof. The potential role of Sn in clearing sialylated pathogens also needs further evaluation. We may well be looking at the “tip of the iceberg” regarding roles of Siglecs in bacterial pathogenesis.
What is the Fate of Orally Ingested Neu5Gc?
We need to know mechanisms by which Neu5Gc is absorbed from the human gut and delivered to tissues. Early studies in rodents showed that the fate of ingested Neu5Gc may differ, based on the form in which it is presented (98). We can now study these issues by feeding Cmah-null mice different forms of Neu5Gc and looking at its fate in the gut, body fluids, and tissues. At this time, we cannot assume that ingestion of a certain amount of Neu5Gc will deliver a corresponding amount to tissues. A related issue is the fate of Neu5Gc during food processing and cooking.
Mechanisms of Anti-Neu5Gc Antibody Induction.
We are studying the tempo and mode of appearance of these highly variable antibodies in human samples and the potential mechanisms for their induction, using Cmah-null mice as a model. We also need to address whether Neu5Gc-containing glycans are truly T-cell–independent antigens, whether the antibody response involves a germline V-set domain, and if the antibody-binding pockets undergo affinity maturation. A related issue is whether these antibodies have any positive value (e.g., potentially protecting against enveloped viruses originating from other species).
Prognostic Value of Anti-Neu5Gc Antibodies.
The highly variable anti-Neu5Gc antibody response of humans is further complicated because Neu5Gc itself is not the entire epitope recognized (i.e., the underlying glycan structures to which it is attached influences binding specificity). Thus, there are many possible Neu5Gc epitopes, and each human has a different response to each of them (80). Because some of these epitopes are differentially expressed in different tissues, only some of the antibodies may have pathogenic roles, and the antibody subclasses may also make a difference. Perhaps one or more of these anti-Neu5Gc-antibodies will prove to be a predictive, prognostic, or diagnostic marker for one or more diseases. We are pursuing this possibility using a glycan microarray that contains matched Neu5Gc and Neu5Ac glycans as targets.
Complexity of the “Sialome” in the Cell Surface.
The manner in which Sias are presented within the context of a complex cell-surface “landscape” can affect the way they interact with Sia-binding proteins (99). In other words, such proteins recognize not only linear glycan sequences but also more complex structures presented on “clustered saccharide patches” (100) on cell surfaces, involving glycans of different types (99). Thus, even specific epitopes in glycan arrays may not be representative of the “sialome” at the cell surface. These considerations apply not only to Siglecs but also to anti-Neu5Gc antibody epitopes. Another unexplored issue is whether loss of CMP-Neu5Gc in the Golgi has other consequences for competing biosynthetic pathways (e.g., we found an increase in Sia O-acetylation in the Cmah-null Neu5Gc-deficient mouse) (75). Finally, relative differences in biophysical properties between Neu5Gc and Neu5Ac could have consequences. Overall, the Sia biology changes in humans could alter more cell phenomena than we can currently imagine. One approach to exploring this issue is to feed different types of human cells with Neu5Gc (or Neu5Ac as a control) and then study interactions of anti-Neu5Gc antibodies or Siglecs, looking for differential binding by these proteins that cannot be explained by cell-surface glycan sequences.
Additional Phenotypes of CMAH-null mice.
The genomic lesion in our Cmah-null mice is almost identical to that of humans (75). The mice are viable and capable of reproduction, a situation that is not surprising, because the same is true of humans. Further studies of fertility are underway to look for any subtler differences. We have already reported that these mice show delayed wound healing and age-dependent hearing loss, similar to humans (75). We have preliminary evidence of metabolic differences that also deserve further study. Detailed neurobiological and cognitive studies are required to see if any known differences between human and NHH brains might be manifest. Of course, mice shared a common ancestor with primates more than 60 Mya, and the impact of this biochemical change in a rodent brain may not necessarily reflect what occurred in a hominid ancestor ∼2–3 Mya. In this regard, it is fascinating that, even in animals with intact CMAH genes, the levels of brain Neu5Gc expression always seem very low (20).
Conclusions and Perspectives
The fact that so many genes related to Sia biology show human-specific differences from NHHs supports the notion that this system was a “hot spot” for evolutionary changes in the human lineage. Discussed here are some specific ways in which these changes would have impacted the immune system and human pathogen regimes. Although this discussion focuses on current human diseases, it also suggests a role for infectious diseases during human evolution. Of course, Sias and Siglecs are involved in many other biological pathways. Thus, Sia-related differences between humans and NHHs are worthy of continued investigation.
This Sackler Symposium focused on understanding “The Human Condition” “In the Light of Evolution.” Since we reported these genetic differences between humans and NHHs (28), many others have been found (101, 102). Any explanation of human evolution and the human condition must take into account all the available data. Indeed, there are many approaches to anthropogeny (explaining the origin of humans) (101, 102), including studies of the fossil and archaeological record since our last common ancestors with other primates; exploring the impact of the environment (biological, physical, and cultural) on humans and other animals; comparisons of the ontogeny of each species; and, of course, species comparisons. All these approaches must be combined in a transdisciplinary manner if we are eventually to explain human origins and human uniqueness.
Acknowledgments
I gratefully acknowledge helpful comments from Miriam Cohen, Sandra Diaz, Jeff Esko, Pascal Gagneux, Chris Gregg, and Nissi Varki and Dr. Gagneux's contributions to drawing the figures.
Footnotes
Conflict of interest statement: The author is Co-Founder of Gc-Free, Inc. a startup biotechnology company interested in the biomedical implications of human Neu5Gc deficiency.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution IV: The Human Condition,” held December 10–12, 2009, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/SACKLER_Human_Condition.
This article is a PNAS Direct Submission.
*The term “Great Ape” refers to chimpanzees, bonobos, gorillas, and orangutans, and the term “Hominoid” also includes lesser apes. Neither term is now taxonomically valid. The term “Hominid” is now being used for the clade including humans and “Great Apes.” I here mostly use the term “Non-Human Hominid” (NHH) in place of “Great Ape” and the term “Hominin” for branches of the human-like lineages after the common ancestor with chimpanzees.
†There is a discrepancy between the two original reports, one of which claimed N-terminal protein truncation (27), and the other, which concluded that the N terminus is intact, and a frame shift resulted (28) from the 92-base pair exon deletion. The second scenario appears more likely correct, as the first assumed an “in frame” start codon.
References
- 1.Darwin C. On the Origin of Species by Means of Natural Selection. London: J. Murray; 1859. [Google Scholar]
- 2.Huxley TH. Evidence as to Man’s place in Nature. London: Williams and Norgate; 1863. [Google Scholar]
- 3.Darwin C. The Descent of Man, and Selection in Relation to Sex. New York: D. Appleton; 1871. [Google Scholar]
- 4.Varki A. A chimpanzee genome project is a biomedical imperative. Genome Res. 2000;10:1065–1070. doi: 10.1101/gr.10.8.1065. [DOI] [PubMed] [Google Scholar]
- 5.Varki A, Altheide TK. Comparing the human and chimpanzee genomes: Searching for needles in a haystack. Genome Res. 2005;15:1746–1758. doi: 10.1101/gr.3737405. [DOI] [PubMed] [Google Scholar]
- 6.Finch CE. Evolution in Health and Medicine Sackler Colloquium: Evolution of the human lifespan and diseases of aging: Roles of infection, inflammation, and nutrition. Proc Natl Acad Sci USA. 2010;107(Suppl 1):1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crick F. Central dogma of molecular biology. Nature. 1970;227:561–563. doi: 10.1038/227561a0. [DOI] [PubMed] [Google Scholar]
- 8.Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126:841–845. doi: 10.1016/j.cell.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Varki A, Lowe JB. Biological roles of glycans. In: Varki A, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. pp. 75–88. [Google Scholar]
- 10.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 11.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis AL, et al. Innovations in host and microbial sialic acid biosynthesis revealed by phylogenomic prediction of nonulosonic acid structure. Proc Natl Acad Sci USA. 2009;106:13552–13557. doi: 10.1073/pnas.0902431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarzkopf M, et al. Sialylation is essential for early development in mice. Proc Natl Acad Sci USA. 2002;99:5267–5270. doi: 10.1073/pnas.072066199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pangburn MK, Pangburn KL, Koistinen V, Meri S, Sharma AK. Molecular mechanisms of target recognition in an innate immune system: Interactions among factor H, C3b, and target in the alternative pathway of human complement. J Immunol. 2000;164:4742–4751. doi: 10.4049/jimmunol.164.9.4742. [DOI] [PubMed] [Google Scholar]
- 15.Varki A, Angata T. Siglecs—the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 16.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 17.Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev. 2004;68:132–153. doi: 10.1128/MMBR.68.1.132-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlin AF, et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanetta JP, et al. Diversity of sialic acids revealed using gas chromatography/mass spectrometry of heptafluorobutyrate derivatives. Glycobiology. 2001;11:663–676. doi: 10.1093/glycob/11.8.663. [DOI] [PubMed] [Google Scholar]
- 20.Gottschalk A. The Chemistry and Biology of Sialic Acids and Related Substances. Cambridge, UK: Cambridge Univ Press; 1960. [Google Scholar]
- 21.Higashi H, Naiki M, Matuo S, Okouchi K. Antigen of “serum sickness” type of heterophile antibodies in human sera: Identification as gangliosides with N-glycolylneuraminic acid. Biochem Biophys Res Commun. 1977;79:388–395. doi: 10.1016/0006-291x(77)90169-3. [DOI] [PubMed] [Google Scholar]
- 22.Merrick JM, Zadarlik K, Milgrom F. Characterization of the Hanganutziu-Deicher (serum-sickness) antigen as gangliosides containing n-glycolylneuraminic acid. Int Arch Allergy Appl Immunol. 1978;57:477–480. doi: 10.1159/000232140. [DOI] [PubMed] [Google Scholar]
- 23.Malykh YN, Schauer R, Shaw L. N-Glycolylneuraminic acid in human tumours. Biochimie. 2001;83:623–634. doi: 10.1016/s0300-9084(01)01303-7. [DOI] [PubMed] [Google Scholar]
- 24.Shaw L, Schauer R. Detection of CMP-N-acetylneuraminic acid hydroxylase activity in fractionated mouse liver. Biochem J. 1989;263:355–363. doi: 10.1042/bj2630355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takematsu H, et al. Reaction mechanism underlying CMP-N-acetylneuraminic acid hydroxylation in mouse liver: Formation of a ternary complex of cytochrome b5, CMP-N-acetylneuraminic acid, and a hydroxylation enzyme. J Biochem(Tokyo) 1994;115:381–386. doi: 10.1093/oxfordjournals.jbchem.a124347. [DOI] [PubMed] [Google Scholar]
- 26.Muchmore EA, Diaz S, Varki A. A structural difference between the cell surfaces of humans and the great apes. Am J Phys Anthropol. 1998;107:187–198. doi: 10.1002/(SICI)1096-8644(199810)107:2<187::AID-AJPA5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273:15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 28.Chou HH, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayakawa T, Satta Y, Gagneux P, Varki A, Takahata N. Alu-mediated inactivation of the human CMP- N-acetylneuraminic acid hydroxylase gene. Proc Natl Acad Sci USA. 2001;98:11399–11404. doi: 10.1073/pnas.191268198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou HH, et al. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci USA. 2002;99:11736–11741. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood B, Collard M. The human genus. Science. 1999;284:65–71. doi: 10.1126/science.284.5411.65. [DOI] [PubMed] [Google Scholar]
- 32.Kyogashima M, Ginsburg V, Krivan HC. Escherichia coli K99 binds to N-glycolylsialoparagloboside and N-glycolyl-GM3 found in piglet small intestine. Arch Biochem Biophys. 1989;270:391–397. doi: 10.1016/0003-9861(89)90042-8. [DOI] [PubMed] [Google Scholar]
- 33.Schwegmann-Wessels C, Herrler G. Sialic acids as receptor determinants for coronaviruses. Glycoconj J. 2006;23:51–58. doi: 10.1007/s10719-006-5437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolsma MD, Kuhlenschmidt TB, Gelberg HB, Kuhlenschmidt MS. Structure and function of a ganglioside receptor for porcine rotavirus. J Virol. 1998;72:9079–9091. doi: 10.1128/jvi.72.11.9079-9091.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campanero-Rhodes MA, et al. N-glycolyl GM1 ganglioside as a receptor for simian virus 40. J Virol. 2007;81:12846–12858. doi: 10.1128/JVI.01311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin MJ, Rayner JC, Gagneux P, Barnwell JW, Varki A. Evolution of human-chimpanzee differences in malaria susceptibility: Relationship to human genetic loss of N-glycolylneuraminic acid. Proc Natl Acad Sci USA. 2005;102:12819–12824. doi: 10.1073/pnas.0503819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rich SM, et al. The origin of malignant malaria. Proc Natl Acad Sci USA. 2009;106:14902–14907. doi: 10.1073/pnas.0907740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varki A, Gagneux P. Human-specific evolution of sialic acid targets: Explaining the malignant malaria mystery? Proc Natl Acad Sci USA. 2009;106:14739–14740. doi: 10.1073/pnas.0908196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki Y, et al. Sialic acid species as a determinant of the host range of influenza A viruses. J Virol. 2000;74:11825–11831. doi: 10.1128/jvi.74.24.11825-11831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniels RS, et al. Antigenic analyses of influenza virus haemagglutinins with different receptor-binding specificities. Virology. 1984;138:174–177. doi: 10.1016/0042-6822(84)90158-2. [DOI] [PubMed] [Google Scholar]
- 41.Baum LG, Paulson JC. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem (Jena) 1990;89(Suppl. 40):35–38. [PubMed] [Google Scholar]
- 42.Snyder MH, London WT, Tierney EL, Maassab HF, Murphy BR. Restricted replication of a cold-adapted reassortant influenza A virus in the lower respiratory tract of chimpanzees. J Infect Dis. 1986;154:370–371. doi: 10.1093/infdis/154.2.370-a. [DOI] [PubMed] [Google Scholar]
- 43.Gagneux P, et al. Human-specific regulation of alpha 2-6-linked sialic acids. J Biol Chem. 2003;278:48245–48250. doi: 10.1074/jbc.M309813200. [DOI] [PubMed] [Google Scholar]
- 44.Appenheimer MM, et al. Biologic contribution of P1 promoter-mediated expression of ST6Gal I sialyltransferase. Glycobiology. 2003;13:591–600. doi: 10.1093/glycob/cwg066. [DOI] [PubMed] [Google Scholar]
- 45.Orlandi PA, Klotz FW, Haynes JD. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(alpha 2-3)Gal− sequences of glycophorin A. J Cell Biol. 1992;116:901–909. doi: 10.1083/jcb.116.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao H, et al. Comparative genomics indicates the mammalian CD33rSiglec locus evolved by an ancient large-scale inverse duplication and suggests all Siglecs share a common ancestral region. Immunogenetics. 2009;61:401–417. doi: 10.1007/s00251-009-0372-0. [DOI] [PubMed] [Google Scholar]
- 47.Angata T, Margulies EH, Green ED, Varki A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci USA. 2004;101:13251–13256. doi: 10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonnenburg JL, Altheide TK, Varki A. A uniquely human consequence of domain-specific functional adaptation in a sialic acid-binding receptor. Glycobiology. 2004;14:339–346. doi: 10.1093/glycob/cwh039. [DOI] [PubMed] [Google Scholar]
- 49.Altheide TK, et al. System-wide genomic and biochemical comparisons of sialic acid biology among primates and rodents: Evidence for two modes of rapid evolution. J Biol Chem. 2006;281:25689–25702. doi: 10.1074/jbc.M604221200. [DOI] [PubMed] [Google Scholar]
- 50.Jones C, Virji M, Crocker PR. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol Microbiol. 2003;49:1213–1225. doi: 10.1046/j.1365-2958.2003.03634.x. [DOI] [PubMed] [Google Scholar]
- 51.Khatua B, et al. Sialic acids acquired by Pseudomonas aeruginosa are involved in reduced complement deposition and s\Siglec mediated host-cell recognition. FEBS Lett. 2009;584:555–561. doi: 10.1016/j.febslet.2009.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Crocker PR, Hartnell A, Munday J, Nath D. The potential role of sialoadhesin as a macrophage recognition molecule in health and disease. Glycoconj J. 1997;14:601–609. doi: 10.1023/a:1018588526788. [DOI] [PubMed] [Google Scholar]
- 53.Oetke C, Vinson MC, Jones C, Crocker PR. Sialoadhesin-deficient mice exhibit subtle changes in B- and T-cell populations and reduced immunoglobulin M levels. Mol Cell Biol. 2006;26:1549–1557. doi: 10.1128/MCB.26.4.1549-1557.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brinkman-Van der Linden ECM, et al. Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by siglecs. J Biol Chem. 2000;275:8633–8640. doi: 10.1074/jbc.275.12.8633. [DOI] [PubMed] [Google Scholar]
- 55.Biesen R, et al. Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus. Arthritis Rheum. 2008;58:1136–1145. doi: 10.1002/art.23404. [DOI] [PubMed] [Google Scholar]
- 56.Junt T, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 57.Rempel H, Calosing C, Sun B, Pulliam L. Sialoadhesin expressed on IFN-induced monocytes binds HIV-1 and enhances infectivity. PLoS ONE. 2008;3:e1967. doi: 10.1371/journal.pone.0001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angata T, Varki NM, Varki A. A second uniquely human mutation affecting sialic acid biology. J Biol Chem. 2001;276:40282–40287. doi: 10.1074/jbc.M105926200. [DOI] [PubMed] [Google Scholar]
- 59.Angata T, Hayakawa T, Yamanaka M, Varki A, Nakamura M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 2006;20:1964–1973. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- 60.Angata T, et al. Cloning and characterization of human Siglec-11. A recently evolved signaling that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J Biol Chem. 2002;277:24466–24474. doi: 10.1074/jbc.M202833200. [DOI] [PubMed] [Google Scholar]
- 61.Hayakawa T, et al. A human-specific gene in microglia. Science. 2005;309:1693. doi: 10.1126/science.1114321. [DOI] [PubMed] [Google Scholar]
- 62.Lu YZ, Lin CH, Cheng FC, Hsueh CM. Molecular mechanisms responsible for microglia-derived protection of Sprague-Dawley rat brain cells during in vitro ischemia. Neurosci Lett. 2005;373:159–164. doi: 10.1016/j.neulet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Cao H, et al. SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur J Immunol. 2008;38:2303–2315. doi: 10.1002/eji.200738078. [DOI] [PubMed] [Google Scholar]
- 64.Brinkman-Van der Linden EC, et al. Human-specific expression of Siglec-6 in the placenta. Glycobiology. 2007;17:922–931. doi: 10.1093/glycob/cwm065. [DOI] [PubMed] [Google Scholar]
- 65.Winn VD, et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009;150:452–462. doi: 10.1210/en.2008-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J Biol Chem. 2004;279:43117–43125. doi: 10.1074/jbc.M403538200. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen DH, Hurtado-Ziola N, Gagneux P, Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci USA. 2006;103:7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soto PC, Stein LL, Hurtado-Ziola N, Hedrick SM, Varki A. Relative over-reactivity of human versus chimpanzee lymphocytes: Implications for the human diseases associated with immune activation. J Immunol. 2010 doi: 10.4049/jimmunol.0903420. 10.4049/jimmunol.0903420, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rutjens E, et al. Lentivirus infections and mechanisms of disease resistance in chimpanzees. Front Biosci. 2003;8:d1134–d1145. doi: 10.2741/1125. [DOI] [PubMed] [Google Scholar]
- 70.Bettauer RH. Chimpanzees in hepatitis C virus research: 1998-2007. J Med Primatol. 2010;39:9–23. doi: 10.1111/j.1600-0684.2009.00390.x. [DOI] [PubMed] [Google Scholar]
- 71.Stebbings R, Poole S, Thorpe R. Safety of biologics, lessons learnt from TGN1412. Curr Opin Biotechnol. 2009;20:673–677. doi: 10.1016/j.copbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 72.Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- 73.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 74.Yamanaka M, Kato Y, Angata T, Narimatsu H. Deletion polymorphism of SIGLEC14 and its functional implications. Glycobiology. 2009;19:841–846. doi: 10.1093/glycob/cwp052. [DOI] [PubMed] [Google Scholar]
- 75.Hedlund M, et al. N-glycolylneuraminic acid deficiency in mice: Implications for human biology and evolution. Mol Cell Biol. 2007;27:4340–4346. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tangvoranuntakul P, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280:4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen DH, Tangvoranuntakul P, Varki A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J Immunol. 2005;175:228–236. doi: 10.4049/jimmunol.175.1.228. [DOI] [PubMed] [Google Scholar]
- 79.Hedlund M, Padler-Karavani V, Varki NM, Varki A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci USA. 2008;105:18936–18941. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Padler-Karavani V, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: Potential implications for disease. Glycobiology. 2008;18:818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pham T, et al. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood. 2009;114:5225–5235. doi: 10.1182/blood-2009-05-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr. 1999;70(3, Suppl):532S–538S. doi: 10.1093/ajcn/70.3.532s. [DOI] [PubMed] [Google Scholar]
- 83.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Proc Nutr Soc. 2008;67:253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 84.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: A prospective study of over half a million people. Arch Intern Med. 2009;169:562–571. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song Y, Manson JE, Buring JE, Liu S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: The women's health study. Diabetes Care. 2004;27:2108–2115. doi: 10.2337/diacare.27.9.2108. [DOI] [PubMed] [Google Scholar]
- 86.Chong EW, et al. Red meat and chicken consumption and its association with age-related macular degeneration. Am J Epidemiol. 2009;169:867–876. doi: 10.1093/aje/kwn393. [DOI] [PubMed] [Google Scholar]
- 87.Yin J, et al. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res. 2006;66:2937–2945. doi: 10.1158/0008-5472.CAN-05-2615. [DOI] [PubMed] [Google Scholar]
- 88.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 89.Lammey ML, et al. Interstitial myocardial fibrosis in a captive chimpanzee (Pan troglodytes) population. Comp Med. 2008;58:389–394. [PMC free article] [PubMed] [Google Scholar]
- 90.Varki N, Anderson D, Herndon JG, Pham T, Gregg CJ, Cheriyan M, Murphy J, Strobert E, Fritz J, JG E, et al. Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evol Appl. 2009;2:101–112. doi: 10.1111/j.1752-4571.2008.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Denton D, et al. The effect of increased salt intake on blood pressure of chimpanzees. Nat Med. 1995;1:1009–1016. doi: 10.1038/nm1095-1009. [DOI] [PubMed] [Google Scholar]
- 92.Byres E, et al. Incorporation of a non-human glycan mediates human susceptibility to a bacterial toxin. Nature. 2008;456:648–652. doi: 10.1038/nature07428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Löfling JC, Paton AW, Varki NM, Paton JC, Varki A. A dietary non-human sialic acid may facilitate hemolytic-uremic syndrome. Kidney Int. 2009;76:140–144. doi: 10.1038/ki.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Finch CE, Stanford CB. Meat-adaptive genes and the evolution of slower aging in humans. Q Rev Biol. 2004;79:3–50. doi: 10.1086/381662. [DOI] [PubMed] [Google Scholar]
- 95.Carmody RN, Wrangham RW. The energetic significance of cooking. J Hum Evol. 2009;57:379–391. doi: 10.1016/j.jhevol.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 96.Tishkoff SA, et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet. 2007;39:31–40. doi: 10.1038/ng1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carlin AF, et al. Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J Exp Med. 2009;206:1691–1699. doi: 10.1084/jem.20090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nöhle U, Schauer R. Metabolism of sialic acids from exogenously administered sialyllactose and mucin in mouse and rat. Hoppe Seylers Z Physiol Chem. 1984;365:1457–1467. doi: 10.1515/bchm2.1984.365.2.1457. [DOI] [PubMed] [Google Scholar]
- 99.Cohen M, Hurtado-Ziola N, Varki A. ABO blood group glycans modulate sialic acid recognition on erythrocytes. Blood. 2009;114:3668–3676. doi: 10.1182/blood-2009-06-227041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Varki A, Nelson D. Genomic differences between humans and chimpanzees. Annu Rev Anthropol. 2007;36:191–209. [Google Scholar]
- 102.Varki A, Geschwind DH, Eichler EE. Explaining human uniqueness: Genome interactions with environment, behaviour and culture. Nat Rev Genet. 2008;9:749–763. doi: 10.1038/nrg2428. [DOI] [PMC free article] [PubMed] [Google Scholar]