Abstract
The image that best expresses Darwin’s thinking is the tree of life. However, Darwin’s human evolutionary tree lacked almost everything because only the Neanderthals were known at the time and they were considered one extreme expression of our own species. Darwin believed that the root of the human tree was very deep and in Africa. It was not until 1962 that the root was shown to be much more recent in time and definitively in Africa. On the other hand, some neo-Darwinians believed that our family tree was not a tree, because there were no branches, but, rather, a straight stem. The recent years have witnessed spectacular discoveries in Africa that take us close to the origin of the human tree and in Spain at Atapuerca that help us better understand the origin of the Neanderthals as well as our own species. The final form of the tree, and the number of branches, remains an object of passionate debate.
Keywords: Darwin, modes of evolution, body size and shape, taxonomy of Homo, Atapuerca
In The Descent of Man (1871) (1) Darwin paid little attention to the only human fossils known at the time that were not modern humans, the Neanderthals. Only two references to them can be found in its pages, and one of them refers to the same fossil that gave its name to this form of humanity: “Nevertheless, it must be admitted that some skulls of very high antiquity, such as the famous one of Neanderthal, are well developed and capacious” (ref. 1: Vol. I, p. 146). The second reference to a Neanderthal fossil mentions the mandible from the Belgian site of La Naulette: “Considering how few ancient skulls have been examined in comparison with recent skulls it is an interesting fact that in at least three cases the canines project largely; and in the Naulette jaw they are spoken of as enormous” (ref. 1: p. 126). Darwin had actually held the Forbes’ Quarry Neanderthal skull from Gibraltar in his hands. This specimen was found in 1848, before the discovery in 1856 of the skeleton at the Feldhofer grotto in the Neander Valley for which the species Homo neanderthalensis (or subspecies, Homo sapiens neanderthalensis, according to some researchers) was named. In a letter to J. D. Hooker dated September 1, 1864, Darwin wrote: “F. (Falconer) brought me the wonderful Gibraltar skull” (2). The reason behind Darwin’s lack of interest in the Neanderthals may stem from the judgment made previously of these same specimens by Thomas Henry Huxley in his Evidence as to Man’s Place in Nature in 1863: “(…) the Neanderthal cranium is by no means so isolated as it appears to be at first, but forms, in reality, the extreme term of a series leading gradually from it to the highest and best developed of human crania.”
Darwin was in search of a “missing link,” a transitional form between modern humans and the chimpanzee or gorilla. At that time, a fossil fulfilling the role of linking two large zoological groupings had already been found. Archaeopteryx was incorporated into the third edition (1866) of The Origin of Species: “… and still more recently, that strange bird, the Archeopteryx, with a long lizard-like tail, bearing a pair of feathers on each joint, and with its wings furnished with two free claws, has been discovered in the oolitic slates of Solenhofen. Hardly any recent discovery shows more forcibly than this how little we as yet know of the former inhabitants of the world.” (3)
The discovery of Homo erectus in 1891 in Java might have satisfied Darwin. Alternatively, he may have considered it too human and, with its reduced cranial capacity, only slightly more primitive than the Neanderthals. It is possible that the authentic missing link (or, more appropriately, “fossil link”) for Darwin would have been the Taung child, discovered in 1924 and of such a primitive aspect that it took 20 years until it was finally accepted as our ancestor by the majority of the scientific community.
Darwin believed that the origins of humanity most likely lay in Africa, although the discovery of fossil apes in Europe made him question this. In The Descent of Man (ref. 1: p. 199) he reflects on the topic: “It is therefore probable that Africa was formerly inhabited by extinct apes closely allied to the gorilla and chimpanzee; and as these two species are now man’s nearest allies, it is somewhat more probable that our early progenitors lived on the African continent than elsewhere. But it is useless to speculate on this subject, for an ape nearly as large as a man, namely the Dryopithecus of Lartet, which was closely allied to the anthropomorphous Hylobates, existed in Europe during the Upper Miocene period; and since so remote a period the earth has certainly undergone many great revolutions, and there has been ample time for migration on the largest scale.”
It is interesting to note that Darwin considered modern humans more closely related to chimpanzees and gorillas, African apes, than to orangutans and gibbons. This must have led him to include us with the African group and consequently to consider us at least as much an ape as the orangutans and gibbons, which had separated previously from a common root. We would therefore be a highly evolved form of African ape. The same resemblance between us and the African apes had been noted previously in 1863 by Huxley (4): “It is quite certain that the Ape which most nearly approaches man, in the totality of its organization, is either the Chimpanzee or the Gorilla.” Nevertheless, this did not lead Huxley to group us in the same taxonomic category with the African apes. Rather, it was they who were grouped with the other great ape, the orangutan, along with the lesser apes, the gibbons, in the same category: “The structural differences between Man and the Man-like apes certainly justify our regarding him as constituting a family apart from them.”
The only way that modern humans are included within the group of apes, in which we are considered apes, is if we are more closely related phylogenetically to some of them (the African apes) than to others (the Asian apes). If this were not the case, the apes would form one clade (a natural group with an exclusive common ancestor) and we would form another, the human clade (sister group), connected with them at a lower node. This is what Darwin believed (ref. 1: p. 197): “If the anthropomorphous apes be admitted to form a natural sub-group, then as man agrees with them, not only in all those characters which he possesses in common with the whole Catarhine group, but in other peculiar characters, such as the absence of a tail and of callosities and in general appearance, we may infer that some ancient member of the anthropomorphous sub-group gave birth to man. It is not probable that a member of one of the other lower sub-groups should, through the law of analogous variation, have given rise to a man-like creature, resembling the higher anthropomorphous apes in so many respects. No doubt man, in comparison with most of his allies, has undergone an extraordinary amount of modifications, chiefly in consequence of his greatly developed brain and erect position.”
However, how could we be closer to chimpanzees and gorillas than to orangutans and gibbons and not share an exclusive common ancestor (which is not also common to the Asian apes) with them? There are two ways to explain this paradox. One of these is that Darwin and Huxley believed that the resemblances between apes and modern humans evolved in parallel. We just saw that Darwin did not believe much in the “law of analogous variation” when explaining our great similarity with apes, which he generally attributed to descent from a common ancestor. However, he did resort to parallel evolution to explain certain features: “It must not be supposed that the resemblances between man and certain apes in the above and many other points—such as having a naked forehead, long tresses on the head, etc.—are all necessarily the result of unbroken inheritance from a common progenitor thus characterized, or of subsequent reversion. Many of these resemblances are more probably due to analogous variation, which follows, as I have elsewhere attempted to show, from co-descended organisms having a similar constitution and having being acted on by similar causes inducing variability” (ref. 1: p. 194). It is not easy, then, to decide if Darwin attributed our great similarity with the great apes (which he recognized as being greater than with the lesser apes), and in particular with the chimpanzee and gorilla, to parallel evolution.
The other explanation is that both Darwin and Huxley maintained the separation between the apes (greater and lesser) on the one hand and humans on the other as a separate branch, on the basis of a notion of evolutionary grade, that is, resemblance between different species of ape, even while realizing that this was not a natural classification. Nevertheless, Darwin (at least according to certain researchers) was a proponent of classification based on genealogy (5). In On the Origin of Species (1859) (3), he writes: “We can understand why a species of a group of species may depart, in several of its most important characteristics, from its allies, and yet be classed with them.”
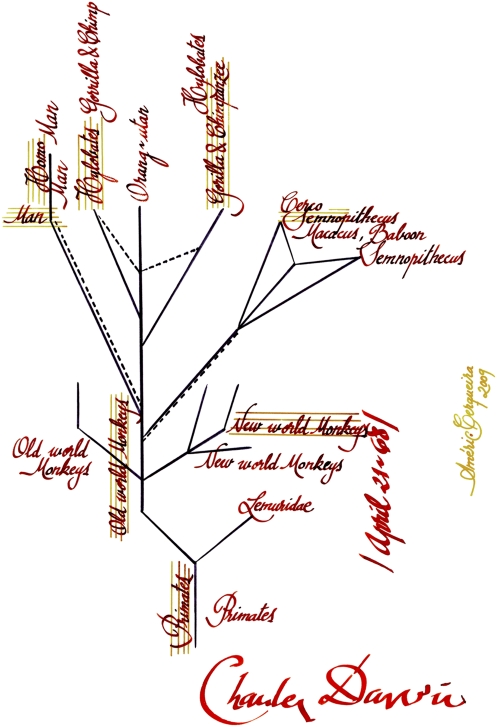
Nevertheless, Darwin does not seem to have recognized our common origin with the African apes because he drew, on April 21 in 1868, a schematic genealogical tree of the primates (Fig. 1) that was never published, but that corresponds with the classification he used in 1871 in The Descent of Man. Interestingly, the human lineage does not occupy the central axis of radiation of the primates, an eloquent confirmation of his vision of evolution as nonteleological. At the same time he relates us closely with the apes, albeit as a separate group. Even the gibbons are slightly more closely related with the chimpanzees and gorillas than with humans.
Fig. 1.
Transcription of the genealogical tree of primates in Darwin’s sketch of 1868. Original is in The Complete Work of Charles Darwin on Line: http://darwin-online.org.uk/. Identifier: CUL-DAR80, image 107.
Looking more closely at the drawing, we see numerous entries that are crossed out but they can still be discerned. Surprisingly, the gibbons were initially placed as the closest to humans, with the African apes further removed. These respective positions were subsequently changed so that the chimpanzee and gorilla are closer to humans, although they do not share a common ancestor with us. Did Darwin know that we are African apes (members of the same clade) but not dare to say it? It does not seem so, on the basis of the existence of the drawing, which he kept to himself. Might it be better to say he did not dare to think it? It is more likely that he simply gave greater importance to the enormous differences between modern humans—as a “consequence of his greatly developed brain and erect position” (ref. 1: p. 197)—and apes than to the similarities between modern humans and African apes. The truth is that without clearly separating primitive and derived features it is not possible to carry out a phylogenetic analysis, and neither Darwin nor T. H. Huxley went further in this regard than other evolutionary biologists of the time.
Years before the fundamental work of W. Hennig (Phylogenetic Systematics) (6) was published in English (in 1966), W. E. Le Gros Clark, in 1959 (7), had already distinguished between “characters of common inheritance” (i.e., primitive characters or plesiomorphies, in cladistic jargon) and “characters of independent acquisition” (derived characters or apomorphies). According to Le Gros Clark, in spite of sharing many primitive features with the great apes, the australopithecines are classified within the hominids on the basis of sharing a few derived characters. Although their grade was largely ape-like, the australopithecines already belonged to the human clade. “Since the pongid sequence of evolution has been much more conservative than the progressive hominid sequence, its terminal products (the modern anthropoid apes) have preserved more of the original characters of the ancestral stock. As divergent evolution proceeds, characters of common inheritance will become progressively supplemented or replaced by characters of independent acquisition in each line” (7). The australopithecines, then, were recognized as primitive ancestors of our own species, and both Raymond Dart (the discoverer of the Taung child) and Darwin were vindicated. Finally, a missing link connecting humans with the great apes had been provided, but with which ones: All of the apes in general, only with the great apes, or only with some of the great apes in particular?
That modern humans shared some derived features only with African apes (but not the orangutans) was not realized at the time by Le Gros Clark. But things would soon change. At a summer conference in 1962 organized by the Wenner-Gren Foundation, three biologists presented the results of studies that grouped modern humans with African apes in particular and excluded orangutans and gibbons. The evidence relied upon was both cytogenetic (8) and molecular: serum proteins (9) and hemoglobin (10). In fact, the study of chromosomes went even further because it showed a closer relationship between modern humans and chimpanzees than between chimpanzees and gorillas, but Morris Goodman had published his results one year earlier (11).
George Gaylord Simpson, Theodosius Dobzhansky, and Ernst Mayr, all present at the conference, accepted the inclusion of humans in the African ape clade. However, the great primatologist Adolph H. Schultz (12) continued to consider the great apes as forming a clade that excluded humans, who had branched off previously (more or less at the same time as the hylobatids and certainly before the orangutans separated from the African apes): “Such evidence [“of extremely close similarities between man and chimpanzee and (or) gorilla”], although of greatest interest, is more than counterbalanced by the mass of profound differences found in all sorts of other characters of recognized reliability.” On the other hand, Simpson (13) preferred to maintain the division between pongids (divided into hylobatines and pongines) and hominids (in spite of clearly realizing this was not a natural or phylogenetic classification) mainly on the basis of notions of evolutionary grade. The human line occupied a new adaptive zone that warranted its own family, even though the pongids were a paraphyletic group. According to Simpson, this will inevitably occur when a new family emerges from an old one: “Classification cannot be based on recency of common ancestry alone.” Today some authors still use the term hominid—in the traditional way—to refer to all taxa of the human lineage after its separation from chimpanzees, whereas other authors prefer to call them hominins.
Human Origins and Quantum Evolution
Once Darwinism (in the strict selectionist sense) was returned to a central place in evolutionary theory, the neo-Darwinians could turn their attention to other matters. The paleontologist G. G. Simpson distinguished in 1944 (14) three patterns of evolution based on the fossil record: speciation, phyletic evolution, and quantum evolution. The first was responsible for the appearance of the lower taxonomic categories and explained the enormous proliferation of species that exist in the biosphere. The second produced the intermediate-level taxonomic categories and accounted for the evolutionary tendencies that paleontologists found everywhere when organizing fossils in progressive series that seemed to reflect gradual and directional changes. The third pattern was the cause of large-scale changes in the adaptive types (biological designs or body plans) that were produced in relatively short periods of geological time and that gave rise to large evolutionary novelties in the highest-level taxonomic categories.
Quantum evolution, as its name suggests, seemed opposed to the fundamental idea of evolutionary synthesis (i.e., that natural selection governed evolution), because passing from one adaptive plateau to another implied a loss of fitness, and natural selection never favors the less adapted. Because of this, in 1953 Simpson (15) presented this mode of evolution in a more orthodox form, as a case of phyletic evolution that proceeded at a more rapid rate than normal (due to an increase in the selection pressure).
But already by 1950, at the Cold Spring Harbor Symposia on Quantitative Biology, Simpson (16) did not use the term “quantum evolution,” but rather considered human evolution to represent a “change from one adaptive type to another.” To explain how a change from one adaptive plateau to another was possible, Simpson no longer held that a maladaptive valley had to be traversed. The intermediate forms could now enjoy the advantages of both adaptive types, the old one that was being abandoned and the new one to which it was directed (which raises the question as to why natural selection would keep pushing the intermediate forms toward the new, more specialized adaptive type). This is what happened in Simpson’s favorite evolutionary example, that of horses passing from browsers to grazers, as well as in the postural changes in our ancestors: “The new feature, for which the specialization was adaptive, was the ability to graze, to eat harshly abrasive food. Nevertheless the ability to eat less abrasive food, to browse, was not thereby lost. The development of upright posture in man and utilization of the hands for manipulation, only, and not locomotion, perhaps provide a better example of specialization that broadened rather than restricted the general adaptive type” (16).
Nevertheless, other attendees of the symposium did use the expression quantum evolution, and in a manner very close to its original meaning, to explain the origin of human bipedal posture. W. W. Howells (17) argued: “It is true that bipedal walking was the more radical line of change. As Washburn says, this was undoubtedly a case of quantum evolution, a conceptual contribution of Simpson (1944).” According to S. L. Washburn (18) “The derivation of this type from an ape is best regarded as a case of rapid or quantum evolution (Simpson, 1944).” For Washburn, the key was in the iliac blade and the gluteus maximum muscle: “The argument runs as follows: among apes who were living at the edge of the forests and coming to the ground, were some who had shorter ilia. These ilia had to be more bent back for obstetrical reasons and in some this carried gluteus maximus far enough so that it became effective in finishing extension. This started a new selection which favored bigger gluteus muscles and ilia still further bent.”
In the australopithecines, the pelvis had already undergone the necessary changes for obligate bipedalism, but other parts of the skeleton reflected this new posture. The unavoidable question is whether the passing from a nonbipedal adaptive type (that of the common ancestor of humans and chimpanzees) to an obligate bipedal one (like the australopithecines) occurred directly and only once or whether a transitional form had previously existed with a generally primitive skeleton but with some particular key feature (perhaps in the iliac blade as suggested by Washburn) that made an early form of facultative bipedal locomotion, still compatible with some degree of life in the trees, possible.
Currently, there are three known genera that predate Australopithecus and that, according to their respective discoverers, are our ancestors and were facultative bipeds: Sahelanthropus (19, 20), Orrorin (21, 22), and Ardipithecus (23, 24). To date, only a single preaustralopithecine pelvis has been recovered, belonging to the Ardipithecus ramidus skeleton found at Aramis (Middle Awash, Ethiopia) and dated to 4.4 mya. According to the researchers who described the specimen, some features characteristic of the modern human pelvis that are strongly related to bipedal posture—because they permit abduction during walking—can already be appreciated and are also found in the australopithecines and later hominids: a short iliac isthmus, a slightly broadened and sagittally oriented ilium with a weak greater sciatic notch, and a strong, anterior inferior iliac spine formed by a separate ossification center. In addition, the pubic symphysis would have been superoinferiorly short, differing from the tall symphysis in chimpanzees.
The authors of the study of the skeleton of A. ramidus maintain that the common ancestor of humans and chimpanzees was not a brachiator like living chimpanzees, but “was probably a palmigrade quadrupedal arboreal climber/clamberer that lacked specializations for suspension, vertical climbing or knucle-walking” (23). It was also claimed that A. ramidus occupied a different ecological niche than extant chimpanzees because the study of stable isotopes has shown that they consumed some C4 plants (a type mostly represented in East Africa by grasses and sedges) as part of their diet (∼10–25%), whereas extant chimpanzees are almost pure C3 (forest green plants) feeders.
Although bipedal posture had been established, it would have undoubtedly been a more primitive form than that of Australopithecus. The postcranial skeleton of A. ramidus is, in general, very different from that of Australopithecus afarensis. If Australopithecus anamensis resembles A. afarensis postcranially, and A. ramidus is the direct ancestor, the passage from one adaptive plateau to another would have occurred in a relatively short period, ≤200 kya (from 4.4 to 4.2 mya). Thus, we could speak of a rapid evolution, at least in comparison with the subsequent stability in the body plan, which would not change during at least the subsequent 2 million years of evolution. But it is also possible that the skeleton of A. ramidus from Aramis corresponds to a later population than the population (of the same species) that gave rise to Australopithecus. In this case, the mother and daughter species would have coexisted, implying that this transition is not an example of the phyletic mode of evolution but rather of speciation or ramification (branching evolution) and further, of a special type (“like a parental Hydra buds off young individuals” in the words of Eldredge and Cracraft) (5), because only a part of the ancestral species would have given rise to the descendant.
Heads and Bodies
The neo-Darwinians, in general, gave more weight to the phyletic mode of evolution and maintained a very lineal notion of human evolution (25). Theodosius Dobzhansky wrote in 1962 (26): “Following Weidenreich, Dobzhansky (1944) and Mayr (1950) entertained the hypothesis that only one human or prehuman species existed in any one territory at any one time level in evolutionary history.” But some years had passed and Dobzhansky now admitted a dead-end branch in the human genealogy, that of the paranthropines, or robust australopithecines: “In view of Robinson’s (1954) fairly convincing demonstration that two species of australopithecines may have lived in South Africa within a relatively short period of time, if not simultaneously, this hypothesis remains now probable only for the representatives of the genus Homo.” For Dobzhansky, anagenesis predominated over cladogenesis in human evolution: “Both cladogenetic and anagenetic changes took place in man’s ancestry but the latter predominated. Mankind was and is a single inclusive Mendelian population and is endowed with a single, corporate genotype, a single gene pool.” Whether or not the evolution of the genus Homo represents a single lineage (that is, a single panmictic unit) that changes through time, passing through different evolutionary grades is a question that has been debated ever since, and it is the topic I deal with in the rest of this article.
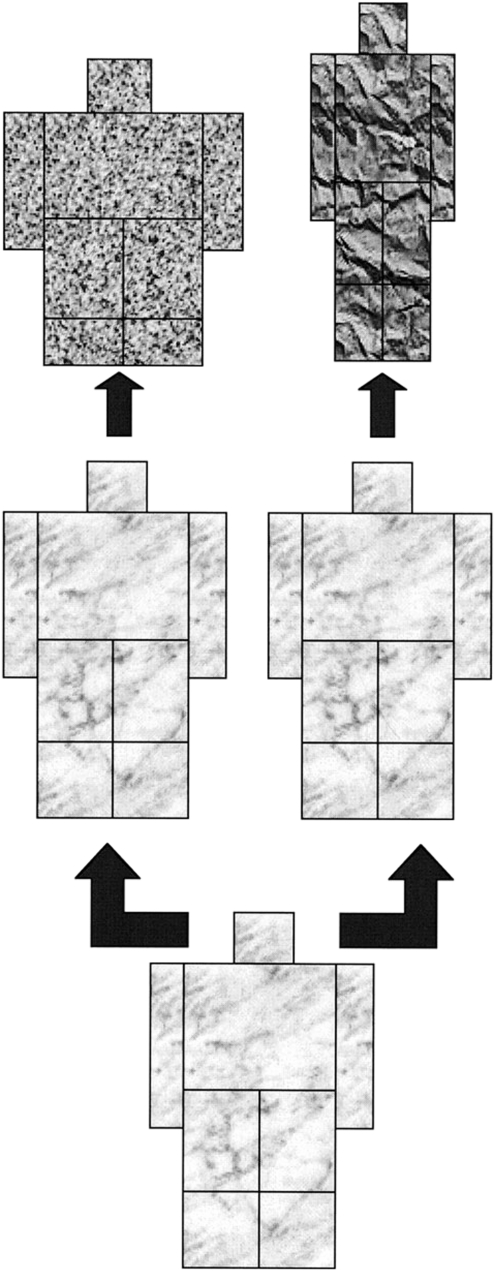
Historically, most of the species of the genus Homo that have been proposed have been based on craniodental anatomy. The postcranial skeleton has barely played any role in the respective diagnoses, mainly because it is more poorly preserved in the fossil record. But we now have a sufficiently large sample to attempt a synthesis of evolutionary changes in the hominid (or hominin, as other authors prefer) body (27) (Fig. 2). The results of this analysis, based on body plan, suggest very few species in the genus Homo. Why, then, should the cranium be privileged when classifying the hominids?
Fig. 2.
Changes in body shape in Homo (26).
The first known hominid postcranial morphotype would then be that of A. ramidus, which could be the same (or not) as the other preaustralopithecines: Ardipithecus kadabba, Orrorin tugenensis, and Sahelanthropus tchadensis.
The subsequent morphotype would be that of the australopithecines and paranthropines, as well as Homo habilis. To this, we also have to add the surprising Homo floresiensis from the late Late Pleistocene (28). This morphotoype is characterized by small stature, markedly wide relative width of the pelvis, and short legs. In fact, the poverty of the fossil record for the postcranial skeleton is such that the attribution of the australopithecine morphotype to H. habilis is based on only a single, very incomplete skeleton (OH 62 from Olduvai Gorge, dated to ∼1.8 mya) that, craniodentally, preserves mainly the palate.
At a later point during the Early Pleistocene (now considered to begin at 2.6 mya), a morphotype appears within the genus Homo that is characterized by taller stature, long legs, and wide pelvis (although, relative to stature, not as wide as in the australopithecines). This change would have occurred in the species known either as Homo ergaster, a term that applies exclusively to African fossils, or H. erectus, a term that includes both the African specimens and Asian fossils from Java, where the species was defined, and Zhoukoudian (and some other sites) in China. The juvenile skeleton from Lake Turkana (KNM-WT 15000) dates to 1.5–1.6 mya and belongs to what we might call this “large hominid” to differentiate it from other earlier and contemporaneous specimens. There appears to have been a long period of coexistence of H. habilis and H. ergaster in East Africa, which would indicate not a lineal (anagenetic) evolutionary pattern, but a branching (cladogenetic) pattern of evolution. Some authors further recognize a third sympatric and synchronic species of Homo: Homo rudolfensis.
The tall and wide morphotype first seen in H. ergaster/erectus was maintained until the end of the Middle Pleistocene, when two new morphotypes appear. One, seen in modern humans, shows a narrower body cylinder and the other, seen in Neanderthals, shows a shortening of the distal segments of the extremities. The earliest (well-dated) modern human fossils are from Ethiopia and are represented by the skeleton of Omo I (∼196 kya) (29) and the crania from Herto (∼150–160 kya) (30).
From the point of view of the postcranial skeleton, then, there would be only four morphotypes within the genus Homo, although the fossils from Dmanisi (31–33) in Georgia (∼1.75 mya) might represent an intermediate form between the primitive, australopithecine, morphotype of H. habilis and the tall and wide morphotype of H. ergaster/erectus. The skull of Homo georgicus is morphologically intermediate between H. habilis and H. ergaster or, alternatively, is a primitive form of the latter, which some authors, in turn, consider to be a primitive grade within H. erectus.
In reality, however, postcranial remains are abundant only at the Middle Pleistocene site of the Sima de los Huesos in the Sierra de Atapuerca, dated to at least 530 kya. Nevertheless, the isolated fossils from other sites such as the East African pelvises KNM-ER 3228 (perhaps older than the Turkana Boy) and OH 28 (<1.0 mya) as well as the Middle Pleistocene skeleton from Jinniushan in China (34) do not differ from those at the Sima de los Huesos.
This overview has necessarily glossed over numerous details. Although living modern human populations are considered “tall hominids” compared with the early hominids, a wide variation in stature exists across the globe today. In Africa alone, there are modern human populations with average male stature <150 cm and others with averages ∼180 cm. This variation in height could have also characterized human species in the past. A recently published pelvis from the site of Gona in Ethiopia (35) dates to 0.9–1.4 mya and shows a very wide maximum width between the iliac crests. At the same time, the size of the acetabulum indicates a very small body, like the australopithecines and paranthropines. Although this specimen has been attributed to H. erectus, its chronology, geographic location, and some aspects of its anatomy are also consistent with an assignment to Paranthropus boisei (36). If it represents H. ergaster/erectus, it would have to be a very small-bodied population, considerably smaller than living pygmies.
The size of the brain is not without importance in hominid taxonomy, and it has been extensively used. In principal component analyses of neurocranial variables, the first factor is always size (by far explaining the most variation) and this is strongly correlated with brain volume. Standardizing the raw values for size does not solve the problem because there is a tight relationship between size and shape. That is, most of the differences in the neurocranial architecture of fossils attributed to Homo are simply related to the size of the brain. Other features used in taxonomy are related to bone thickness, cranial superstructures (e.g., tori), or more or less subtle features of the temporal bone.
However, this is not always the case. Neanderthals and modern humans show similar cranial capacities but differ markedly in neurocranial morphology. The brain of Homo sapiens seems to follow a very different pattern from that of other species (37). At the same time, some of the H. erectus fossils from Ngandong (Java) have cranial capacities similar to that of Cranium 5 from the Sima de los Huesos but the neurocranial anatomy is very different.
Consideration of the neurocranium, then, should complement the study of the postcranial morphotype. When brain size is related to body size using allometric equations (to eliminate the size factor), an encephalization curve is obtained that can be used for systematics (38). Compared with the australopithecines, H. habilis shows an increase in encephalization because the brain size increases whereas the body size does not. The shift to the subsequent cranial and postcranial combined morphotype (which starts with H. ergaster/erectus) involves an important increase in both brain size and body size, but the increase in encephalization is small. However, this may still indicate an advance in cognitive abilities. Comparison between chimpanzees and gorillas indicates that closely related species may show large differences in body size but little difference in brain mass and intelligence. In contrast, the cranial capacity in H. ergaster/erectus is much larger than that of the australopithecines.
Although the body cylinder does not differ among Middle Pleistocene fossils from Africa and Europe, an important brain expansion does occur by at least 500 kya, leading to a clear increase in encephalization. The range of cranial capacities from the Sima de los Huesos varies from 1,100 to 1,390 cm3. Combining body size and shape and absolute and relative brain size, these Middle Pleistocene fossils represent a different morphotype from that of their Early Pleistocene ancestors as well as from that of Asian H. erectus. Those from Zhoukoudian in China have been dated to between 300 and 550 kya (39), but new dates using a different technique yielded much older results (∼780 kya), at least for the lower levels (40). Thus, it is possible that by 500 kya, H. erectus survived only in Indonesia. The Neanderthals and modern humans show the highest encephalization because the increase in brain size is coupled with a decrease in body size, although by two different means. The Neanderthals underwent a shortening of the distal segments of the extremities, whereas H. sapiens shows a narrowing of the body cylinder.
In the middle of the Early Pleistocene at least two cranial and postcranial combined morphotypes coexisted, whereas during the late Middle and Late Pleistocene four morphotypes coexisted: that of Neanderthals, that of modern humans, the fossils from Ngandong (smaller brain size and assigned to a late population of H. erectus), and the australopithecine morphotype of H. floresiensis. If in the Late Pleistocene, when the fossil record is more complete, we find that four different human lineages coexisted, why not think that this has been the general trend?
It is important to point out here that, although this encephalization can be represented as a curve, it does not necessarily imply a steady, continuous rate of increase through time. In fact, body size, which is one of the variables involved in calculating the encephalization quotient, shows long periods of stability for each morphotype. Gould and Eldredge (41) warn: “We have learned as a received truth of evolution, for example, that human brain size increased at an extraordinary (many say unprecedented) rate during later stages of our lineage. But this entrenched belief may be a chimera born of an error in averaging rates over both punctuations and subsequent periods of stasis.” Hominid taxonomy within the genus Homo could be refined further if body size and shape and brain size were considered along with craniodental features.
Paleontological Species
To approach a cranial analysis, I adopt a paleontological species definition based on an operational criterion: Two or more populations represent different paleontological species if the variation between them is clearly larger than the variation within each of them. Under these circumstances it is relatively easy to recognize the affinities of an isolated specimen, something which would not be the case if the intrapopulation variation greatly exceeded the interpopulation variation.
The different geographic human populations alive today would clearly not be identified as different species using this criterion because it is very difficult to establish, visually, the geographic provenience of a modern human cranium (much less an isolated postcranial bone). However, in spite of the large overlap between the frequency distributions of local populations, certain differences do exist between the average values. Thus, it is possible to make a probable diagnosis of population affinity using a large number of cranial dimensions and relying on discriminant functions calculated on samples of known population affiliation. Nevertheless, in practice, the results of these analyses are often far from satisfactory.
One simple example comes from Ubelaker et al. (42), who tried to classify the remains from a 16th–17th century ossuary near the city of Valladolid in the north of Spain. It is important to point out that this region of Spain was the least affected by the Arab incursions of the eighth century (which were primarily composed of Berbers from North Africa), so this population is unlikely to have been particularly heterogeneous. Twenty cranial measurements were taken on 95 individual skulls and compared, in turn, with the data in the Forensic Data Bank and with the collections studied previously by W. W. Howells (43). In the first case, 29 individuals were classified as white, 40 as black, 17 as Hispanic, 2 as Chinese, 3 as Japanese, 2 as Amerindian, and 2 as Vietnamese. Using the Howells database, the 95 individuals were classified into 21 different groups.
Nevertheless, as Howells himself noted, a Neanderthal cranium is something else. It cannot be confused with a modern human, even at first glance. In this case, even the postcranial bones are often diagnostic. This criterion (variation between populations clearly larger than variation within each of them) can be applied to other hominoids, comparing, for example, the two chimpanzee species to one another or the different subspecies (or species, according to some researchers) of orangutans and gorillas, to determine the taxonomic level at which it is useful. At the same time, accumulating evidence from the Neanderthal genome has not documented any significant level of gene flow between them and us.
For authors who consider the Neanderthals to represent a subspecies of H. sapiens that appears at the end of the Middle Pleistocene in Europe, the last ancestral population (earlier in time, yet still undifferentiated) should also be called H. sapiens and it has been informally recognized as “archaic H. sapiens” (44). If, on the other hand, the species Homo neanderthalensis is accepted, their last ancestor could still be H. sapiens (or the reverse) if the mode of speciation favored under the punctuated equilibrium model of evolution (i.e., with survival of the mother species) is used. But the fossils from the early Pleistocene, as well as those of the early and middle Middle Pleistocene across the globe, are so different from Neanderthals and modern humans that a last common ancestor of a different species must be sought.
One available, although impractical, name is Homo heidelbergensis, with the Mauer mandible from Germany (∼500 kya) as the holotype (45). For some researchers, this is the last common ancestor, and it would have inhabited Europe, Africa, and perhaps even Asia (in more recent times than the H. erectus fossils from Zhoukoudian). The problem is that many researchers have recognized derived Neanderthal features, developed to a greater or a lesser degree, in the European middle Middle Pleistocene fossils, including the Mauer mandible, but not in the African specimens. These European specimens, then, could either be included within the species H. neanderthalensis (46) or maintained as an earlier more primitive chronospecies, H. heidelbergensis.
One practical limitation in the previously mentioned criterion is that good samples of fossils are needed to compare the intra- and interpopulation variation. These kinds of samples are rare in paleoanthropology, unless fossils that span a considerable temporal and geographic range (and may therefore represent more than one species) are grouped together. However, this is not the case with the “classic” Neanderthals of the second half of the Late Pleistocene, which represent a relatively temporally and geographically restricted sample. Of course, the ideal situation would be to find contemporaneous fossils from the same geographic region and, if possible, even from the same site or geological strata.
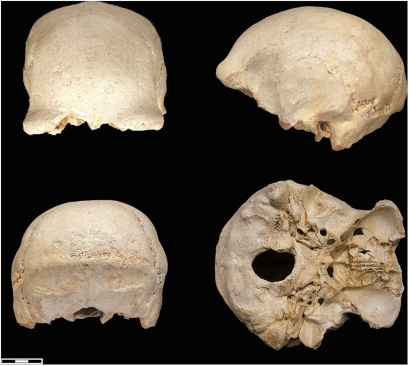
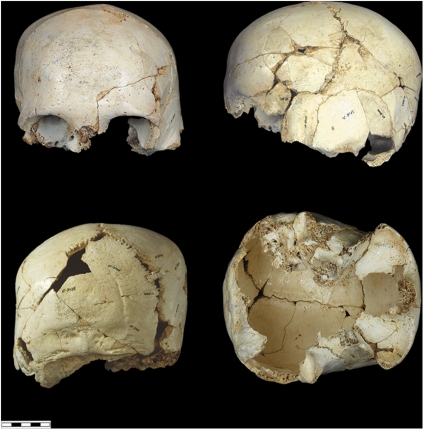
The site of Dmanisi (Georgia) represents one of these rare circumstances. Another is in the Sierra de Atapuerca, where the site of the Gran Dolina, dated to ∼900 kya (47), has yielded a sample of human fossils that to date represent a minimum of 11 individuals. Additional individuals may eventually be recovered given that the accumulation represents one or more episodes of cannibalism. These fossils have been designated a new species, Homo antecessor (48), that predates H. heidelbergensis and is close to the last common ancestor of Neanderthals and modern humans. There are three mandibles and a parietal from the Algerian site of Ternifine (∼700 kya) that have been designated Homo mauritanicus and could be the same species as those from the Gran Dolina (49). However the Gran Dolina specimens are quite different (50). The site of the Sima del Elefante, also in the Sierra de Atapuerca, has yielded a human mandible dated to 1.2–1.4 mya (51). In the near future, it will be possible to study the intraspecific variation within the sample from the Gran Dolina, but currently this type of study can only be carried out on a different sample from Atapuerca. At the Sima de los Huesos, a little less than half of at least 28 individuals have been recovered, dating to >530 kya (52–54). There are presently 17 crania and an equal number of mandibles in different states of reconstruction, ranging from complete specimens to more fragmentary remains (Figs. 3–6).
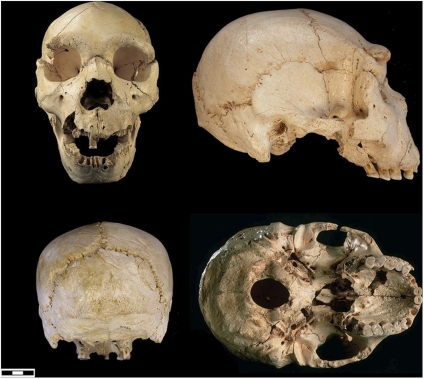
Fig. 3.
Sima de los Huesos (Atapuerca) cranium 4.
Fig. 6.
Sima de los Huesos (Atapuerca) cranium 14.
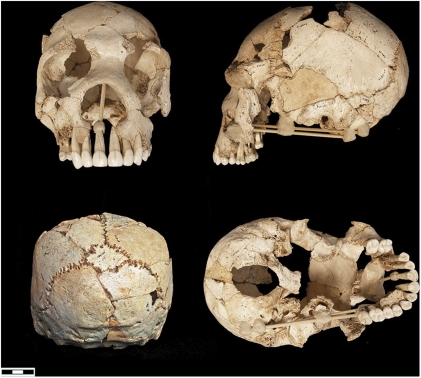
Fig. 4.
Sima de los Huesos (Atapuerca) cranium 5.
Fig. 5.
Sima de los Huesos (Atapuerca) cranium 6.
The fossils from the Sima de los Huesos are neither phenetically nor cladistically H. sapiens. When they are compared with Middle Pleistocene fossils from Zhoukoudian, from other parts of China, or in Java, they are also clearly not H. erectus, nor are they Neanderthals, but their sister group. Because the Mauer mandible is such an uninformative specimen, it is worth taking a closer look at the characteristics of this large collection of remains.
The population from the Sima de los Huesos can be identified by a combination of various types of features (55, 56):
(i) Features shared with Neanderthals and modern humans, but absent in H. erectus. Among these is a very convex superior border of the temporal squama.
(ii) Features that are neither plesiomorphies (they are not present in H. erectus) nor aporporphies of Neanderthals and modern humans, but intermediate character states that could give rise to one or the other. For example, cranial wall sides slightly convergent upward or vertical (parallel) in rear view, which could transform into the high pentagonal shape displayed by H. sapiens as well as the rounded contour exhibited by H. neanderthalensis. In addition, the location of opisthocranion on the occipital plane of the occipital squama precedes the bulging occipital bones (although different in form) of Neanderthals and modern humans. These features are linked with a cranial capacity that is larger than that of H. erectus but smaller than in modern humans and Neanderthals.
(iii) Features exclusive to this and other European Middle Pleistocene populations. These features are not interpreted as a late stage in the transformation sequence of a derived character state but as intermediate character states in a postulated sequence of change (morphocline) that leads to the apomorphies of the Neanderthals. They are thus both primitive and derived. The anatomy of the occipital torus and that of the suprainiac area are a good example. The midface and the supraorbital torus are another.
(iv) Primitive features retained in H. sapiens but lost in the Neanderthals, such as the size and shape of the mastoid process.
(v) Primitive features lost in H. sapiens but retained in the Neanderthals, such as the absence of a chin.
(vi) Derived features unique to the Neanderthals (autapomorphies), such as the retromolar space of the mandible.
The postcranial skeleton, in particular the pelvis, is primitive and does not show the modifications from the archaic design seen in Neanderthals, such as the thin superior pubic ramus. In principle, autapomorphies have not been found in the Sima de los Huesos, which would exclude them from forming part of a chronospecies in the evolution of the Neanderthals, but this is because the unique features that are found (in this and other European middle Middle Pleistocene fossils) are interpreted as character states that are intermediate in their polarity. The amount of time between the fossils from the Gran Dolina (terminal Early Pleistocene) and the appearance of Neanderthals and modern humans (toward the end of the Middle Pleistocene) is sufficiently long (≥500 kyr) to be able to recognize other similar entities, like that at the Sima de los Huesos, in Europe or Africa.
Although we cannot compare the Sima de los Huesos with any other collection (because they do not exist), we can ask whether it is possible to find a fossil within the Sima de los Huesos sample with characteristics like those seen in the mandible of H. antecessor from the Gran Dolina or the Mauer mandible (Germany) or in the crania from Ceprano (Italy), Petralona (Greece), Swanscombe (England), or Broken Hill (Zambia). The fossil from Ceprano was even designated as a new species (Homo cepranensis) when it was thought to be contemporaneous with the fossils from the Gran Dolina (57). It is now considered to be a possible contemporary of the Sima de los Huesos population (58).
Let me be clear. There is no fossil in the Sima de los Huesos that could be confused with Ceprano. The same could be said for Broken Hill, Arago, or Mauer. Others, including Swanscombe, Reilingen, Steinheim, and Petralona are more similar, but not the same. Reilingen, for example, already shows an “en bombe” profile (59) and the Petralona extraordinary sinuses in the face and the supraorbital torus are out of the Sima de los Huesos range of variation. It is my impression that if these other sites had yielded more fossils, they would be essentially the same as those already known (i.e., there would be more remains of “cepranensis,” “petralonensis,” and “swanscombensis,” etc.) as occurred in the Sima de los Huesos and happens in any living human population, even across the entire species. If this is correct, and we may know when there are additional samples discovered, we would have other “entities” like the Sima de los Huesos. What taxonomic category should these hypothetical entities be given? Relying on the criterion of inter- vs. intrapopulation variation, they should be given that of species. If that of demes (subspecies) is preferred, it should be borne in mind that they would be demes of a strongly polytypic species, much more so than modern humans and perhaps more so than any of the extant hominoid species.
If we also have a fine chronological control for these entities, it would be possible to establish whether the evolutionary pattern that led to Neanderthals and modern humans was characterized by anagenesis or by successive speciation events. On the basis of the current state of our knowledge, reducing the human variability in Europe and Africa from the late Early Pleistocene to the middle Middle Pleistocene to a single species seems to be an exaggerated simplification.
Acknowledgments
I am grateful to Francisco J. Ayala and John C. Avise for inviting me to participate in the A. M. Sackler Colloquium “In the Light of Evolution IV: The Human Condition”; to Rolf Quam for the English translation and valuable comments; to Ignacio Martínez, Ana Gracia, and Alejandro Bonmatí for suggestions and criticism; to Américo Cerqueira for the drawing of Fig. 1; and to Javier Trueba for providing pictures of fossils. The Atapuerca field work is supported by Junta de Castilla y León and Fundación Atapuerca and the Atapuerca Research Project by Ministerio de Ciencia y Tecnología, Spanish Government CGL2006-13532-C03-02.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, "In the Light of Evolution IV: The Human Condition," held December 10–12, 2009, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/SACKLER_Human_Condition.
References
- 1.Darwin C. The Descent of Man and Selection in Relation to Sex. London: John Murray; 1871. [Google Scholar]
- 2.Menez A, editor. Human Evolution. 150 Years After Darwin. Gibraltar Museum, Gibraltar: Calpe Conference; 2009. pp. 9–16. [Google Scholar]
- 3.Darwin C. On the Origin of Species. London: John Murray; 1859, 1866. [Google Scholar]
- 4.Huxley TH. Man’s Place in Nature. Ann Arbor, MI: Univ of Michigan Press; 1959. [Google Scholar]
- 5.Eldredge N, Cracraft J. Phylogenetic Patterns and the Evolutionary Process. New York: Columbia Univ Press; 1980. [Google Scholar]
- 6.Hennig W. Phylogenetic Systematics. Champaign, IL: Univ of Illinois Press; 1966. [Google Scholar]
- 7.Le Gros Clark, WE . The Antecedents of Man. Edinburgh: Edinburgh Univ Press; 1959. [Google Scholar]
- 8.Klinger PH, Hamerton JL, Mutton D, Lang EM. In: Classification and Human Evolution. Washburn SL, editor. Vol. 37. Chicago: Viking Fund Publications in Anthropology; 1963. pp. 235–242. [Google Scholar]
- 9.Goodman M. In: Classification and Human Evolution. Washburn SL, editor. Vol. 37. Chicago: Viking Fund Publications in Anthropology; 1963. pp. 204–234. [Google Scholar]
- 10.Zuckerkandl E. In: Classification and Human Evolution. Washburn SL, editor. Vol. 37. Chicago: Viking Fund Publications in Anthropology; 1963. pp. 243–272. [Google Scholar]
- 11.Goodman M. Immunochemistry of the primates and primate evolution. Ann N Y Acad Sci. 1962;102:219–234. doi: 10.1111/j.1749-6632.1962.tb13641.x. [DOI] [PubMed] [Google Scholar]
- 12.Schultz A. In: Classification and Human Evolution. Washburn SL, editor. Vol. 37. Chicago: Viking Fund Publications in Anthropology; 1963. pp. 85–115. [Google Scholar]
- 13.Simpson GG. In: Classification and Human Evolution. Washburn SL, editor. Vol. 37. Chicago: Viking Fund Publications in Anthropology; 1963. pp. 1–31. [Google Scholar]
- 14.Simpson GG. Tempo and Mode in Evolution (A Columbia Classic in Evolution) New York: Columbia Univ Press; 1984. [Google Scholar]
- 15.Simpson GG. The Major Features of Evolution. New York: Columbia Univ Press; 1953. [Google Scholar]
- 16.Simpson GG. Cold Spring Harbor Symposia on Quantitative Biology XV. New York: The Biological Laboratory; 1950. pp. 55–66. [DOI] [PubMed] [Google Scholar]
- 17.Howells WW. Cold Spring Harbor Symposia on Quantitative Biology XV. New York: The Biological Laboratory; 1950. pp. 79–86. [DOI] [PubMed] [Google Scholar]
- 18.Washburn SL. Cold Spring Harbor Symposia on Quantitative Biology XV. New York: The Biological Laboratory; 1950. pp. 67–78. [DOI] [PubMed] [Google Scholar]
- 19.Brunet M, et al. A new hominid from the Upper Miocene of Chad, Central Africa. Nature. 2002;418:145–151. doi: 10.1038/nature00879. [DOI] [PubMed] [Google Scholar]
- 20.Zollikofer CPE, et al. Virtual cranial reconstruction of Sahelanthropus tchadensis. Nature. 2005;434:755–759. doi: 10.1038/nature03397. [DOI] [PubMed] [Google Scholar]
- 21.Senut B, et al. First hominid from the Miocene (Lukeino Formation, Kenya) C R Acad Sci Paris, Sciences de la Terre et des Planètes. 2001;332:137–144. [Google Scholar]
- 22.Pickford M, Senut B, Gommery D, Treil J. Bipedalism in Orrorin tugenensis revealed by its femora. C R Palevol. 2002;4:191–203. [Google Scholar]
- 23.White T, et al. Ardipithecus ramidus and the Paleobiology of Early Hominids. Science. 2009;326:75–86. [PubMed] [Google Scholar]
- 24.Lovejoy CO, Suwa G, Spurlock L, Asfaw B, White TD. The pelvis and femur of Ardipithecus ramidus: The emergence of upright walking. Science. 2009;326:71e1–71e6. [PubMed] [Google Scholar]
- 25.Tattersall I. Paleoanthropology: The last half century. Evol Anthropol. 2000;9:2–16. [Google Scholar]
- 26.Dobzhansky T. Mankind Evolving. The Evolution of the Human Species. New Haven, CT: Yale Univ Press; 1975. 17th printing. [Google Scholar]
- 27.Carretero JM, et al. In: Homage to Emiliano Aguirre. Paleoanthropology. Baquedano E, Rubio S, editors. Spain: Museo Arqueológico Regional de la Comunidad de Madrid, Alcalá de Henares; 2004. pp. 120–135. [Google Scholar]
- 28.Brown P, et al. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature. 2004;431:1087–1091. doi: 10.1038/nature02999. [DOI] [PubMed] [Google Scholar]
- 29.Pearson OM, Royer DF, Grine FE, Fleagle JG. A description of the Omo I postcranial skeleton, inlcuidng newly discovered fossils. J Hum Evol. 2008;55:421–437. doi: 10.1016/j.jhevol.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 30.White T, et al. Pleistocene Homo sapiens from Middle Awash, Ethiopia. Nature. 2003;423:742–747. doi: 10.1038/nature01669. [DOI] [PubMed] [Google Scholar]
- 31.Lordkipanidze D, et al. The earliest toothless hominin skull. Nature. 2005;434:717–718. doi: 10.1038/434717b. [DOI] [PubMed] [Google Scholar]
- 32.Lordkipanidze D, et al. Postcranial evidence from early Homo from Dmanisi, Georgia. Nature. 2007;449:305–310. doi: 10.1038/nature06134. [DOI] [PubMed] [Google Scholar]
- 33.Rightmire GP, Lordkipanidze D, Vekua A. Anatomical descriptions, comparative studies and evolutionary significance of the hominin skulls from Dmanisi, Republic of Georgia. J Hum Evol. 2006;50:115–141. doi: 10.1016/j.jhevol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg K, Zuné L, Ruff CB. Body size, body proportions, and encephalization in a Middle Pleistocene archaic human from northern China. Proc Natl Acad Sci USA. 2006;103:3552–3556. doi: 10.1073/pnas.0508681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson SW, et al. A female Homo erectus pelvis from Gona, Ethiopia. Science. 2008;322:1089–1091. doi: 10.1126/science.1163592. [DOI] [PubMed] [Google Scholar]
- 36.Ruff C. Body size and body shape in early hominins—implications of the Gona pelvis. J Hum Evol. 2009;58:166–178. doi: 10.1016/j.jhevol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Bruner E, Manzi G, Arsuaga JL. Encephalization and allometric trajectories in the genus Homo: Evidence from the Neandertal and modern lineages. Proc Natl Acad Sci USA. 2003;100:15335–15340. doi: 10.1073/pnas.2536671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arsuaga JL, Martínez I. The origin of mind. Investig Cienc. 2001;302:4–12. [Google Scholar]
- 39.Grün R, et al. ESR anaylisis of teeth from the paleoanthropological site of Zhoukoudian, China. J Hum Evol. 1997;32:83–91. doi: 10.1006/jhev.1996.0094. [DOI] [PubMed] [Google Scholar]
- 40.Shen G, Gao X, Gao B, Granger DE. Age of Zhoukoudian Homo erectus determined with 26AL/10Be burial dating. Nature. 2009;458:198–200. doi: 10.1038/nature07741. [DOI] [PubMed] [Google Scholar]
- 41.Gould SJ, Eldedge N. Punctuated equilibrium comes of age. Nature. 1993;366:223–227. doi: 10.1038/366223a0. [DOI] [PubMed] [Google Scholar]
- 42.Ubelaker DH, Ross AH, Graver SM. Application of forensic discriminant functions to a Spanish cranial sample. Forensic Sci Commun. 2002;4:1–6. [Google Scholar]
- 43.Howells WW. Skull Shapes and the Map. Vol. 79. Cambridge, MA: Papers of the Peabody Museum of Archaeology and Ethnology, Harvard University; 1989. [Google Scholar]
- 44.Stringer CB, editor. The Great Ideas Today. Chicago: Encyclopedia Britannica; 1992. pp. 42–94. [Google Scholar]
- 45.Harvati K. 100 years of Homo heidelbergensis—life and times of a controversial taxon. Mitt Gesselschaft Ungersichte. 2007;16:85–94. [Google Scholar]
- 46.Hublin JJ. The origin of Neandertals. Proc Natl Acad Sci USA. 2009;106:16022–16027. doi: 10.1073/pnas.0904119106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger GW, et al. Luminiscence chronology of cave sediments at the Atapuerca paleoanthropological site, Spain. J Hum Evol. 2008;55:300–311. doi: 10.1016/j.jhevol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Bermúdez de Castro JM, et al. A hominid from the lower Pleistocene of Atapuerca, Spain: Possible ancestor to Neandertals and modern humans. Science. 1997;276:1392–1395. doi: 10.1126/science.276.5317.1392. [DOI] [PubMed] [Google Scholar]
- 49.Hublin JJ. Northwestern African Middle Pleistocene hominids and their bearing on the emergence of Homo sapiens. In: Barham L, Robson-Brown K, editors. Human Roots. Africa and Asia in the Middle Pleistocene. Bristol, UK: Western Academic & Specialist Press; 2001. pp. 99–121. [Google Scholar]
- 50.Bermúdez de Castro JM, Martinón-Torres M, Gómez-Robles A, Prado L, Sarmiento S. Bull Mem Soc Anthropol Paris. 2007;19:149–167. [Google Scholar]
- 51.Carbonell E, et al. The first hominin of Europe. Nature. 2008;452:465–470. doi: 10.1038/nature06815. [DOI] [PubMed] [Google Scholar]
- 52.Arsuaga JL, Martínez I, Gracia A, Carretero JM, Carbonell E. Three new human skulls from the Sima de los Huesos Middle Pleistocene site in Sierra de Atapuerca, Spain. Nature. 1993;362:534–536. doi: 10.1038/362534a0. [DOI] [PubMed] [Google Scholar]
- 53.Arsuaga JL, et al. A complete human pelvis from the Middle Pleistocene of Spain. Nature. 1999;399:255–258. doi: 10.1038/20430. [DOI] [PubMed] [Google Scholar]
- 54.Bischoff JL, et al. High-resolution U-series dates from the Sima de los Huesos hominids yields 600+-66 kyrs: Implications for the evolution of the early Neanderthal lineage. J Archaeol Sci. 2007;33:763–770. [Google Scholar]
- 55.Arsuaga JL, Martínez I, Gracia A, Lorenzo C. The Sima de los Huesos crania (Sierra de Atapuerca, Spain). A comparative study. J Hum Evol. 1997;33:219–281. doi: 10.1006/jhev.1997.0133. [DOI] [PubMed] [Google Scholar]
- 56.Martínez A, Arsuaga JL. The temporal bones from Sima de los Huesos Middle Pleistocene site (Sierra de Atapuerca, Spain). A phylogenetic approach. J Hum Evol. 1997;33:283–318. doi: 10.1006/jhev.1997.0155. [DOI] [PubMed] [Google Scholar]
- 57.Mallegni F, et al. Homo cepranensis sp. nov. and the evolution of African-European Middle Pleistocene hominids. C R Palevol. 2003;2:153–159. [Google Scholar]
- 58.Muttoni G, et al. Pleistocene magnetochronology of early hominin sites at Ceprano and Fontana Ranuccio, Italy. Earth Planet Sci Lett. 2009;286:255–268. [Google Scholar]
- 59.Schwartz J, Tattersall I. The Human Fossil Record. Vol. 1. New York: Wiley-Lyss; 2002. [Google Scholar]